Abstract
Purpose
Prostate cancer (PCa) patients often have dendritic cell (DC) function defects, but the mechanism is not clear. The aim of this study was to detect the effect of vascular endothelial growth factor (VEGF) in mature DCs.
Patients and methods
In this study, we chose 30 PCa patients, 10 prostatic intraepithelial neoplasia (PIN) patients and 30 benign prostatic hyperplasia (BPH) patients, and compared the composition of peripheral blood T cells, the composition and function of local dendritic cells in prostate tissue, and the density of local VEGF.
Results
The results showed that the numbers of total DCs, mature and functional DCs, and CD4+ T cells were inhibited in PCa, and the inhibitory effect was enhanced with increased malignancy. In addition, the infiltration density of VEGF-positive cells was increased in PCa, and this increase was associated with an increased malignant degree of PCa. The inhibition of tumor immunity in patients with PCa is achieved by inhibiting the function of dendritic cells.
Conclusion
VEGF plays an important role in the inhibition of the maturation and function of dendritic cells, and this inhibition is gradually increased with an increasing malignant degree of PCa.
Keywords: prostate cancer, vascular endothelial growth factor, dendritic cell, immunology, T cell
Introduction
Prostate cancer (PCa) is the most frequently diagnosed cancer and the second leading cause of cancer death in males in the United States, accounting for 27% (233,000) of total new cancer cases and 10% (29,480) of total cancer deaths in males in 2014.1 Beyond traditional surgery, radiotherapy and hormone deprivation methods, many studies focus on dendritic cell-based immunotherapy since it is a novel treatment for advanced PCa.2 Dendritic cells (DCs) are the most important antigen-presenting cell population for regulating innate and adaptive immune responses. They are required to convert naïve resting T cells into potent tumor-reactive cytotoxic CD8+ and helper CD4+ T cells. The activation of DCs can effectively stimulate B lymphocytes to produce a specific antibody – T cell transformation into cytotoxic T cells produces an effective immune response,3 and plays an important role in tumor immunity. The immune response of DCs is mainly mediated by CD8+ and CD4+ T cells. The secretion of various cytokines produced by CD4+ cells, and a part of them influence CD8+ cells differentiation development to CD8+ cytotoxic T cells, and by CD8+ cytotoxic T cells, leads to the activation of the perforin/pathway and Fas/FasL pathways and the killing of target cells. DCs also play a major role in the immune memory, which can stimulate the formation of T cell differentiation and expansion to form antigen-specific killer T cells.4
Early theories did not consider the prostate to be an organ monitored by the body’s immune system,5 but nearly 7 years of research has overthrown this viewpoint, suggesting that the treatment of PCa may potentially work by activating the immune system.6 Therefore, increasing attention to the clinical treatment of PCa, especially in late androgen-independent or distant metastasis, focuses on activating the human immune system to treat PCa. Sipuleucel-T was approved by the United States Food and Drug Administration for PCa therapy in 2010.7
Previous studies have demonstrated that the quantity and activity of DCs in PCa patients are significantly lower than in healthy controls,8 and in vitro experiments verified that PCa cells induce apoptosis, as well as suppress the regeneration and differentiation of DCs.9 Studies also showed that the numbers of DCs are reduced in PCa tissues compared to prostatic hyperplasia (BPH) tissues, and the levels of infiltrating DCs are further decreased with the advancement of atypia.10 Current immunotherapies are confined to relieve the symptoms in advanced PCa patients and cannot cure the disease, and the immunosuppressive tumor microenvironment may limit their effectiveness.11 Therefore, it is necessary to determine the mechanism of immune inhibition to overcome the immune restriction and improve the efficacy of immunotherapy.
Vascular endothelial growth factor (VEGF), which induces neoangiogenesis and the blockade of angiogenesis, plays an important role in the development and metastasis of solid tumors, and targeting VEGF has become a useful approach in cancer therapy.12 The inhibition of VEGF in a mouse model leads to increased antigen uptake and the migration of tumor-associated DCs.13 In the human tongue carcinoma, cancer cells could be inhibited in proliferation and be increased in apoptosis by knocking down the expression of VEGF, and maturation of DCs also could be improved.14 VEGF also influences the effect of DCs on therapy in the model of colon cancer-induced malignant ascites.15 VEGF expression in the peripheral blood and neoplasm nest from patients with oral squamous cell carcinoma (OSCC) was positively correlated with the course of the disease, while an inverse correlation between VEGF expression and DCs was identified in the peripheral blood.14 Some clinical research has been carried out that blocks VEGF to improve the antitumor responses in treating PCa patients.16 PCa is divided into three categories, which fit the following standard definitions: low-risk PCa (biopsy Gleason grade < 7 or prostate-specific antigen (PSA) < 10 ng/mL), intermediate-risk PCa (biopsy Gleason score 7 or PSA 10–20 ng/mL), and high-risk PCa (biopsy Gleason score > 7 or PSA > 20 ng/mL).17–20 Determining the status of DC and VEGF expression in different degrees of PCa patients is needed.
