Abstract
After information is encoded into memory, it undergoes an offline period of consolidation that occurs optimally during sleep. The consolidation process not only solidifies memories, but also selectively preserves aspects of experience that are emotionally salient and relevant for future use. Here, we provide evidence that an afternoon nap is sufficient to trigger preferential memory for emotional information contained in complex scenes. Selective memory for negative emotional information was enhanced after a nap compared to wakefulness in two control conditions designed to carefully address interference and time-of-day confounds. Although prior evidence has connected negative emotional memory formation to rapid eye movement (REM) sleep physiology, we found that non-REM delta activity and the amount of slow wave sleep (SWS) in the nap were robustly related to the selective consolidation of negative information. These findings suggest that the mechanisms underlying memory consolidation benefits associated with napping and nighttime sleep are not always the same. Finally, we provide preliminary evidence that the magnitude of the emotional memory benefit conferred by sleep is equivalent following a nap and a full night of sleep, suggesting that selective emotional remembering can be economically achieved by taking a nap.
Keywords: emotion, memory, consolidation, sleep, slow-wave sleep
Not all events are remembered with equal probability. Although there are several forms of selectivity in memory, one form is determined by the emotionality of stimuli (see Payne & Kensinger, 2010; Walker, 2009 for review). Emotional events often are more likely to be remembered than neutral ones, because of processes that unfold during both the initial encoding of the event and its subsequent consolidation (see Hamann, 2001 for review). Emotional events, however, are not stored as complete replicas of the original experience. Rather, central, emotional information is often remembered at the expense of background detail. Such ‘trade-offs’ in memory are an established phenomenon in memory research (Kensinger, 2009), and are seen in the real world as well. For example, the weapon-focus effect involves vivid memory for an assailant’s weapon following an attack, but little memory for relevant background details such as the face of one’s perpetrator (Stanny & Johnson, 2000).
Although this memory selectivity was once attributed primarily to attentional factors at encoding, recent evidence has demonstrated that such selectivity is not predicted simply by the time spent looking at emotional scene elements (e.g., Steinmetz & Kensinger, 2013; Riggs et al., 2011). Moreover, our prior research has demonstrated that the degree of selectivity can vary as a function of delay interval (Payne, Chambers, & Kensinger, 2012b), a pattern of results that is inconsistent with an isolated effect of emotion on encoding. Taken together, this research suggests that memory selectivity requires the consideration of factors that act beyond the initial encoding stage of memory, likely during its consolidation.
Sleep is thought to provide ideal conditions for memory consolidation, or the time-dependent, off-line process that stabilizes memories after initial acquisition (Dudai, 2004; McGaugh, 2000). Evidence from molecular and cellular, as well as behavioral and cognitive, levels of analysis demonstrates that sleep benefits the consolidation of most types of memory (Bendor & Wilson, 2012; Diekelmann & Born, 2010; Ellenbogen, Payne & Stickgold, 2006; Payne, 2011a; Walker and Stickgold, 2004). This benefit is usually observed quantitatively; procedural memories are enhanced by sleep relative to a period of wakefulness (e.g. one gets faster and more accurate on a motor sequence task – Walker et al., 2002), while declarative memories, though typically not enhanced, become more resistant to deterioration (e.g. one forgets fewer word pair associates – Payne et al., 2012a). To date, most studies of sleep and memory have used one-dimensional, un-nuanced measures of memory performance (e.g. number of word-pairs corrected recalled) that conceptualize memory as simply ‘more’ or ‘less’. Yet abundant evidence demonstrates that memories can be qualitatively transformed over time (Moscovitch et al., 2005), and studies have begun to suggest that sleep plays an important role in this process as well (Oudiette & Paller, 2013; Payne, 2011a, b; Payne & Kensinger, 2010; Stickgold & Walker, 2013 for review).
Although the majority of these studies have concerned nocturnal sleep, emerging evidence suggests that a nap can be nearly as good as a night at stabilizing some forms of new knowledge (Conte & Ficca, 2012 for review; Mednick et al., 2002, 2003; Schabus et al., 2005). However, in spite of clear practical and theoretical importance, it remains unclear whether naps are of sufficient length and quality and have the proper sleep stage properties to trigger some of the more nuanced memory effects that underlie adaptive processing, such as transformation and selectivity, especially in the emotional domain.
In a previous study (Payne et al., 2008), we demonstrated that a full night of sleep plays a key role in the selective consolidation of negative emotional aspects of complex scenes. Participants studied scenes consisting of either negative (e.g. a snake) or neutral (e.g. a chipmunk) objects on a neutral background (e.g. a forest). While a day spent awake led to forgetting of emotional scenes in their entirety, with both objects and backgrounds deteriorating at similar rates, a night of sleep selectively preserved negative objects, but not their backgrounds, suggesting that these components undergo different post-encoding processing during sleep. This finding suggests that negative scene memories evolve differentially across time delays containing sleep and wakefulness, and that, rather than preserving intact representations of scenes, sleep-based consolidation processes may further “unbind” scenes to retain only their most emotionally salient, and perhaps adaptive, elements (Payne & Kensigner, 2010).
However, we did not record sleep via polysomnography in our prior study (Payne et al., 2008), and, in general, the underlying mechanisms of selective memory effects during sleep remain unclear. Although the aspects of sleep that benefit memory selectivity are likely to depend on the specific task used, evidence suggests that while slow-wave sleep (SWS) is most essential for neutral episodic memory consolidation, and perhaps particularly for the veridical details of neutral memories (Payne et al., 2009; Alger et al., 2012), REM sleep may be more important for emotional memory consolidation and various types of memory transformation and selectivity (Diekelmann & Born, 2010; Cai et al., 2009; Payne, 2011a,b; Stickgold & Walker, 2013). Complementing the classification of sleep stages, spectral analysis of the EEG provides a more quantitative measure of the amount of oscillatory activity in different frequency ranges. Largely concurring with the sleep stage evidence, delta activity (slow, synchronized activity between 1and 4Hz, which is the dominant rhythm of SWS) is typically associated with neutral episodic memory consolidation, while theta activity (faster, desynchronized activity between 4 and 7Hz) during REM sleep has been associated with emotional memory consolidation (Diekelmann & Born, 2010 for review; Nishida, Pearsall, Buckner, & Walker, 2009). In line with this reasoning, we recently showed that the amount of REM sleep obtained overnight correlated positively with the selective consolidation of central, negative objects in this task (Payne, Chambers & Kensinger, 2012b), a finding that coheres with previous evidence for a REM sleep-emotional memory connection (see Payne & Kensinger, 2010 and Walker, 2009 for review), including a nap study demonstrating that emotional memory performance was positively correlated with both time spent in REM sleep and theta activity within REM sleep (Nishida, Pearsall, Buckner, & Walker, 2009).
