Abstract
Purpose
Because a tracheal cartilaginous sleeve (TCS) confers a significant mortality risk that can be mitigated with appropriate intervention, we sought to describe the prevalence and associated genotypes in a large cohort of children with syndromic craniosynostosis.
Methods
Chart review of patients with syndromic craniosynostosis across two institutions.
Results
In a cohort of 86 patients with syndromic craniosynostosis, 31 required airway evaluation under anesthesia. TCS was found in 19, for an overall prevalence of 22%. FGFR2, TWIST1, and FGFR3 mutations were identified in children with TCS. All five children with a W290C mutation in FGFR2 had TCS, and most previously reported children with W290C had identification of TCS or early death. In contrast, TCS was not associated with other mutations at residue 290.
Conclusion
There is an association between TCS and syndromic craniosynostosis, and it appears to be particularly high in individuals with the W290C mutation in FGFR2. Referral to a pediatric otolaryngologist and consideration of operative airway evaluation (i.e., bronchoscopy or rigid endoscopy) in all patients with syndromic craniosynostosis should be considered to evaluate for TCS. Results from genetic testing may help providers weigh the risks and benefits of early airway evaluation and intervention in children with higher-risk genotypes.
Keywords: Crouzon syndrome, Pfeiffer syndrome, Saethre-Chotzen syndrome, sudden death, tracheal cartilaginous sleeves
INTRODUCTION
Syndromic craniosynostosis syndromes (e.g., Pfeiffer, Crouzon, Apert, Beare-Stevenson, Saethre-Chotzen) are associated with significant morbidity and mortality, including increased risk of infantile death. Early mortality estimates were approximately 25–85% for Pfeiffer syndrome.1–3 With advances in early surgical interventions, more recent estimates have ranged from 7 to 22%.4,5 Some deaths have been attributed to complications of known malformations. However, there is also an increased risk of death due to sudden respiratory arrest.6
A tracheal cartilaginous sleeve (TCS) occurs when typical segmentation of the C-shaped tracheal rings is absent and the trachea consists of a long tube of solid cartilage anteriorly and laterally, with a normal membranous tracheal wall posteriorly.7 Typical C-shaped rings permit airway distention for effective airway clearance with coughing and normal tracheal lumen enlargement. As a child with TCS grows, the tubelike tracheal cartilage fails to appropriately increase in size, resulting in a relatively stenotic airway. This phenomenon places patients at risk for sudden death due to tracheal occlusion. Most children with TCS also have syndromic craniosynostosis, although TCS has been reported in a patient with suspected Goldenhar syndrome and a child with Opitz G syndrome.3,6–31 Lertsburapa and colleagues performed a meta-analysis of all previously reported cases of TCS and reported a 90% risk of death by age 2 years without a tracheostomy.8 Of note, no patients in that study had undergone surgical airway reconstruction, which may be an effective surgical alternative to tracheostomy.17 TCS is often diagnosed by bronchoscopy, although many cases are diagnosed at the time of autopsy. An operative airway evaluation with general anesthesia in young infants also carries significant risk, making it difficult to balance the anesthesia and procedure-related risks with the risk of delaying TCS diagnosis.
Approximately 50 children with TCS have been reported, with varying amounts of phenotypic information and inconsistent genetic testing. Although craniosynostosis syndromes such as Apert and Beare-Stevenson syndromes are easily distinguishable based on clinical examination, there is significant overlap between the clinical features of Crouzon and Pfeiffer syndromes, and some overlap with Saethre-Chotzen syndrome. Identical mutations in FGFR2 have been reported in children with clinical diagnoses of either Crouzon syndrome or Pfeiffer syndrome.32 Moreover, most of these conditions share common medical problems and management issues, and there is a significantly increased risk of infantile death with all of these syndromes. Many providers do not routinely perform genetic testing in children with syndromic craniosynostosis because management recommendations are similar for all syndromes.
No prevalence estimates are currently available for TCS in syndromic craniosynostosis, and genotypes have been reported for only 18 patients in the literature (see Table 1). Therefore, neonatologists and otolaryngologists caring for children with syndromic craniosynostosis have limited ability to make a personalized risk assessment for TCS in a child with syndromic craniosynostosis. This forces providers to use clinical information to balance the procedure and anesthesia-related risks of operative airway evaluation with the risk of missing a TCS.
Table 1.
