Abstract
Rapid improvements in the detection and tracking of early-stage tumor progression aim to guide decisions regarding cancer treatments as well as predict metastatic recurrence in patients following surgery. Mathematical models may have the potential to further assist in estimating metastatic risk, particularly when paired with in vivo tumor data that faithfully represent all stages of disease progression. Herein we describe mathematical analysis that uses data from mouse models of spontaneous metastasis developing after surgical removal of orthotopically implanted primary tumors. Both presurgical (primary tumor) and postsurgical (metastatic) growth was quantified using bioluminescence and was then used to generate a mathematical formalism based on general laws of the disease (i.e. dissemination and growth). The model was able to fit and predict pre-/post-surgical data at the level of the individual as well as the population. Our approach also enabled retrospective analysis of clinical data describing the probability of metastatic relapse as a function of primary tumor size. In these data-based models, inter-individual variability was quantified by a key parameter of intrinsic metastatic potential. Critically, our analysis identified a highly nonlinear relationship between primary tumor size and postsurgical survival, suggesting possible threshold limits for the utility of tumor size as a predictor of metastatic recurrence. These findings represent a novel use of clinically relevant models to assess the impact of surgery on metastatic potential and may guide optimal timing of treatments in neoadjuvant (presurgical) and adjuvant (postsurgical) settings to maximize patient benefit.
Major findings
A mathematical model was used to connect presurgical primary tumor volume and postsurgical metastatic burden and survival in two clinically relevant animal models of spontaneous metastasis and one clinical dataset of metastatic relapse probability in breast cancer patients. This model used one specific parameter to quantify differential metastatic aggressiveness, which could be of help for personalizing adjuvant therapy. Simulations revealed a highly nonlinear relationship between resected primary tumor size and metastatic recurrence. These results uncover a computable and patient-dependent threshold for evaluating the efficacy of surgery on overall survival.
Introduction
Surgical removal of an early-stage localized tumor remains one of the most effective strategies in reducing the probability of systemic metastatic disease spread (1). Improved technologies of early cancer detection aim to classify primary tumor stage to identify whether potential treatment modalities – such as presurgical ‘neoadjvuant’ or postsurgical ‘adjuvant’ – should be considered to complement surgery and reduce metastatic potential. However the relationship between primary tumor growth and eventual metastasis remains enigmatic (2). Metastatic seeding was initially thought to occur only during late stages of primary tumor growth and invasion (3), however, recent evidence suggests systemic dissemination is a much earlier event (4). Indeed even the direction of tumor spread, initially thought to occur unidirectionally from primary to secondary sites, has been replaced by more complex and dynamic theories of interaction. These include models where primary and secondary lesions grow (and evolve) in parallel (2) and the possibility that cell seeding can be bi-directional, with metastasis potentially ‘re-seeding’ back to original primary location (5,6).
To assist in understanding this complexity, mathematical modeling has been used to determine the relationship between primary (localized) and secondary (metastatic) tumor dissemination and growth. Early studies used statistical analyses only (7,8), while later work included experimentally-derived data to validate models using biological information that aimed to more faithfully represent the metastatic process (9). In 2000, Iwata and colleagues used imaging data from one patient with metastatic hepatocellular carcinoma to introduce a more formalistic and biologically-based approach that relied on the description of the temporal dynamics of a population of metastatic colonies, with equations written at the organ or organism scale (10). In parallel, several studies have sought to include additional variables when modeling tumor growth, such as angiogenesis (11), stem cell behavior (12), tumor-immune interactions (13) and microenvironment influences (14), among numerous others. To date, the majority of mathematical studies in cancer modeling have focused on primary tumor and relatively few have investigated the metastatic development (15–22).
This dearth in metastatic data stems largely from the complexity of studying metastasis itself. Metastasis starts with localized primary tumor growth which then invades and intravasates into the bloodstream which, in turn, spreads systemically until extravating into tissue at a distant (hospitable) site (23,24). While clinical (retrospective) data has value (2,7,20,25,26), mouse tumor models have typically aimed to mimic (and distinguish between) several stages of the metastatic process. In certain mouse models, metastasis can derive from a tumor that is implanted ectopically or orthotopically into a primary or metastatic site (‘ectopic’, ‘orthotopic’ or ‘ortho-metastatic’ models, respectively (27)) and can involve various immune states (i.e., human xenograft or mouse isograft). Although more rarely performed, models can also include surgical resection of the primary tumor which allows for progression of clinically relevant spontaneous metastatic disease. These can include surgery following ectopic implantation (i.e., ‘ecto-surgical’, such as tumors grown in the ear or limb that are later amputated), or orthotopic implantation and resection (i.e., ‘ortho-surgical’), which more faithfully represent patient disease. To date, no studies have utilized data from ortho-surgical metastasis models for mathematical analysis.
