Abstract
Background
Chronic shoulder pain (CSP) is a common disease causing pain and functional limitation, which is highly prevalent and has substantial negative effects on the quality of life. Acupuncture has gained popularity and has been accepted gradually by many countries because it can successfully treat patients with chronic pain, but the specific brain mechanisms under acupuncture treatment for CSP remain unclear. Therefore, in this study, we aimed to 1) compare the clinical effects between acupuncture at the contralateral and ipsilateral Tiaokou (ST 38) point in patients with unilateral shoulder pain and 2) explore how contralateral- and ipsilateral-acupuncture modulates the regional homogeneity (ReHo) of patients with CSP.
Patients and methods
This was a pilot functional magnetic resonance imaging (fMRI) trial. Twenty-four patients with CSP were recruited and randomized to the contralateral acupuncture group (contra-group) and the ipsilateral acupuncture group (ipsi-group). All patients completed resting-state functional magnetic resonance imaging (fMRI) scans before and after acupuncture treatment. Shoulder pain intensity (visual analog scale [VAS]) and shoulder joint function (Constant–Murley score [CMS]) were used to evaluate clinical efficiency of treatment. ReHo was used to assess resting-state brain activity.
Results
We found clinical improvement in decreasing pain intensity and increasing shoulder function in both groups, and the mean objective shoulder functional improvement in contra-group was better than that in ipsi-group (p = 0.010). Interestingly, the brain mechanism of contra-acupuncture at ST 38 was distinguishable from ipsi-acupuncture regarding ReHo values.
Conclusion
Anterior cingulate cortex (ACC) may play a direct role in the regulation of brain by the contralateral acupuncture at ST 38 in patients with shoulder pain. On the contrary, the pathway of brainstem-thalamus-cortex may be likely to work in mechanism of acupuncture at ipsilateral ST 38.
Significance
Our results indicate that the clinical effects and brain mechanisms are different between the stimulation given at contralateral and ipsilateral acupoints in patients with CSP and imply that the selection of either contralateral or ipsilateral acupuncture therapy to treat some chronic pain conditions is necessary.
Keywords: region homogeneity, anterior cingulate cortex, brainstem, resting-state fMRI, ST 38
Introduction
Chronic shoulder pain (CSP) is the third most frequently reported type of musculoskeletal pain.1 It is a chronic condition that causes pain along with functional limitations in the affected persons and causes substantial negative effect on the quality of life2 with a heavy social and economic burden.3,4 Significant differences in the prevalence of CSP (from 6.9% to 26%) have been noted for point prevalence, and it has up to a 66.7% lifetime prevalence in the general population.2,5
In recent years, acupuncture, as a widely used form of complementary and alternative medicine, has gained popularity and has been accepted by countries around the world because it has been shown to successfully treat patients with chronic pain.6 In addition, a large number of clinical studies7–11 and a meta-analysis12 have demonstrated that acupuncture is effective and safe in the treatment of shoulder pain.
Resting-state functional magnetic resonance imaging (rs-fMRI) has been extensively used to explore the underlying brain mechanisms of pain management and relative disorders due to its advantages such as having high resolution, absence of radiation, and ease of application. Among all kinds of rs-fMRI data analysis methods, regional homogeneity (ReHo) is an advanced technique to measure the characteristics of regional activity of each voxel within the brain rather than to measure the generalized neuronal activity.13,14 Alterations in ReHo may serve as a neural marker which reflect the progress of a particular disease.15 Numerous studies have shown the difference in brain function between patients with chronic pain and healthy subjects, involving the limbic system (especially anterior cingulated cortex [ACC]), brainstem, and the somatosensory system (especially supplementary motor area, parietal cortices, and primary and secondary somatosensory cortex [S1, S2]), insular cortex, and superior frontal gyrus.15–19
Previous neuroimaging studies have demonstrated that acupuncture can significantly modulate brain activation patterns in both healthy subjects and in patients with pain, but the results were found to be inconsistent. A study20 discovered more pronounced activation of insula and S2 as well as deactivation of precuneus during stimulation at Zusanli (ST 36) than that of nonacupuncture point locations. A recent study21 suggested that acupuncture regulates the dysfunction of the ascending input/descending modulation system. Furthermore, a study on CSP showed that values of ReHo were found to be significantly increased bilaterally in the middle frontal gyrus and decreased in the left middle cingulate gyrus, supplementary motor area, superior frontal gyrus, right postcentral gyrus, insula, and superior parietal lobule.22
In the clinical treatment of CSP, acupuncture at contralateral acupoints has better effect,8,23 though some studies have suggested that there were no statistically significant differences between the contralateral and ipsilateral acupuncture groups.24 Appropriate selection of contralateral and ipsilateral acupuncture (corresponding to the pain site) makes a difference in achieving a better treatment effect. Nevertheless, the relevant brain mechanism still remains unclear. Therefore, we explored the different brain mechanisms of acupuncture by stimulating Tiaokou (ST 38) on the contralateral or affected side for left shoulder pain by using rs-fMRI combined with ReHo. In this pilot study, we hypothesized that 1) compared with acupuncture at the ipsilateral ST 38, there might be a superior effect with the contralateral point in patients with unilateral shoulder pain and that 2) there are different modulatory mechanisms between acupuncture at contralateral and ipsilateral ST 38 point.
