Abstract
Background
The effect of amylases combined with exogenous carbohydrase and protease in a newly harvested corn diet on starch digestibility, intestine health and cecal microbiota was investigated in broiler chickens.
Methods
Two hunderd and eighty-eight 5-day-old female chickens were randomly divided into six treatments: a newly harvested corn-soybean meal diet (control); control supplemented with 1,500 U/g α-amylase (Enzyme A); Enzyme A + 300 U/g amylopectase + 20,000 U/g glucoamylase (Enzyme B); Enzyme B + protease 10,000 U/g (Enzyme C); Enzyme C + xylanase 15,000 U/g (Enzyme D); and Enzyme D + cellulase 200 U/g + pectinase 1,000 U/g (Enzyme E). Growth performance, starch digestibility, digestive organ morphology, and intestinal microbiota were evaluated in the birds at 16 and 23 d of age.
Results
Compared with the control diet, supplementation with Enzyme A significantly decreased ileum lesion scoring at 16 d of age (P < 0.05); supplementation with Enzyme B or Enzyme C showed positive effects on ileal amylopectin and total starch digestibility (P < 0.05); Broilers fed with a diet supplemented with Enzyme D had a tendency to decrease body weight gain at 23 d. Enzyme E supplementation improved lesion scoring of jejunum and ileum at 16 d (P < 0.05), and increased ileal amylopectin or total starch digestibility at 23 d (P < 0.05). Supplementation of enzymes changed cecal microbiota diversity. High numbers of Campylobacter, Helicobacter and Butyricicoccus, Anaerostipes and Bifidobacterium, Sutterella and Odoribacter were the main genera detected in supplementations with Enzymes B, C, D, and E respectively.
Conclusions
Supplementation with amylase combined with glucoamylase or protease showed a beneficial effect on starch digestibility and intestinal microbiota diversity, and increased growth of broilers fed with newly harvested corn.
Electronic supplementary material
The online version of this article (10.1186/s40104-018-0238-0) contains supplementary material, which is available to authorized users.
Keywords: Amylase, Broiler, Glucoamylase, Microbiota diversity, Newly harvested corn, Protease
Background
In China, the production of corn is insufficient to meet the requirements for animal production, so a large amount of newly harvested corn is directly processed as animal feed each autumn. However, this material often causes rapid feed passage, incomplete grain digestion in the small intestine, and reduced performance in livestock.
The proportion of the components of plant feedstuffs for which there is no corresponding endogenous digestive secretions limits the nutrient and energy digestibility by poultry [1]. Cornstarch is composed of amylose (AM) and amylopectin (AP). Amylopectin makes up 70–80% of most starch sources. Amylopectin not only contains α-1, 4 -glucosidic bonds, but also α-1, 6-glucosidic bonds. In animals, only α-amylase can degrade α-1, 4 -glucosidic bonds, and amylopectase is required to hydrolyze amylopectin [1].
Additionally, corn has a high concentration of insoluble non-starch polysaccharides (NSP) such as xylans and cellulose [2], which limit the ability of digestive enzymes to access and fully digest the starch and protein components enclosed within the plant cells [3]. A previous study showed that starch granules are different in size and composition depending on the type and variety of cereal as well as the age of the cells in the developing endosperm [4]. Corn has large amounts of native trypsin inhibitors, ranging between 0.56 and 1.87 mg/g dry matter (DM) [5], which imposes restrictions on enzyme access to substrates, and is associated with high digesta viscosity in broiler chickens [6]. Slower digesta passage rate and impaired nutrient digestion, as well as greatly increased water intake provides greater opportunities for growth of Clostridium perfringens in the upper gut of broiler chickens [7, 8], which has a negative influence on gut health [9].
Supplementation with α-amylase shows a positive effect on digestibility of starch and organic matter, AME (apparent metabolizable energy) of diet, as well as feed conversion [10, 11]. However, no improvement in starch digestibility was observed by Mahagna [12] and Kaczmarek, [13] when α-amylase alone was supplemented to feed for broiler chicks during the first 14 d of age, and no beneficial effect on performance was observed by Yegani and Korver [14] and Kaczmarek [13].
Supplementation with appropriate NSP-degrading enzymes in new season grains diets could help hydrolyze NSP, reduce digesta viscosity and improve nutrient digestibility, thus increasing body-weight gain and feed conversion of broilers fed a corn-based diet [15]. Xylanase may increase the access of cell contents in feed to endogenous digestive enzymes due to hydrolysis of cell wall arabinoxylans and thus reduce the anti-nutritional effect of some polysaccharides [2, 16, 17]. Moreover, mixed enzyme preparations can effectively degrade indigestible cell constituents [18] or a large array of feed ingredients [19]. Supplementation with amylase and xylanase increases starch digestibility in the jejunum and ileum, AME and growth performance in broilers [1].
The gastrointestinal tract is populated with diverse assemblages of microbiota that play critical roles in the overall well-being of an animal [20]. The diversity and abundance of gut microbiota are affected by the dietary composition as well as by its digestibility [2, 20, 21]. NSPase supplementation improves digesta passage and nutrient digestion rate, leading to fewer opportunities for interaction between pathogens and substrates [22], and reducing levels of Clostridium perfringens [7]. Traditional identification methods for bacteria have limitations, wherase 16S rDNA analysis can identify all species present in a habitat including those that cannot be cultivated by conventional techniques [23]. 16S rRNA gene sequencing has been employed in a range of studies to assess the diversity and phylogenetic relationships of gut microbes and this has been proven to be a powerful tool for understanding the factors that shape microbial communities due to its informative and predictive potential [24]. Supplementation with NSPase can alter microbial profiles, as detected using Miseq Illumina sequencing [19].
Although much research has studied the effect of multienzyme preparations in corn/wheat-soy bean (SBM) diets, little is known of multienzyme preparations in newly harvested corn-SBM diets. The purpose of this experimental design was to use feed supplemented with amylase and combined with exogenous carbohydrases and/or protease to study the effect of different enzyme cocktails on the performance, nutrient utilization, hormone secretion, gut health and microbial profiles in broiler chickens.
Methods
The present study was approved by China Agricultural University and conducted in accordance with the Guidelines for Experimental Animals.
Enzymes
The enzymes were all purchased from Beijing Huameiyuan Biotechnology Co., LTD (Beijing). Alpha-amylase (Aspergillus oryzae) 3,000 U/g, amylopectase (Bacillus amyloliquofaciens) 2,000 U/g, glucoamylase (Aspergillus usamil) 200,000 U/g, protease (Bacillus subtilis) 800,000 U/g, xylanase (Trichoderma longibrachiatum) 240,000 U/g, cellulase (Trichoderma harziamum) 10,000 U/g, and pectinase (Aspergillus niger) 30,000 U/g (according to the manufacturer’s declaration).
Birds and treatment diets
One-day-old Cobb 500 female-chicks were purchased from a commercial hatchery. Birds were raised in battery cages, fed ad libitum with commercial broiler starter diet without enzyme for the first 5 d. At 5 d, the chickens were individually weighed, and 288 chicks with similar body weight were then randomly allotted to six dietary treatments (six replicates per diet with eight chicks). The basal diet (control) composition and nutrient content is provided in Table 1. The corn used in the diet was newly harvested Zhengdan 958, sun dried for 1 wk post-harvest and contained about 14% moisture. The other five treatments were: Enzyme A (1,500 U/g α-amylase); Enzyme B (Enzyme A + 300 U/g amylopectase+ 20,000 U/g glucoamylase); Enzyme C (Enzyme B + protease 10,000 U/g); Enzyme D (Enzyme C + 15,000 U/g xylanase); and Enzyme E (Enzyme D + 200 U/g cellulase + 1,000 U/g pectinase). The nutrient values met or exceeded the recommendations of NY/T33–2004 [25].
Table 1.
Composition of basal diet
| Ingredients | Inclusion rate | Nutrient content | |
|---|---|---|---|
| Corn | 51.60 | AME, Mcal/kg | 2.95 |
| Soybean meal | 36.20 | CP, % | 21.00 |
| Corn gluten meal | 5.00 | Lys, % | 1.20 |
| Soy oil | 2.50 | Met, % | 0.50 |
| Calcium hydrophosphate | 2.10 | M + C | 0.85 |
| Limestone | 1.00 | Thr | 0.85 |
| Salt | 0.30 | Ca, % | 1.00 |
| Minerals Premixa | 0.20 | AP, % | 0.44 |
| Vitamin Premixb | 0.03 | ||
| 50% Choline chloride | 0.20 | ||
| DL-Methionine, 98% | 0.20 | ||
| L-Lysine HCl, 98% | 0.14 | ||
| Butylated hydroxytoluene (33%) | 0.03 | ||
| Titanium dioxide | 0.50 |
aProvided per kg diet: Cu (CuSO4·5H2O), 8 mg; Zn (ZnSO4·H2O), 75 mg; Fe (FeSO4·H2O), 80 mg; Mn (MnSO4·H2O), 100 mg; Se (Na2SeO3), 0.30 mg; I [Ca(IO3)2·H2O], 0.35 mg
bProvided per kg diet: vitamin A (as retinyl acetate), 12,500 U; cholecalciferol, 2500 U; vitamin E (as DL-α-tocopherol acetate), 18.75 mg; menadione, 2.65 mg; thiamine, 2.5 mg; riboflavin, 6.0 mg; pyridoxine, 4.9 mg; pantothenic acid, 12 mg; niacin, 50 mg; folic acid, 1.25 mg; biotin, 0.0325 mg; cobalamine, 0.025 mg
Animal rearing conditions
All birds were housed in two tier cages (100 cm × 70 cm) in an environmentally controlled house. The room temperature was initially set at 33°C then gradually reduced according to the age of the birds until reaching 23 °C at 21 d. Birds were kept on a 23:1 Light/Dark regimen with free access to feed and water. At d 16 post-treatment, one bird per replicate was removed for sampling. Between d 16 and 23, diets in all treatments were supplemented with 0.5% TiO2 (an inert marker). Body weight and feed intake per cage were recorded weekly to determine growth and feed efficiency. On d 23 birds were killed by intracardial administration of sodium pentobarbital (50 mg/kg of body weight) into the wing vein. Immediately following euthanasia the abdominal cavity was opened, the pancreas, liver and small intestines were removed, and the organ weights relative to body weight (BW) were calculated.