However a strict REM sleep/SWS dichotomy seems unlikely given several studies that implicate REM sleep in neutral memory consolidation (e.g. Guerrien et al., 1989; Tilley & Empson, 1978), including a nap study (Muto et al., 2005), and findings pointing to a role for SWS (and SWS associated norepinephrine activity) in amygdala-dependent emotional memory consolidation (Groch et al., 2011; see also Eschenko & Sara, 2008). Thus, in addition to uncertainty about whether a daytime nap can go beyond memory stabilization to produce more nuanced selectivity effects in emotional memory, it remains unclear whether SWS might benefit emotional memory consolidation under some conditions. Here, we aimed to shed light on these questions by asking whether an afternoon nap, containing both SWS and REM sleep, would be sufficient to provoke selective consolidation effects in emotional memory. For both theoretical and practical reasons, we were interested in whether a nap, which occurs at a different circadian phase than nocturnal sleep, would confer a similar emotional selectivity benefit as more lengthy sleep at night, and whether this benefit would depend on the same underlying (REM) sleep physiology (Payne, Chambers & Kensinger, 2012b). As in these prior studies, we focused exclusively on negative emotional memory in this experiment.
We predicted that an afternoon nap would be sufficient for the selective consolidation of negative, emotional objects relative to two wake control conditions that were carefully designed to control for time-of-day and interference effects. We also predicted that the sleep-dependent modulation of the trade-off effect would be similar in magnitude to that observed in our overnight study (Payne et al., 2012b). Further, following previous findings (Nishida et al., 2009; Payne et al., 2012b), we predicted that this consolidation benefit would be related to theta activity as well as the amount of REM sleep obtained in the nap. However, given arguments that 1) SWS may be essential for the consolidation of specific memory for neutral episodic details, but not for semantic generalities, within the declarative memory system (see Payne, 2011a; Payne et al., 2009), and that 2) SWS plays a role in emotional memory consolidation (e.g. Eschenko & Sara, 2008; Groch et al., 2011), we also predicted that delta activity and SWS would benefit memory consolidation in this task.
Methods
Participants and Experimental Design
Sixty students from Harvard University (18–26 years of age, M= 20.6 years) participated for payment. They were native English speakers who had normal or corrected-to-normal vision, screened to ensure they had no neurological, psychiatric, or sleep disorders and were not taking medications affecting sleep architecture or the central nervous system. This screen took place three days in advance of the study, at which time participants were asked to formally agree to sleep at least 7 hours on each night leading up to their participation in order to minimize sleep debt.
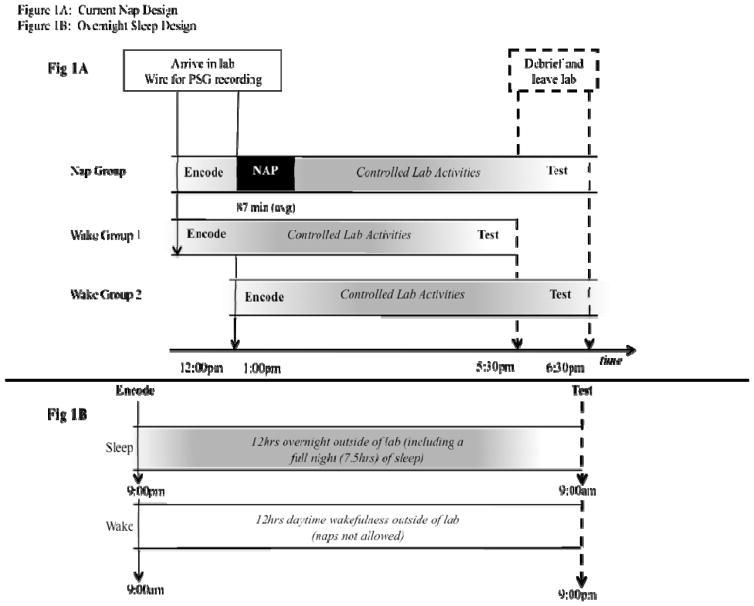
Participants were assigned to either a nap condition (n=24), or to one of two wake conditions (n=18, in each). See Figure 1A for experimental timeline. Participants were randomly assigned to the nap and the first wake control condition (Wake 1). Participants in both of these groups were wired for PSG recording and then a coin was flipped to determine group membership. We were unable to randomly assign participants to the second wake control condition (Wake 2) for scheduling reasons; however, there were no performance differences between the two wake groups (see Table 1), thus ameliorating concerns about time of day preferences. All participants were asked about their prior night’s sleep (quantity and quality) and given the Stanford Sleepiness Scale upon arrival in the lab (Hoddes et al., 1973).
Figure 1.
A. Experimental design for the Nap group and two Wake control groups (Wake1 and Wake2). B. Experimental design of our prior overnight study, for purposes of comparison. Note different time scale.
Table 1.
| Emotional | |||||
|---|---|---|---|---|---|
|
| |||||
| Objects | Backgrounds | ||||
|
| |||||
| Specific Recognition | General Recognition | Specific Recognition | General Recognition | ||
| Wake 1 (noon control) | Mean | 0.73 | 0.87 | 0.44 | 0.67 |
| SEM | 0.05 | 0.02 | 0.05 | 0.05 | |
| Wake 2 (1:30pm control) | Mean | 0.67 | 0.86 | 0.33 | 0.55 |
| SEM | 0.04 | 0.03 | 0.05 | 0.07 | |
|
| |||||
| Neutral | |||||
|
| |||||
| Objects | Backgrounds | ||||
|
| |||||
| Specific Recognition | General Recognition | Specific Recognition | General Recognition | ||
|
| |||||
| Wake 1 (noon control) | Mean | 0.55 | 0.78 | 0.55 | 0.77 |
| SEM | 0.04 | 0.04 | 0.07 | 0.06 | |
| Wake 2 (1:30pm control) | Mean | 0.58 | 0.74 | 0.48 | 0.65 |
| SEM | 0.04 | 0.04 | 0.05 | 0.07 | |
No Differences in Recognition Memory Performance in the Wake Control Groups. All p’s > .1 for Wake 1 vs. Wake 2 comparisons of individual scores.
