Abstract
Acinetobacter baumannii is a rare but emerging cause of fulminant community-acquired pneumonia (CAP-AB). We describe a patient from a rural area who developed acute respiratory distress syndrome and septic shock. We describe risk factors and characteristics of this syndrome and review published cases of CAP-AB from North America.
Keywords: Acinetobacter baumannii, community-acquired pneumonia
CASE PRESENTATION
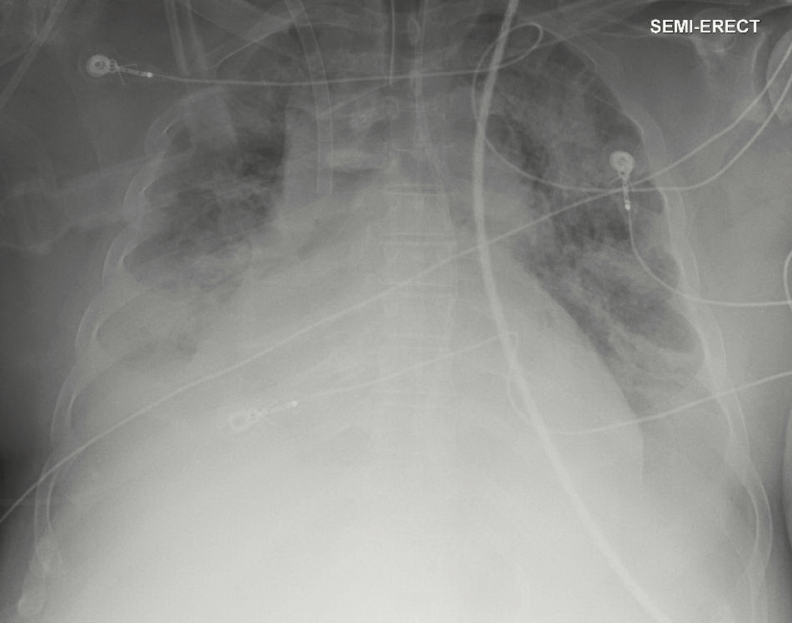
A 41-year-old man with severe alcohol use disorder was admitted to a hospital in Alabama in January with 2 days of productive cough, shortness of breath, and fever. He presented with septic shock, hypoxemic respiratory failure, and bilateral pulmonary infiltrates (Figure 1). Laboratory results were notable for neutropenia (absolute neutrophil count 450 cells/mcL), thrombocytopenia (58 000 cells/mcL), and acute kidney injury (creatinine 1.9 mg/dl). He had a history of alcohol use disorder with prior alcohol withdrawal seizures. His level of recent alcohol consumption was unknown, but he did not have evidence of alcohol withdrawal during the hospitalization. He lived with his father in rural Alabama and was unemployed. He smoked approximately 5 cigarettes per day and did not use illicit drugs. He had no travel outside the eastern United States, no recent health care exposures, and no sick contacts.
Figure 1.
Anterior–posterior chest x-ray showing bilateral infiltrates consistent with multifocal pneumonia.
He was started on vancomycin, piperacillin-tazobactam, levofloxacin, and oseltamivir. Hypoxemia progressed rapidly, and within the first 24 hours of hospitalization he was intubated with high ventilatory support requirements (FiO2 of 100% and positive-end expiratory pressure of 18 cm of water) despite paralysis with cisatracurium. Blood cultures from admission grew Gram-negative bacilli in both sets in the aerobic bottles after 12 hours of incubation. The organism was identified as Acinetobacter baumannii by matrix-assisted laser desorption ionization time of flight mass spectroscopy (MALDI-TOF). Drug resistance testing, performed by an automated biochemical testing system (MicroScan Walkaway, Beckman Coulter, Inc. Brea, CA), showed sensitivity to all antimicrobials tested, including ceftazidime, levofloxacin, ampicillin-sulbactam, and meropenem.
The patient was transferred to our institution for initiation of venovenous extracorporeal membrane oxygenation (ECMO). On arrival, he required norepinephrine and vasopressin for blood pressure support, and antimicrobials were changed to intravenous meropenem and levofloxacin. Bronchoalveolar lavage showed many Gram-negative coccobacilli on Gram stain with growth of A. baumannii in culture with similar sensitivity to the previously obtained blood cultures. Antimicrobials were changed to intravenous ampicillin-sulbactam and levofloxacin, and he completed a 14-day course of therapy. His ECMO was discontinued after 9 days of therapy. He continued to require ventilatory support, necessitating tracheostomy, and was transferred to a subacute rehabilitation facility with eventual recovery.