Histo-morphology of the intestinal mucosa
At d 23, the duodenum, jejunum, and ileum were located and opened lengthwise. The intestine was examined microscopically and scored for gross lesions following a 0 to 6 scoring system described by Keyburn et al. [26]. Approximately 1 cm from each segments of the intestine were cut and placed separately in a 10% formalin solution for further processing to examine histo-morphological changes in the intestinal mucosa. The light microscopy evaluation and measurement of intestinal mucosal villus height and crypt depth were performed on a Zeiss Axiophot microscope (Carl Zeiss, Oberkochen, Germany). Measurements of villus height and crypt depth were made on 10 villi at 100× and 200× magnifications with imaging software (Image Pro Plus, Version 4.1.0.9; Media Cybernetics, Silver Spring, MD).
Microbiota analysis
Fresh cecal samples were collected and stored at − 80 °C for further analysis. DNA was extracted from 0.18 to 0.22 g of cecal digesta using a QIA amp DNAStool Mini Kit (Qiagen). The DNA was recovered with 30 mL of AE buffer (10 mmol/L Tris-Cl, 0.5 mmol/L EDTA, pH 9.0; Qiagen). The 16S ribosomal RNA (rRNA) gene was analyzed to evaluate the bacterial diversity using Illumina Miseq (Novogene Bioinformatics Technology Co., Ltd.).
Pyrosequencing
The normalized concentration of purified genomic DNA was used as a template to analyzed microbial communities. PCR amplification was performed using Takara Ex-Taq polymerase (Takara Bio, Shiga, Japan). The V3-V4 region of the 16S rRNA gene was amplified using eubacterial primers (341F: ACTCCTACGGGAGGCAGCAG, 806R: GGACTACHVGGGTWTCTAAT). PCR reactions were performed by initial denaturation at 94 °C for 3 min and then 28 cycles of 94 °C for 30 s, 53 °C for 40 s and 72 °C for 1 min, followed by a final elongation step at 72 °C for 5 min. Amplicon libraries were separated by agarose gel electrophoresis and purified using a QIA quick Gel Extraction Kit (Qiagen, Valencia, CA, USA). The DNA libraries were sequenced on Illumina HiSeq 2500 PE250 platform (Illumina, San Diego, US) for paired-end reads of 300 bp. The 16S rRNA gene sequences determined in this study were deposited in the NCBI Sequence Read Archive (SRA) database.
Data processing
Sequence reads were analyzed by the quantitative insights into microbial ecology (QIME) software package (Uparse v7.0.1001, http://drive5.com/uparse/). Briefly, the raw sequence reads were quality trimmed and demultiplexed. The sequence reads were then clustered into operational taxonomic units (OTUs) by de novo OTU picking at a 97% level of sequence similarity. The taxonomic assignments for each representative sequence were obtained using the uclust consensus taxonomic classifier using the GreenGenes 13_8 database.
The microbial diversity indices of the samples (alpha diversity) were determined using the abundance-based coverage estimator (ACE), Chao1, observed OTUs, Shannon, and Simpson methods. Principal component analysis (PCA) was performed at the phylum and genus level. The abundance of microbial taxa was expressed as a percentage of the total 16S rRNA gene sequences, and the differences between the treatments were compared.
Digestibility of nutrients
At 23 d of age, three birds per replicate were randomly selected and killed by intracardial administration of 5% sodium pentobarbital. Digesta of proximal and distal of ileum were collected gently, mixed and then freeze-dried, ground and analyzed for total starch, amylopectin, amylose and TiO2. The TiO2 was performed by the spectrophotometic method AOAC (975.21). Starch was determined by the α-amylase glucosidase method (996.11), as described by the Association of Official Analytical Chemists (2000) [27]. Amylose (AM) and amylopectin (AP) were determined using the method of Lin et al. [28]. The apparent ileal digestibility of nutrient or energy was calculated as described by Edwards and Gillis [29].
Blood biochemistry
At 16 d and 23 d of age, one bird per pen was selected. Blood samples were taken from the wing vein and centrifuged at 3,600×g for 10 min to obtain serum to determine glucose (GLU), triglyceride (TG), total cholesterol (TC) and total bile acid (TBA) by using commercial analytical kits, according to manufacturer’s recommendations (Jian Cheng Bioengineering Institute, Nanjing, China). The content of insulin (INS), proglucagon (PG), triiodothyronine (T3) and tetraiodothyronine (T4) was determined by radioimmunoassay at the Beijing Sino-UK Institute of Biological Technology (Beijing, China).
Statistical analysis
The results were analyzed using PROC ANOVA and Duncan’s Multiple Range Test using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL) [30]. Percentage values were arcsine-transformed before analysis. Differences among means with P ≤ 0.05 were accepted as statistically significant.
Results
Growth and feed efficiency
Supplemented enzymes did not affect body weight gain (BWG) over 5–16 d of age (Table 2). There was a tendency difference between treatments on BWG during 17–23 d of age (P < 0.1). Supplemented Enzyme B and Enzyme C improved BWG of broilers between 17 and 23 d of age compared with Enzyme A or Enzyme D (P ≤ 0.05). Supplemented enzymes did not affect the average feed intake or feed conversion ratio (FCR) of the broilers (P > 0.05).
Table 2.
Effect of enzyme1 supplementation in poultry diet on performance2
| Treatments | BWG, g | AFI, g | FCR | |||
|---|---|---|---|---|---|---|
| 5-16 d | 17-23 d | 5-16 d | 17-23 d | 5-16 d | 17-23 d | |
| Control | 295 | 272 | 447 | 843 | 1.52 | 1.51 |
| Enzyme A | 303 | 247 | 458 | 845 | 1.51 | 1.56 |
| Enzyme B | 313 | 280 | 464 | 897 | 1.48 | 1.51 |
| Enzyme C | 318 | 277 | 461 | 887 | 1.45 | 1.48 |
| Enzyme D | 310 | 242 | 456 | 850 | 1.47 | 1.55 |
| Enzyme E | 313 | 255 | 454 | 859 | 1.45 | 1.52 |
| SEM | 4.0 | 5.0 | 4.6 | 4.6 | 0.017 | 0.024 |
| P-value | 0.527 | 0.061 | 0.877 | 0.416 | 0.794 | 0.769 |
1Enzyme A (1,500 U/g α-amylase; Enzyme B (Enzyme A + 300 U/g amylopectase + 20,000 U/g glucoamylase); Enzyme C (Enzyme B + protease 10,000 U/g); Enzyme D (Enzyme C + 15,000 U/g xylanase); Enzyme E (Enzyme D + 200 U/g cellulase + 1,000 U/g pectinase)
2ABW Average body weight, BWG Body weight gain, AFI Average feed intake, FCR Feed conversion ratio
Digestibility and serum metabolites
Supplementation of enzymes did not affect amylose and amylopectin digestibility in proximal ileum, but supplemented Enzymes B, C and E increased distal ileal amylopectin and total starch digestibility in broilers at 23 d of age (P ≤ 0.05) (Table 3). Supplementation with Enzymes B, C, D or E significantly (P ≤ 0.05) increased serum glucose at d 16 compared to the control (Table 4). Similarly, supplementation with Enzymes C, D or E significantly increased serum TC content of broilers at 16 d of age compared with the control (P ≤ 0.05). Supplemented Enzyme E significantly decreased serum TBA content compared with the control (P ≤ 0.05) of broilers at 16 d of age. However, at d 23, supplementation with Enzyme B or Enzyme D significantly decreased serum TBA content of broilers compared with the control (P ≤ 0.05). There were differences in hormonal changes among treatments at d 16, but not d 23. There was no pattern of change of serum INS with respect to the treatments. However, plasma PG was significantly reduced in all treatment groups compared with the control. Both T3 and T4 appeared to increase in the treatment groups compared to the control (Table 5).
Table 3.