In this study, we compared the composition and functional status of DCs in prostate tissues of patients with BPH, prostatic intraepithelial neoplasia (PIN) and PCa and compared the expression of VEGF in these prostate tissues. We also compared the changes of T-lymphocyte CD4+ T cells, CD8+ T cells, and the CD4+/CD8+ ratio in the BPH, PIN and PCa groups. We examined the DCs in the PCa microenvironment, the T lymphocytes in peripheral blood, and the association of VEGF expression with Gleason scores and PSA levels in PCa patients. In addition, we studied the correlation between S100 expression, VEGF and CD208 in PCa patients.
Materials and methods
This study obtained permission from the ethics committee of the Shanghai Jiao Tong University Affiliated 6th People’s Hospital and the Shanghai Institute of Ultrasound in Medicine, and written informed consent was obtained from each patient. Mice blood was used in this research and permission was also obtained from the ethics committee of the Shanghai Jiao Tong University Affiliated 6th People’s Hospital and the Shanghai Institute of Ultrasound in Medicine. All experiments were performed following relevant Shanghai Jiao Tong University Affiliated 6th People’s Hospital and national guidelines and regulations.
Patients
An ultrasound-guided 10- to 12-core transrectal systematic prostate biopsy was obtained from all patients based on the European Association of Urology (EAU) guideline suggestions. The patients had no history of blood transfusions, no acute or chronic infectious diseases, and they did not use any immunomodulatory drugs or treatment, receive radiation therapy, or have autoimmune diseases. The peripheral blood levels of PSA, CD4+ T cells, and CD8+ T cells, as well as the CD4+/CD8+ ratio, were measured before the procedure.
Groups
The patients were divided into three groups according to their pathological results: BPH, PIN and PCa. The PCa patients were divided into three groups according to their PSA levels: group A, PSA < 10 ng/mL; group B, PSA 10–20 ng/mL; and group C, PSA > 20 ng/mL.
PCa patients were also stratified according to Gleason score: group D, Gleason grade < 7; group E, Gleason grade 7; and group F, Gleason grade > 7.
Phenotypic analysis of CD4+, CD8+, and CD4/CD8 by flow cytometry
Fresh peripheral venous blood was collected from all patients 1 day before biopsy. Anticoagulant (100 μL) was added to each of the two test tubes containing either 20 μL CD4-FITC (BD, Franklin Lakes, NJ, USA) or 20 μL CD8-PE (BD) and mixed evenly. Additionally, a test tube with blood from Control mice and IgG1-FITC (BD) or IgG1-PE (BD) monoclonal antibodies (20 μL) was also mixed. All test tubes were incubated at room temperature after mixing for 20 min; then, 2 mL of red cell lysate (BD Biosciences, San Jose, CA, USA) was added. After incubation at room temperature for 10 min, samples were centrifuged at 12,000 rpm for 5 min, the supernatant was discarded, and 2 mL of phosphate buffer pH 7.4 (0.05% PBS) was added. The samples were mixed well and washed 1–2 times. Cells were resuspended in 500 μL of PBS, and immediately tested by flow cytometry. We gated for small lymphocytes (R1 gate) based on the forward scatter and side scatter of the cells. Mouse IgG1-FITC and IgG1-PE monoclonal antibodies were used as isotype-negative controls.
The data were acquired using a FACScan flow cytometer (BD) and analyzed with CellQuest software (BD).
PSA detection
Fasting venous blood (4 mL), which had been placed in sterile centrifuge tubes, was centrifuged at 3,000 rpm for 15 min, and collected sera were placed in a sterile tube. PSA detection was done using a Swiss Roche E601 automatic electrochemical luminescence. Test results were automatically determined based on the standard curve.