DISCUSSION
Acinetobacter baumannii is an aerobic, oxidase-negative nonfermenting Gram-negative coccobacillus most often associated with hospital-acquired infections, particularly ventilator-associated pneumonia (VAP). Hospital-acquired A. baumannii is associated with extended length of hospital stay and high mortality [1]. However, during the last 25 years, there has been a growing body of literature describing severe community-acquired pneumonia due to A. baumannii (CAP-AB) in patients without health care exposure or classic risk factors for this organism [2]. The majority of these cases come from Northern Australia and Asia, including Thailand, China, Taiwan, and are more common in tropical and subtropical areas during the summer months. Throat and skin carriage of Acinetobacter has been identified in areas of endemicity, and soil, livestock, and other animals have also been shown to serve as community reservoirs for Acinetobacter [3]. Based on pulse-field gel electrophoresis analyses, community-acquired isolates represent a distinct lineage from health care–associated Acinetobacter infections and often do not harbor the same resistance mechanisms [3]. Although less drug-resistant than hospital-acquired A. baumannii bacteremia, community-acquired infection has been associated with increased mortality (odds ratio, 5.72; 95% confidence interval, 1.02–32.00) [4].
Epidemiologic studies have linked the syndrome of severe CAP-AB with alcohol use disorder and recent alcohol binges prior to symptom onset [5]. Animal studies have shown that alcohol has wide-ranging effects on the innate immune system in relation to pulmonary Acinetobacter infection [6–8]. Gandhi et al. compared ethanol-exposed mice with unexposed mice after pulmonary inoculation of Acinetobacter [6]. They showed that ethanol-exposed mice demonstrated decreased neutrophil- mediated phagocytosis, increased lung inflammation, and higher mortality. Asplund et al. similarly showed decreased alveolar macrophage phagocytosis of Acinetobacter in the presence of alcohol [7]. Other significant risk factors include tobacco use, chronic pulmonary disease, and diabetes mellitus [2]. Onset of symptoms is typically rapid, with fulminant disease developing over 48–72 hours. Bilateral infiltrates, ARDS, leukopenia, and bacteremia are all common. A right-lung predominance has been noted, which likely implicates an element of aspiration to the pathogenesis of this condition [9]. Taking into account characteristics from multiple series, a distinct clinical syndrome of CAP-AB emerges (Box 1).
BOX 1. Characteristics Of The Cap-Ab Syndrome
| Rapid onset of symptoms with fulminant disease |
| Alcohol use disorder, especially binge episode |
| Leukopenia |
| Middle-aged men |
| Tobacco use |
| Right > left lung infiltrates |
| Warm moist environments |
To date, 19 cases of CAP-AB have been reported in North America (Table 1). While most cases of CAP-AB in Southeast Asia and Australia have been reported since the late 1990s, most North American cases were published between 1959 and 1981, with the most recent report from 1999 [10–19]. Overall, these North American cases conform to the typical presentation of the “CAP-AB syndrome” described outside of North America. Most patients were middle-aged (median age, 54 years), male (15/19), and reported a history of alcohol use (10/19). All but two patients presented with a rapid-onset of illness with ≤3 days of symptoms and fulminant disease. Eleven of 15 patients with reported information on respiratory support required mechanical ventilation. Our patient was the only case with the use of ECMO for cardiopulmonary support. Mortality was high (42%), although many of the cases occurred prior to the advent of modern diagnostic and therapeutic advances, which is exemplified by the frequent use of aminoglycosides as definitive therapy (13/19 cases).
Table 1.