Reported mutations in patients with tracheal cartilaginous sleeve
| Gene | Mutation | Number in literature |
Present report |
Total |
|---|---|---|---|---|
| FGFR2 | C278F | – | 1 | 1 |
| FGFR2 | C278L | – | 1 | 1 |
| FGFR2 | C342G | 1 | – | 1 |
| FGFR2 | C342R | 2 | 4 | 6 |
| FGFR2 | C342S | 2 | – | 2 |
| FGFR2 | C342W | 1 | – | 1 |
| FGFR2 | C342Y | – | 1 | 1 |
| FGFR2 | S252W | – | 1 | 1 |
| FGFR2 | S347C | – | 1 | 1 |
| FGFR2 | S351C | 6 | – | 6 |
| FGFR2 | S354C | 1 | – | 1 |
| FGFR2 | W290C | 3* | 5 | 8 |
| FGFR2 | Y375C | 1 | – | 1 |
| FGFR3 | A391E | 1 | 1 | 2 |
| TWIST1 | Tyr103term | – | 1 | 1 |
| (FGFR2) | Apert, presumed S252W/P253R | 7 | 4 | 11 |
| (FGFR2) | Beare-Stevenson, presumed Y375C/S372Y | 1 | – | 1 |
| Total with confirmed mutations only | 18 | 16 | 34 | |
| Total including presumed mutations due to specific phenotype | 25 | 20 | 45 |
The goals of the present study were (i) to describe the prevalence of TCS in a population of children with syndromic craniosynostosis and (ii) to explore genotype–phenotype correlations by combining data from patients with known genotypes in our cohort and the literature.
MATERIALS AND METHODS
Prevalence estimate
Patient selection and population characteristics
We performed a retrospective chart review for children with syndromic craniosynostosis who received care at Seattle Children’s Hospital (IRB#10925) and had their most recent visit within the past 3 years. Seattle Children’s Hospital is the only craniofacial team referral site for syndromic craniosynostosis for a five-state region (Washington, Alaska, Montana, Idaho, and Wyoming). Because there are no other centers in the five-state region that provide care for children with syndromic craniosynostosis, we are fairly confident that there are few, if any, patients living in the region who have not been evaluated in this center. In addition, few patients move out of the catchment area after initiating treatment. At our center, all children with syndromic craniosynostosis are evaluated by experienced pediatric otolaryngologists due to the high rate of midface hypoplasia and obstructive sleep apnea. Children are evaluated at least annually; in the majority of cases, children receive all of their craniosynostosis-related care through our hospital. Examination of our database revealed that two otolaryngologists (J.P., K.C.Y.S.) had seen 100% of patients eligible for this study at Seattle Children’s Hospital. For this study, we also excluded patients with isolated unicoronal craniosynostosis and a diagnosis of Muenke syndrome because it is common, well described, and generally not associated with airway anomalies. However, many other genetic syndromes were represented, including children with multiple congenital anomalies whose genetic testing had not yet revealed a unifying diagnosis.
Methods
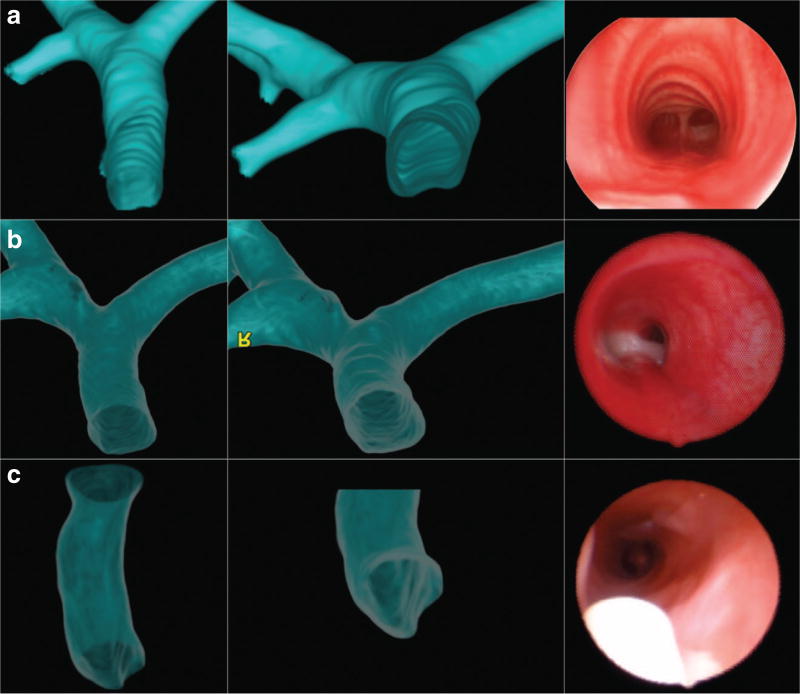
Chart review was performed for all children. When available, all operative airway reports and videos of bronchoscopies or rigid endoscopies (recorded as part of clinical care) were also reviewed by a second examiner (J.D., A.F.I.). Endoscopic criteria for TCS included the absence of tracheal rings with the presence of a normal membranous posterior tracheal wall (Figure 1). High-resolution computerized tomography and/or direct visualization of the anterior tracheal wall at tracheostomy were used to confirm endoscopic findings.