Immunohistochemistry
The composition and functional status of DCs and the expression of VEGF (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) in prostate tissues of patients were detected by immunohistochemistry. S100 (Santa Cruz Biotechnology, Inc.) is a specific marker of DCs, and CD208 (Santa Cruz Biotechnology, Inc.) is a specific marker for mature and functional DCs.21–23 Routine formalin-fixed paraffin-embedded 3–4-μm-thick sections from each patient were attached to silanized slides, sequentially deparaffinized and rehydrated. Access to tissue antigen sites for antibody attachment was enhanced by microwaving slides, which were treated with citrate buffer for 20 min. The primary antibody was diluted 1:100 in Tris buffered saline (TBS)/1% BSA/1% human serum and incubated for 30 min. The EnVision technique and development with the chromogen 3,3′-diaminobenzidine tetrachloride was used for visualization. Sections were lightly counterstained with hematoxylin.24 All slides were examined by two trained pathologists. Negative staining slices also were completed.
Brown granular staining in the cytoplasm or nucleus was defined as S100- and CD208-positive results. VEGF-positive cells showed brown granular staining in the cytoplasm.
Measurements of DC and VEGF-positive cell infiltration density are expressed as the mean ± standard deviation. The saturation density of DCs/VEGF-positive cells = 10 × the high power field of view of positive cells/2.5 mm2.
Statistical analysis
SPSS16.0 statistical software was used for statistical analyses. The data were subjected to single factor variance analysis. Further pairwise comparisons were conducted using the Student-Newman-Keuls test, and the correlation between VEGF and DCs was analyzed by correlation analysis. P-values < 0.05 were considered statistically significant.
Results
Age and PSAlevels of the three groups (Table 1)
Table 1.
Age and PSA levels in patients with different pathological types
| Group | Age (year) | PSA (ng/mL) |
|---|---|---|
| BPH | 67.47 ± 10.39 | 11.67 ± 12.70 |
| PIN | 69.50 ± 11.13 | 22.42 ± 12.32 |
| PCa | 67.97 ± 11.91 | 25.07 ± 26.07* |
Notes:
P = 0.010 vs BPH. Data are presented as mean ± standard deviation.
Abbreviations: PSA, prostate-specific antigen; BPH, benign prostatic hyperplasia; PIN, prostatic intraepithelial neoplasia; PCa, prostate cancer.
A total of 30 BPH patients, 10 PIN patients and 30 PCa patients were included in this study.
The PSA levels in patients with PCa and PIN were significantly different from those with BPH (P = 0.010). There was no significant difference in PSA levels between BPH and PIN patients or PCa and PIN patients (P > 0.05). No significant differences in age were seen between the three groups (P > 0.05).
Changes of peripheral blood CD4, CD8 and CD4/CD8 levels in three groups (Table 2)
Table 2.
Changes of serum CD4, CD8 and CD4/CD8 levels in patients with different pathological types
| Group | CD4 (%) | CD8 (%) | CD4/CD8 |
|---|---|---|---|
| BPH | 37.14 ± 5.44 | 26.67 ± 6.63 | 1.51 ± 0.54 |
| PIN | 34.48 ± 9.46 | 23.73 ± 6.29 | 1.57 ± 0.81 |
| PCa | 28.61 ± 8.00a,b | 23.30 ± 7.83 | 1.36 ± 0.60 |
Notes:
P = 0.000 vs BPH;
P = 0.030 vs PIN. Data are presented as mean ± standard deviation.
Abbreviations: BPH, benign prostatic hyperplasia; PIN, prostatic intraepithelial neoplasia; PCa, prostate cancer.
The expression level of CD4 in each group reduced with the malignant degree of disease, and there was a significant difference in CD4 levels between BPH and PCa patients (P = 0.000) and PCa and PIN patients (P = 0.030). There were no significant differences in the levels of CD8 and CD4/CD8 between groups (P > 0.05).
Differences in S100, CD208 and VEGF expression in biopsy tissues of each group (Table 3 and Figure 1)
Table 3.
Expression density changes of S100, CD208 and VEGF of three groups patients with local tissue biopsy
| Group | S100 | CD208 | VEGF |
|---|---|---|---|
| BPH | 26.97 ± 4.35 | 2.23 ± 0.68 | 60.21 ± 7.22 |
| PIN | 21.70 ± 2.38c | 1.60 ± 0.37e | 71.75 ± 4.37h |
| PCa | 13.20 ± 5.08a,b | 1.12 ± 0.58d | 83.77 ± 5.48f,g |
Notes:
P = 0.000 vs BPH;
P = 0.000 vs PIN;
P = 0.015 vs BPH;
P = 0.000 vs BPH;
P = 0.045 vs BPH;
P = 0.000 vs BPH;
P = 0.000 vs PIN;
P = 0.000 vs BPH. Data are presented as mean ± standard deviation.