Effect of enzyme1 supplementation in poultry diet on starch digestibility
| Treatments | Proximal ileum | Distal ileum | ||||
|---|---|---|---|---|---|---|
| Amylose, % | Amylopectin, % | Total starch, % | Amylose, % | Amylopectin, % | Total starch, % | |
| Control | 95.64 | 93.38 | 93.82 | 97.86 | 93.89c | 94.87c |
| Enzyme A | 97.06 | 95.13 | 95.38 | 97.61 | 96.12abc | 96.51abc |
| Enzyme B | 97.47 | 97.09 | 97.20 | 97.80 | 97.08ab | 97.29ab |
| Enzyme C | 97.93 | 96.27 | 96.71 | 97.93 | 97.58a | 97.67a |
| Enzyme D | 96.33 | 94.71 | 94.74 | 97.29 | 94.12bc | 94.94bc |
| Enzyme E | 97.39 | 96.51 | 96.73 | 98.47 | 97.30a | 97.60a |
| SEM | 0.550 | 0.647 | 0.607 | 0.416 | 0.592 | 0.492 |
| P-value | 0.266 | 0.256 | 0.080 | 0.671 | 0.033 | 0.025 |
1Enzyme A (1,500 U/g α-amylase); Enzyme B (Enzyme A + 300 U/g amylopectase + 20,000 U/g glucoamylase); Enzyme C (Enzyme B + protease 10,000 U/g); Enzyme D (Enzyme C + 15,000 U/g xylanase); Enzyme E (Enzyme D + 200 U/ g cellulase + 1,000 U/g pectinase)
Table 4.
Effect of enzyme1 supplementation in poultry diet on serum biochemical indices
| Treatments | Glu, mmol/L | TG, mmol/L | TC, mmol/L | TBA, mmol/L | ||||
|---|---|---|---|---|---|---|---|---|
| 16 d | 23 d | 16 d | 23 d | 16 d | 23 d | 16 d | 23 d | |
| Control | 11.69c | 13.31 | 0.77 | 0.46 | 2.51c | 2.85 | 4.45a | 5.60a |
| Enzyme A | 12.40bc | 12.96 | 0.48 | 0.39 | 2.84bc | 2.91 | 3.48ab | 3.84abc |
| Enzyme B | 12.63ab | 12.22 | 0.52 | 0.44 | 2.86bc | 2.71 | 4.05a | 2.16c |
| Enzyme C | 13.30a | 12.28 | 0.55 | 0.48 | 3.24ab | 2.81 | 2.75ab | 5.03ab |
| Enzyme D | 12.67ab | 13.66 | 0.53 | 0.44 | 2.98ab | 3.08 | 3.17ab | 3.09bc |
| Enzyme E | 12.88ab | 12.56 | 0.54 | 0.39 | 3.34a | 3.14 | 1.86b | 3.70abc |
| SEM | 0.132 | 0.173 | 0.033 | 0.014 | 0.070 | 0.081 | 0.246 | 0.350 |
| P-value | 0.010 | 0.080 | 0.123 | 0.327 | 0.004 | 0.664 | 0.033 | 0.047 |
1Enzyme A(1,500 U/g α-amylase); Enzyme B(Enzyme A + 300 U/g amylopectase + 20,000 U/g glucoamylase); Enzyme C (Enzyme B + protease 10,000 U/g); Enzyme D (Enzyme C + 15,000 U/g xylanase); Enzyme E (Enzyme D + 200 U/ g cellulase + 1,000 U/g pectinase)
Glu glucose, TG triglyceride TC total cholesterol, TBA total bile acid
Table 5.
Effect of enzyme1 supplementation of in poultry diet on serum endocrine hormone levels
| Treatments | INS, μIU/mL | PG, pg/mL | T3, ng/mL | T4, ng/mL | ||||
|---|---|---|---|---|---|---|---|---|
| 16 d | 23 d | 16 d | 23 d | 16 d | 23 d | 16 d | 23 d | |
| Control | 12.75bc | 11.55 | 121.52a | 86.25 | 0.65b | 0.69 | 18.47b | 20.55 |
| Enzyme A | 13.21b | 12.49 | 90.11b | 78.60 | 0.80ab | 0.68 | 19.54b | 22.55 |
| Enzyme B | 15.55a | 12.23 | 85.86b | 84.84 | 0.97a | 0.64 | 28.97a | 24.52 |
| Enzyme C | 10.85c | 12.69 | 90.14b | 73.07 | 0.92a | 0.68 | 22.36b | 22.41 |
| Enzyme D | 13.08b | 13.59 | 98.52b | 79.97 | 0.84ab | 0.72 | 19.05b | 23.55 |
| Enzyme E | 12.79bc | 11.77 | 88.89b | 82.26 | 0.79ab | 0.75 | 23.33ab | 28.37 |
| SEM | 0.345 | 0.258 | 3.053 | 1.511 | 0.029 | 0.025 | 0.991 | 1.219 |
| P-value | 0.002 | 0.306 | 0.002 | 0.131 | 0.017 | 0.884 | 0.011 | 0.580 |
1Enzyme A(1,500 U/g α-amylase); Enzyme B(Enzyme A + 300 U/g amylopectase + 20,000 U/g glucoamylase); Enzyme C (Enzyme B + protease 10,000 U/g); Enzyme D (Enzyme C + 15,000 U/g xylanase); Enzyme E (Enzyme D + 200 U/ g cellulase + 1,000 U/g pectinase)
INS insulin, PG proglucagon, T3 triiodothyronine, T4 tetraiodothyronine
Organ weight, lesion scores and intestine anatomy
There were no differences in liver and pancreas weights at 16 and 23 d of age with treatment, except for the significant difference between Enzymes B and E regarding pancreas weight at 23 d (Table 6). At 16 d of age, there were minor weight differences in duodenum, jejunum and ileum among treatments. However, the weights of the different sections of the intestines were all significantly heavier in the control group compared with the treatment groups. By d 23, there were no significant differences in the duodenum and ileum between the control and enzyme supplementation groups. The jejunal weight in the group supplemented with Enzyme E was significantly lighter than the control and all treatment groups except Enzyme A. Supplemented enzymes significantly affected lesion scoring of the intestine of broilers at 16 d but not 23 d of age (Table 7). Enzyme supplementation generally reduced intestinal lesion scores at d 16. Supplemented Enzyme D significantly decreased lesion scoring of the duodenum compared with the control (P ≤ 0.05). In the jejunum and ileum, supplementation with Enzymes C, D and E significantly reduced lesion scores compared with the control. Villi height was significantly higher in the enzyme-treated groups compared to the control at 16 d, but at 23 d the differences had disappeared (Table 8). Consequently, the ratio of villi height and crypt depth was significantly higher in the treatment groups compared with the control at d 16, but not at d 23.
Table 6.
Effect of enzyme1 supplementation in poultry diet on the relative weight of digestive organs (%)
| Treatments | d 16 | d 23 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Duodenum | Jejunum | Ileum | Liver | Pancreas | Duodenum | Jejunum | Ileum | Liver | Pancreas | |
| Control | 1.48a | 2.60 | 1.93 | 1.08 | 0.45 | 1.27 | 2.22ab | 1.82 | 2.31 | 0.40ab |
| Enzyme A | 1.37a | 2.18 | 1.73 | 1.13 | 0.41 | 1.10 | 1.92cd | 1.33 | 2.31 | 0.35bc |
| Enzyme B | 1.20b | 2.29 | 1.83 | 1.19 | 0.42 | 1.12 | 2.35a | 1.65 | 2.41 | 0.42a |
| Enzyme C | 1.40a | 2.30 | 1.57 | 1.03 | 0.40 | 1.14 | 2.06abc | 1.66 | 2.42 | 0.37abc |
| Enzyme D | 1.41a | 2.14 | 1.65 | 1.14 | 0.41 | 1.21 | 2.05bc | 1.67 | 2.46 | 0.35bc |
| Enzyme E | 1.38a | 2.19 | 1.56 | 1.13 | 0.40 | 1.09 | 1.73d | 1.50 | 2.34 | 0.34c |
| SEM | 0.021 | 0.050 | 0.047 | 0.024 | 0.010 | 0.030 | 0.049 | 0.057 | 0.037 | 0.009 |
| P-value | 0.001 | 0.072 | 0.077 | 0.542 | 0.848 | 0.488 | 0.001 | 0.180 | 0.805 | 0.033 |
1Enzyme A(1,500 U/g α-amylase); Enzyme B(Enzyme A + 300 U/g amylopectase + 20,000 U/g glucoamylase); Enzyme C (Enzyme B + protease 10,000 U/g); Enzyme D (Enzyme C + 15,000 U/g xylanase); Enzyme E (Enzyme D + 200 U/ g cellulase + 1,000 U/g pectinase), respectively
Table 7.