Figure 1. Three-dimensional computed-tomography reconstructions and midtracheal bronchoscopy images.
Images from (a) a normal patient and (b and c) two patients with TCS. Note the presence of C-shaped rings and a flat posterior tracheal wall in the normal trachea (a) and the absence of rings and a flat posterior tracheal wall in the TCS patients (b and c). The white object in the endoscopic image in panel c is the tip of the tracheostomy tube.
Genotype determination
After initial determination of prevalence of TCS, we sought to determine whether there were genotype–phenotype correlations. To do this, we included patients with genotypes of interest from a cohort at the Children’s Hospital of Philadelphia because there were not a sufficient number of patients at Seattle Children’s Hospital to make an adequate comparison. A comparison cohort of patients with FGFR2 mutations at residue 290 were identified by chart review of clinical and/or research testing at Seattle Children’s Hospital (IRB#10925) or through clinical testing by the molecular laboratory at Children’s Hospital of Philadelphia. Parents of patients who had genetic testing performed on a research basis provided informed consent.
RESULTS
Eighty-six children with syndromic craniosynostosis met inclusion criteria for this study. Of these 86, 31 had undergone bronchoscopy or rigid endoscopy for evaluation of respiratory symptoms or other clinical concerns. Overall, 19 of 86 (22%) were found to have TCS, including 19 of 31 (61%) who underwent operative airway evaluation. Therefore, a conservative prevalence of 22% was reached; however, it is possible that this is an underestimate because it is based on patients who had clinical indications for operative airway evaluation, and there may be patients with TCS who do not have respiratory symptoms.
We identified two additional patients with TCS from the Children’s Hospital of Philadelphia, for a total of 21 patients with TCS. The syndromes diagnosed in these patients can be found in Table 2.
Table 2.
Patients by clinical diagnosis and genotype
| Diagnosis | No. patients with tracheal sleeve |
No. patients with genetic testing |
Gene | Genotype |
|---|---|---|---|---|
| Pfeiffer | 9 | 8 | FGFR2 | W290C (n = 5) |
| C342R (n = 2) | ||||
| C278L (n = 1) | ||||
|
| ||||
| Crouzon | 5 | 5 | FGFR2 | C342R (n = 2) |
| C278F (n = 1) | ||||
| C342Y (n = 1) | ||||
| S347C (n = 1) | ||||
|
| ||||
| Apert | 5 | 1 | FGFR2 | S252W (n = 1) |
|
| ||||
| Crouzon with acanthosis nigricans | 1 | 1 | FGFR3 | A371E (n = 1) |
|
| ||||
| Saethre-Chotzen | 1 | 1 | TWIST1 | Tyr103term (n = 1) |
Of the 21 patients with TCS, genetic testing was available for 16. Of the patients without genetic testing results available, 4 were diagnosed with Apert syndrome, which is generally due to one of two mutations (S252W or P253R). Therefore, 20 individuals had known or presumed mutations based on phenotype. Previously reported patients with known or presumed genotype and TCS are also included in Table 1 for comparison.
We identified five patients with W290C, four with W290R, and two with W290G mutations. A summary of these patients can be found in Table 3. All patients with W290C had TCS, whereas no patients with W290R or W290G were found to have TCS. Of note, although each of these patients was categorized as having either Pfeiffer or Crouzon syndrome, there appeared to be an intermediate phenotype, with the clinical geneticist making a determination of classification. In some cases there was disagreement between some features (e.g., broad thumbs absent in patients 5 and 10 who were classified as having Pfeiffer syndrome, with broad thumbs noted in patients 6, 7, and 11, who were classified as having Crouzon syndrome). This suggests that patients with mutations at residue 290 may have an intermediate phenotype, with some having an overall gestalt consistent with Pfeiffer syndrome and others with that consistent Crouzon syndrome.
Table 3.