Herein we describe a mathematical approach developed using data derived from two ortho-surgical metastasis models representing competent and incompetent immune systems with luciferase-tagged human breast (LM2-4LUC+) and mouse kidney (RENCALUC+) cell lines. We first defined a mathematical formalism from basic laws of the disease (dissemination and growth). Then we confronted the mathematical outputs to longitudinal measurements of primary tumor size, metastatic burden and survival using a population approach (nonlinear mixed-effects) for statistical estimation of the parameters. Minimally parameterized models of each experimental system were generated and used to fit and predict pre-/post-surgical data at the individual and population levels. Next we used clinical datasets to assess metastatic relapse probability from primary tumor size and show that, in both cases (preclinical and clinical), one specific parameter (μ) allowed quantification of inter-animal/individual variability in metastatic propensity. Critically, our models confirm a strong dependence between presurgical primary tumor size and postsurgical metastatic growth and survival. However, quantitative analysis revealed a highly nonlinear pattern in this dependency and identified a range of tumor sizes (either large or small) where variation of tumor size did not significantly impact on survival. These represent potential threshold limits for the utility of primary size as a predictor of metastatic disease (i.e., if small, then surgical cure; if large, then surgical redundancy). These findings represent the first time clinically relevant surgical models have been integrated with data-based mathematical models to inform the quantitative impact of presurgical primary tumor size on subsequent metastatic disease.
Quick guide to equations and assumptions
The metastatic modeling approach we employed follows the formalism initiated by Iwata et al. (10), which was further developed/expanded in recent works in two key ways: 1) effect of systemic therapies (28,29), and 2) use in a (non-surgical) in vivo human xenograft model involving orthotopic primary tumors (PTs) and metastasis (21). Metastatic development is reduced to two main components:
Growth: includes presurgical primary (gp) and secondary (g) tumor growth rates
Dissemination: includes metastatic dissemination rate (d).
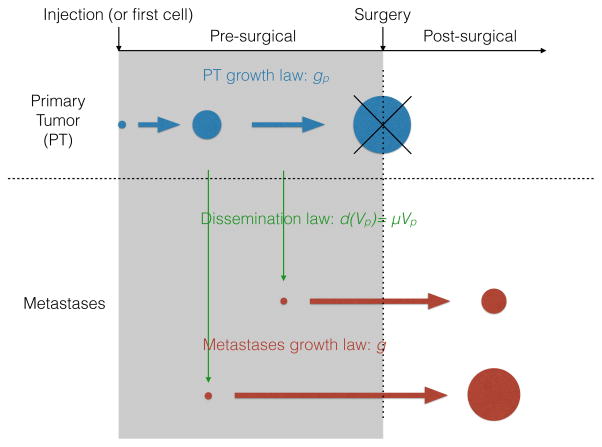
A schematic description of the model is depicted in Figure 1. More complex considerations on the biology (1,30) and modeling (31) of the metastatic process have been considered elsewhere.
Figure 1.
Growth dynamics
The PT volume Vp(t)P solves the following equations
| (1) |
The initial condition for the PT, denoted by Vi, was determined either by the number of injected cells (preclinical case) or the initial tumor size at inception (clinical case, Vi = 1 cell). Metastases were assumed to start from one cell. For each case, the optimal structure resulting from our investigations was to assume the same structural law for the PT and the metastases, although with possibly different parameter values.