Abbreviations: VEGF, vascular endothelial growth factor; BPH, benign prostatic hyperplasia; PIN, prostatic intraepithelial neoplasia; PCa, prostate cancer.
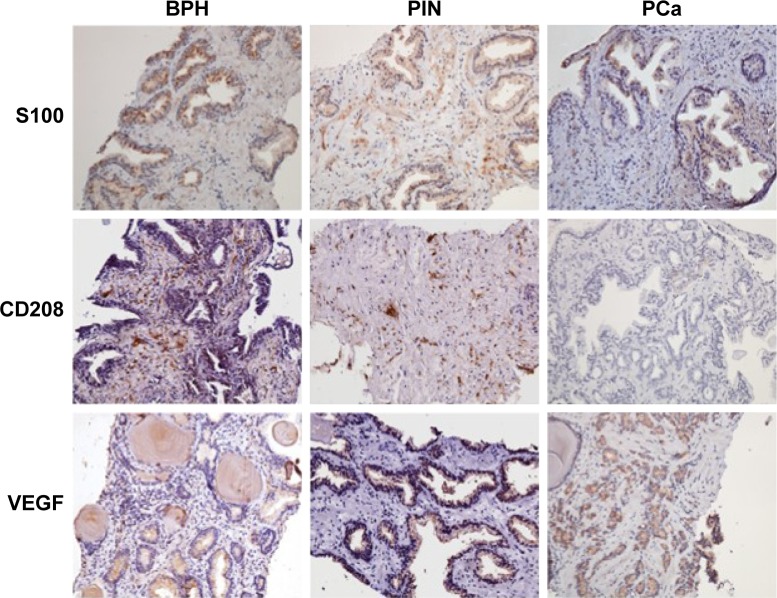
Figure 1.
Immunohistochemistry results of S100, CD208 and VEGF expression in biopsy tissues of each group (×200 original magnification).
Abbreviations: PCa, prostate cancer; BPH, benign prostatic hyperplasia; PIN, prostatic intraepithelial neoplasia; VEGF, vascular endothelial growth factor.
The expression level of S100 in each group decreased gradually with the increase of the degree of malignancy. PCa patients showed significantly lower expression of S100 than the other two groups (P = 0.000). The expression level of CD208 in each group also decreased gradually with the progression of the disease. The expression of CD208 in PCa patients was the lowest of the three groups, and the difference was statistically significant between PCa and BPH patients (P = 0.000). The difference in CD208 expression in PIN and BPH patients was also statistically significant (P = 0.045). The expression level of VEGF in each group increased gradually with the increase of the malignant degree of the disease statistical significance, P = 0.000.
Differences in CD4, CD8 and CD4/CD8 levels in 30 patients with PCa based on PSA levels and Gleason score (Table 4)
Table 4.
Differences in CD4, CD8 and CD4/CD8 levels in 30 PCa patients based on PSA levels and different Gleason score
| Group (N) | CD4 (%) | CD8 (%) | CD4/CD8 |
|---|---|---|---|
| A (10) | 38.03 ± 4.10 | 27.75 ± 7.11 | 1.58 ± 0.45 |
| B (10) | 27.72 ± 2.53c | 19.90 ± 5.95d | 1.56 ± 0.73 |
| C (10) | 20.09 ± 1.60a,b | 23.84 ± 7.83 | 0.97 ± 0.35e,f |
| D (8) | 38.74 ± 4.60 | 27.47 ± 5.51 | 1.50 ± 0.24 |
| E (9) | 30.42 ± 2.44i | 20.68 ± 6.45 | 1.58 ± 0.52 |
| F (13) | 21.12 ± 2.57g,h | 22.46 ± 8.84 | 1.16 ± 0.72 |
Notes: Group A, PSA < 10 ng/mL; group B, PSA 10–20 ng/mL; group C, PSA > 20 ng/mL; group D, Gleason grade < 7; group E, Gleason grade 7; group F, Gleason grade > 7.
P = 0.000 vs group A;
P = 0.000 vs group B;
P = 0.000 vs group A;
P = 0.025 vs group A;
P = 0.016 vs group A;
P = 0.019 vs group B;
P = 0.000 vs group D;
P = 0.000 vs group E;
P = 0.000 vs group D. N is the numbers of the cases. Data are presented as mean ± standard deviation.