Clinical features of all patients with mutations at residue 290 at our centers
| W290C (c.G870C) | W290R (c.T898C) | W290G (c.868G) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|
|
|
|
|||||||||
| Diagnosis | Pfeiffer | Pfeiffer | Pfeiffer | Pfeiffer | Pfeiffer | Crouzon | Crouzon | Crouzon | Crouzon | Pfeiffer | Crouzon |
| Age at last visit (years) | 6 | 4a | 4 | 2 | 8 | 22 | 12 | 13 | 7 | 18 | 7 |
|
| |||||||||||
| Craniosynostosis sutures involved | Bicoronal, R lambdoid | Bicoronal | All (cloverleaf skull) | Bicoronal, lambdoid | All (cloverleaf skull) | All, late fusion, repair at 7 | − | − | All | All | Sagittal, coronal |
|
| |||||||||||
| Choanal atresia/stenosis | + | + | + | − | − | − | − | − | − | − | − |
|
| |||||||||||
| Broad thumbs/toes | + | + | + | + | − | + | + | − | − | − | + |
| Elbow ankyloses | + | + | + | + | + | − | − | − | − | − | − |
|
| |||||||||||
| Genitourinary anomalies | Sacral appendage | − | − | Cloacal malformation, anterior anus | − | − | Anterior anus | − | − | − | − |
|
| |||||||||||
| Ear anomalies | + | + | + | + | + | − | − | − | − | − | − |
|
| |||||||||||
| Aural atresia | + | Aural stenosis | + | + | + | − | − | − | − | − | − |
|
| |||||||||||
| Middle ear atresia | + | − | − | − | − | − | − | − | − | − | − |
|
| |||||||||||
| Tracheal cartilaginous sleeve | + | + | + | + | + | − | − | − | − | − | − |
|
| |||||||||||
| Tracheostomy | + | + | + | + | + | − | − | − | − | − | − |
|
| |||||||||||
| Vertebral anomalies | + | − | + | + | + | − | − | − | − | − | − |
|
| |||||||||||
| Ophthalmologic anomalies | Lagophthalmos, sclerocornea | Proptosis, exotropia | Severe proptosis | Recurrent subluxation of globe | Recurrent subluxation of globe | − | Bilateral canthopexy | Ptosis | Proptosis, strabismus | Exotropia | Proptosis |
|
| |||||||||||
| Other | Natal teeth | Hydrocephalus | Lower limb contractures | Hydronephrosis, natal teeth, lower limb contractures | Chiari I | Chiari I | Anomalous carotid artery | Inherited from undiagnosed father | Chiari I | − | Inherited from undiagnosed father |
|
| |||||||||||
| Development | Global delay, some signs | At 3.5, nonverbal but used signs | At 4, many signs and is conversant, although restricted by mobility | Interactive, uses signs | Normal, above average in all subjects except math | Normal | Above average | Normal | Normal | Normal | Normal |
Died at age 4.
Among children in whom no TCS was identified, a wide range of genetic syndromes was identified (Table 4). Of note, no TCS cases were found in children with syndromic craniosynostosis in syndromes other than those associated with FGFR2, FGFR3 and TWIST1 in our cohort. Mutations associated with clinical diagnoses of Apert, Crouzon, and Pfeiffer syndromes in children who did not have TCS are listed in Supplementary Table S1 online.
Table 4.
Diagnoses of patients without TCS.
| Syndrome |
n, Clinical diagnosis |
n, Confirmation of diagnosis with genetic testing |
n, Total |
|---|---|---|---|
| Apert | 9 | 6 | 15 |
|
| |||
| Carpenter | – | 1 | 1 |
|
| |||
| Christian | 1 | – | 1 |
| Chromosomal | – | 4 | |
| 7p15p21 deletion | – | 1 | |
| Mosaic trisomy 9 | – | 1 | |
| 17q21.31 deletion | – | 1 | 4 |
| Unbalanced translocation, chr 11 and 8 | – | 1 | |
|
| |||
| Craniofrontonasal dysplasia | – | 2 | 2 |
|
| |||
| Crouzon with acanthosis nigricans | – | 3 | 3 |
|
| |||
| Crouzon | 3 | 12 | 15 |
|
| |||
| Kabuki | 1 | – | 1 |
|
| |||
| Manitoba-oculo-tricho-anal | 1 | – | 1 |
|
| |||
| Otopalatodigital | 1 | – | 1 |
|
| |||
| Pfeiffer | – | 1 | 1 |
|
| |||
| Saethre-Chotzen | 2 | 5 | 7 |
|
| |||
| Multiple congenital anomalies, no unifying diagnosis | 15 | 0 | 15 |
|
| |||
| Total | 33 | 34 | 67 |
DISCUSSION
This study found a prevalence of 22% with a diagnosis of TCS in syndromic craniosynostosis in children who had been evaluated by two pediatric otolaryngologists. It is possible that this is an overestimate because these children had been referred to otolaryngology; however, we do not believe this represents a significant bias because it is the standard of care at many centers (including SCH) for patients with syndromic craniosynostosis to be evaluated by a pediatric otolaryngologist. Although the majority of TCS were found in patients with Pfeiffer syndrome, TCS were also identified in children with other FGFR2-associated craniosynostosis syndromes (i.e., Crouzon, Apert) as well as in craniosynostosis syndromes associated with mutations in FGFR3 (i.e., Crouzon syndrome with acanthosis nigricans) and TWIST1 (Saethre-Chotzen). This suggests a need for heightened concern for TCS in all children with syndromic multisuture craniosynostosis, with the greatest risk in Pfeiffer syndrome.