Preclinical: Human breast (LM2-4LUC+) metastasis model
Growth dynamics were defined by
Gomp-Exp (32) growth model (see expression below)
Growth parameters for PT and metastases treated identically (g = gp)
In a previous study quantifying the descriptive power of several growth kinetics models using data from the same breast animal model (33), the Gompertz model accurately described primary tumor growth curves, in accordance with a large body of literature (see references in (33)). However, a limitation of this model is that the tumor doubling time could become arbitrarily small for small volumes, a feature that we considered biologically irrelevant for small volumes at metastatic initiation (of the order of the cell). A lower bound to this doubling time might be expressed by the in vitro doubling time of the cell line, which can be experimentally determined. Consequently, we adopted the Gomp-Exp model (32), defined by
| (2) |
Under this model, growth is divided between two phases: an initial exponential phase, followed by a Gompertz growth phase. Parameter λ is the maximal proliferation rate, taken here to be equal to the value inferred from in vitro proliferation assays (see supplementary Figure 1A and Table 2). The second term in the min function is the Gompertz growth rate, defined by two parameters. Parameter α is the intrinsic relative (specific) growth rate at the size V0 of one cell. Parameter β is the exponential decay rate of the relative (specific) growth rate.
Table 2.
Parameters inferred from the models
| Data | Growth model | Location | Par. | Unit | Estimate (CV) | 95 % CI |
|---|---|---|---|---|---|---|
| In vitro (Breast) | Exp. | λ | day−1 | 0.837 (−) | (0.795 – 0.879) | |
|
| ||||||
| Preclinical Breast | Gomp-Exp. | PT | Vi | cell | 1.00 × 106 (−) | - |
| α | day−1 | 1.9 (5.73) | (1.84 – 1.96) | |||
| β | day−1 | 0.0893 (21.3) | (0.0791 – 0.101) | |||
|
| ||||||
| Met | V0 | p/s | 10 (−) | - | ||
| μ | cell−1 · day−1 | 4.43 × 10−11 (176) | (2.70 × 10−11 – 7.27 × 10−11) | |||
|
| ||||||
| Preclinical Kidney | Exp. | PT | Vi | p/s | 1.63 × 105 (45.5) | (9.40 × 104 – 2.83 × 105) |
| αp | day−1 | 0.21 (60.3) | (0.151 – 0.292) | |||
|
| ||||||
| Met | V0 | p/s | 10 (−) | - | ||
| α | day−1 | 0.0307 (201) | (0.0133 – 0.0707) | |||
| μ | cell−1 · day−1 | 0.0415 (397) | (0.0181 – 0.0948) | |||
|
| ||||||
| Clinical Breast | Gomp. | PT | Vi | cell | 1 (−) | |
| α | day−1 | 0.013 (−) | ||||
| β | day−1 | 0.000471 (−) | ||||
|
| ||||||
| Met | V0 | cell | 1 (−) | |||
| μ | cell−1 · day−1 | 7.00 × 10−12 (1.04 × 104) | ||||
Parameters corresponding to the preclinical data were obtained using nonlinear mixed-effects modeling. Inter-animal variability of each parameter is captured by its respective coefficient of variation (CV). Parameter values for the clinical data are those that produced the fit to the clinical data of metastatic relapse probability from (26), reported in Table 1. For these data, only parameter μ was allowed to vary between the individuals in this setting and consequently it is the only parameter having a coefficient of variation (CV). , with std the standard deviation of the lognormal distribution of the parameter and est the population estimate.
CI = Confidence Interval on the population estimate inferred from the standard errors on the fit.
Preclinical: Mouse kidney (RENCALUC+) metastasis model
Growth dynamics were defined by
Exponential growth model.
Growth parameters for PT and metastases treated differently.
In mathematical terms, this is expressed by
| (3) |
Clinical: Human metastatic breast data
Growth dynamics were defined by
Gompertz growth model
Growth parameters for PT and metastases treated identically (gp = g)
Metastatic dissemination
The formation of new metastases was assumed to occur at a PT volume-dependent rate d(Vp) having the following parametric expression
| (4) |
where parameter μ is an intrinsic parameter of metastatic aggressiveness. This critical coefficient is the daily probability for a given tumor cell to successfully establish a metastasis. Therefore it is the product of several probabilities: 1) the probability of having evolved the necessary genetic mutations to ensure the phenotypic abilities required at each step of the metastatic process, 2) the survival probability of all adverse events occurring in transit including survival in the blood or immune escape, among others, and 3) the probability to generate a functional colony at the distant site. Following reported observations (34), we assumed that all the metastases were growing at the same volume (v)-dependent rate g(v) and that they all started from the same volume corresponding to the volume of one cell. The population of metastases was then formalized by means of a time (t)-dependent volume distribution ρ(t,v), solving the following problem (10):
| (5) |
The first equation is a continuity equation expressing conservation of the number of metastases when they grow. The second equation is a Neumann boundary condition on the flux of entering metastases at size V = V0. The third equation describes the initial condition (no metastases at the initial time). From the solution of this problem two main macroscopic quantities can be derived, the metastatic burden M(t) and the number of metastases N(t). In the convolution formula for M(t) (35), V(s)represents a solution to the Cauchy problem (1) with g instead of gp and V0 as initial condition. This formula allows fast simulation of the model using the fast Fourier transform algorithm (35), which was essential for estimation of the parameters that required a very large number of model evaluations.