One sleep participant was excluded due to an inauspiciously timed fire alarm in the building. Two wake participants were also excluded, one for failure to comply with the prescreening requirements and another due to a computer failure and subsequent loss of data, leaving a total of 57 participants for analysis (Nap, n=23, 13 female; Wake, n=34, 16 female).
Given concerns about interference and time-of-day confounds in sleep and memory research (Wixted, 2004), we ensured that participants in each of the wake groups experienced the same total time of active waking interference as the nap group (Figure 1A). Participants in the nap group arrived for the encoding task at noon, were put to bed at 1pm (napping for 87 ± 30min on average, see Table 1), and performed the memory test at 6:30pm. To control for time-of-day effects on memory encoding, participants in the first wake control condition encoded the images at noon, at the same time as the nap participants; however, their memory was tested 90min earlier than nap participants (i.e. at 5pm instead of 6:30pm, see Figure 1A), which ensured that subjects in both groups spent an equal amount of time awake (and thus exposed to interference). To control for time-of-day effects on memory retrieval, participants in the second wake control condition arrived 90min later than nap participants (i.e. at 1:30pm instead of noon), but were tested at the same time as nap participants (at 6:30pm). Note that this design not only controls for interference effects as well as time-of-day influences on both encoding and retrieval, but it is also a rather conservative test of sleep-based memory consolidation given that nap participants experienced a longer memory retention interval (6.5 hours) than participants in either of the control groups (5 hours, Figure 1A).
Materials
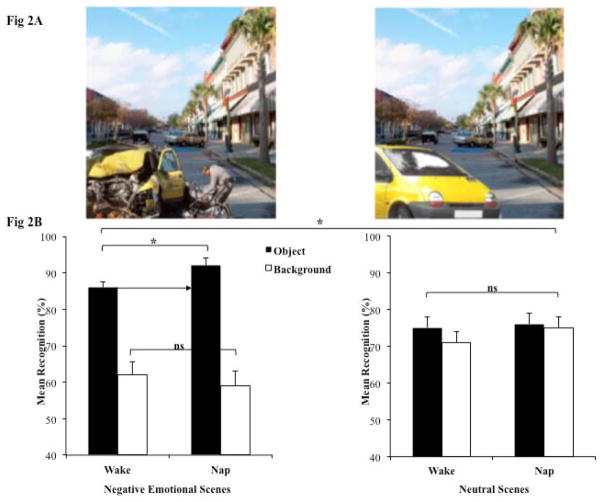
Scenes consisted of either a negative (e.g., a crashed car) or neutral object (e.g., an intact car) on a plausible neutral background (e.g., a street). For each of 64 scenes (e.g., a car on a street), we created eight different versions by placing each of two similar neutral objects (e.g., two images of a car) and each of two related negative objects (e.g., two images of a crashed car) on each of two neutral backgrounds (e.g., two images of a street). An additional 32 scenes served as ‘new’ items on a recognition memory test. See Figure 2A for sample scenes. Objects and backgrounds were previously rated for valence and arousal using 7-point scales (Kensinger, Garoff-Eaton, & Schacter, 2006). All negative objects had arousal ratings of 5–7 (with high scores signifying an exciting or arousing image) and valence ratings lower than 3 (with low scores signifying a negative image). All neutral items (objects and backgrounds) were rated as non-arousing (arousal values lower than 4) and neutral (valence ratings of 3–5).
Figure 2.
A. Sample Stimuli. Sample stimuli with a neutral background and either a neutral object (intact car) or negative object (crashed car) in the foreground. B. A nap selectively benefits memory for emotional components of scenes. This figure depicts mean general recognition memory for objects and backgrounds in the nap and combined wake groups. Separate graphs show results for negative scenes (left), and neutral scenes (right). The arrow highlights the benefit conferred selectively to negative objects following the nap compared to wakefulness. Note, however, that no such differences emerge for the backgrounds on which these negative objects are embedded, or for either component (objects or backgrounds) of neutral scenes. Significant differences are denoted by asterisks, *p<.05
Procedure
During the (incidental) encoding session, participants viewed a set of 64 scenes (32 with a neutral object and 32 with a negative object, all on neutral backgrounds) for 5sec each, and then indicated on a 7-point scale whether they would approach or move away from the scene if they encountered it in real life. This task was used to maximize attention to the scene and promote deep encoding. The studied version of each scene (of the eight possible versions) was counterbalanced across participants.
Immediately after viewing the scenes, Nap participants were put to bed, while Wake participants watched a low-arousing, black-and-white movie. Participants in all conditions remained in the laboratory performing similar controlled activities until their memory for the scenes was tested in a recognition memory task (Figure 1A).
In the self-paced recognition task, participants viewed objects and backgrounds, presented separately and one at a time. This memory test used a combination of items that were either identical (i.e. the same objects and backgrounds seen at encoding) or, alternatively, similar but non-identical (e.g. a picture of a snake similar to the snake seen at encoding) to studied items, which allowed us to examine the role of napping in memory specificity. Participants either saw the identical or similar version of a particular item at test, never both, and were asked to indicate whether each item was the “same” to the one seen previously, “similar” (but not identical) to the one seen previously, or entirely “new” (neither similar nor identical to a previously viewed item). Items included on the recognition test were: 32 identical objects, 32 similar objects, and 32 new objects (16 negative and 16 neutral, each), as well as 32 identical backgrounds, 32 similar backgrounds (16 previously shown with a negative item and 16 with a neutral item, each), and 32 new backgrounds.
Memory Analysis
Because we were interested in the specificity of memory following the nap vs. wake consolidation intervals, we assessed both corrected specific and corrected general recognition memory for all items (hits minus false alarms). Consistent with prior studies, the more conservative specific recognition score (i.e., a “same” response to an identical item, divided by the total number of identical items) was computed to capture veridical memory for the details of the studied object or background (Kensinger et al., 2006, 2007; Payne et al., 2008; Waring, Payne, Schacter & Kensinger, 2010). The less conservative general recognition score, on the other hand, was computed by summing the proportion of “same” and “similar” responses to identical items (Kensinger et al., 2006, 2007). This score is considered to reflect memory for at least some aspects of the studied item (Kensinger et al., 2006, 2007; Payne et al., 2008); that is, for identical items given either a “same” or “similar” response, participants had to remember at least that a particular type of object or background had been studied (e.g., that they had seen a crashed car or a street scene), because otherwise they would have instead indicated that the item was “new”. Thus, the general recognition score reflects a participant’s tendency to remember at least the gist of the items (with or without specific detail). The specific and general recognition memory scores were computed separately for negative and neutral objects, as well as for the neutral backgrounds on which they were placed.