Effect of enzyme1 supplementation in poultry diet on lesion scoring of the intestine
| Treatments | d 16 | d 23 | ||||
|---|---|---|---|---|---|---|
| Duodenum | Jejunum | Ileum | Duodenum | Jejunum | Ileum | |
| Control | 2.50a | 2.60a | 2.40a | 1.17 | 2.00 | 1.67 |
| Enzyme A | 1.33ab | 1.50ab | 1.00bc | 0.40 | 2.17 | 1.33 |
| Enzyme B | 1.50ab | 1.50ab | 1.67ab | 1.00 | 1.83 | 1.83 |
| Enzyme C | 1.17ab | 0.60b | 0.33c | 0.80 | 2.00 | 2.40 |
| Enzyme D | 0.67b | 0.83b | 0.67bc | 1.20 | 2.00 | 1.60 |
| Enzyme E | 1.40ab | 0.50b | 1.00bc | 1.00 | 2.17 | 1.83 |
| SEM | 0.197 | 0.224 | 0.258 | 0.174 | 0.213 | 0.207 |
| P-value | 0.033 | 0.023 | 0.005 | 0.838 | 0.998 | 0.828 |
1Enzyme A (1,500 U/g α-amylase); Enzyme B (Enzyme A + 300 U/g amylopectase + 20,000 U/g glucoamylase); Enzyme C (Enzyme B + protease 10,000 U/g); Enzyme D (Enzyme C + 15,000 U/g xylanase); Enzyme E (Enzyme D + 200 U/ g cellulase + 1,000 U/g pectinase)
Table 8.
Effect of enzyme1 supplementation in poultry diet on intestinal histomorphology
| Treatments | Duodenum | Jejunum | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Villus height, μm | Crypt depth, μm | V/C | Villus height, μm | Crypt depth, μm | V/C | ||||
| 16 d | 23 d | 16 d | 23 d | 16 d | 23 d | 23 d | 23 d | 23 d | |
| Control | 1189a | 1542 | 280 | 241 | 4.42a | 6.60b | 943 | 194 | 5.43 |
| Enzyme A | 1774b | 1822 | 296 | 257 | 6.43b | 7.31ab | 1050 | 207 | 5.29 |
| Enzyme B | 1717 b | 1648 | 254 | 267 | 7.08b | 6.47b | 953 | 211 | 4.93 |
| Enzyme C | 1736b | 1768 | 268 | 250 | 7.06b | 7.51ab | 977 | 218 | 4.66 |
| Enzyme D | 1828b | 1875 | 275 | 271 | 7.02b | 7.39ab | 880 | 195 | 4.82 |
| Enzyme E | 1894b | 1816 | 257 | 212 | 7.68b | 8.89a | 1037 | 182 | 5.92 |
| SEM | 49.2 | 37.8 | 9.3 | 7.1 | 0.260 | 0.238 | 19 | 5.8 | 0.195 |
| P-value | < 0.001 | 0.092 | 0.817 | 0.185 | 0.002 | 0.041 | 0.089 | 0.515 | 0.468 |
1Enzyme A (1,500 U/g α-amylase); Enzyme B (Enzyme A + 300 U/g amylopectase + 20,000 U/g glucoamylase); Enzyme C (Enzyme B + protease 10,000 U/g); Enzyme D (Enzyme C + 15,000 U/g xylanase); Enzyme E (Enzyme D + 200 U/ g cellulase + 1,000 U/g pectinase), respectively
Microbial profile
A total of 1,364,610 sequence reads were recovered from a total of 24 samples from 6 treatments. Average Rarefaction curves indicated that a high coverage had been achieved in all samples (Additional file 1: Figure S1). The number of OTUs and sample richness and diversity are shown in Table 9. Good’s coverage index was almost constant (0.995~ 0.996) in the six treatments, which indicated a high coverage. The samples from the group supplemented with Enzyme E had lowest number (529) of OTUs while samples from the control group showed the highest number (595) of OTUs among treatments.
Table 9.
The number of OTUs and sample richness and diversity of cecal microbiota
| Treatments | OTUs per sample | Good’s coverage | Chao1 (richness) | Shannon (diversity) | Simpson (diversity) |
|---|---|---|---|---|---|
| Control | 595 | 0.995 | 581.59 | 5.89 | 0.95 |
| Enzyme A | 568 | 0.995 | 631.27 | 5.78 | 0.96 |
| Enzyme B | 537 | 0.995 | 571.41 | 5.45 | 0.94 |
| Enzyme C | 555 | 0.996 | 586.81 | 5.72 | 0.95 |
| Enzyme D | 546 | 0.996 | 524.78 | 5.86 | 0.95 |
| Enzyme E | 529 | 0.996 | 492.43 | 5.64 | 0.94 |
Enzyme A (1,500 U/g α-amylase); Enzyme B (Enzyme A + 300 U/g amylopectase + 20,000 U/g glucoamylase); Enzyme C (Enzyme B + protease 10,000 U/g); Enzyme D (Enzyme C + 15,000 U/g xylanase); Enzyme E (Enzyme D + 200 U/ g cellulase + 1,000 U/g pectinase)
The richness of the bacterial community is reflected by Chao 1 index, which ranged from 492.43 to 631.27 among all treatments (Additional file 1:Figure S2 ). The Shannon and Simpson indices, reflecting the bacterial community diversity, ranged from 5.45 to 5.89 and 0.94 to 0.96, respectively (Additional file 1: Figure S2 ). However, based on those sequence metrics and sample richness and diversity parameters, there were no significant differences among all treatments. As shown in Fig. 1, the relationships among bacterial communities from different treatments were represented by principal component analysis (PCA). Based on Bray-Curtis dissimilarity, ANOSIM showed statistically significant differences in bacterial community among the treatments (P ≤ 0.05).
Fig. 1.
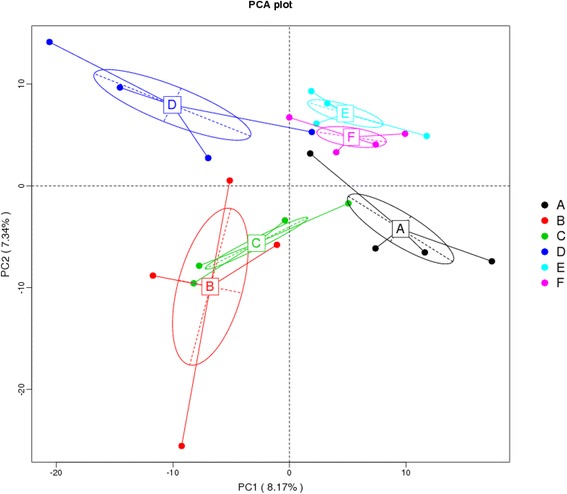
Principal component analysis (PCA) of microbiota community by Bray-Curtis distance. The circles were drawn around microbiota from the same treatment. Treatment information: (a): control; (b): Enzyme A (1,500 U/g α-amylase; C: Enzyme B(Enzyme A + 300 U/g amylopectase + 20,000 U/g glucoamylase); D: Enzyme C (Enzyme B + 10,000 U/g protease); E: Enzyme D (Enzyme C + 15,000 U/g xylanase); F: Enzyme E (Enzyme D + 200 U/g cellulase + 1,000 U/g pectinase)
The most abundant phylum was Fimicutes, followed by Bacteroidetes (Fig. 2a). The relative abundances of Fimicutes and Bacteroidetes ranged from 45.24% to 60.28% and 25.98% to 32.90%, respectively (S1 Table). Those two dominant phyla dominated around 80% of the bacterial community. The remaining 20% of the bacterial population belonged to Proteobacteria, Cyanobacteria, Actinobacteria, Tenericutes, Acidobacteria, Synergistetes, Euryarchaeota, Verrucomicrobia and others (Fig. 2a and Additional file 1: Table S1). The number of OTUs classified as Acidobacteria phylum increased (P ≤ 0.05) in the group supplemented with Enzyme C compared with supplementation of Enzyme D or Enzyme E. Supplementation of Enzyme D significantly decreased the number of OTUs classified as Synergistetes phylum, while the relative abundance of Synergistetes phylum was increased in the control group (P ≤ 0.05). Most OTUs were classified within the families Ruminococcaceae and Porphyromonadaceae, followed by Lachnospiraceae, Veillonellaceae, Lactobacillaceae, Rikenellaceae, Bacteroidaceae and Acidaminococcaceae (Fig. 2b and Additional file 1: Table S2). The OTUs, belonging to the family Lactobacillaceae, were significantly enriched in the groups supplemented with Enzyme A or Enzyme B, and those OTUs were uniformly depleted in the presence of Enzyme D or Enzyme E. Adding Enzyme B or Enzyme C to diets decreased the number of OTUs classified as family Acidaminococcaceae when compared with the control group. Similarly, the abundance of Lactobacillus, belonging to Lactobacillaceae family (Additional file 1: Figure S3), was significantly increased with supplemented Enzyme A or Enzyme B while those bacteria were depleted in the groups with Enzyme D or Enzyme E (Fig. 2c and Additional file 1: Table S3). Meanwhile, the administration of Enzyme B or Enzyme C resulted in a decreased representation of the abundance of Phascolarctobacterium (Fig. 2c and Additional file 1: Table S3), which was most dominant in Acidaminococcaceae family (Additional file 1: Figure S3).
Fig. 2.