Materials and methods
Preclinical Methodology
Cell lines
The human LM2-4LUC+ cells are a luciferase-expressing metastatic variant of the MDA-MB-231 breast cancer-cell line derived after multiple rounds of in vivo lung metastasis selection in mice, as previously described (see (36) (37)). Mouse kidney RENCALUC+ cells expressing luciferase were a kind gift from R. Pili, Roswell Park Cancer Institute and described previously (38). LM2-4LUC+ and RENCALUC+ were maintained in Dulbecco’s modified Eagle’s medium (Corning, Cat. #MT10-013-CV) and in RPMI (Roswell Park Memorial Institute) medium (Corning, Cat. #MT15-041-CV), respectively, with 5% heat-inactivated fetal bovine serum (Corning, Cat. #MT35-010-CV). Cells were authenticated by STR profile comparison to ATCC parental cell database (for LM2-4LUC+) or confirmation of species origin (for RENCALUC+) (DDC Medical, USA). All cells were incubated at 37°C and 5% CO2 in a humidified incubator.
Cell Proliferation assay
LM2-4LUC+ cells were plated in 35mm plates (5×105 cells per plate) and were manually counted using trypan blue staining every 24 hours for 72 hours total (cellgro, Cat. #25-900-CI).
Photon-to-cell ratio
LM2-4LUC+ cells were trypsinized and counted. 5×106 cells were serial diluted 2 fold down to 9.77×103 cells and processed with Bright-Glo Luciferase Assay System (Promega, Cat. #E2610) following manufacture’s protocol.
Ortho-surgical models of metastasis
Animal tumor model studies were performed in strict accordance with the recommendations in the Guide for Care and Use of Laboratory Animals of the National Institutes of Health and according to guidelines of the institutional Animal Care and Use Committee (IACUC) at Roswell Park Cancer Institute (Protocol: 1227M, to JMLE).
The optimization and use of animal models of breast and kidney metastasis orthotopic primary tumor implantation and surgical resection have been extensively detailed elsewhere (39). Briefly, LM2-4LUC+ cells (2×106 cells in 50μL) and RENCALUC+ (4×104 cells in 5μL) were implanted, respectively, into the right inguinal mammary fat pad (right flank) or kidney (subcapsular space) of 6–8 week old female CB-17 SCID or Balb/c mice(39). Primary breast tumor size was assessed regularly with Vernier calipers using the formula width2(length×0.5) and in both tumor models animals were monitored bi-weekly for bioluminescence to quantify tumor growth (40). See Supplementary preclinical methodology section for more details.
Mathematical Methodology: Fit procedures
Preclinical data: primary tumor and metastatic burden dynamics
Three fit procedures were investigated: 1) fitting the population average time series, 2) individual fits of each mouse’s primary tumor (PT) and metastatic burden (MB) kinetics and 3) a mixed-effect population approach. Due to the high variability in the data, the first approach was not considered relevant. The second approach showed that the model was able to describe individual dynamics but, due to the relative scarcity of the data in a given animal, led to very poor identifiability of the coefficients, in particular the metastatic dissemination parameter μ. The third approach was considered the most appropriate to our case. Indeed, nonlinear mixed-effect modeling (41) is a statistical technique specifically tailored for sparse serial measurements in a population. It assumes that inter-animal variability can be described by a parametric distribution on the model’s parameters (here assumed to be lognormal, consistently with other works (20,42)). Multiple strategies were tested in order to find the appropriate formalism to fit the data. These included fitting PT and MB separately or together. The strategy fitting PT and MB was ultimately selected because it resulted in more accurate fits and allowed for possible correlations between the primary and secondary tumors growth parameters in a same animal.