Patients and methods
Population
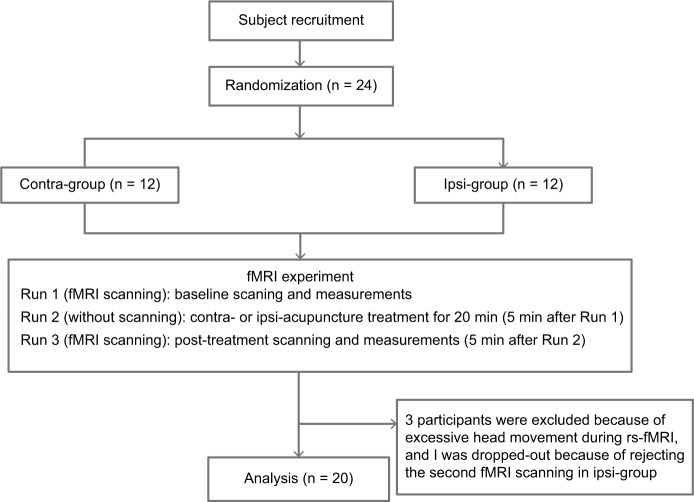
Patients with CSP were recruited from acupuncture clinics at the Beijing Hospital of Traditional Chinese Medicine, Affiliated to Capital Medical University. The trial was performed from December 2016 to July 2017. Screening and clinical assessment were accomplished via a face-to-face interview prior to participation. Twenty-four patients with CSP experiencing pain in their left shoulder were randomly assigned, and 20 patients were finally analyzed (data from three participants were excluded because of excessive head movement during rs-fMRI, and one was dropped-out because of refusing the second fMRI scanning; all these belonged to the ipsilateral group).
All of the participants met the following criteria: 1) age between 45 and 65 years, inclusive, and right-handed; 2) left-sided shoulder pain for at least 6 weeks and no more than 1 year; 3) visual analog scale (VAS) score of 50–100 mm inclusive (0–100 mm, 0 mm indicated “no pain at all” and 100 mm indicated “worst pain imaginable”) in the past week; 4) no other chronic pain conditions or a history of shoulder trauma; 5) no other current therapy involving analgesics for the pain; 6) no clear organic lesion or obvious asymmetry of brain structures on MRI scan; 7) no history of neurological or psychiatric disorders such as major depressive disorder, bipolar disorder, schizophrenia, or obsessive compulsive disorder; and 8) no history of alcohol or substance abuse.
Ethics
This study was approved by the Research Ethical Committee of Beijing Hospital of Traditional Chinese Medicine, Affiliated to Capital Medical University (reference: 201315). We provided with a description of the study procedures and obtained written informed consent from all the participants.
Procedures
We performed a process of scan–acupuncture–scan (Figure 1) after randomization and accomplished a clinical assessment before and after acupuncture treatment respectively. Briefly, 5 min after the first scan, the subjects received acupuncture at contra- or ipsi- ST 38 for 20 min. Then, 5 min after the treatment, the subjects had the second scan. Twenty-four participants with left chronic shoulder pain were randomized in two groups: the contralateral group (on the contralateral side with traditional acupuncture at ST 38, n = 12) or the ipsilateral group (on the affected side with traditional acupuncture at ST 38, n = 12), according to a computerized randomization schedule generated with SPSS19.0 software.
Figure 1.
Experimental design. A total of 24 subjects were recruited in this experiment. Finally, 20 subjects completed the study and their data were analyzed.
Imaging procedure
All patients underwent the fMRI scan on a 3.0 T whole body MRI scanner (Symphony; Siemens Medical System, Erlangen, Germany) with a standard head coil in the Imaging Center for Brain Research. Participants were scanned in a supine position with their heads snugly fixed by straps and foam pads to minimize the head movement.
Rs-fMRI data were acquired using a gradient echo planar imaging (EPI) sequence with the following parameter: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, field of view (FOV) = 220 mm × 220 mm, flip angle (FA) = 90°, slice thickness/gap = 3.5/0.6 mm, axial slices = 33, in-plane resolution = 64 × 64, and 240 volumes covering the whole brain. All of the participants were asked to close their eyes without thinking or sleeping during the entire rs-fMRI scan. After scanning, all of the participants reported that they were fully awake during the procedure.