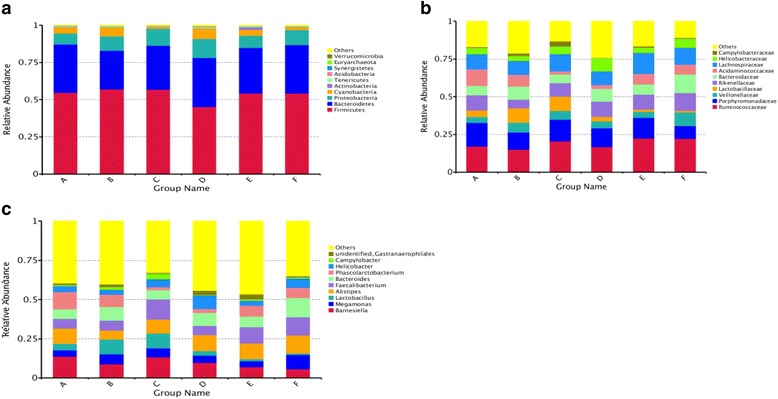
Relative abundance of the dominant bacterial communities in the cecal digesta of 23-day-old broiler chickens at phylum (a), family (b) and genus (c) level
Each bar represents the relative abundance of each treatment. Each color represents a particular bacterial phylum. A: the control; B: Enzyme A (1,500 U/g α-amylase; C: Enzyme B(Enzyme A + 300 U/g amylopectase + 20,000 U/g glucoamylase); D: Enzyme C (Enzyme B+ 10,000 U/g protease); E: Enzyme D (Enzyme C + 15,000 U/g xylanase); F: Enzyme E (Enzyme D + 200 U/g cellulase + 1,000 U/g pectinase).
The heatmap in Fig. 3 shows the relative abundances of various bacteria at the genus level in the different treatments. The primary genera of bacteria in the control group were in the Ruminococcaceae, phylum Firmicutes, and included Anaerotruncus, Tyzzerella and Phascolarctobacterium. Additionally, the number of Chloroplast and Barnesiella also increased in the control group. Supplementation with Enzyme A shifted the bacterial community, and many Coprobacter and Parasutterella were detected. Many Campylobacter were associated with supplementation by Enzyme B. The primary genera were Helicobacter and Butyricicoccus in the group supplemented with Enzyme C. In contrast to other groups, the group supplemented with Enzyme D was enriched in Anaerostipes, Bifidobacterium, Collinsella, Staphylococcus, Parabacteroides, Sutterella and Odoribacter. In the group supplemented with Enzyme E, Bacteroides and Megamonas were the primary genera.
Fig. 3.
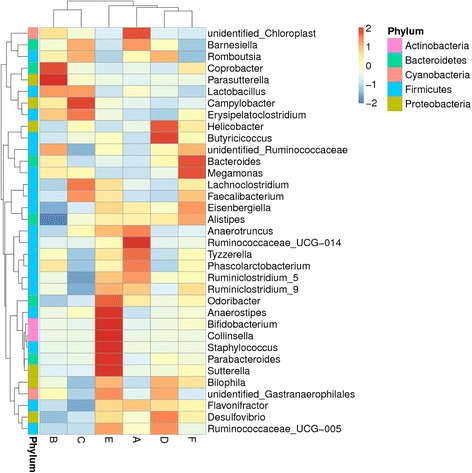
Hierarchically clustered heatmap analysis of the highly represented bacterial taxa (at the genus level) found in the cecal digesta communities of 23-day-old broiler chickens. The relative percentages (%) of the bacterial genus are indicated by varying color intensities according to the legend at the top of the figure. Bacterial genus names are listed on the right side of the heatmap, and the phylum names are listed on the left. Dendrograms at the top and left of the map indicate the phylogenetic relationship between the treatments and genus; the intensity of the cell color represents the abundance of the amplicons belonging to each genus. (a): the control; (b): Enzyme A (1,500 U/g α-amylase; (c): Enzyme B (Enzyme A + 300 U/g amylopectase + 20,000 U/g glucoamylase); (d): Enzyme C (Enzyme B+ 10,000 U/g protease); (e): Enzyme D (Enzyme C + 15,000 U/g xylanase); (f): Enzyme E (Enzyme D + 200 U/g cellulase + 1,000 U/g pectinase)
Discussion
Corn (grain) contains approximately 9% NSP [31], and improved digestibility of NSP is expected to increase the nutrient availability. When the broiler diet was supplemented with only α-amylase, the total starch digestibility improved slightly, but the improvement was not significant. Based on the literature, data on diet supplementation with α-amylase are conflicting. Whereas Mahagna et al. [12], Ritz et al. [26] and Shapiro and Nir [28] observed no improvement in digestibility, Jiang et al. [11] observed significant improvements in performance due to α-amylase supplementation. Svihus [32] argues that specific diet properties, cereal type, inclusion level and bird related factors might contribute to the variation in the outcomes of supplementing poultry diets with α-amylase. Glucoamylase is expected to hydrolyze both α-1, 4- and α-1, 6-glucosidic linkage bonds, and amylopectase is expected to hydrolyze α-1, 6 bonds; thus, Enzyme B should be most likely to improve total starch digestion. In fact, Enzyme B slightly improved the digestibility of amylopectin in the proximal ileum. Because starch is composed of 70–80% amylopectin, a slight improvement in amylopectin digestion suggests that including a combination of amylopectase and α-amylase in poultry diets could improve starch digestibility compared with α-amylase alone. However, the inclusion of Enzyme B did not significantly change growth, feed intake or feed efficiency. We observed that Enzymes D and E decreased intestine and pancreas weights. Exogenous supplementation with certain enzymes could be antagonistic to secretion of some endogenous enzymes. Mahagna et al. [12] reported a similar observation.
The degree to which exogenous enzymes added to poultry diet may affect performance depends on the type and amount of cereal in the diet, the level of anti-nutritional factors in the diet, the spectrum and level of enzymes and the type of animal receiving the feed [33]. Choct et al. [7] found that corn contains protease and other inhibitors. A bi-functional Hageman factor inhibitor from corn inhibits endogenous protease and amylase activities [5, 34], demonstrating that the average native trypsin inhibitor activity (TIA) was 1.27 ± 0.33 mg/g DM and ranged between 0.56 and 1.87 mg/g DM in 12 corn kernel samples from Germany and Austria. Therefore, supplementing feed with proteases could improve the digestion of protein-based anti-nutrients. In the current study, protease was included in the Enzyme C supplementation, and this treatment significantly improved distal ileum starch digestibility compared with that in the control diet. Including proteases in the diet may change the starch-protein interface and affect digestibility [35]. Anti-nutrition factors are primary determinants of the availability of energy and other nutrients for poultry [6], which can form a viscous gel in the gut and affect the rates of digestion and absorption of nutrients, leading to diarrhea and reducing animal performance [36]. Although we did not study the rate of passage of digesta, we observed some stickiness among the fecal droppings in the control.
A large proportion of starch is entrapped in cell wall material, particularly from areas of the endosperm close to the aleurone layer [37]; therefore, including enzymes with the capability of digesting cell wall material should make starch more easily accessible to starch digesting enzymes. Xylanase may increase access to entrapped nutrient components by destroying some fractions of the plant cell walls of grains, allowing α-amylase access to starch fractions [38, 39]. Additional amylase and protease can effectively degrade indigestible cell constituents and increase the digestibility of nutrients in the broiler feed [18]. In previous studies, for birds fed corn-soybean diets supplemented with exogenous enzymes, nutrient digestibility increased [15, 40], nutritive variation of ingredients was eliminated and performance improved [41]. Munyaka et al. [19] showed that xylanase and β-glucanase increased starch digestibility in birds fed a wheat-based diet. Supplementation with amylase and xylanase increase starch digestibility in the jejunum and ileum by 3.5% and 2.4%, respectively, leading to improvements in growth performance, energy and starch digestibility in broilers [1]. However, in the current study, supplementation with NSP-degrading enzymes D (xylanase) and E (pectinase) did not benefit performance, although Enzyme E marginally increased digestibility of amylopectin and total starch. Others show that the effects of enzymes may not always be beneficial and that some low-molecular-weight NSP hydrolysis products may adversely affect protein digestion by chickens. A multi-carbohydrase enzyme not only hydrolyzed water-soluble NSP but also partially depolymerized the water-insoluble NSP fraction of feed ingredients, which significantly increased water-soluble and decreased water-insoluble NSP concentrations in the ileal digesta of birds fed enzyme-supplemented diets [16]. This result may explain why no significant benefits were derived from supplementing diets with multienzymes in the current study.