Polysomnography Recordings
Polysomnographic (PSG) sleep recording was performed in accordance with standardized techniques, using digital electroencephalographic (EEG), electrooculographic (EOG), and electromyographic (EMG) signals acquired with an Embla A10 digital recording system (200Hz sampling rate). The recording montage included frontal (F1, F2) and central (C3, C4) electrodes, with each electrode referenced to the contralateral mastoid. Each 30sec epoch of sleep was scored blind to participants’ behavioral task performance according to the standards of Rechtschaffen and Kales (1968). The PSG recording was scored visually as NREM stages 1–4, REM sleep, or wakefulness. Slow-wave sleep (SWS) was calculated by combining NREM stages 3 and 4. Next, EEG data were filtered at 0.3–35Hz (with a 60Hz notch filter), and artifacts were visually identified and removed. Spectral analysis was then applied to all artifact-free epochs of NREM and REM sleep during the nap. Fast Fourier transforms were carried out in BrainVision Analyzer 2.0 to calculate mean absolute power spectral density (μV2/Hz) in the slow oscillation (0.5–1Hz), delta (1–4Hz), theta (4–7Hz), and sigma (12–15Hz) frequency bands. Sleep spindle counts were obtained by filtering all artifact-free epochs of Stage 2 sleep and SWS at 12–15Hz and then applying the established automatic detection algorithm of Ferrarelli et al. (2007) using MATLAB 8.0 (The MathWorks; Natick, Massachusetts). Total number of spindles and spindle density (spindles/total analyzed time in S2 and SWS) were calculated for each scalp electrode.
Results
We assessed all data for potential outliers prior to conducting comparisons according to the outlier labeling calculations detailed in Hoaglin et al., (1986). Because performance in the two wake control conditions did not significantly differ on any memory measure (all p’s > .1, see Table 1), they were collapsed into a single ‘wake’ group in the following analyses. Participants in the nap and wake groups were similarly well rested when they arrived in the laboratory, as the sleep log data revealed no significant differences between groups in total sleep time the night before the study (7.8 hours on average in the nap group vs. 7.9 hours in the wake group, t = .75, p = .46, 95% CI [−0.67, 0.31]), or in the time participants woke up in the morning on the day of the study (t = .77, p = .45, 95% CI [−0.95, 0.42]). Similarly, there was no significant difference between groups in perceived sleep quality, with all subjects reporting at least average quality sleep on the night prior to the experiment (likely due to the fact that all subjects agreed to sleep for at least 7hr on the three nights leading up to the experiment during screening). There was also no difference between groups in perceived sleepiness levels prior to the experiment (t = .16, p = .87, 95% CI [−0.41, 0.35]), as determined via the Stanford Sleepiness Scale (Hoddes et al., 1973).
Memory performance, however, differed considerably between the nap and wake conditions. A condition (nap, wake) x scene component (object, background) x valence (negative, neutral) mixed ANOVA on general recognition, with scene component and valence as repeated measures variables, revealed a main effect of scene component, F(1,55) = 85.37, p < .001, ηp2 = .61, whereby objects (M = .82 ± .01) were remembered better than backgrounds (M = .67 ± .02). This was qualified by a 2-way interaction between scene component and valence, F(1,55) = 88.70, p < .001, ηp2 = .62, which reflects the expected trade-off effect (Kensinger et al., 2007; Payne & Kensinger, 2010; Waring, Payne, Schacter, & Kensinger, 2010); paired t-tests confirmed that negative objects (M=.89 ± .01) were well remembered at the expense of their associated neutral backgrounds (M=.60 ± .03), t = 11.5, p < .001, 95% CI [0.23, 0.33], whereas neutral objects (M = .75 ± .02) and their associated backgrounds were remembered equivalently (M = .73 ± .03), p > .15. Most importantly, however, there was a significant 3-way interaction among the factors demonstrating that the trade-off effect was enhanced by the nap, F(1,55) = 4.5, p = .039, ηp2 = .075 (see Figure 2B). As predicted, participants in the nap group remembered negative objects (M = .92 ± .02) better than those in the wake group (M = .87 ± .02), F(1,56) = 5.0, p = .029, 95% CI [0.01, 0.10]. However, there was no group difference for neutral object memory, F(1,56) = .13, p = .71 or memory for backgrounds, regardless of whether they were paired with negative or neutral objects at encoding (Fs < 1.1, ps > .30). Thus, the selective memory benefit for emotional information observed following a full night of sleep (Payne et al., 2008) was also observed following a daytime nap. A similar pattern of results emerged in analyses of specific recognition memory (see Table 2), where a main effect of scene component F(1,55) = 87.2, p < .001, ηp2 = .61, and a scene component by valence interaction, F(1,55) = 121.5, p < .001, ηp2 = .69 further interacted with condition (3-way interaction, F(1,55) = 4.3, p = .043, ηp2 = .07).
Table 2.
| Emotional | |||
|---|---|---|---|
|
| |||
| Objects | Backgrounds | ||
|
| |||
| Nap | Mean | 71 | 34 |
| SEM | 3 | 3 | |
| Wake (Combined) | Mean | 68 | 41 |
| SEM | 3 | 4 | |
| Neutral | |||
|
| |||
| Objects | Backgrounds | ||
|
|
|||
| Nap | Mean | 55 | 55 |
| SEM | 3 | 3 | |
| Wake (Combined) | Mean | 54 | 52 |
| SEM | 3 | 3 | |
Mean specific recognition memory (%) for objects and backgrounds in the nap and combined wake groups. The pattern of specific memory performance is similar overall to that for general recognition memory. However, here participants in the nap group did not have significantly better memory for negative emotional objects than participants in the wake group.
It is interesting to note that the magnitude of the selective emotional memory effect in the nap group is very similar to that observed in our prior overnight study (Figures 1B and 3). We calculated the magnitude of the trade-off effect by subtracting emotional objects from their backgrounds in the nap group from the present study and in the overnight sleep group from Payne et al (2012b). The magnitude of the trade-off effect in the nap group (M = .33) was not significantly different from the magnitude of the trade-off effect in the overnight sleep group (M = .27), F(1, 49) = 1.3, p = .26, suggesting that a nap may be as good as a night for producing a selective memory benefit for emotional components of scenes (see Figure 3).
Figure 3.