There are two limitations regarding the prevalence and clinical significance of TCS. The conservative estimate in this population was 22%, but TCS was found in 61% of those who underwent operative airway evaluation. It is clinical practice in our center to perform operative airway evaluation for children with clinical symptoms. It is possible that there were children in our cohort who had TCS but were asymptomatic and therefore did not undergo operative airway evaluations. Conversely, it is possible that the meta-analysis by Lertsburapa and colleagues overestimated the risk of TCS because they ascertained cases in patients who presumably had enough clinical symptoms to warrant operative airway evaluation, or cases were identified on autopsy.8 The only treatment evaluated by Lertsburapa and colleagues was tracheostomy,8 although airway reconstruction, in the hands of an experienced pediatric otolaryngologist, may be another suitable treatment for TCS.17
The overall estimate of TCS in syndromic craniosynostosis was 22%, and it was 100% in children with a W290C mutation in FGFR2. Previous reports of children with W290C in the literature include three patients with TCS, an additional patient with no TCS but with tracheal stenosis (which could represent a TCS) and fibrous cartilaginous rings, and children with early death due to respiratory distress.6,15,28 The majority of the remaining patients with W290C in the literature were younger than age 2 years and operative evaluation of the airway was not documented. Without documentation of an airway evaluation, occult TCS cannot be ruled out in infants and toddlers. One patient with W290C was still living at age 15 years without reported TCS or history of tracheostomy.33 Between our cohort and the literature, there were also 11 patients with TCS who had mutations in FGFR2 at site 342. Among our cohort alone, 5 patients with mutations at site 342 had TCS but 4 did not. Because more than 50% in our cohort had TCS, airway evaluation should be strongly considered for patients with mutations at this site.
All patients with W290C had TCS and a severe phenotype, whereas no patients with W290R and W290G had TCS. Our cohort did not include sufficient numbers of patients with Pfeiffer syndrome with other mutations to evaluate the mutation-specific risk at other residues. It is difficult to extrapolate the rate of TCS from case reports of children with Pfeiffer due to other mutations, because a standardized airway evaluation cannot be assumed. However, the differential risk for patients with a mutation at residue 290 is clear. Moreover, the diagnosis of Crouzon compared with Pfeiffer in the neonatal period and beyond can be challenging; therefore, we believe early genetic testing would be beneficial for identification of children with W290C who carry a very high risk of TCS.
We believe that all children with syndromic craniosynostosis should be referred to a center where they can be evaluated by a team of experienced specialists. Identification of TCS can be challenging and should be evaluated by an experienced pediatric otolaryngologist. Airway evaluation should be considered for all patients with syndromic craniosynostosis, especially those with clinical suspicion of any FGFR2-associated craniosynostosis syndrome, Crouzon syndrome with acanthosis nigricans, or Saethre-Chotzen syndrome. To balance the risks and benefits of anesthesia and procedure-related risks, this airway evaluation could be coordinated with craniosynostosis repair or another surgical procedure. Images from bronchoscopy and 3D reconstruction of normal trachea and TCS are shown in Figure 1. In the event that a TCS is identified, timing and placement of tracheostomy and tracheostomy-related care, or airway reconstruction, should be undertaken by a pediatric otolaryngologist and anesthesiologist with expertise in the treatment of patients with syndromic craniosynostosis. This need is compounded by the elevated rate of choanal atresia/stenosis in patients with FGFR2-associated craniosynostosis syndromes, because there is risk for both upper and lower airway obstruction. These patients should also be evaluated by a pediatric geneticist or practitioner comfortable with pretest counseling and determination of appropriate clinical genetic testing (e.g., next-generation sequencing panel for FGFR1, FGFR2, FGFR3, and TWIST1) to aid in the interpretation and delivery of results.