Nutritional factors affect intermediary metabolism, resulting in changes in serum metabolite levels in poultry [42]. Blood GLU concentrations affect serum total cholesterol and triglyceride concentrations [43], and plasma GLU levels reflect the rate of starch digestion [44]. We observed that supplementing the diet with Enzymes B, C, D or E increased serum GLU and TC at d 16. Supplementing diets with enzymes has the potential to change digesta properties and release more simple sugars, amino acids and di-peptides, increasing their metabolic availability. A previous study showed that NSP could affect bile salts and decrease the digestibility of oil [45]. However, no clear pattern was observed for the level of TBA with supplementation of poultry diets with exogenous enzymes. A close relationship between the somatotropic and thyrotropic axes in the regulation of growth and development of broiler chickens plays an important role in poultry growth [46]. Nutritional status is an important factor in the regulation of blood hormones and intermediary metabolism in broiler chickens [47]. The addition of dietary enzymes increases the digestion of feed and the absorption of nutrients, which affect hormone concentrations. Glucose is the primary trigger for the postprandial changes in metabolic and endocrine functioning [47]. Insulin levels may affect growth and nutrient utilization [48]. Gao et al. [49] reported that increases in nutrient digestion and absorption resulting from enzyme supplementation affected hormonal levels. We observed that Enzyme B increased the INS level, whereas Enzyme C affected T3 and T4 levels. Other studies show a relationship between T3 and growth [50]; however, we did not observe any growth trends associated with levels of T3 and T4 in the current study. Enzyme supplementation changed the villi height:crypt depth ratio, similar to the observations of Ritz et al. [51] and Onderci et al. [10]. Changes in intestinal anatomy can affect lesion scores and morphology. We observed that lesion scores were reduced at d 16 with enzyme supplementation. Gut anatomy and morphology invariably change the surface area of the gut and improve digestibility of NSP, and as a consequence, a reduction in digesta viscosity is expected. Substrate modification in the gut by enzyme supplementation can also change the dynamics of intestinal microbes relying on these substrates. Wagner and Thomas [52] demonstrated that the growth dynamics of over 600 species of organisms harbored in the gut respond to changes in the diet.
Gut microbiota perform diverse functions and participate in carbohydrate metabolism, fiber degradation, and immune maintenance; however, the functions may be influenced by diet, genotype, feeding pattern and inflammation [53]. Thus, changes in the intestinal environment can affect the microbial population dynamics in the gut and thereby influence functions. According to Torok et al. [54], digesta flow rate, pH, anoxic conditions, types of endogenous and dietary substrates, inhibitory factors such as bacteriocins and short-chain fatty acids (SCFA), and competition influence gut microflora. For example, incomplete digestion of starch in the small intestine favored growth of Clostridium perfringens in the upper gut [8], and resistant starch is also associated with increased numbers of Brachyspira hyodysenteriae and increased incidence of clinical dysentery in swine [55]. Supplementation of diets with exogenous enzymes can alter the microbial profile [56]. In the current study, the most abundant phylum was Fimicutes, followed by Bacteroidetes (Fig. 2a), and those two dominant phyla composed approximately 80% of the entire bacterial community. The other approximately 20% of bacteria were in the phyla Proteobacteria, Cyanobacteria, Actinobacteria, Tenericutes, Acidobacteria, Synergistetes, Euryarchaeota, Verrucomicrobia and others (Fig. 2a and Additional file 1: Table S1), as also reported by Oakley et al. [57] and Neumann and Suen [58].
The PCA axis 1 accounted for 8.17% of the intertreatment variation, and the axis 2 accounted for 7.34% of the intersample variation. Although the intertreatment variability explained was small, clear differences were observed in the gut microbiota populations among the treatments. In the current study, enzyme supplementation altered the relative abundance of cecal microbiota at the phylum and genus levels. The degradation products of the different treatments were expected to be different, with different enzyme combinations causing different changes in the substrate composition and digesta viscosity. In the current study, the number of OTUs classified as Campylobacter, Helicobacter and Megamonas was apparently enriched in treatments supplemented with Enzymes B, C and E, respectively. Sergeant et al. [59] suggested that these enzymes might improve uptake of hydrogenases and consequently be beneficial to both the bacterial community and to energy absorption.
With changes in the diet, some microbes can grow opportunistically to high levels or have growth suppressed to low levels, which in each case, can lead to certain maladies [60]. The number of OTUs and diversity apparently declined with enzyme supplementation, particularly in the treatment with Enzyme E. The diversity of gut microbiota may be associated with the proportion of indigestible components in the diet. Megamonas contains several genes that encode a series of polysaccharide degradation enzymes, such as cellobiose phosphotransferase and endo-glucanases, which aid in degrading complex polysaccharides to cellobiose and subsequent digestion to glucose [59]. Similarly, Megamonas and Bacteroides were also enriched in the treatment with Enzyme E. Bacteroides can secrete enzymes that participate in cellobiose and xylan degradation and that are also involved in polysaccharide utilization systems [59, 61] and short-chain fatty acid metabolism [60]. Bacteroides are indole negative, resistant to 20% bile, and produce acetic acids and a smaller amount of succinic acids as the primary end products of glucose metabolism [62]. Vahjen et al. [63] demonstrated that exogenous supplementation with xylanase led to increased counts of lactobacilli, which was a result also confirmed in a study by Nian et al. [64] in which, simultaneously, coliform and Salmonella were reduced. However, in the current study, coliform and Salmonella were not members of the enriched populations. Bacteroides may deconjugate bile acids, which can lead to poor fat absorption and storage [65]. Thus, the relationship between Bacteroides and the level of TBA should be explored further. For Megamonas, Scupham et al. [66] presented some data that suggest this genus is antagonistic to Campylobacter. Apparently, changes in gut microbial diversity may distort homeostasis, which affects the immune state of the host [53].
Conclusions
It was concluded from the present study that a newly harvested corn diet of broilers requires supplementation with carbohydrase and protease to increase starch digestibility and increase the levels of INS and T3 to improve broiler body weight. Supplementation with dietary exogenous enzymes modulates the relative abundance of specific bacteria. Increases in population levels of polysaccharide-producing bacteria and decreases in destructive bacteria in chickens may be an intestinal health-promoting attribute and may contribute to starch utilization. Amylase combined with glucoamylase or protease is recommended for broiler diets with newly harvested corn.
Additional file
Table S1. Relative abundance of the dominant bacterial in the cecal digesta communities of 23-day-old broiler chickens at phylum level. Table S2. Relative abundance of the dominant bacterial community in the cecal digesta of 23-day-old broiler chickens at family level. Table S3. Relative abundance of the dominant bacterial community in the cecal digesta of 23-day-old broiler chickens at genus level. Figure S1. Rarefaction curves of samples clustered at 95% sequences identity. Figure S2. Richness and diversity of microbiome in cecal content. Chao 1 (a), Shannon diversity (b) and Simpson (c) indices are represented by box plots. Figure S3 Detailed taxon tree (DOCX 403 kb)
Acknowledgements
This research was supported by the System for Poultry Production Technology, Beijing Innovation Research Team of Modern Agriculture (CARS-PSTP, Beijing, China). We are grateful to Dr. Valeria Torok for some comments on the manuscript, and Dr. Maxwell Hincke for assistance with editing of the manuscript.
Funding
This research was supported by the System for Poultry Production Technology, Beijing Innovation Research Team of Modern Agriculture (BAIC04–2016).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- ABW
Average body weight
- ACE
Abundance-based coverage estimator
- AFI
Average feed intake
- AM
Amylose
- AP
Amylopectin
- BWG
Body weight gain
- DM
Dry matter
- FCR
Feed conversion ratio
- GLU
Glucose
- INS
Insulin
- NSP
Non-starch polysaccharides
- OUTs
Operational taxonomic units
- PCA
Principal component analysis
- PG
Proglucagon
- SBM
Soybean meal
- SCFA
Short chain fatty acid
- T3
Triiodothyronine
- T4
Tetraiodothyronine
- TBA
Total bile aid
- TC
Total cholesterol
- TG
Triglyceride
- TIA
Trypsin inhibitor activity
Authors’ contributions
JY and YG conceived and designed the experiments. WN, XW, FW, ZL and DY performed the experiments. XY analyzed the data. XY and SEA contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All protocols and procedures were performed according to the Beijing Regulations of Laboratory Animals, and the animal study proposal was approved by The Laboratory Animal Ethical Committee of China Agricultural University (permit number SYXK20130013). All experiments used in this study were performed in accordance with protocols approved guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors confirm they have read Biomed Central’s guidelines on competing interests and declare no competing interests.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s40104-018-0238-0) contains supplementary material, which is available to authorized users.
Contributor Information
Wei Nie, Email: caunw@163.com.
Jianmin Yuan, Email: yuanjm@cau.edu.cn.