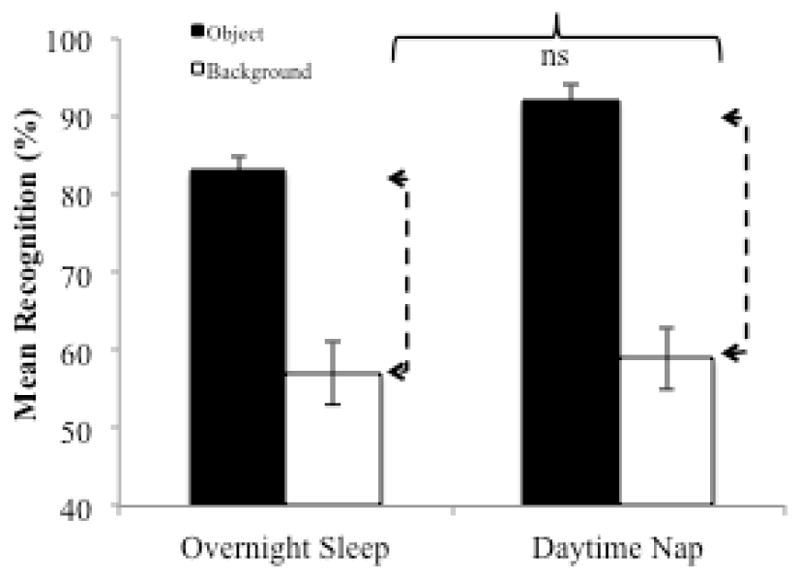
Mean recognition memory for emotional objects and paired neutral backgrounds in the overnight sleep group from Payne et al (2012b) compared to the daytime nap group from the current study. Dashed arrows denote the magnitude of the trade-off effect, which is statistically equivalent between groups.
EEG Spectral Power and Sleep Stage Parameters in Relation to Memory Performance
Given our predictions and the prior literature (e.g. Groch et al., 2011; Nishida et al., 2009), we focused a priori on measures of REM sleep and SWS in our correlational analyses exploring relationships between sleep parameters and memory performance.
A summary of sleep measures is provided in Table 3. One nap participant’s sleep data failed to record, leaving 22 subjects for analysis. Sixteen of these subjects obtained REM sleep. Although we anticipated that REM sleep physiology would play a key role in the selective retention of emotional aspects of scene memories (i.e., emotional objects), we did not find evidence for this. There was no relationship between recognition of emotional objects and theta power in REM sleep averaged across electrode sites (general recognition, r(16)=.33, p=.22; specific recognition, r(16)=.17, p=.52). Similarly, neither the amount of REM sleep, nor the percentage of REM sleep, in the nap correlated with general or specific memory for emotional objects (all ps > .50). Theta activity and REM sleep also did not correlate significantly with any of the other memory measures.
Table 3.
| Sleep Parameters
| ||
|---|---|---|
| Minutes (SEM) | % of TST (SEM) | |
| SL | 14.45 (1.7) | |
| TST | 87.36 (6.5) | |
| WASO | 19.61 (4.6) | |
| S1 | 12.57 (1.3) | 15.42 (1.6) |
| S2 | 44.61 (4.0) | 52.80 (3.2) |
| SWS (S3 + S4) | 14.52 (2.2) | 16.76 (2.6) |
| REM | 15.66 (2.8) | 15.01 (2.6) |
Note. SL, latency to sleep onset (first epoch of sleep); TST, total sleep time; WASO, wake after sleep onset, S1-S2, Sleep stages 1 & 2, SWS, slow wave sleep, REM, rapid eye movement sleep
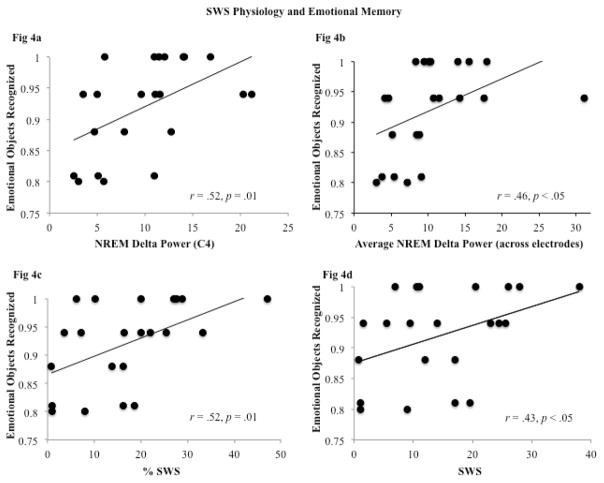
Although there is a substantial literature pointing to a beneficial effect of slow-wave sleep (SWS) physiology on neutral episodic memory consolidation (Marshall & Born, 2007), recent studies suggest that SWS may also play a role in emotional memory consolidation (Eschenko & Sara, 2008; Groch et al., 2011). In line with these studies, we found that general recognition of emotional objects was associated with spectral power in the delta frequency band when averaged across all electrode sites, r(22) = .46, p < .05 (Figure 4B). To rule out the possibility that this correlation was driven by activity at a single electrode site, we confirmed that strong correlations emerged between absolute delta activity and general emotional object memory at both C3, r(22)=.51, p<.01 and C4, r(22) = .52, p < .01 (Figure 4A). Similarly, delta power in the frontal electrode sites (F3 and F4) was positively correlated with general recognition of emotional objects, although these correlations did not reach significance, r(22) = .33, p= .14, and r(22) = .39, p = .07, respectively. To rule out a generally beneficial effect of delta power on memory, we confirmed that power in the delta frequency band was not correlated with any other measure of memory, either when averaging across electrodes, or when examining individual electrodes separately. Similarly, sigma power, total sleep spindles, spindle density, and slow oscillation activity were not significantly related to any measure of memory, whether specific or general.
Figure 4.
Correlations of SWS and delta power with general recognition memory
In agreement with the spectral analyses, general recognition of emotional objects was positively correlated with the percentage of the nap spent in SWS (SWS percent), r(22) = .52, p = .01, and with overall time spent in SWS, r(22) = .43, p < .05 (Figure 4c and 4d). Correlations between specific recognition of emotional objects and SWS did not quite reach significance (SWS%, r(22) = .41, p = .06; SWS, r(22) = .40, p = .06). Together, these findings suggest that the ability to selectively remember emotional information following a daytime nap is likely related to delta activity in the nap and not to activity in the other frequency bands, including the theta band, and to time spent in SWS as opposed to REM sleep.