Acupuncture manipulation
The patients were asked to remain seated and to relax their shoulders. In this trial, the acupuncturist inserted disposable needle (Huatuo disposable acupuncture needle; Suzhou Medical Co. Ltd., Jiangsu, People’s Republic of China) to a depth of 10–15 mm at the subject’s Tiaokou (ST 38, contralateral or ipsilateral, Figure 2) between the two MRI scans, lifting, thrusting, and twirling approximately 180° to induce the deqi sensation for 30 s. Needle insertions were maintained for 20 min. During the period of manipulation, the subjects were asked to perform active mobilization of the left shoulder, in abduction, internal, and external rotation. At the end of manipulation, participants were asked to complete the clinical data evaluation.
Figure 2.
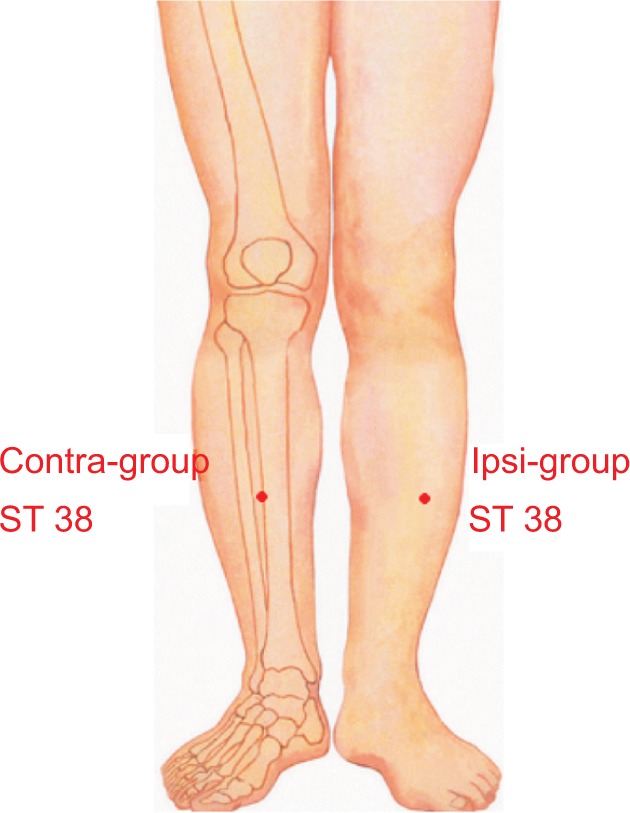
Acupuncture stimulation point. Tiaokou (ST 38) belongs to Stomach Meridian, on the lower leg, midway between the tibiofemoral joint line (level with the popliteal crease) and the prominence of the lateral malleolus, one finger-breadth lateral to the anterior crest of the tibia.
Clinical assessment
The researchers responsible for clinical assessment were blinded to the treatment group allocation. Personal data and details of the subjects’ medical history and present condition were acquired in the out-patient office including age, gender, education, residence, and duration of shoulder pain. The VAS and the Constant–Murley score (CMS)25 were evaluated before and after acupuncture to indicate the pain intensity and function. All the evaluations and imaging were performed on the same day.
Imaging data preprocessing
The rs-fMRI data were preprocessed using the Data Processing Assistant and Resting-State fMRI (DPARSF) toolbox for MATLAB.26 The first 10 volumes for each subject were discarded to attain the signal equilibrium. The remaining volumes were processed with the slice timing, motion correction, spatially realigned, and spatial normalization to the Montreal Neurological Institute (MNI) for each subject. Then the processed data were re-sampled to 3 × 3 × 3 mm3. The linear trend of the signals was removed, and a filter with a frequency range of 0.01–0.1 Hz was applied. Subjects were eliminated if head motion was more than 3 mm or more than 3° in translation or rotation.
ReHo analysis
ReHo was computed based on the Kendall’s coefficient of concordance (KCC) of the time series of the voxel with its nearest 26 neighboring voxels using DPARSF, followed by a smoothing procedure with a Gaussian kernel with a full-width-half-maximum (FWHM, 6 mm) to minimize the noise and residual differences of the preprocessed images.
Statistical analysis
A two-sample independent t-test was used to compare the clinical data variables, and a paired-samples t-test was used to examine within-group changes using SPSS 19.0. On the individual ReHo maps, statistical analysis was performed using the rs-fMRI Data Analysis Toolkit (Song et al, Beijing Normal University, Beijing, People’s Republic of China, http://www.restfmri.net). A two-sample independent t-test was performed to compare the results of ReHo between the two groups (p < 0.05). Then, a paired-samples t-test (p < 0.05) was performed to extract the results of ReHo across the subjects within each group.
Results
Demographic and clinical characteristics
Table 1 shows the demographic characteristics of all participants in each group. Except for education level (p = 0.030), there were no significant differences in demographic variables between the two groups (p > 0.05). The average baseline pain intensity score on VAS and function evaluation score on CMS were found to be respectively 67.92 ± 16.16 mm and 50.67 ± 9.94 in the contra-group, whereas it was found to be respectively 60.63 ± 7.29 mm and 56.88 ± 16.69 in the ipsi-group (Table 1).