References
- 1.Stefanello C. Starch digestibility, energy utilization, and growth performance of broilers fed corn-soybean basal diets supplemented with enzymes. Poult Sci. 2015;94(10):2472–2479. doi: 10.3382/ps/pev244. [DOI] [PubMed] [Google Scholar]
- 2.Kiarie E, Romero LF, Ravindran V. Growth performance, nutrient utilization, and digesta characteristics in broiler chickens fed corn or wheat diets without or with supplemental xylanase. Poult Sci. 2014;93(5):1186–1196. doi: 10.3382/ps.2013-03715. [DOI] [PubMed] [Google Scholar]
- 3.Bedford MR, McNab JM, Boorman KN. The role of carbohydrases in feedstuff digestion. 2002. pp. 319–336. [Google Scholar]
- 4.South JB, Morrison WR, Nelson OE. A relationship between the amylose and lipid contents of starches from various mutants for amylose content in maize. J Cereal Sci. 1991;14(3):267–278. doi: 10.1016/S0733-5210(09)80045-3. [DOI] [Google Scholar]
- 5.Brugger D, Loibl P, Schedle K, Windisch WM, Fahn C. In-silico and in-vitro evaluation of the potential of maize kernels to inhibit trypsin activity. Anim Feed Sci Technol. 2015;207:289–294. doi: 10.1016/j.anifeedsci.2015.06.023. [DOI] [Google Scholar]
- 6.Choct M, Annison G. The inhibition of nutrient digestion by wheat pentosans. Brit. J Nutr. 1992;67(1):123. doi: 10.1079/BJN19920014. [DOI] [PubMed] [Google Scholar]
- 7.Choct M, Sinlae M, Aljassim RAM, Pettersson D. Effects of xylanase supplementation on between-bird variation in energy metabolism and the number of Clostridium Perfringens in broilers fed a wheat-based diet. Aust J Agr Res. 2006;57(9):1017–1021. doi: 10.1071/AR05340. [DOI] [Google Scholar]
- 8.Timbermont L, Haesebrouck F, Ducatelle R, Van IF. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40(4):341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- 9.Teirlynck E, Bjerrum L, Eeckhaut V, Huygebaert G, Pasmans F, Haesebrouck F, et al. The cereal type in feed influences gut wall morphology and intestinal immune cell infiltration in broiler chickens. Brit J Nutr. 2009;102(10):1453–1461. doi: 10.1017/S0007114509990407. [DOI] [PubMed] [Google Scholar]
- 10.Onderci M, Sahin N, Sahin K, Cikim G, Aydín A, Ozercan I, et al. Efficacy of supplementation of α-amylase-producing bacterial culture on the performance, nutrient use, and gut morphology of broiler chickens fed a corn-based diet. Poult Sci. 2006;85(3):505–510. doi: 10.1093/ps/85.3.505. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Z, Zhou Y, Lu F, Han Z, Wang T. Effects of different levels of supplementary alpha-amylase on digestive enzyme activities and pancreatic amylase mRNA expression of young broilers. Asian Austral J Anim. 2008;21(1):97–102. doi: 10.5713/ajas.2008.70110. [DOI] [Google Scholar]
- 12.Mahagna M, Nir I, Larbier M, Nitsan Z. Effect of age and exogenous amylase and protease on development of the digestive tract, pancreatic enzyme activities and digestibility of nutrients in young meat-type chicks. Reprod Nutr Dev. 1995;35(2):201–212. doi: 10.1051/rnd:19950208. [DOI] [PubMed] [Google Scholar]
- 13.Kaczmarek SA, Rogiewicz A, Mogielnicka M, Rutkowski A, Jones RO, Slominski BA. The effect of protease, amylase, and nonstarch polysaccharide-degrading enzyme supplementation on nutrient utilization and growth performance of broiler chickens fed corn-soybean meal-based diets. Poult Sci. 2014;93(7):1745–1753. doi: 10.3382/ps.2013-03739. [DOI] [PubMed] [Google Scholar]
- 14.Yegani M, Korver DR. Effects of corn source and exogenous enzymes on growth performance and nutrient digestibility in broiler chickens. Poult Sci. 2013;92(5):1208–1220. doi: 10.3382/ps.2012-02390. [DOI] [PubMed] [Google Scholar]
- 15.Kaczmarek S, Bochenek M, Józefiak D, Rutkowski A. Effect of enzyme supplementation of diets based on maize or hominy feed on performance and nutrient digestibility in broilers. Cotton. Outlook. 2009;18(1):113–123. [Google Scholar]
- 16.Meng X, Slominski BA, Nyachoti CM, Campbell LD, Guenter W. Degradation of cell wall polysaccharides by combinations of carbohydrase enzymes and their effect on nutrient utilization and broiler chicken performance. Poult Sci. 2005;84(1):37–47. doi: 10.1093/ps/84.1.37. [DOI] [PubMed] [Google Scholar]
- 17.Francesch M, Geraert PA. Enzyme complex containing carbohydrases and phytase improves growth performance and bone mineralization of broilers fed reduced nutrient corn-soybean-based diets. Poult Sci. 2009;88(9):1915–1924. doi: 10.3382/ps.2009-00073. [DOI] [PubMed] [Google Scholar]
- 18.Tahir M, Saleh F, Amjed M, Ohtsuka A, Hayashi K. An effective combination of carbohydrases that enables reduction of dietary protein in broilers: importance of hemicellulase. Poult Sci. 2008;87(4):713–718. doi: 10.3382/ps.2007-00340. [DOI] [PubMed] [Google Scholar]
- 19.Munyaka PM, Nandha NK, Kiarie E, Nyachoti CM, Khafipour E. Impact of combined β-glucanase and xylanase enzymes on growth performance, nutrients utilization and gut microbiota in broiler chickens fed corn or wheat-based diets. Poultry Sci. 2016;95(3):528-40. [DOI] [PubMed]
- 20.Kiarie E, Romero LF, Nyachoti CM. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr Res Rev. 2013;26(01):71–88. doi: 10.1017/S0954422413000048. [DOI] [PubMed] [Google Scholar]
- 21.Geier MS, Torok VA, Allison GE, Ophel-Keller K, Hughes RJ. Indigestible carbohydrates alter the intestinal microbiota but do not influence the performance of broiler chickens. J Appl Microbiol. 2009;106(5):1540–1548. doi: 10.1111/j.1365-2672.2008.04116.x. [DOI] [PubMed] [Google Scholar]
- 22.Huyghebaert G, Ducatelle R, Immerseel FV. An update on alternatives to antimicrobial growth promoters for broilers. Vet J. 2011;187(2):182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Amann RI, Ludwig W, Phylogenetic SKH. Identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59(59):143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waite DW, Taylor MW. Characterizing the avian gut microbiota: membership, driving influences, and potential function. Front Microbiol. 2014;5(4):223. doi: 10.3389/fmicb.2014.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministry of agriculture of China. Chinese Chicken Feeding Standard NY/T 33–2004. Standard Press of China,2004.
- 26.Keyburn AL, Boyce JD, Vaz P, Bannam TL, Ford ME, Parker D, et al. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium Perfringens. PLoS Pathog. 2008;4(2). doi: ARTN e26 10.1371/journal.ppat.0040026. PubMed PMID: WOS:000255415900031. [DOI] [PMC free article] [PubMed]
- 27.AOAC . Official methods of analysis. 16. Washington, DC: Assoc. Off. Anal. Chem; 2000. [Google Scholar]
- 28.Lin CJ, Li CY, Lin SK, Yang FH, Huang JJ, Liu YH, et al. Influence of high temperature during grain filling on the accumulation of storage proteins and grain quality in Rice (Oryza Sativa L.) J Agric Food Chem. 2010;58(19):10545–10552. doi: 10.1021/jf101575j. [DOI] [PubMed] [Google Scholar]
- 29.Edwards HM, Gillis MB. A chromic oxide balance method for determining phosphate availability. Poult Sci. 1959;38(3):569–574. doi: 10.3382/ps.0380569. [DOI] [Google Scholar]
- 30.Spss 15.0 Command Syntax Reference. Chicago IL: SPSS Inc.2006.