One of the model parameters for Gomp-Exp growth was the in vitro proliferation rate, which was determined by an exponential fit to an in vitro proliferation assay. Maximization of the likelihood function under nonlinear mixed-effect formalism was solved using the function nlmefitsa implemented in Matlab (43), which is based on the stochastic approximation of expectation maximization (SAEM) algorithm. Specific assumptions were: log-transformation of the parameters (i.e. log-normal population distribution), proportional error model and full covariance matrix. For individual fits, weighted least squares minimization corresponding to individual likelihood maximization was performed using the function fminsearch of Matlab (Nelder-Mead algorithm), following previously reported methods (33).
Clinical data: Calculation of metastatic relapse probability
Our methodology for fitting the clinical data followed the same format as (44), although here the model was simplified (only parameter μ was allowed to vary among individuals) and PT size at diagnosis was considered to be uniformly distributed within each size range. Parameters for the growth of the primary and secondary tumors were fixed (not subject to optimization) and corresponded to a maximal volume of 1012 cells (≃ 1 kg) and a doubling time of 7.5 months at 1 g, consistently with clinical values reported in the literature (8,25).
The data reported in (26) consisted of metastatic relapse probabilities during the next 20 years post-surgery, for patients stratified by PT size (see Table 1). Diameter data from PT sizes at diagnosis were converted into volumes under the assumption of a spherical shape and then converted to number of cells using the conversion rule 1 mm3 ≃ 106 cells (45). Parameter μ was assumed log-normally distributed in the population, with mean μm and standard deviation μσ.
Table 1.
Descriptive power of the mathematical model: clinical data of metastatic relapse probability
| Diameter (cm) | No. patients | Prop. of relapse (Data) | Prop. of relapse (Model) |
|---|---|---|---|
| 1 ≤ D ≤ 2.5 | 317 | 27.1 | 25.5 |
| 2.5 < D ≤ 3.5 | 496 | 42.0 | 42.4 |
| 3.5 < D ≤ 4.5 | 544 | 56.7 | 56.3 |
| 4.5 < D ≤ 5.5 | 422 | 66.5 | 65.9 |
| 5.5 < D ≤ 6.5 | 329 | 72.8 | 74.3 |
| 6.5 < D ≤ 7.5 | 192 | 83.8 | 80.8 |
| 7.5 < D ≤ 8.5 | 136 | 81.3 | 85.7 |
Fit of the model was significant for Pearson’s χ2 test for goodness-of-fit (p = 0.023).
The probability of having a metastatic relapse in the next 20 years for a primary tumor diagnosed with a given size was assumed to be equal to the probability of already having one distant tumor at the time of diagnosis. For a given volume range of PT sizes at diagnosis (Vk,Vk+1), k ∈ {1, …, 7}, we considered the diagnosis volume as a random variable uniformly distributed in (Vk,Vk+1). Then, we computed the corresponding age of the tumor at diagnosis (i.e. the time elapsed from the first cancer cell) from the assumption of Gompertzian growth with the parameter values previously mentioned. This quantity was denoted . Under our formalism, the probability of having a disseminated metastasis at time then writes
| (6) |
where Metk stands for the event of having one metastasis at diagnosis when the PT volume is in (Vk,Vk+1). For any volume range and value of μm and μσ, this formalism allowed us to compute a probability to be compared to the respective empirical proportion of relapsing patients reported in (26), by simulating the two random variables involved ( and μ). We then determined the best-fit parameters by minimizing the sum of squared errors to the data, using the function fminsearch from Matlab.