Table 1.
Demographics and characteristics at baseline, and clinical outcomes before and after acupuncture treatment
| Characteristics | Contra-group (n = 12) | Ipsi-group (n = 8) | p-value | |
|---|---|---|---|---|
| Age (in years)a | 53.33 ± 5.26 (47–65) | 54.13 ± 7.45 (45–65) | 0.783 | |
| Education (in years)a | 14.92 ± 2.64 (9–19) | 11.63 ± 3.62 (9–16) | 0.030 | |
| Gender (M:F)b | 6:6 | 4:4 | 1.000 | |
| Illness duration (months)a | 4.92 ± 3.40 (2–12) | 5.38 ± 3.20 (2–12) | 0.766 | |
| Pain intensity (VAS)a | Baseline | 67.92 ± 16.16 | 60.63 ± 7.29 | 0.250 |
| Post-treatment | 47.50 ± 17.26 | 51.88 ± 5.30 | – | |
| Change (post–pre) | −20.42 ± 5.62 | −8.75 ± 2.27 | 0.122 | |
| Function (CMS)a | Baseline | 50.67 ± 9.94 | 56.88 ± 16.69 | 0.309 |
| Post-treatment | 66.33 ± 11.17 | 61.38 ± 18.05 | – | |
| Change (post–pre) | 15.67 ± 3.07 | 4.50 ± 1.00 | 0.010 |
Notes: Values are presented as mean ± SD (range of minimum–maximum);
p values for two-sample independent t-tests;
p values for Pearson chi-square test.
Abbreviations: M, male; F, female; SD, standard deviation; VAS, visual analog scale score, 0–100 mm; CMS, the Constant–Murley score, 0–100 points.
Clinical outcomes, pain intensity and shoulder function of patients with left shoulder pain in contra- and ipsi-group were found to be significantly improved (contra-group VAS −20.42 [95% CI: −32.79 – −8.04], p = 0.004; contra-group CMS 15.67 [95% CI 8.90–22.43], p = 0.000; ipsi-group VAS −8.75 [95% CI: −14.11 – −3.39], p = 0.006; ipsi-group CMS 4.50 [95% CI: 2.24–6.87], p = 0.003). The mean objective shoulder function improvement in patients who underwent contra-acupuncture had an obvious advantage compared with those who underwent ipsi-acupuncture (p = 0.010), whereas pain intensity was not found to be insignificantly different (p = 0.122).
ReHo analysis
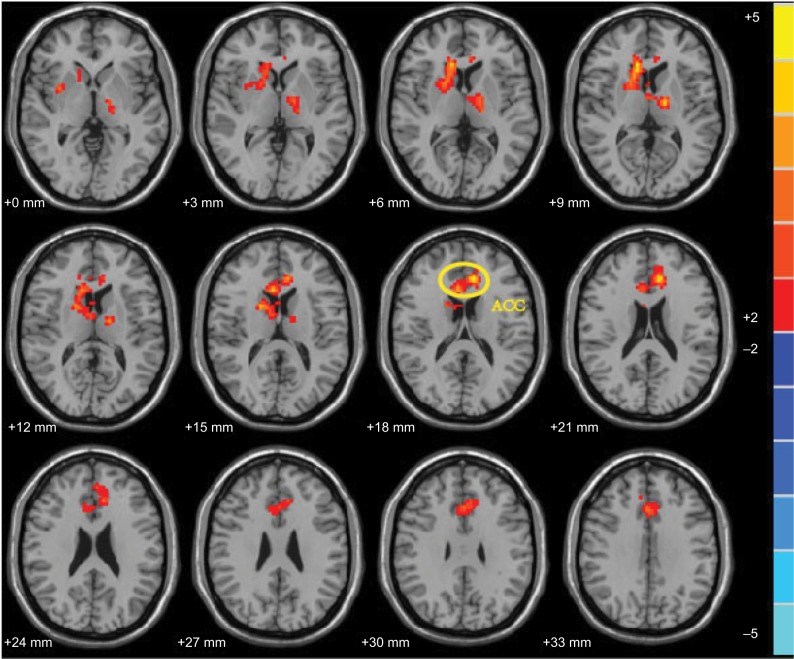
To explore the effects of the two treatments in the contra-group (post minus pre) and ipsi-group (post minus pre), the differences in ReHo values between pre and posttreatment were assessed and compared between the two groups. It revealed that the ReHo values in the ACC were found to be significantly more increased by treatment in the contra-group than that of ipsi-group (Figure 3).
Figure 3.
Comparison of the effect of acupuncture in contra-group and ipsi-group. In this study, the acupuncture effect was post minus preacupuncture in contra-group or ipsi-group. The comparison result was the effect in the contra-group minus the effect in the ipsi-group. The threshold is set to p = 0.05 with 738 continuous voxels for whole brain with Gaussian Random Field (GRF) correction. Peak coordinates refer to the Montreal Neurological Institute (MNI) atlas. The bilateral Anterior Cingulate Cortex (ACC, MNI coordinates: −9, 33, 18, voxels: 793, peak intensity: 4.8894, which was extended to Thalamus, callosum, Supper and Medial Frontal Gyrus).