Summary of North American Cases of Community-Acquired Pneumonia due to Acinetobacter baumannii
| Year (Reference) | Location | Age | Sex | Risk Factors | Mech. Vent. | Site of Positive Cultures | Final Antibiotics Used | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1959 [10] | Chicago, IL | 50 | M | None | No | Blood and sputum | Chloramphenicol and oxytetracycline | Survived |
| 1968 [11] | Chapel Hill, NC | 49 | M | Alcohol | No | Blood and lung tissue (autopsy) | Penicillin G | Died |
| 1973 [12] | Baltimore, MD | 69 | M | CKD | Yes | Tracheal aspirate and pleural fluid | Tetracycline | Died |
| 1976 [13] | Houston, TX | 50 | M | Alcohol, tobacco | Yes | Blood and tracheal aspirate | Gentamicin and carbenicillin | Survived |
| 1977 [14] | Philadelphia, PA | 33 | F | Alcohol, tobacco | No | Sputum | Gentamicin and cephalothin | Died |
| 58 | M | Alcohol, cirrhosis, tobacco | Yes | Sputum | Gentamicin | Survived | ||
| 1979 [15] | Dallas, TX | 58 | M | Alcohol, asthma | ? | Blood | Clindamycin and gentamicin | Died |
| 41 | M | Alcohol, pancreatitis, hepatitis | Yes | Blood and tracheal aspirate | Penicillin, gentamicin, and carbenicillin | Survived | ||
| 35 | F | Alcohol | Yes | Blood and tracheal aspirate | Penicillin and gentamicin | Died | ||
| 51 | M | Lymphoma | ? | Blood and sputum | Gentamicin and carbenicillin | Survived | ||
| 74 | F | None | ? | Blood and sputum | Penicillin and gentamicin | Survived | ||
| 44 | M | Alcohol, chronic bronchitis | ? | Blood and sputum | Penicillin, gentamicin, and carbenicillin | Survived | ||
| 1981 [16] | Hartford, CT | 54 | M | Pneumoconiosis | Yes | Blood and sputum | Penicillin G | Died |
| 63 | M | Alcohol, tobacco | Yes | Blood and sputum | Gentamicin and carbenicillin | Survived | ||
| 56 | M | Pneumoconiosis | Yes | Blood and sputum | Ticarcillin and tobramycin | Died | ||
| 1987 [17] | San Antonio, TX | 56 | M | Tobacco | Yes | Sputum | Gentamicin and clindamycin | Died |
| 1993 [18] | Chicago, IL | 74 | F | None | Yes | Blood | Gentamicin, ticarcillin-clavulanate, and erythromycin | Survived |
| 1999 [19] | Tampa, FL | 80 | M | None | No | BAL | Trimethoprim- sulfamethoxazole | Survived |
| 2017 | Atlanta, GA | 41 | M | Alcohol | Yes | Blood and BAL | Ampicillin-sulbactam and levofloxacin | Survived |
Abbreviations: ?, unreported data; BAL, bronchoalveolar lavage; CKD, chronic kidney disease; F, female; M, male.
It is possible that CAP-AB in North America occurs more frequently but is underreported because identification of Acinetobacter is not noteworthy outside of the community-acquired context. It is also possible that climate plays a role in identification of only a small number of cases in the northern hemisphere, as the majority of cases have been identified in tropical locations [2]. Studies have demonstrated that even health care–associated cases may have some seasonal variation. For example, when surveillance data on Acinetobacter infections were collected at Yale–New Haven Hospital from 1990–1992, the incidence during the summer months was more than double the incidence during the remainder of the year [20]. The fact that our patient became critically ill during the winter is therefore unusual, although multiple cities in Alabama documented record high average temperatures throughout the year of this patient’s illness [21]. The etiology of this association of cases with warmer temperatures remains unknown and is a potential area for further study, especially as it suggests that climate change could ultimately influence disease prevalence.
CONCLUSION
This case is a representative example of the CAP-AB syndrome in a patient in the Southeast United States in 2017. While much focus is appropriately directed toward multidrug-resistant nosocomial Acinetobacter infections, we present this case to raise awareness of the presence of CAP-AB and document its contemporary existence outside of its typical area of endemicity. Previous reported cases in North America have been sporadic and infrequent since the 1950s, but globalization, climate change, and increasing prevalence of alcohol use disorder [22] could lead to emergence of this syndrome in the United States.