Given the wide range of biological activities attributed to members of the fibroblast growth factor family, a number of mouse models have been developed to better understand the role(s) of the fibroblast growth factor receptors in embryogenesis and development. Early studies utilizing gene-targeting approaches in mice demonstrated that complete disruption of the FGFR2 gene resulted in early embryonic lethality in homozygous animals.34 This clear association between FGFR2 mutations and human disease led to the development of additional mouse models to help better understand the pathophysiology underlying these syndromes. Interestingly, the FGFR2+/S252W mouse model of Apert syndrome demonstrated complete tracheal rings and abnormal thickening of the tracheal cartilage. The tracheal phenotype seen in the FGFR2+/S252W mouse is very similar to that in the patients with TCS described in this article.35 A second knock-in mouse model of Apert syndrome FGFR2+/P250R failed to report any abnormalities of the tracheobronchial tree.35 Because patients with Apert syndrome are presumed to have one of two mutations in FGFR2, clinical genetic testing is typically not performed. It is interesting that our patient with TCS and genetically confirmed Apert syndrome had the S252W mutation, which is the same mutation that caused TCS in mice. In contrast, of those who underwent genetic testing and who did not have TCS, five had P250R and only one had a S252W mutation. Genetic testing of the remaining patients in the present series and previously reported patients would be needed to determine whether S252W confers a greater risk for TCS than P250R, because mouse models suggest a possible differential risk between the two mutations and would provide a clinical indication for genetic testing in patients with Apert syndrome.
Interestingly, a mouse model of Beare-Stevenson syndrome (FGFR2+/Y394C) failed to describe any lower airway anomalies, whereas two recently reported patients with Beare-Stevenson syndrome were found to have TCS, with a suspected TCS in a third patient.10,21,36 Given the findings of the present study, it would be very interesting to revisit these knock-in mouse models and perform a more in-depth analysis of the airway anatomy to look for more subtle changes that may provide insight into the respiratory issues seen in patients with craniosynostosis syndromes caused by mutations in the FGFR2 genes, in addition to the other genes implicated in the present study (i.e., FGFR3 and TWIST1). Further exploration of the phenotype in mouse models of the condition as related to phenotypes in patients with the disorders may provide insight into the pathogenesis of the conditions.
Conclusions
Children with syndromic craniosynostosis are at significant risk for TCS, which has been estimated in a recent meta-analysis to have a 90% risk of death by age 2 years without tracheostomy.8 The prevalence of TCS in our cohort was approximately 22%, and it was not limited to a particular diagnostic category or gene. There was a very high rate of TCS in our series of patients with a W290C mutation in FGFR2, as well as in previous reports of individuals with W290C with TCS and infantile deaths attributed to respiratory distress. Neonatologists should consider genetic testing and referral to a hospital with a pediatric otolaryngologist experienced in the identification and treatment of TCS for all children with syndromic multisuture craniosynostosis. Otolaryngologists should consider operative airway evaluation for all children with suspected craniosynostosis to identify patients at risk for sudden death. Genetic testing may help providers to weigh anesthesia and procedure-related risks of early operative airway evaluation with the risk of sudden death due to missed TCS. Furthermore, operative airway evaluation should be considered the standard of care for all children identified to have W290C in FGFR2. Future studies are needed to determine the mutation-related risk for children with syndromic craniosynostosis due to other genetic mutations.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Victor Ghioni for providing 3D reconstructions of normal and abnormal tracheas for Figure 1. This study was funded in part by the Jean Renny Endowment for Craniofacial Research and NIHT32 GM008628
Footnotes
Supplementary material is linked to the online version of the paper at http://www.nature.com/gim
DISCLOSURE
The authors declare no conflict of interest.