- 31.Knudsen KE. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult Sci. 2014;93(9):2380–2393. doi: 10.3382/ps.2014-03902. [DOI] [PubMed] [Google Scholar]
- 32.Svihus B. Starch digestion capacity of poultry. Poultry Sci. 2014;93(9):2394–2399. doi: 10.3382/ps.2014-03905. [DOI] [PubMed] [Google Scholar]
- 33.Bedford MR. The effect of enzymes on digestion. J Appl Poult Res. 1996;5(4):370–378. doi: 10.1093/japr/5.4.370. [DOI] [Google Scholar]
- 34.Behnke CA, Yee VC, Trong IL, Pedersen LC, Stenkamp RE, Kim SS, et al. Structural determinants of the bifunctional corn Hageman factor inhibitor: x-ray crystal structure at 1.95 a resolution. Biochemistryus. 1998;37(44):15277–15288. doi: 10.1021/bi9812266. [DOI] [PubMed] [Google Scholar]
- 35.Pusztai A, Grant G, Duguid T, Brown DS, Peumans WJ, Van Damme EJ, et al. Inhibition of starch digestion by alpha-amylase inhibitor reduces the efficiency of utilization of dietary proteins and lipids and retards the growth of rats. J Nutr. 1995;125(6):1554–1562. doi: 10.1093/jn/125.6.1554. [DOI] [PubMed] [Google Scholar]
- 36.Pettersson D, Aman P. Enzyme supplementation of a poultry diet containing rye and wheat: Paradigme. 1985. pp. 139–149. [DOI] [PubMed] [Google Scholar]
- 37.Péron A, Gomez J, Mignon-Grasteau S, Sellier N, Besnard J, Derouet M, et al. Effects of wheat quality on digestion differ between the D+ and D- chicken lines selected for divergent digestion capacity. Poult Sci. 2006;85(3):462–469. doi: 10.1093/ps/85.3.462. [DOI] [PubMed] [Google Scholar]
- 38.Alfonso THD. Sources of variance of energy digestibility in corn-soy poultry diets and the effect on performance : starch, protein, oil and fiber. Krmiva. 2005;47(2):83–86. [Google Scholar]
- 39.Leslie MA, Jr ME, Bedford MR. The effect of phytase and glucanase on the ileal digestible energy of corn and soybean meal fed to broilers. Poult Sci. 2007;86(11):2350–2357. doi: 10.3382/ps.2007-00115. [DOI] [PubMed] [Google Scholar]
- 40.Troche C, Sun X, McElroy AP, Remus J, Novak CL. Supplementation of Avizyme 1502 to corn-soybean meal-wheat diets fed to turkey tom poults: the first fifty-six days of age. Poult Sci. 2007;86(3):496–502. doi: 10.1093/ps/86.3.496. [DOI] [PubMed] [Google Scholar]
- 41.Cowieson AJ, Adeola O. Carbohydrases, protease, and phytase have an additive beneficial effect in nutritionally marginal diets for broiler chicks. Poult Sci. 2005;84(12):1860–1867. doi: 10.1093/ps/84.12.1860. [DOI] [PubMed] [Google Scholar]
- 42.Swennen Q, Janssens GP, Millet S, Vansant G, Decuypere E, Buyse J. Effects of substitution between fat and protein on feed intake and its regulatory mechanisms in broiler chickens: endocrine functioning and intermediary metabolism. Poult Sci. 2005;84(7):1051–1057. doi: 10.1093/ps/84.7.1051. [DOI] [PubMed] [Google Scholar]
- 43.Denardin CC, Walter M, LPD S, Souto GD, CAA F. Effect of amylose content of rice varieties on glycemic metabolism and biological responses in rats. Food Chem. 2007;105(4):1474–1479. doi: 10.1016/j.foodchem.2007.05.028. [DOI] [Google Scholar]
- 44.Englyst HN, Veenstra J, Hudson GJ. Measurement of rapidly available glucose (RAG) in plant foods: a potential in vitro predictor of the glycaemic response. Brit J Nutr. 1996;75(3):327–337. doi: 10.1079/BJN19960137. [DOI] [PubMed] [Google Scholar]
- 45.Bao YM, Cowieson LFRAJ. Functional patterns of exogenous enzymes in different feed ingredients. Worlds Poult Sci J. 2013;69(4):759–774. doi: 10.1017/S0043933913000792. [DOI] [Google Scholar]
- 46.Cogburn LA, Mao NC, Agarwal S, Burnside J. Interaction between somatotropic and thyrotropic axes in regulation of growth and development of broiler chickens. Archiv Fuer Gefluegelkunde. 1995.
- 47.Buyse J, Janssens K, Van dGS, Van AP DE, Darras VM. Pre- and postprandial changes in plasma hormone and metabolite levels and hepatic deiodinase activities in meal-fed broiler chickens. Brit. J Nutr. 2002;88(6):641–653. doi: 10.1079/BJN2002741. [DOI] [PubMed] [Google Scholar]
- 48.Józefiak D, Ptak A, Kaczmarek S, Mackowiak P, Sassek M, Slominski BA. Multi-carbohydrase and phytase supplementation improves growth performance and liver insulin receptor sensitivity in broiler chickens fed diets containing full-fat rapeseed. Poult Sci. 2010;89(9):1939–1946. doi: 10.3382/ps.2010-00694. [DOI] [PubMed] [Google Scholar]
- 49.Gao F, Jiang Y, Zhou GH, Han ZK. The effects of xylanase supplementation on performance, characteristics of the gastrointestinal tract, blood parameters and gut microflora in broilers fed on wheat-based diets. Anim Feed Sci Technol. 2008;142(1):173–184. doi: 10.1016/j.anifeedsci.2007.07.008. [DOI] [Google Scholar]
- 50.Buys N, Scheele CW, Kwakernaak C, Decuypere E. Performance and physiological variables in broiler chicken lines differing in susceptibility to the ascites syndrome: 2. Effect of ambient temperature on partial efficiencies of protein and fat retention and plasma hormone concentrations. Brit Poultry Sci. 1999;40(1):140–144. doi: 10.1080/00071669987980. [DOI] [PubMed] [Google Scholar]
- 51.Ritz CW, Hulet RM, Self BB, Denbow DM. Endogenous amylase levels and response to supplemental feed enzymes in male turkeys from hatch to eight weeks of age. Poult Sci. 1995;74(8):1317–1322. doi: 10.3382/ps.0741317. [DOI] [PubMed] [Google Scholar]
- 52.Wagner DD, Thomas OP. Influence of diets containing rye or pectin on the intestinal flora of chicks. Poult Sci. 1978;57(4):971–975. doi: 10.3382/ps.0570971. [DOI] [PubMed] [Google Scholar]
- 53.Mu C, Yang Y, Zhu W. Gut Microbiota: The Brain Peacekeeper. Front Microbiol. 2016;7(22):345. doi: 10.3389/fmicb.2016.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torok VA, Dyson C, McKay A, Ophelkeller K. Quantitative Molecular assays for evaluating changes in broiler gut microbiota linked with diet and performance. Anim Prod Sci. 2013;53(12):1260–1268. [Google Scholar]
- 55.Pluske JR, Durmic Z, Pethick DW, Mullan BP, Hampson DJ. Confirmation of the role of rapidly fermentable carbohydrates in the expression of swine dysentery in pigs after experimental infection. J Nutr. 1998;128(10):1737–1744. doi: 10.1093/jn/128.10.1737. [DOI] [PubMed] [Google Scholar]
- 56.Högberg A, Lindberg JE. Influence of cereal non-starch polysaccharides and enzyme supplementation on digestion site and gut environment in weaned piglets. Anim Feed Sci Technol. 2004;116(1–2):113–128. doi: 10.1016/j.anifeedsci.2004.03.010. [DOI] [Google Scholar]
- 57.Oakley BB, Lillehoj HS, Kogut MH, Kim WK, Maurer JJ, Pedroso A, et al. The chicken gastrointestinal microbiome. FEMS Microbiol Lett. 2014;360(2):100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- 58.Neumann AP, Suen G. Differences in major bacterial populations in the intestines of mature broilers after feeding virginiamycin or bacitracin methylene disalicylate. J Appl Microbiol. 2015;119(6):1111–1113. doi: 10.1111/jam.12960. [DOI] [PubMed] [Google Scholar]
- 59.Sergeant MJ, Constantinidou C, Cogan TA, Bedford MR, Penn CW, Pallen MJ. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS One. 2014;9(3):e91941. doi: 10.1371/journal.pone.0091941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaakoush NO, Sodhi N, Chenu JW, Cox JM, Riordan SM, Mitchell HM. The interplay between campylobacter and helicobacter species and other gastrointestinal microbiota of commercial broiler chickens. Gut Pathog. 2014;6(1):1–10. doi: 10.1186/1757-4749-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lan PT, Sakamoto M, Sakata S, Benno Y. Bacteroides barnesiae sp. nov., Bacteroides salanitronis sp. nov. and Bacteroides Gallinarum sp. nov., isolated from chicken caecum. Int J Syst Evol Microbiol. 2006;56(12):2853–2859. doi: 10.1099/ijs.0.64517-0. [DOI] [PubMed] [Google Scholar]
- 62.Song Y, Liu C, Lee J, Bolanos M, Vaisanen ML, Finegold SM. "Bacteroides goldsteinii sp. nov." isolated from clinical specimens of human intestinal origin. J Clin Microbiol. 2005;43(9):4522–4527. doi: 10.1128/JCM.43.9.4522-4527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vahjen W, Gläser K, Schäfer K, et al. Influence of xylanase-supplemented feed on the development of selected bacterial groups in the intestinal tract of broiler chicks. J Agric Sci. 1998;130(4):489–500. doi: 10.1017/S0021859698005498. [DOI] [Google Scholar]
- 64.Nian F, Guo YM, Ru YJ, Li FD, Peron A. Effect of exogenous Xylanase supplementation on the performance, net energy and gut microflora of broiler chickens fed wheat-based diets. Asian Austral J Anim. 2011;24(3):400–406. doi: 10.5713/ajas.2011.10273. [DOI] [Google Scholar]
- 65.Begley M, Hill C, Gahan CG. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol. 2006;72(3):1729–1738. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scupham AJ, Jones JA, Rettedal EA, Weber TE. Antibiotic manipulation of intestinal microbiota to identify microbes associated with campylobacter jejuni exclusion in poultry. Appl Environ Microbiol. 2010;76(24):8026–8032. doi: 10.1128/AEM.00678-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Relative abundance of the dominant bacterial in the cecal digesta communities of 23-day-old broiler chickens at phylum level. Table S2. Relative abundance of the dominant bacterial community in the cecal digesta of 23-day-old broiler chickens at family level. Table S3. Relative abundance of the dominant bacterial community in the cecal digesta of 23-day-old broiler chickens at genus level. Figure S1. Rarefaction curves of samples clustered at 95% sequences identity. Figure S2. Richness and diversity of microbiome in cecal content. Chao 1 (a), Shannon diversity (b) and Simpson (c) indices are represented by box plots. Figure S3 Detailed taxon tree (DOCX 403 kb)
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.