Results
Quantitative and differential modeling of metastasis in ortho-surgical models
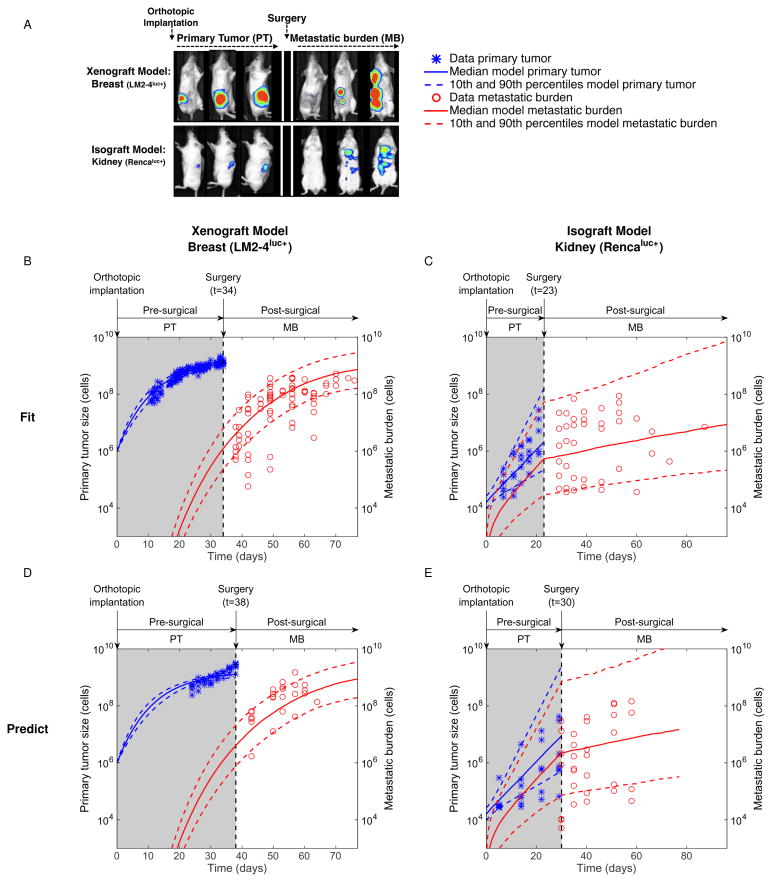
To mimic clinical progression of spontaneous systemic metastatic disease, two models involving orthotopic tumor implantation and surgical resection (ortho-surgical) were employed. These included a xenograft breast model (LM2-4LUC+ cells implanted into the mammary fat pad) and an isograft kidney model (RENCALUC+ implanted into the subcapsular kidney space) (38) (see Methods). Presurgical primary tumor (PT) and postsurgical metastatic burden (MB) were tracked by bioluminescence (BL) emission, expressed in photons/second (p/s) (Figure 2A).
Figure 2.
In the breast model, simultaneous BL and gross tumor volume measurements (caliper) were performed. The former only quantifies living cells whereas the latter computes a total volume indifferently of its composition. Volume and BL emission were significantly correlated (supplementary Figure 1B), as observed by others (46). Determination of the signal corresponding to one cell was required in our modeling for the value assigned to V0. Based on linear regression between BL emission and tumor volume, we established that BL = 2.19·106 V + 7.89·107, where BL is the bioluminescence in p/s and V is the volume in mm3. This relationship, evaluated at V = 10 mm3 ≃ 107 cells gives 1 cell ≃ 10.08 p/s, which was approximated to 10 p/s. Using this value gave reasonable fits to the PT growth data (supplementary Figure 2).
Validation and calibration of the mathematical model
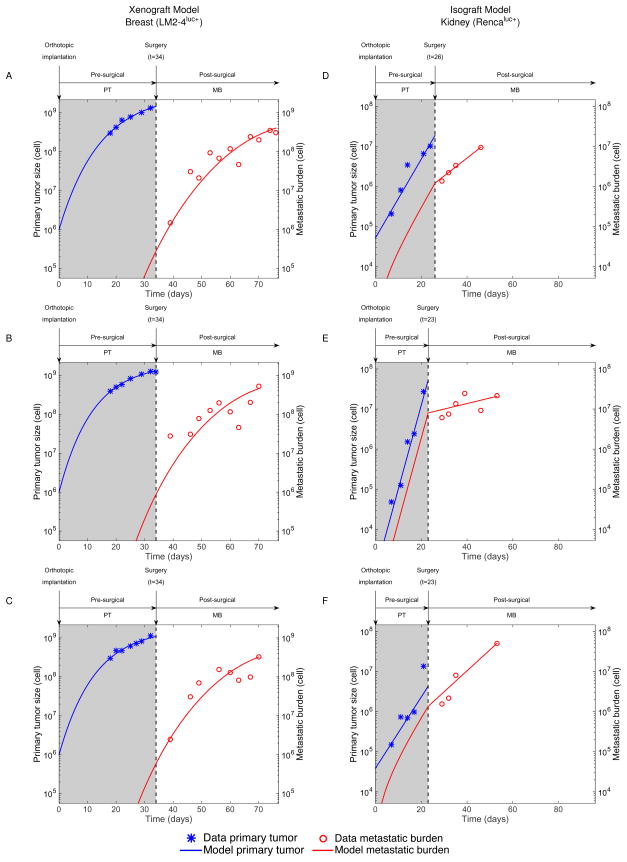
We assessed the ability of the models to describe and predict the experimental data of postsurgical MB dynamics. Several model designs were evaluated to define the optimal structure and methodology that would allow accurate and reliable data description. Specifically, for each in vivo experimental system, multiple structural expressions and parametric dependences between the growth rate of the PT and MB were tested. We refer to supplementary Figures 3 and 4 for direct comparison of goodness-of-fit and identifiability under different modeling setups. Population and individual fits of the best models to the data are shown in Figures 2B–C (and supplementary Figure 5), and Figure 3, respectively. The parameter values inferred from the population fits are reported in Table 2. The mathematical models – combined with the population distribution of the parameters inferred from the nonlinear mixed-effects statistical procedure – were able to give reasonable descriptions of the presurgical PT and postsurgical MB growth. Importantly, these combinations could quantify the dynamics of the process as well as the inter-animal variability. The latter was better characterized by the metastatic potential parameter μ (large coefficients of variation in Table 2). The models could also fit individual dynamics of longitudinal data of pre-surgical PT and post-surgical MB (see Figure 3 for some representative examples of growth dynamics in particular mice and supplementary Figures 6 and 7 for fits of all mice).