References
- 1.Plomp AS, Hamel BC, Cobben JM, et al. Pfeiffer syndrome type 2: further delineation and review of the literature. Am J Med Genet. 1998;75:245–251. [PubMed] [Google Scholar]
- 2.Cohen MM, Jr, Kreiborg S. Upper and lower airway compromise in the Apert syndrome. Am J Med Genet. 1992;44:90–93. doi: 10.1002/ajmg.1320440121. [DOI] [PubMed] [Google Scholar]
- 3.Hockstein NG, McDonald-McGinn D, Zackai E, Bartlett S, Huff DS, Jacobs IN. Tracheal anomalies in Pfeiffer syndrome. Arch Otolaryngol Head Neck Surg. 2004;130:1298–1302. doi: 10.1001/archotol.130.11.1298. [DOI] [PubMed] [Google Scholar]
- 4.Koga H, Suga N, Nakamoto T, Tanaka K, Takahashi N. Clinical expression in Pfeiffer syndrome type 2 and 3: surveillance in Japan. Am J Med Genet A. 2012;158A:2506–2510. doi: 10.1002/ajmg.a.35590. [DOI] [PubMed] [Google Scholar]
- 5.Fearon JA, Rhodes J. Pfeiffer syndrome: a treatment evaluation. Plast Reconstr Surg. 2009;123:1560–1569. doi: 10.1097/PRS.0b013e3181a2057e. [DOI] [PubMed] [Google Scholar]
- 6.Lajeunie E, Heuertz S, El Ghouzzi V, et al. Mutation screening in patients with syndromic craniosynostoses indicates that a limited number of recurrent FGFR2 mutations accounts for severe forms of Pfeiffer syndrome. Eur J Hum Genet. 2006;14:289–298. doi: 10.1038/sj.ejhg.5201558. [DOI] [PubMed] [Google Scholar]
- 7.Inglis AF, Jr, Kokesh J, Siebert J, Richardson MA. Vertically fused tracheal cartilage. An underrecognized anomaly. Arch Otolaryngol Head Neck Surg. 1992;118:436–438. doi: 10.1001/archotol.1992.01880040102017. [DOI] [PubMed] [Google Scholar]
- 8.Lertsburapa K, Schroeder JW, Jr, Sullivan C. Tracheal cartilaginous sleeve in patients with craniosynostosis syndromes: a meta-analysis. J Pediatr Surg. 2010;45:1438–1444. doi: 10.1016/j.jpedsurg.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Sagehashi N. An infant with Crouzon’s syndrome with a cartilaginous trachea and a human tail. J Craniomaxillofac Surg. 1992;20:21–23. doi: 10.1016/s1010-5182(05)80191-x. [DOI] [PubMed] [Google Scholar]
- 10.Wenger TL, Bhoj EJ, Wetmore RF, et al. Beare-Stevenson syndrome: two new patients, including a novel finding of tracheal cartilaginous sleeve. Am J Med Genet A. 2015;167A:852–857. doi: 10.1002/ajmg.a.36985. [DOI] [PubMed] [Google Scholar]
- 11.Devine P, Bhan I, Feingold M, Leonidas JC, Wolpert SM. Completely cartilaginous trachea in a child with Crouzon syndrome. Am J Dis Child. 1984;138:40–43. doi: 10.1001/archpedi.1984.02140390032010. [DOI] [PubMed] [Google Scholar]
- 12.Noorily MR, Farmer DL, Belenky WM, Philippart AI. Congenital tracheal anomalies in the craniosynostosis syndromes. J Pediatr Surg. 1999;34:1036–1039. doi: 10.1016/s0022-3468(99)90787-x. [DOI] [PubMed] [Google Scholar]
- 13.Stone P, Trevenen CL, Mitchell I, Rudd N. Congenital tracheal stenosis in Pfeiffer syndrome. Clin Genet. 1990;38:145–148. doi: 10.1111/j.1399-0004.1990.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 14.Lin SY, Chen JC, Hotaling AJ, Holinger LD. Congenital tracheal cartilaginous sleeve. Laryngoscope. 1995;105:1213–1219. doi: 10.1288/00005537-199511000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Zackai EH, McDonald-McGinn DM, Stolle C, Huff DS. Craniosynostosis with tracheal sleeve: a patient with Pfeiffer syndrome, tracheal sleeve and additional malformations in whom an FGFR2 mutation was found. Clin Dysmorphol. 2003;12:209. doi: 10.1097/01.mcd.0000080414.95344.ae. [DOI] [PubMed] [Google Scholar]
- 16.Alli A, Gupta S, Elloy MD, Wyatt M. Laryngotracheal anomalies in children with syndromic craniosynostosis undergoing tracheostomy. J Craniofac Surg. 2013;24:1423–1427. doi: 10.1097/SCS.0b013e3182953b43. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton J, Clement WA, Kubba H. Management of congenital cartilaginous sleeve trachea in children. Int J Pediatr Otorhinolaryngol. 2014;78:2011–2014. doi: 10.1016/j.ijporl.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 18.Mixter RC, David DJ, Perloff WH, Green CG, Pauli RM, Popic PM. Obstructive sleep apnea in Apert’s and Pfeiffer’s syndromes: more than a craniofacial abnormality. Plast Reconstr Surg. 1990;86:457–463. doi: 10.1097/00006534-199009000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Antón-Pacheco JL, Cano I, García A, Martínez A, Cuadros J, Berchi FJ. Patterns of management of congenital tracheal stenosis. J Pediatr Surg. 2003;38:1452–1458. doi: 10.1016/s0022-3468(03)00495-0. [DOI] [PubMed] [Google Scholar]