Abbreviations: PCa, prostate cancer; PSA, prostate-specific antigen.
With the increase in PSA, the expression of peripheral blood CD4 in patients with PCa was gradually reduced. The difference was statistically significant between the two groups (P = 0.000). The expression level of CD8 in group B was significantly lower than that in group A (P = 0.025). No significant differences in expression levels of CD8 were seen between group C and group A or group B (> 0.05). The ratio of CD4/CD8 decreased with the increase of PSA, and the differences between group C and group B and between group C and group B were statistically significant (P < 0.05). There was no significant difference between group B and group C (P > 0.05).
The expression of CD4 in PCa patients decreased with the increase in Gleason score, and the difference was statistically significant between the two groups (P = 0.000). The expression level of CD8 in PCa patients was not statistically significant between groups, (P > 0.05), and there was no significant difference in the CD4/CD8 ratio between the two groups (P > 0.05).
Differences in S100, CD208 and VEGF expression in PCa patients based on PSA levels and Gleason score (Table 5)
Table 5.
Differences in S100, CD208 and VEGF expression in PCa patients based on PSA levels and different Gleason score
| Group | S100 (N) | CD208 (N) | VEGF (N) |
|---|---|---|---|
| A | 18.77 ± 4.10 (6) | 1.90 ± 0.37 (6) | 76.60 ± 3.98 (7) |
| B | 12.57 ± 1.91 (7)c | 0.97 ± 0.31 (7)e | 83.27 ± 3.24 (9)h |
| C | 9.06 ± 2.43 (9)a,b | 0.68 ± 0.10 (8)d | 88.38 ± 3.62 (9)f,g |
| D | 18.56 ± 3.81 (5) | 1.84 ± 0.57 (5) | 76.60 ± 4.16 (6) |
| E | 14.16 ± 4.50 (8)k | 1.13 ± 0.42 (8)n | 82.40 ± 4.26 (8)q |
| F | 9.33 ± 2.65 (9)i,j | 0.68 ± 0.10 (8)l,m | 87.78 ± 3.56 (11)o,p |
Notes: Group A, PSA < 10 ng/mL; group B, PSA 10–20 ng/mL; group C, PSA > 20 ng/mL; group D, Gleason grade < 7; group E, Gleason grade 7; group F, Gleason grade > 7.
P = 0.000 vs group A;
P = 0.024 vs group B;
P = 0.001 vs group A;
P = 0.000 vs group A;
P = 0.000 vs group A;
P = 0.000 vs group A;
P = 0.006 vs group B;
P = 0.001 vs group A;
P = 0.000 vs group D;
P = 0.014 vs group E;
P = 0.049 vs group D;
P = 0.000 vs group D;
P = 0.029 vs group E;
P = 0.004 vs group D;
P = 0.000 vs group D;
P = 0.007 vs group E;
P = 0.012 vs group D. N is the numbers of the cases. Data are presented as mean ± standard deviation.
Abbreviations: PCa, prostate cancer; VEGF, vascular endothelial growth factor; PSA, prostate-specific antigen.
With increased PSA levels, the expression of S100 in PCa patients decreased significantly. The differences in S100 expression was significant between all groups (P < 0.05). The expression of CD208 decreased with increased PSA levels in PCa patients, and the differences between group B and group A and between group C and group A were statistically significant (P = 0.000). However, there was no significant difference in the expression of CD208 between group C and group B (P > 0.05). The expression level of VEGF increased gradually with the increase of PSA, and this difference was statistically significant in both groups (P < 0.05).
The expression of S100 in PCa patients decreased significantly with the increase in Gleason score (P < 0.05). In addition, the expression level of CD208 in patients with PCa decreased significantly with the increase in Gleason score (P < 0.05). The expression level of VEGF in patients with PCa increased with the increase in Gleason score, and the difference was statistically significant between the two groups (P < 0.05).
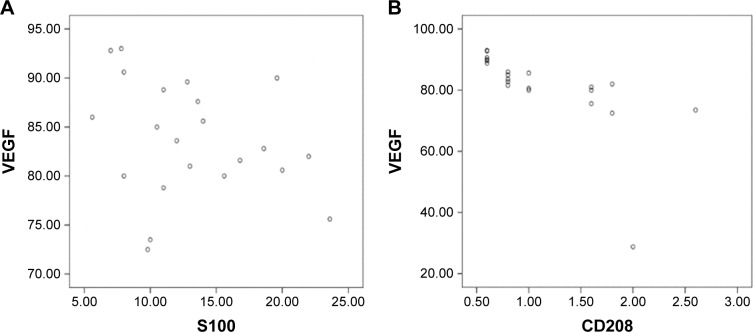
Correlation between expression of VEGF and S100 and CD208 expression levels in PCa patients
There was no correlation between VEGF and S100 expression levels (P = 0.839) (Figure 2A). However, there was a negative correlation between VEGF and CD208 expression levels (P = 0.001) (Figure 2B).