Abbreviation: ACC, anterior cingulate cortex.
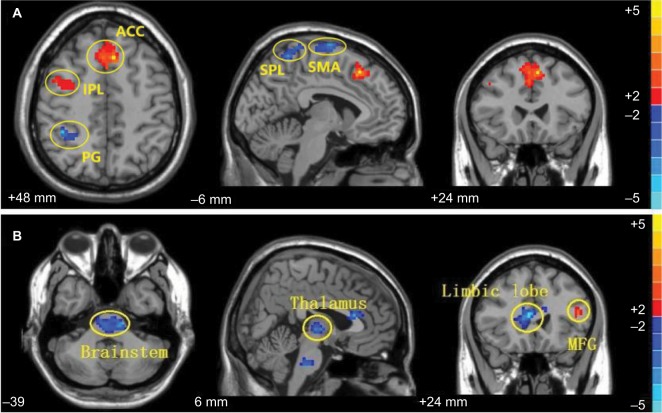
Further analysis showed that the effect of acupuncture stimulating in contralateral or ipsilateral ST 38 displayed great diversity in ReHo signal changes within each group (post minus pre acupuncture) (Table 2), revealing the underlying different brain effects between contra- and ipsi-acupuncture. The brain map of pre and postacupuncture in both groups have been shown in Figure S1. In the contra-group, the ReHo signals of bilateral anterior cingulate gyrus, right inferior parietal lobule, and inferior frontal gyrus were found to be significantly increased, and the left superior parietal lobule, right supplementary motor area, and postcentral gyrus were found to be decreased. However, the ipsi-group demonstrated a different pattern. The ReHo was found to be increased in left middle frontal gyrus and were found to be decreased in the bilateral brainstem, right thalamus, and limbic lobe (Figure 4).
Table 2.
Alterations in ReHo values (post minus pretreatment) in contra- and ipsi-group
| Group | Brain regions | Side | MNI coordinates
|
Cluster size | t | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Contra-group | Post > pre | ||||||
| Inferior parietal lobule | R | 51 | −42 | 21 | 378 | 5.3138 | |
| Anterior cingulate gyrus | L, R | −6 | 24 | 48 | 250 | 4.9075 | |
| Inferior frontal gyrus | R | 42 | 9 | 39 | 93 | 4.3645 | |
| Pre > post | |||||||
| Supplementary motor area | R | 9 | −6 | 69 | 248 | −5.4010 | |
| Superior parietal lobule | L | −15 | −42 | 72 | 209 | −5.2592 | |
| Postcentral gyrus | R | 45 | −39 | 60 | 119 | −4.6164 | |
| Ipsi-group | Post > pre | ||||||
| Middle frontal gyrus | L | −48 | 30 | 24 | 158 | 4.8663 | |
| Pre > post | |||||||
| Limbic lobe | R | 6 | 24 | 12 | 278 | −6.4421 | |
| Thalamus | R | 12 | −24 | −3 | 194 | −6.7245 | |
| Brainstem | L, R | −9 | −27 | −39 | 92 | −5.1631 | |
Notes: The threshold is set at p = 0.05 with cluster-level correction for each brain region. Peak coordinates refer to the atlas of MNI.
Abbreviations: ReHo, regional homogeneit; R, right; L, left; MNI, Montreal Neurological Institute.
Figure 4.
Comparison of the post and preacupuncture scans in contra-group (A) and ipsi-group (B). (A) Alterations in ReHo in the contra-group (post minus pre, n = 12). (B) Alterations in ReHo in the ipsi-group (post minus pre, n = 8).
Abbreviations: ACC, anterior cingulate gyrus; IPL, inferior parietal lobule; PG, postcentral gyrus; ReHo, regional homogeneit; SPL, superior parietal lobule; SMA, supplementary motor area; MFG, middle frontal gyrus.
Discussion
In this study, we investigated the clinical changes including shoulder pain intensity and function in patients with CSP before and after acupuncture treatment, as well as the different modulatory mechanisms between contra- and ipsi-acupuncture by means of the voxel-based analysis of MRI-derived ReHo maps. According to our results, both pain intensity and shoulder function improved in the two groups. Acupuncture treatment at the contra-group showed a significantly greater improvement in shoulder function than ipsi-group, but no obvious difference was found in changes of pain intensity in this study. We observed that contra- and ipsi-acupuncture have different modulation effects on ReHo of ACC in patients with CSP. To elucidate the reasons for the different mechanisms, further analysis was conducted. We found alterations in ReHo in the ACC, supplementary motor area, inferior parietal lobule, inferior frontal gyrus, superior parietal lobule, and postcentral gyrus after acupuncture at the contralateral ST 38 site, whereas alterations in ReHo in the brainstem, thalamus, limbic lobe, and middle frontal gyrus were seen after acupuncture at ipsilateral ST 38.