It is also worth noting that SWS correlated positively with specific memory for neutral items. For example, SWS percent was significantly correlated with specific memory for neutral objects, r(22) = .54, p < .01 (r(22) = .39, p = .07 for specific memory for neutral backgrounds). The pattern was similar for overall time spent in SWS, although these correlations did not reach significance, r(22) = .36, p = .10 for neutral objects and r(22) = .33, p = .14 for neutral backgrounds. These exploratory findings are of interest because while it has been argued that specific, detailed episodic memories for neutral stimuli may be related to slow-wave sleep in a way that more general neutral memories are not (e.g. Payne et al., 2009, Payne, 2011a for review), direct evidence for such a notion has been lacking. That SWS may be key for neutral memory specificity is further supported by the fact that SWS was unrelated to general memory for neutral objects (SWS percent, r(22) = .22, p = .32; SWS amount, r(22) = −.03, p = .90) and backgrounds (SWS percent, r(22) = .02, p = .92; SWS amount, r(22) = .07, p = .77), and no other stage of sleep was related to specific memory for neutral images.
Notably, SWS was the only sleep stage to correlate with any aspect of memory performance in this study. Also, in support of the notion that sleep plays an active as opposed to passive role in these selective consolidation effects, total sleep time did not correlate with any of the memory measures (all p’s > .4). This suggests that the memory benefits are specific to SWS, and are not simply a byproduct of protection from interference during sleep.
Discussion
To remember a complex experience in a precise and detailed manner, its features must be bound together and maintained as an intact, unified representation in memory. However, emerging evidence suggests that in addition to promoting such veridical representations, the consolidation process can transform memories, allowing individual elements of experience to be selectively enhanced or preserved at the expense of features deemed to be of less importance (e.g. Payne, Swanberg, et al., 2008). Sleep has been shown to facilitate this preferential processing of information (Oudiette & Paller, 2013; Stickgold & Walker, 2013), especially when that information is emotionally salient (Payne & Kensinger, 2010). However, in spite of growing theoretical and practical interest in napping (Ficca et al., 2010), it is largely unknown whether such selective emotional consolidation can be achieved by such a brief period of sleep.
Here we show that a daytime nap is sufficient for the selective consolidation of specifically negative emotional components of scene memories. Relative to neutral scenes, the nap preferentially preserved memory for the arousing central focus of emotional scenes while leaving the neutral backgrounds completely unaffected. Such trade-off effects in memory are well established (Kensinger, 2009; Payne et al., 2004 for review), but are typically described in terms of attentional narrowing at encoding. Indeed, having a visually evocative “attention magnet” in a scene can increase the likelihood that such trade-offs occur (Reisberg & Heuer, 2004). However, the current study adds support to the idea that details of emotional experiences can also be influenced by post-encoding consolidation processes that occur during sleep. Notably, we further show that this effect can be economically achieved during a nap and that, although we acknowledge that the comparison was retrospective, the magnitude of the trade-off effect appears comparable to that seen after a full night of sleep. Comparable memory benefits for a nap and a full night of sleep have also been reported for a procedural motor discrimination task (e.g. Mednick et al, 2002, 2003). A direct experimental comparison would of course be necessary to confirm this finding, and a separate experiment should confirm whether the findings reported here would also extend into the positive emotional memory domain. Future studies would also benefit from the use of actigraphy in the days, if not weeks, preceeding the experiment to control for sleep debt.
There is a growing consensus that the consolidation of hippocampus-dependent neutral episodic memories is modulated by deep, slow-wave sleep (SWS) (Marshall & Born, 2007). SWS is characterized by slow (delta 1–4Hz), high amplitude waves in the EEG. It is associated with hippocampal sharp wave-ripple events (SPW-Rs), which are thought to participate in reactivating recent patterns of neural activity (i.e. those representing a new memory) and to lead to strengthening and stabilization of memory. The properties of the brain during SWS support communication between hippocampal and neocortical memory stores as memories undergo this process of consolidation (Buzsáki, 1989, 1996). Conversely, emotionally salient information, especially when that information is negative, is thought to mainly benefit from rapid eye movement (REM) sleep, a period of high activity in the hippocampus and amygdala, brain regions cooperatively involved in emotional memory benefits.
Although there has been a tendency to associate SWS and REM sleep with neutral declarative and emotional memory consolidation, respectively (Plihal & Born, 1997; Wagner et al., 2001; Marshall & Born, 2007; Nishida et al., 2009), this strict separation seems increasingly unlikely as literature emerges implicating REM sleep in neutral memory consolidation (Muto et al., 2005) and SWS in emotional memory processing (Eschenko & Sara, 2008; Groch et al., 2011). Much of what is considered emotional memory has both a declarative, hippocampus-dependent component as well as memory of the emotional reaction to the experience. Therefore, it makes sense that a state of facilitated communication between the hippocampus and the neocortex (i.e. SWS) would benefit this type of memory. In line with this, we have here reported evidence that SWS facilitates selective consolidation of the negative emotional components of complex visual memories. While additional experimental work is needed, the current evidence adds to a growing literature suggesting that SWS physiology, at least in some cases, plays a role in emotional memory consolidation.
The current nap study found a different sleep stage-memory relationship than the positive correlation between REM sleep and selective negative emotional memory consolidation that we recently found, using the same task, across a night of sleep (Payne, Chambers & Kensinger, 2012b). In that study, every subject obtained several cycles of both SWS and REM sleep, while in the current nap study, only 16 of the nap subjects obtained REM sleep at all. Moreover, given the brevity of the nap, subjects averaged only 15min in REM sleep, compared to over 90min in our overnight study – perhaps not enough to allow this sleep stage to make its typical contribution to memory performance. However, this logic does not easily align with Nishida et al (2009), who found correlations between emotional memory performance and several measures of REM sleep in a similar afternoon nap design in which subjects got even less REM sleep (10min on average). The difference may lie in the type of memory probed in the two experiments. Nishida et al. assessed participants’ memory for intact negative and neutral images, rather than the more nuanced, selective memory for negative emotional components of complex scenes examined in the current study. Therefore, perhaps the contribution of SWS to this type of preferential processing was greater within our naps. Indeed, it is possible that any type of selective remembering, even if non-emotional, would produce the same SWS mechanisms in a nap.