- 20.Davis S, Bove KE, Wells TR, Hartsell B, Weinberg A, Gilbert E. Tracheal cartilaginous sleeve. Pediatr Pathol. 1992;12:349–364. doi: 10.3109/15513819209023315. [DOI] [PubMed] [Google Scholar]
- 21.Stater BJ, Oomen KP, Modi VK. Tracheal cartilaginous sleeve association with syndromic midface hypoplasia. JAMA Otolaryngol Head Neck Surg. 2015;141:73–77. doi: 10.1001/jamaoto.2014.2790. [DOI] [PubMed] [Google Scholar]
- 22.Ulualp SO, Wright ST, Font JP. Tracheal cartilaginous sleeve contributing to obstructive sleep apnea in a child with Crouzon syndrome. Int J Pediatr Otorhinolaryngol Extra. 2006;1:76–80. [Google Scholar]
- 23.D’Anza B, Canto-Helwig C. Tracheal cartilaginous sleeve in Apert syndrome. Otolaryngol Head Neck Surg. 2014;150:1086–1087. doi: 10.1177/0194599814522591. [DOI] [PubMed] [Google Scholar]
- 24.Nakano T, Aiba T, Kubo T, Kusuki M, Hirano A, Matsushita N. [Tracheal cartilaginous sleeve in craniosynostosis] Nihon Jibiinkoka Gakkai Kaiho. 2008;111:623–627. doi: 10.3950/jibiinkoka.111.623. [DOI] [PubMed] [Google Scholar]
- 25.Scheid SC, Spector AR, Luft JD. Tracheal cartilaginous sleeve in Crouzon syndrome. Int J Pediatr Otorhinolaryngol. 2002;65:147–152. doi: 10.1016/s0165-5876(02)00132-5. [DOI] [PubMed] [Google Scholar]
- 26.Elloy MD, Cochrane LA, Wyatt M. Tracheal cartilaginous sleeve with cricoid cartilage involvement in Pfeiffer syndrome. J Craniofac Surg. 2006;17:272–274. doi: 10.1097/00001665-200603000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Gonzales M, Heuertz S, Martinovic J, et al. Vertebral anomalies and cartilaginous tracheal sleeve in three patients with Pfeiffer syndrome carrying the S351C FGFR2 mutation. Clin Genet. 2005;68:179–181. doi: 10.1111/j.1399-0004.2005.00477.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen CP, Lin SP, Su YN, Chien SC, Tsai FJ, Wang W. Craniosynostosis and congenital tracheal anomalies in an infant with Pfeiffer syndrome carrying the W290C FGFR2 mutation. Genet Couns. 2008;19:165–172. [PubMed] [Google Scholar]
- 29.Faust RA, Stroh B, Rimell F. The near complete tracheal ring deformity. Int J Pediatr Otorhinolaryngol. 1998;45:171–176. doi: 10.1016/s0165-5876(98)00100-1. [DOI] [PubMed] [Google Scholar]
- 30.Freeman L, Elakis G, Watson G, et al. Pfeiffer syndrome with neonatal death secondary to tracheal obstruction owing to the FGFR2 Glu565Ala mutation. Clin Dysmorphol. 2008;17:223–224. doi: 10.1097/MCD.0b013e3282fdcc86. [DOI] [PubMed] [Google Scholar]
- 31.Shimada H, Misugi K. Anomalies of the tracheal cartilage. Pathol Int. 1979;29:1001–1011. [Google Scholar]
- 32.Rutland P, Pulleyn LJ, Reardon W, et al. Identical mutations in the FGFR2 gene cause both Pfeiffer and Crouzon syndrome phenotypes. Nat Genet. 1995;9:173–176. doi: 10.1038/ng0295-173. [DOI] [PubMed] [Google Scholar]
- 33.Shotelersuk V, Ittiwut C, Srivuthana S, Mahatumarat C, Lerdlum S, Wacharasindhu S. Distinct craniofacial-skeletal-dermatological dysplasia in a patient with W290C mutation in FGFR2. Am J Med Genet. 2002;113:4–8. doi: 10.1002/ajmg.10449. [DOI] [PubMed] [Google Scholar]
- 34.Xu X, Weinstein M, Li C, et al. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development. 1998;125:753–765. doi: 10.1242/dev.125.4.753. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Sun M, Uhlhorn VL, et al. Activation of p38 MAPK pathway in the skull abnormalities of Apert syndrome Fgfr2(+P253R) mice. BMC Dev Biol. 2010;10:22. doi: 10.1186/1471-213X-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Zhou X, Oberoi K, et al. p38 Inhibition ameliorates skin and skull abnormalities in Fgfr2 Beare-Stevenson mice. J Clin Invest. 2012;122:2153–2164. doi: 10.1172/JCI62644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.