Previous studies have suggested that most of these regions are involved in the analgesic effects in the brain.27 Interestingly, the ACC may be the most important area in contralateral acupuncture treatment, whereas the brainstem may be the most important region in ipsilateral acupuncture treatment.
This finding is in line with many brain imaging studies reporting that the ACC is a key region of the central pain modulatory system. Previous studies and meta-analysis28 have suggested that the ACC is involved in pain information processing in the brain and is associated with the affective component of pain. Furthermore, a previous study29 has shown that lesions of the rostral ACC completely inhibited the anti-nociceptive effects of contra- but not ipsilateral electro-acupuncture. This suggests that the ACC exerts a direct role in the mechanism of acupuncture at contra-lateral ST 38 in patients with CSP. In a previous study,30 researchers have found that patients with migraine in the interictal phase showed lower BOLD-fMRI signals in the brainstem compared with healthy controls. Furthermore, recent studies31–34 have shown that the periaqueductal gray matter (PAG), located within the brainstem, is a key region involved in the endogenous pain inhibition by descending modulation of spinal cord neurons. It can be inferred that it regulates the dysfunction of the ascending input/descending modulation system when we acupuncture at ipsilateral ST 38 in patients with CSP.
Many studies have indicated that acupuncture treatment regulates the impaired descending pain modulatory system in CSP34,35 and other chronic pain conditions.36,37
Different needling positions in different empirical acupuncture treatment paradigms may play a role in modulating pain-related neuronal functions.35 Contra-acupuncture led to a change in supplementary motor area which is clearly associated with shoulder mobility function (CMS scores), and CMS scores are obviously superior compared with ipsi-acupuncture. On the contrary, limbic lobe was found to be decreased on ReHo signal after ipsi-acupuncture. This result was consistent with the previous finding that acupuncture could modulate the limbic–paralimbic–neocortical network in healthy volunteers.38 Moreover, we also found some frontal lobe or parietal lobe brain regions, which are associated with memory and cognition process in both groups. Many previous studies39–43 have shown that acupuncture in healthy subjects or in patients with pain evokes deactivation in the frontal lobe and parietal lobe regions. Combining our results and the previous findings, it is apparent that pain is a complex process with factors related to sensory perception combined cognition. Future studies are needed to shed light on the complex neural mechanism of pain.
After the comparative analysis, we concluded that acupuncture exerted its analgesic effects by directly regulating the ACC and other brain regions with contralateral acupuncture at ST 38, whereas ipsilateral acupuncture may exert its effect on the pathway of brainstem-thalamus-cortex.43
Limitations
It is noteworthy that there are several limitations in our study. First, the generalization of the results from our study to a larger population should be made cautiously, because our sample size is relatively small, and the nonsignificant dif ference in pain intensity may be a result of the small sample size. Future studies with larger sample size may provide larger statistical effect. Second, there was a different educational level between the two groups, but it likely that it did not directly affect the results on shoulder pain. Third, we did not quantitatively evaluate the intensity of deqi sensation, which is thought to have an impact on acupuncture effect. Studies with deqi sensation quantification are needed in future studies. Finally, future studies are needed to examine the clinical effects and illuminate the neural mechanism underlying the contra- and ipsi-acupuncture at ST 38 in healthy subjects.
Conclusion
Our results demonstrate that compared with ipsi-acupuncture, contra-acupuncture at ST 38 has a more specific effect in patients with CSP. In addition, there are different modulation effects on ReHo between contra- and ipsi-acupuncture treatments. Contra-acupuncture showed analgesic effects by directly regulating the ACC and other brain regions, whereas ipsi-acupuncture regulated the dysfunction of the ascending input/descending modulation system in patients with CSP.
Supplementary material
The whole brain map (group mean) of pre and postacupuncture in each group. The color bar indicates a range of ReHo values between 4.6 (red) and 15 (yellow).
Notes: (A) The whole brain map (group mean) of contralateral group with preacupuncture. (B) The whole brain map (group mean) of contralateral group with postacupuncture. (C) The whole brain map (group mean) of ipsilateral group with preacupuncture. (D) The whole brain map (group mean) of ipsilateral group with postacupuncture.
Abbreviations: L, left; R, right; ReHo, regional homogeneit.
Acknowledgments
The authors involved in this project thank the patients who made this study possible. This study was supported by grants from the Beijing Technology Development of Traditional Chinese Medicine Foundation – Annual Planning Project (ref: JJ 2013-40).