Figure 2.
(A) VEGF and S100 in PCa infiltration density scatter diagram. (B) VEGF and CD208 in PCa infiltration density scatter diagram.
Abbreviations: PCa, prostate cancer; VEGF, vascular endothelial growth factor.
Discussion
As important antigen-presenting cells (APCs), DCs function at the interface of the innate and adaptive immune systems. In their role as master APCs, DCs induce adaptive responses by processing and presenting antigens on major histocompatibility complex (MHC) molecules to naïve T lymphocytes in lymphoid organs.25 DCs are the most important APC population for regulating innate and adaptive immune responses. They are required for converting naïve resting T cells into potent tumor-reactive cytotoxic helper CD4+ T cells.26 In this study, we showed that S100, which is expressed by all DCs, and CD208, which is expressed by mature and functional DCs, were lower than in PCa patients than in BPH and PIN patients. The infiltration density level of total DCs and mature and functional DCs decreased with the increase of PSA level and Gleason score. Prostate tumor cells cannot only induce the apoptosis of mature DCs but also inhibit the differentiation and maturation of immature DCs, thereby reducing the number of DCs and inhibiting their function. DC deficiency and dysfunction are immune escape mechanisms of PCa.27 We also found that CD4+ T cells in PCa patients were more suppressed than in BPH and PIN patients. The decrease of CD4+ cells was enhanced with increasing PSA levels and Gleason scores. This indicates that PCa patients have abnormal immune functions and that DC activation and function were inhibited by the cancer cells. Furthermore, cancer cell-induced suppression of CD4+ T-cell numbers leads to the inhibition of immune system functions. This effect was achieved by inhibiting DCs. The number of tumor-infiltrating DCs in tumor patients was reduced and cells had functional defects. Compared with the normal DC phase, tumor-infiltrating DC deficiency stimulates the proliferation of T cells and the ability to produce cytokines. This is one of the mechanisms of tumor immune escape.8 CD4/CD8 ratio is a key marker for clinical outcome and immune dysfunction, and in the results of this study, the ratio of CD4/CD8 in PCa was lower than in BPH, but this difference was not statistically significant. We also got the same result between group F and group D. We thought that the possible reason was that the number of cases was small. Thus, we hope to increase the number of cases in future studies. Furthermore, we did not observe any difference in CD8+ T cell levels with the progression of PCa. Although a lot of research has shown that the CD8+ T cell is one of the important immune T cells involved in tumor immunity, it could inhibit the process of cancer, but some other results have shown that the CD8+ T-cell subpopulation also has potent suppressive activity in prostate cancer, and it could inhibit tumor immunity.28,29
VEGF was initially identified due to its capacity to enhance vascular permeability and endothelial cell growth. It is now recognized as the key inducer of angiogenesis in tumors. VEGF is secreted by most tumors and myeloid infiltrates.30 A high level of VEGF in the serum of cancer patients is correlated with increased metastasis, tumor aggressiveness, and poor prognosis. VEGF suppresses the maturation capacity and the differentiation of DCs and other immune cells from hematopoietic lineages.31 DCs which have been damaged cannot effectively present tumor antigen to the body’s immune system. In the past study, we inhibited VEGF expression and found that it can improve DC and T cells proliferation and also promote activation of anti-tumor immunocytes in the VEGF-inhibited microenvironment.32 In this study, we found that VEGF levels were higher in PCa patients than in PIN and BPH patients. The infiltration density level of VEGF-positive cells increased with increased PSA level and Gleason score. The infiltration density of VEGF-positive cells was negatively correlated with the maturation and function of DCs. VEGF inhibited the number of mature and functional DCs, thereby inhibiting the function of DCs and further inhibiting tumor immunity. VEGF expression had a relationship with the degree of malignancy of prostate cancer.33 With the progress of prostate cancer, DC maturation was inhibited, tumor immunity was inhibited too. VEGF might have an influence on the biophysical properties of DC, including electrophoretic mobility, osmotic fragility, viscoelasticity, and transmigration,34 so the total number of DCs was reduced in prostate cancer, and the number of total DCs reduced with the progress of prostate cancer. VEGF also acts, through the induction of programmed death ligand 1(PDL1 or B7-H1) on myeloid DCs,35 to inhibit the immunity of cancer, but the real mechanism of how VEGF inhibits mature DCs still needs to be studied in the future. The therapeutic efficacies may be improved by blocking the signaling pathway of VEGF in an appropriate manner.