While the main findings of this study are 1) that a daytime nap is sufficient to produce selective emotional memory consolidation, and 2) that SWS appears to play a role in this selective consolidation, it is worth noting that we also found evidence for a relationship between SWS and neutral object memory. Even in the absence of a behavioral difference between nap and wake groups in memory for neutral objects, within the nap group, participants who obtained more SWS also had better neutral object memory. Although the SWS-neutral declarative memory relationship has been observed many times (Marshall & Born, 2007 for review), it typically concerns precise memory for specific episodic or spatial details (Payne, 2011a). Indeed, it has been argued that specific memories for neutral events may benefit from SWS in a way that more general neutral memories do not (e.g. Payne et al., 2009, Payne, 2011a for review). The current findings provide preliminary support for this idea. Specific memory for neutral foreground objects appeared to benefit from SWS in the nap, while general memory for these objects was not. Another potential explanation for this result is that SWS was involved in the consolidation of objects central to these scene memories, regardless of their valence. This argument cannot be definitively ruled out. However, if this were the case, one would expect to also see a strong relationship between general memory for neutral objects and SWS, yet this was not the case. Moreover, in addition to the positive correlation between SWS and specific memory for neutral objects, there was a marginal positive correlation between SWS and memory for neutral backgrounds. As such, this pattern provides preliminary support for the idea that SWS’s contribution to the consolidation of neutral declarative memories is strongest for detailed, veridical memory representations rather than general or “gist” memory representations (Payne et al., 2009; Payne, 2011 for review).
In this study we also addressed two arguments against the proposal that the effects of sleep on memory are due to active processes (Wixted, 2004). One argument is that sleep provides a temporary passive shield against interfering stimulation simply by reducing sensory input. Another is that apparent sleep-dependent benefits are artifacts of time-of-day differences in training and testing, which are unavoidable in comparing nocturnal sleep and daytime wake groups. To address these concerns, we compared the nap condition to two different wake control conditions. This design controlled for potential time-of-day effects, with all groups trained and tested in the afternoon and early evening. Moreover, time of day influences on the nap group’s performance were precisely equated at encoding, by training one of the Wake groups with the Nap group (Wake1), and at retrieval, by testing the other Wake group with the Nap group (Wake2). However, while we pseudo randomly assigned subjects to groups, with complete randomization in the nap and first wake groups, we cannot rule out the possibility that there may have been uneven distribution of subjects who were considered “morning” or “evening” types across groups, as we did not assess chronotype in this study. Future studies would benefit from evenly assigning different chronotypes to all groups.”
Time spent awake, when memories would be subject to interference was also equated across all three groups. In fact, this design is particularly conservative because the nap group maintained the memories for the longest retention period (6.5hrs compared to 5hrs) and yet still demonstrated significantly superior performance. Any concern that the extra 90min in the retention interval for the nap group may have actually benefited emotional memory (i.e. because some studies suggest that emotional memories grow stronger with the passage of time, e.g. Kleinsmith & Kaplan, 1963) should be ameliorated by the fact that we previously observed a benefit of sleep in our design comparing equivalent amounts of nocturnal sleep and daytime wakefulness (Payne et al., 2008, 2012).
Equally important for ruling out potential time-of day and interference confounds, memory performance in our study correlated with a specific sleep stage – SWS – and not total sleep time, as well as with activity in the delta frequency band. Critically, then, it is likely to be features of active sleep physiology, rather than passive time-of-day differences or interference factors, that produced the superior memory seen for emotional items in the nap group.
Our adaptive memory system hones in on the emotional facets of experience, preferentially preserving them in memory so that we can appropriately respond to future emotional situations. If memory functions to predict and prepare for the future as much as to recall the past (Schacter, Addis, & Buckner, 2008), then selective emotional memory consolidation makes good evolutionary sense (Nairne et al., 2007). Our findings provide evidence that sleep-based consolidation processes, achieved in sleep as brief as a nap, are essential for promoting the selective storage of emotional elements over less biologically relevant information.
Conclusion
Although sleep is oftentimes undervalued and napping carries a stigma of laziness, the present results add to a growing body of evidence suggesting that even a short daytime nap is powerful enough to produce marked memory advantages. Importantly, the present results demonstrate that these benefits can be selective for emotionally salient and adaptive information; even over the brief sleep period, memory consolidation processes are not indiscriminate. Interestingly, the type of sleep found to enhance the salient components of memory, SWS, differs from that previously found during overnight sleep. Future studies are needed to determine if there is a fundamental difference in daytime and overnight sleep, perhaps with neurochemical and hormonal levels in combination with sleep stages, that could lead to similar mnemonic effects of sleep over a nap and across a night but to different sleep-stage correlates for those behavioral effects.
Acknowledgments
This study was supported by grant BCS-0963581 from the National Science Foundation (to E.A.K. and J.D.P.).
References
- Alger SE, Lau H, Fishbein W. Slow wave sleep during a daytime nap is necessary for protection from subsequent interference and long-term retention. Neurobiology of Learning and Memory. 2012;98(2):188–196. doi: 10.1016/j.nlm.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Bendor D, Wilson MA. Biasing the content of hippocampal replay during sleep. Nature Neuroscience. 2012;15:1439–1444. doi: 10.1038/nn.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31(3):551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. The hippocampo-neocortical dialogue. Cerebral Cortex. 1996;6(2):81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proceedings of the National Academy of Sciences. 2009;106(25):10130–10134. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte F, Ficca G. Caveats on psychological models of sleep and memory: a compass in an overgrown scenario. Sleep Medicine Reviews. 2012;17(2):105–121. doi: 10.1016/j.smrv.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nature Reviews Neuroscience. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Ellenbogen JM, Payne JD, Stickgold R. The role of sleep in declarative memory consolidation: passive, permissive, active or none? Current Opinion in Neurobiology. 2006;16:716–722. doi: 10.1016/j.conb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Eschenko O, Sara SJ. Learning-Dependent, Transient Increase of Activity in Noradrenergic Neurons of Locus Coeruleus during Slow Wave Sleep in the Rat: Brain Stem–Cortex Interplay for Memory Consolidation? Cerebral Cortex. 2008;18(11):2596–2603. doi: 10.1093/cercor/bhn020. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Huber R, Peterson M, Massimini M, Murphy M, Riedner B, … Tononi G. Reduced sleep spindle activity in schizophrenia patients. American Journal of Psychiatry. 2007;164(3):483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- Ficca G, Axelsson J, Mollicone DJ, Muto V, Vitiello MV. Naps, cognition and performance. Sleep Medicine Reviews. 2010;14:249–258. doi: 10.1016/j.smrv.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Groch S, Wilhelm I, Diekelmann S, Sayk F, Gais S, Born J. Contribution of norepinephrine to emotional memory consolidation during sleep. Psychoneuroendocrinology. 2011;36(9):1342–1350. doi: 10.1016/j.psyneuen.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Guerrien A, Dujardin K, Sockeel MP, Leconte P. Enhancement of memory by auditory stimulation during postlearning REM sleep in humans. Physiology & Behavior. 1989;45(5):947–950. doi: 10.1016/0031-9384(89)90219-9. [DOI] [PubMed] [Google Scholar]