Footnotes
Author contributions
Shuai Zhang contributed to the study implementation and writing the manuscript. Xu Wang contributed to the data analyses and interpretation. Chao-Qun Yan contributed to the examination and selection of the patients and writing of the manuscript. Shang-Qing Hu contributed to the evaluation of clinical results and study implementation. Jian-Wei Huo, Zhong-Yan Wang, and Chun-Hong Liu contributed to the fMRI data acquisition. Ping Zhou contributed to randomization. Cun-Zhi Liu contributed to the study design, treatment of patients, and study implementation and supervision. All authors gave comments on the drafts of the manuscript and read the final version. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Herin F, Vézina M, Thaon I, Soulat JM, Paris C, ESTEV group Predictors of chronic shoulder pain after 5 years in a working population. Pain. 2012;153(11):2253–2259. doi: 10.1016/j.pain.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Whittle S, Buchbinder R. In the clinic. Rotator cuff disease. Ann Intern Med. 2015;162(1):ITC1–ITC15. doi: 10.7326/AITC201501060. [DOI] [PubMed] [Google Scholar]
- 3.Meislin RJ, Sperling JW, Stitik TP. Persistent shoulder pain: epidemiology, pathophysiology, and diagnosis. Am J Orthop (Belle Mead NJ) 2005;34(12 Suppl):5–9. [PubMed] [Google Scholar]
- 4.Parsons S, Breen A, Foster NE, Letley L, Pincus T, Vogel S, Underwood M. Prevalence and comparative troublesomeness by age of musculoskeletal pain in different body locations. Fam Pract. 2007;24(4):308–316. doi: 10.1093/fampra/cmm027. [DOI] [PubMed] [Google Scholar]
- 5.Luime JJ, Koes BW, Hendriksen IJ, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol. 2004;33(2):73–81. doi: 10.1080/03009740310004667. [DOI] [PubMed] [Google Scholar]
- 6.White A, Editorial Board of Acupuncture in Medicine Western medical acupuncture: a definition. Acupunct Med. 2009;27(1):33–35. doi: 10.1136/aim.2008.000372. [DOI] [PubMed] [Google Scholar]
- 7.Asheghan M, Aghda AK, Hashemi E, Hollisaz M. Investigation of the effectiveness of acupuncture in the treatment of frozen shoulder. Mater Sociomed. 2016;28(4):253–257. doi: 10.5455/msm.2016.28.253-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Sun J, Wang C, et al. Randomised controlled trial of contralateral manual acupuncture for the relief of chronic shoulder pain. Acupunct Med. 2016;34(3):164–170. doi: 10.1136/acupmed-2015-010947. [DOI] [PubMed] [Google Scholar]
- 9.Guerra de Hoyos JA, Andrés Martín Mdel C, Bassas y Baena de Leon E, et al. Randomised trial of long term effect of acupuncture for shoulder pain. Pain. 2004;112(3):289–298. doi: 10.1016/j.pain.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Vas J, Ortega C, Olmo V, et al. Single-point acupuncture and physiotherapy for the treatment of painful shoulder: a multicentre randomized controlled trial. Rheumatology (Oxford) 2008;47(6):887–893. doi: 10.1093/rheumatology/ken040. [DOI] [PubMed] [Google Scholar]
- 11.Johansson KM, Adolfsson LE, Foldevi MO. Effects of acupuncture versus ultrasound in patients with impingement syndrome: randomized clinical trial. Phys Ther. 2005;85(6):490–501. [PubMed] [Google Scholar]
- 12.Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture Trialists’ Collaboration Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172(19):1444–1453. doi: 10.1001/archinternmed.2012.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22(1):394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 14.Zuo XN, Di Martino A, Kelly C, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49(2):1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu QZ, Li DM, Kuang WH, et al. Abnormal regional spontaneous neural activity in treatment-refractory depression revealed by resting-state fMRI. Hum Brain Mapp. 2011;32(8):1290–1299. doi: 10.1002/hbm.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu D, Yuan K, Zhao L, et al. Regional homogeneity abnormalities in patients with interictal migraine without aura: a resting-state study. NMR Biomed. 2012;25(5):806–812. doi: 10.1002/nbm.1796. [DOI] [PubMed] [Google Scholar]
- 17.Zhang SS, Wu W, Liu ZP, Huang GZ, Guo SG, Yang JM. Altered regional homogeneity in experimentally induced low back pain: a resting-state fMRI study. J Neuroeng Rehabil. 2014;11:115. doi: 10.1186/1743-0003-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preis MA, Kröner-Herwig B, Schmidt-Samoa C, Dechent P, Barke A. Neural correlates of empathy with pain show habituation effects. An fMRI study. PLoS One. 2015;10(8):e0137056. doi: 10.1371/journal.pone.0137056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duerden EG, Albanese MC. Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum Brain Mapp. 2013;34(1):109–149. doi: 10.1002/hbm.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nierhaus T, Pach D, Huang W, et al. Differential cerebral response to somatosensory stimulation of an acupuncture point vs. two non-acupuncture points measured with EEG and fMRI. Front Hum Neurosci. 2015;9:74. doi: 10.3389/fnhum.2015.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Zeng F, Yin T, et al. Acupuncture modulates the abnormal brain-stem activity in migraine without aura patients. Neuroimage Clin. 2017;15:367–375. doi: 10.1016/j.nicl.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu CX, Ji TT, Song H, et al. Abnormality of spontaneous brain activities in patients with chronic neck and shoulder pain: a resting-state fMRI study. J Int Med Res. 2017;45(1):182–192. doi: 10.1177/0300060516679345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somers DL, Clemente FR. Transcutaneous electrical nerve stimulation for the management of neuropathic pain: the effects of frequency and electrode position on prevention of allodynia in a rat model of complex regional pain syndrome type II. Phys Ther. 2006;86(5):698–709. [PubMed] [Google Scholar]