Conclusion
The numbers of total DCs, mature and functional DCs, and CD4+ T cells were inhibited in PCa, and the inhibitory effect was enhanced with increased malignancy. In addition, the infiltration density of VEGF-positive cells was increased in PCa, and this increase was associated with an increased malignant degree of PCa. The inhibition of tumor immunity in patients with PCa is achieved by inhibiting the function of DCs. VEGF plays an important role in the inhibition of the maturation and function of DCs, and this inhibition is gradually increased with an increasing malignant degree of PCa.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81271597, 81401421) and capacity building project of clinical auxiliary department (SHDC22015001).
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. Wen-kun Bai and Wei Zhang are co-first authors.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Simons JW. Prostate cancer immunotherapy: beyond immunity to curability. Cancer Immunol Res. 2014;2(11):1034–1043. doi: 10.1158/2326-6066.CIR-14-0174. [DOI] [PubMed] [Google Scholar]
- 3.Zhang SY, Thara E, Quinn DI, Dorff TB. Blood cells and their use in active immunotherapy of prostate cancer. Hum Vaccin Immunother. 2012;8(4):528–533. doi: 10.4161/hv.19188. [DOI] [PubMed] [Google Scholar]
- 4.Cintolo JA, Datta J, Mathew SJ, Czerniecki BJ. Dendritic cell-based vaccines: barriers and opportunities. Future Oncol. 2012;8(10):1273–1299. doi: 10.2217/fon.12.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitmore WF, Gittes RF. Studies on the prostate and testis as immunologically privileged sites. Cancer Treat Rep. 1977;61(2):217–222. [PubMed] [Google Scholar]
- 6.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10(8):580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahnisch H, Fussel S, Kiessling A, et al. Dendritic cell-based immunotherapy for prostate cancer. Clin Dev Immunol. 2010;2010:517493. doi: 10.1155/2010/517493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troy A, Davidson P, Atkinson C, Hart D. Phenotypic characterisation of the dendritic cell infiltrate in prostate cancer. J Urol. 1998;160(1):214–219. [PubMed] [Google Scholar]
- 9.Aalamian M, Pirtskhalaishvili G, Nunez A, et al. Human prostate cancer regulates generation and maturation of monocyte-derived dendritic cells. Prostate. 2001;46(1):68–75. doi: 10.1002/1097-0045(200101)46:1<68::aid-pros1010>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Luczynska E, Gasinska A, Blecharz P, Stelmach A, Jereczek-Fossa BA, Reinfuss M. Value of perfusion CT parameters, microvessl density and VEGF expression in differentiation of benign and malignant prostate tumours. Pol J Pathol. 2014;65(3):229–236. doi: 10.5114/pjp.2014.45787. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109(43):17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franovic A, Holterman CE, Payette J, Lee S. Human cancers converge at the HIF-2alpha oncogenic axis. Proc Natl Acad Sci U S A. 2009;106(50):21306–21311. doi: 10.1073/pnas.0906432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5(11):1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 14.Ni YH, Wang ZY, Huang XF, et al. Effect of siRNA-mediated downregulation of VEGF in Tca8113 cells on the activity of monocyte-derived dendritic cells. Oncology Lett. 2012;3(4):885–892. doi: 10.3892/ol.2012.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugiyama M, Kakeji Y, Tsujitani S, et al. Antagonism of VEGF by genetically engineered dendritic cells is essential to induce antitumor immunity against malignant ascites. Molec Cancer Therapeut. 2011;10(3):540–549. doi: 10.1158/1535-7163.MCT-10-0479. [DOI] [PubMed] [Google Scholar]
- 16.Rini BI, Weinberg V, Fong L, Conry S, Hershberg RM, Small EJ. Combination immunotherapy with prostatic acid phosphatase pulsed antigen-presenting cells (provenge) plus bevacizumab in patients with serologic progression of prostate cancer after definitive local therapy. Cancer. 2006;107(1):67–74. doi: 10.1002/cncr.21956. [DOI] [PubMed] [Google Scholar]
- 17.Xu G, Wu J, Yao MH, et al. Parameters of prostate cancer at contrast-enhanced ultrasound: correlation with prostate cancer risk. Int J Clin Exp Med. 2015;8(2):2562–2569. [PMC free article] [PubMed] [Google Scholar]