- Hamman S. Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences. 2001;5(9):394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10(4):431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Hoaglin DC, Iglewicz B, Tukey JW. Performance of some resistant rules for outlier labeling. Journal of the American Statistical Association. 1986;81:991–999. [Google Scholar]
- Kensinger EA. Remembering the details: Effects of emotion. Emotion Review. 2009;1:99–113. doi: 10.1177/1754073908100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Memory for specific visual details can be enhanced by negative arousing content. Journal of Memory and Language. 2006;54(1):99–112. [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. How negative emotion enhances the visual specificity of a memory. Journal of Cognitive Neuroscience. 2007;19:1872–1887. doi: 10.1162/jocn.2007.19.11.1872. [DOI] [PubMed] [Google Scholar]
- Kleinsmith LJ, Kaplan S. Paired-associate learning as a function of arousal and interpolated interval. Journal of Experimental Psychology. 1963;65:190–193. doi: 10.1037/h0040288. [DOI] [PubMed] [Google Scholar]
- Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends in Cognitive Sciences. 2007;11:442–450. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science (New York, NY. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Mednick SC, Nakayama K, Cantero JL, Atienza M, Levin AA, Pathak N, Stickgold R. The restorative effect of naps on perceptual deterioration. Nature Neuroscience. 2002;5(7):677–681. doi: 10.1038/nn864. [DOI] [PubMed] [Google Scholar]
- Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nature Neuroscience. 2003;6(7):697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, … Nadel L. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. Journal of Anatomy. 2005;207(1):35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto V, Arpaia L, De Padova V, Russo E, Ficca G. The effect of naps on the recall of verbal material. Proceedings of the 1st Congress of the World Association of Sleep Medicine, Medimond; Bologna. 2005. pp. 29–34. [Google Scholar]
- Nairne JS, Thompson SR, Pandeirada JN. Adaptive memory: survival processing enhances retention. J Exp Psychol Learn Mem Cogn. 2007;33:263–273. doi: 10.1037/0278-7393.33.2.263. [DOI] [PubMed] [Google Scholar]
- Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cerebral Cortex. 2009;19:1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudiette D, Paller KA. Upgrading the sleeping brain with targeted memory reactivation. Trends in Cognitive Sciences. 2013;17(3):142–149. doi: 10.1016/j.tics.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Payne JD. Sleep on it: Stabilizing and transforming memories during sleep. Nature Neuroscience. 2011a;14(3):272–274. doi: 10.1038/nn0311-272. [DOI] [PubMed] [Google Scholar]
- Payne JD. Learning, memory and sleep in humans. Sleep Medicine Clinics. 2011b;6(1):15–30. [Google Scholar]
- Payne JD, Kensinger EA. Sleep’s role in the consolidation of emotional episodic memories. Current Directions in Psychological Science. 2010;19(5):290–295. [Google Scholar]
- Payne JD, Nadel L, Britton WB, Jacobs WJ. The Biopsychology of Trauma and Memory. In: Reisberg D, Hertel P, editors. Memory and Emotion. Oxford University Press; 2004. pp. 76–128. [Google Scholar]
- Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychological Science. 2008a;19:781–788. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Ellenbogen JM, Walker MP, Stickgold R. The role of sleep in memory consolidation. In: Roediger H, editor. Learning and Memory: A Comprehensive Reference. Vol. 2. New York: Elsevier; 2008b. pp. 663–685. [Google Scholar]
- Payne JD, Schacter DL, Propper RE, Huang LW, Wamsley EJ, Tucker MA, Walker MP, Stickgold R. The role of sleep in false memory formation. Neurobiology of Learning and Memory. 2009;92:327–334. doi: 10.1016/j.nlm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Tucker MA, Ellenbogen JM, Wamsley EJ, Walker MP, Schacter DL, Stickgold R. Memory for semantically related and unrelated declarative information: the benefit of sleep, the cost of wake. PloS one. 2012a;7(3):e33079. doi: 10.1371/journal.pone.0033079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Chambers AM, Kensinger EA. Sleep promotes lasting changes in selective memory for emotional scenes. Frontiers in Integrative Neuroscience. 2012b:6. doi: 10.3389/fnint.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. Journal of Cognitive Neuroscience. 1997;9(4):534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. 1968 doi: 10.1046/j.1440-1819.2001.00810.x. [DOI] [PubMed] [Google Scholar]
- Reisberg D, Heuer F. Remembering emotional events. In: Reisberg D, Hertel P, editors. Memory and Emotion. New York, NY: Oxford University Press; 2004. pp. 3–41. [Google Scholar]
- Riggs L, McQuiggan DA, Farb N, Anderson AK, Ryan JD. The role of overt attention in emotion-modulated memory. Emotion. 2011;11(4):776–85. doi: 10.1037/a0022591. [DOI] [PubMed] [Google Scholar]
- Schabus M, Hodlmoser K, Pecherstorfer T, Klosch G. Influence of midday naps on declarative memory performance and motivation. Somnologie. 2005;9:148–153. [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: Concepts, data, and applications. Annals of the New York Academy of Sciences. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Stanny CJ, Johnson TC. Effects of stress induced by a simulated shooting on recall by police and citizen witnesses. American Journal of Psychology. 2000;113:359–386. [PubMed] [Google Scholar]
- Steinmetz KR, Kensinger EA. The emotion-induced memory trade-off: more than an effect of overt attention? Memory and Cognition. 2013;41(1):69–81. doi: 10.3758/s13421-012-0247-8. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory triage: evolving generalization through selective processing. Nature Neuroscience. 2013;16(2):139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley AJ, Empson JAC. REM sleep and memory consolidation. Biological Psychology. 1978;6(4):293–300. doi: 10.1016/0301-0511(78)90031-5. [DOI] [PubMed] [Google Scholar]
- Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learning & Memory. 2001;8(2):112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Annals of the New York Academy of Sciences. 2009;1156(1):168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44(1):121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35(1):205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Waring J, Payne JD, Schacter DL, Kensinger EA. Impact of individual differences upon emotion-induced memory trade-offs. Cognition and Emotion. 2010;24(1):150–167. doi: 10.1080/02699930802618918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT. The psychology and neuroscience of forgetting. Annual Review of Psychology. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]