Acknowledgments
Financial support. This work is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR002378 and TL1TR002382, both D.P.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Falagas ME, Rafailidis PI. Attributable mortality of Acinetobacter baumannii: no longer a controversial issue. Crit Care 2007; 11:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dexter C, Murray GL, Paulsen IT, Peleg AY. Community-acquired Acinetobacter baumannii: clinical characteristics, epidemiology and pathogenesis. Expert Rev Anti Infect Ther 2015; 13:567–73. [DOI] [PubMed] [Google Scholar]
- 3. Eveillard M, Kempf M, Belmonte O et al. Reservoirs of Acinetobacter baumannii outside the hospital and potential involvement in emerging human community-acquired infections. Int J Infect Dis 2013; 17:e802–5. [DOI] [PubMed] [Google Scholar]
- 4. Chen CT, Wang YC, Kuo SC et al. Community-acquired bloodstream infections caused by Acinetobacter baumannii: a matched case-control study. J Microbiol Immunol Infect 2017; doi: 10.1016/j.jmii.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 5. Davis JS, McMillan M, Swaminathan A et al. A 16-year prospective study of community-onset bacteremic Acinetobacter pneumonia: low mortality with appropriate initial empirical antibiotic protocols. Chest 2014; 146:1038–45. [DOI] [PubMed] [Google Scholar]
- 6. Gandhi JA, Ekhar VV, Asplund MB et al. Alcohol enhances Acinetobacter baumannii-associated pneumonia and systemic dissemination by impairing neutrophil antimicrobial activity in a murine model of infection. PLoS One 2014; 9:e95707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asplund MB, Coelho C, Cordero RJ, Martinez LR. Alcohol impairs J774.16 macrophage-like cell antimicrobial functions in Acinetobacter baumannii infection. Virulence 2013; 4:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eugenin EA. Community-acquired pneumonia infections by Acinetobacter baumannii: how does alcohol impact the antimicrobial functions of macrophages?Virulence 2013; 4:435–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patamatamkul S, Klungboonkrong V, Praisarnti P, Jirakiat K. A case-control study of community-acquired Acinetobacter baumannii pneumonia and melioidosis pneumonia in northeast Thailand: an emerging fatal disease with unique clinical features. Diagn Microbiol Infect Dis 2017; 87:79–86. [DOI] [PubMed] [Google Scholar]
- 10. Glick LM, Moran GP, Coleman JM, O’Brien GF. Lobar pneumonia with bacteremia caused by Bacterium anitratum. Am J Med 1959; 27:183–6. [DOI] [PubMed] [Google Scholar]
- 11. Hammett JB. Death from pneumonia with bacteremia due to mimeae tribe bacterium. JAMA 1968; 206:641–2. [PubMed] [Google Scholar]
- 12. Wands JR, Mann RB, Jackson D, Butler T. Fatal community-acquired Herellea pneumonia in chronic renal disease. Case report. Am Rev Respir Dis 1973; 108:964–7. [DOI] [PubMed] [Google Scholar]
- 13. Wallace RJ Jr, Awe RJ, Martin RR. Bacteremic Acinetobacter herellea pneumonia with survival: case report. Am Rev Respir Dis 1976; 113:695–9. [DOI] [PubMed] [Google Scholar]
- 14. Goodhart GL, Abrutyn E, Watson R et al. Community-acquired Acinetobacter calcoaceticus var anitratus pneumonia. JAMA 1977; 238:1516–8. [PubMed] [Google Scholar]
- 15. Rudin ML, Michael JR, Huxley EJ. Community-acquired Acinetobacter pneumonia. Am J Med 1979; 67:39–43. [DOI] [PubMed] [Google Scholar]
- 16. Cordes LG, Brink EW, Checko PJ et al. A cluster of Acinetobacter pneumonia in foundry workers. Ann Intern Med 1981; 95:688–93. [DOI] [PubMed] [Google Scholar]
- 17. Suchyta MR, Peters JI, Black RD. Chronic Acinetobacter calcoaceticus var anitratus pneumonia. Am J Med Sci 1987; 294:117–9. [DOI] [PubMed] [Google Scholar]
- 18. Bick JA, Semel JD. Fulminant community-acquired Acinetobacter pneumonia in a healthy woman. Clin Infect Dis 1993; 17:820–1. [DOI] [PubMed] [Google Scholar]
- 19. Carter JD, Cutolo EP, Behnke RH, Adelman HM. Pulmonary infiltrates in an elderly man. Hosp Pract (1995) 1999; 34:21–2, 4. [DOI] [PubMed] [Google Scholar]
- 20. Christie C, Mazon D, Hierholzer W Jr, Patterson JE. Molecular heterogeneity of Acinetobacter baumanii isolates during seasonal increase in prevalence. Infect Control Hosp Epidemiol 1995; 16:590–4. [DOI] [PubMed] [Google Scholar]
- 21. Christy J. The Alabama climate report 2017 2017; 8(10). Available at: https://www.nsstc.uah.edu/alclimatereport/. Accessed January 15, 2018. [Google Scholar]
- 22. Grant BF, Chou SP, Saha TD et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry 2017; 74:911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]