- 18.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65(1):124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 19.Roobol MJ, van Vugt HA, Loeb S, et al. Prediction of prostate cancer risk: the role of prostate volume and digital rectal examination in the ERSPC risk calculators. Eur Urol. 2012;61(3):577–583. doi: 10.1016/j.eururo.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Roobol MJ, Zhu X, Schroder FH, et al. A calculator for prostate cancer risk 4 years after an initially negative screen: findings from ERSPC Rotterdam. Eur Urol. 2013;63(4):627–633. doi: 10.1016/j.eururo.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 21.Poliani PL, Kisand K, Marrella V, et al. Human peripheral lymphoid tissues contain autoimmune regulator-expressing dendritic cells. Am J Pathol. 2010;176(3):1104–1112. doi: 10.2353/ajpath.2010.090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thongtharb A, Uchida K, Chambers JK, Kagawa Y, Nakayama H. Histological and immunohistochemical studies on primary intracranial canine histiocytic sarcomas. J Veterin Med Sci. 2016;78(4):593–599. doi: 10.1292/jvms.15-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer K, Michel S, Reuschenbach M, Nelius N, von Knebel Doeberitz M, Kloor M. Dendritic cell and macrophage infiltration in microsatellite-unstable and microsatellite-stable colorectal cancer. Fam Cancer. 2011;10(3):557–565. doi: 10.1007/s10689-011-9449-7. [DOI] [PubMed] [Google Scholar]
- 24.Duque JL, Loughlin KR, Adam RM, Kantoff P, Mazzucchi E, Freeman MR. Measurement of plasma levels of vascular endothelial growth factor in prostate cancer patients: relationship with clinical stage, Gleason score, prostate volume, and serum prostate-specific antigen. Clinics (Sao Paulol) 2006;61(5):401–408. doi: 10.1590/s1807-59322006000500006. [DOI] [PubMed] [Google Scholar]
- 25.Datta J, Terhune JH, Lowenfeld L, et al. Optimizing dendritic cell-based approaches for cancer immunotherapy. Yale J Biol Med. 2014;87(4):491–518. [PMC free article] [PubMed] [Google Scholar]
- 26.Thara E, Dorff TB, Pinski JK, Quinn DI. Vaccine therapy with sipuleucel- T (Provenge) for prostate cancer. Maturitas. 2011;69(4):296–303. doi: 10.1016/j.maturitas.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Pirtskhalaishvili G, Shurin GV, Gambotto A, et al. Transduction of dendritic cells with Bcl-xL increases their resistance to prostate cancer-induced apoptosis and antitumor effect in mice. J Immunol. 2000;165(4):1956–1964. doi: 10.4049/jimmunol.165.4.1956. [DOI] [PubMed] [Google Scholar]
- 28.Kiniwa Y, Miyahara Y, Wang HY, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13(23):6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 29.Yu P, Steel JC, Zhang M, et al. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci U S A. 2012;109(16):6187–6192. doi: 10.1073/pnas.1203479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:13–27. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- 31.Sheng KC, Wright MD, Apostolopoulos V. Inflammatory mediators hold the key to dendritic cell suppression and tumor progression. Curr Medicinal Chem. 2011;18(36):5507–5518. doi: 10.2174/092986711798347207. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Shou WD, Xu YJ, Bai WK, Hu B. Low-frequency ultrasound-induced VEGF suppression and synergy with dendritic cell-mediated anti-tumor immunity in murine prostate cancer cells in vitro. Scientific Rep. 2017;7(1):5778. doi: 10.1038/s41598-017-06242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kostis G, Ioannis L, Helen K, Helen P. The expression of vascular endothelial growth factor-C correlates with lymphatic microvessel density and lymph node metastasis in prostate carcinoma: an immunohistochemical study. Urol Ann. 2014;6(3):224–230. doi: 10.4103/0974-7796.134275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu ZQ, Xue H, Long JH, et al. Biophysical properties and motility of human mature dendritic cells deteriorated by vascular endothelial growth factor through cytoskeleton remodeling. Int J Molec Sci. 2016;17(11):1756. doi: 10.3390/ijms17111756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9(5):562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]