Figure 3.
In addition to their descriptive power, the models were able to predict growth dynamics in external data sets that were not employed for estimation of the parameters (Figure 2D–E). These results emphasize the ability of our general modeling structure to capture MB growth dynamics. Additionally, the modeled post-surgical MB could also be related to empirical survival by means of a lethal burden threshold, which was estimated to be 4×109 p/s (supplementary Figure 8).
Qualitative and quantitative differences across ortho-surgical models
Xenograft Model: Breast metastasis
Using the same growth model (Gomp-Exp) and parameters for both presurgical PT and postsurgical MB, we were able to adequately fit the data, while ensuring reasonable standard errors on the parameters estimates (Table 2). Although more complex structures (e.g. models with one parameter differing between primary and secondary growth) provided marginally better fits, robustness in estimating μ was impaired (supplementary Figure 3). Quantitative inference of μ revealed small metastatic potential (Table 2), which translated into late development of metastases following xenograft and growth of the MB mostly dominated by proliferation (Figures 2B, 3A–C).
Isograft Model: Kidney metastasis
In contrast, the kidney model MB growth curves exhibited a different behavior, with a marked change of regimen at the time of surgery. In the context of the model, this means that most of the presurgical MB increase was driven by the dissemination process, and not by proliferation of the metastases themselves. This was reflected by a very large value of μ (Table 2), with nine orders of magnitude of difference compared to the breast model. This feature was not directly visible, nor quantifiable, by direct examination of the data, and reflects the large metastatic aggressiveness of isograft spontaneous metastasis animal models, since overpassing the immune surveillance is a major challenge in the metastatic process (4). When the PT was removed, dissemination stopped and only proliferation remained for further growth of the MB, which happened at a slower rate than at the primary site (Figures 2C and 3D–F). In some cases, growth of the MB remained constant or even decreased after surgery (see supplementary Figure 7). This result reflects the fact that the competent immune status of the mice might have an important impact on the establishment of durable, fast-growing metastatic colonies at the secondary sites (47).
Together, our data-based quantitative modeling analysis of presurgical PT and postsurgical MB growth kinetics demonstrated the descriptive power of the models, unraveled distinct growth patterns between the two animal models and emphasized the critical role of the parameter μ for quantification of the inter-animal variability.