- 24.Miura K, Ohara T, Zeredo JL, Okada Y, Toda K, Sumikawa K. Effects of traditional “Juci” (contralateral acupuncture) on orofacial nociceptive behavior in the rat. J Anesth. 2007;21(1):31–36. doi: 10.1007/s00540-006-0443-4. [DOI] [PubMed] [Google Scholar]
- 25.Constant CR, Gerber C, Emery RJ, Søjbjerg JO, Gohlke F, Boileau P. A review of the Constant score: modifications and guidelines for its use. J Shoulder Elbow Surg. 2008;17(2):355–361. doi: 10.1016/j.jse.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong J, White NS, Kwong KK, et al. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2006;27(9):715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong J, Loggia ML, Zyloney C, Tu P, Laviolette P, Gollub RL. Exploring the brain in pain: activations, deactivations and their relation. Pain. 2010;148(2):257–267. doi: 10.1016/j.pain.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi M, Zhang H, Lao L, Xing GG, Wan Y. Anterior cingulate cortex is crucial for contra- but not ipsilateral electro-acupuncture in the formalin-induced inflammatory pain model of rats. Mol Pain. 2011;7:61. doi: 10.1186/1744-8069-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stankewitz A, Aderjan D, Eippert F, May A. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci. 2011;31(6):1937–1943. doi: 10.1523/JNEUROSCI.4496-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu CX, Li B, Xu YK, et al. Altered functional connectivity of the periaqueductal gray in chronic neck and shoulder pain. Neuroreport. 2017;28(12):720–725. doi: 10.1097/WNR.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 32.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci U S A. 2010;107(1):355–360. doi: 10.1073/pnas.0906186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulte LH, Sprenger C, May A. Physiological brainstem mechanisms of trigeminal nociception: an fMRI study at 3T. Neuroimage. 2016;124(Pt A):518–525. doi: 10.1016/j.neuroimage.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Liu M, Lan L, et al. Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Sci Rep. 2016;6:20298. doi: 10.1038/srep20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung A, Zhao Y, Shukla S. The effect of acupuncture needle combination on central pain processing—an fMRI study. Mol Pain. 2014;10:23. doi: 10.1186/1744-8069-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Spaeth RB, Freeman SG, et al. The modulation effect of longitudinal acupuncture on resting state functional connectivity in knee osteoarthritis patients. Mol Pain. 2016;11 doi: 10.1186/s12990-015-0071.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egorova N, Gollub RL, Kong J. Repeated verum but not placebo acupuncture normalizes connectivity in brain regions dysregulated in chronic pain. Neuroimage Clin. 2015;9:430–435. doi: 10.1016/j.nicl.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang J, Jin Z, Wang Y, et al. The salient characteristics of the central effects of acupuncture needling: limbic-paralimbic-neocortical network modulation. Hum Brain Mapp. 2009;30(4):1196–1206. doi: 10.1002/hbm.20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckner RL. The serendipitous discovery of the brain’s default network. Neuroimage. 2012;62(2):1137–1145. doi: 10.1016/j.neuroimage.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 40.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salomons TV, Iannetti GD, Liang M, Wood JN. The “Pain Matrix” in pain-free individuals. JAMA Neurol. 2016;73(6):755–756. doi: 10.1001/jamaneurol.2016.0653. [DOI] [PubMed] [Google Scholar]
- 42.Malfliet A, Coppieters I, Van Wilgen P, et al. Brain changes associated with cognitive and emotional factors in chronic pain: a systematic review. Eur J Pain. 2017;21(5):769–786. doi: 10.1002/ejp.1003. [DOI] [PubMed] [Google Scholar]
- 43.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14(7):502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The whole brain map (group mean) of pre and postacupuncture in each group. The color bar indicates a range of ReHo values between 4.6 (red) and 15 (yellow).
Notes: (A) The whole brain map (group mean) of contralateral group with preacupuncture. (B) The whole brain map (group mean) of contralateral group with postacupuncture. (C) The whole brain map (group mean) of ipsilateral group with preacupuncture. (D) The whole brain map (group mean) of ipsilateral group with postacupuncture.
Abbreviations: L, left; R, right; ReHo, regional homogeneit.