Clinical data of metastatic relapse probability
Clinical data reported in the literature generally do not provide detailed information about the untreated growth of the metastatic burden, either because the residual disease is invisible, or because the patients benefit from adjuvant therapy after resection of their PT. Nevertheless, before the generalization of adjuvant therapy for breast cancer, Koscielny et al. (26) reported data from a cohort of 2648 patients followed for 20 years after surgery of the PT, without additional treatment. Their data (reproduced in Table 1) demonstrated that, despite a clear association between PT size at diagnosis and the probability of metastatic relapse, not all the patients having a given PT size were relapsing. For instance, only 42% of patients with a PT diameter at diagnosis between 2.5 and 3.5 centimeters developed metastasis. Based on this observation, we used our model to describe inter-individual variability by means of a limited number of parameters. We considered that the probability of developing a metastasis in the next 20 years was equal to the probability of already having one at the time of diagnosis (see Methods). Using a lognormal population distribution of parameter μ we were able to obtain a significant fit to the data of metastatic relapse for all size ranges (Table 1, p = 0.023). Interestingly, the median value of μ resulting from these human data was close to the value from the preclinical breast data, in comparison to the kidney model.
These results demonstrated that, within our semi-mechanistic modeling approach, parameter μ was able to capture the inter-individual metastatic variability, not only in animal models, but also for patient data.
Assessing the impact of surgery on metastasis and survival: a simulation study
When diagnosis detects only a localized primary tumor, distant occult disease might already be present. In our model, the extent of this invisible metastatic burden depends on: 1) the PT size at diagnosis and 2) the patient’s metastatic potential μ. For instance, if the PT size (or μ) is small then the occult MB might be negligible and surgery would substantially benefit to the patient in terms of metastatic reduction, by stopping further spread of new foci. Conversely, if the PT size (or μ) is large, then the occult MB might already be consequent and removing the PT might only have a marginal impact.
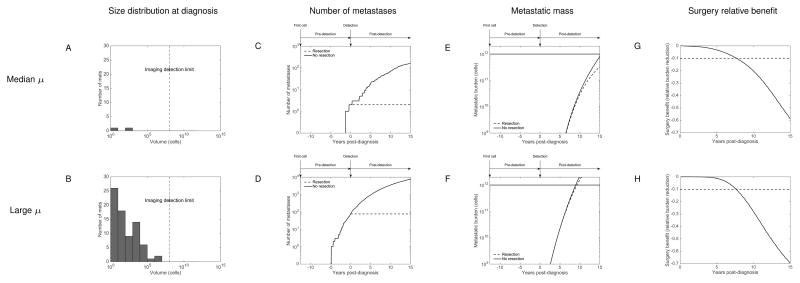
Virtual simulation of two breast cancer patients
We simulated the quantitative impact of PT surgery in two virtual breast cancer patients having a PT diagnosed at 4.32 cm and two values of μ (median and 90th percentile within a population distributed according to our previous estimate). Results are reported in Figure 4 and supplementary movies 1 and 2. A discrete and stochastic version of the metastatic dissemination was employed here for the simulations (see supplementary methods for details). Interestingly, our simulation revealed that at the time of diagnosis, no metastasis was detectable (i.e. below the imaging detection limit, taken here to 108 cells), in both cases (Figure 4A–B). In clinical terms, this means that both patients would have been diagnosed with a localized disease. However, the two size distributions were very different, with a much larger residual burden in the “large μ” case, illustrative of the increased metastatic potential.
Figure 4.
For the “median μ” case, our model predicted the presence of two small metastases, with respective sizes 6 and 278 cells. Not surprisingly, when no surgery was simulated, this number continued to increase, reaching 160 secondary lesions after 15 years (Figure 4C). However, most of the metastatic burden (126 tumors, i.e. 78.8% of the total burden) was composed of lesions smaller than 109 cells (≃1g). Panels E and G of Figure 4 demonstrate that a substantial relative benefit (larger than 10%) in MB reduction was eventually obtained, but only after 7.8 years. Nevertheless, at the end of the simulation (15 years after surgery), the predicted two occult metastases at diagnosis had reached substantial sizes (1.41×1011 and 1.89×1011 cells). Therefore, for this patient with median metastatic potential, the model indicates an important benefit in using adjuvant therapy.
For a patient with higher metastatic potential (at the level of the 90th percentile, see Figure 4 panels B, D, F and H, and supplementary movie 2), even with a PT diagnosed at the same size, the predicted metastatic burden at diagnosis was considerably more important, with 76 lesions and the largest comprising 6.23×106 cells. This consequent occult burden translated into poor outcome and the metastatic mass would have reached a lethal burden of 1012 cells 9.3 years after the initial diagnosis if no therapy would have been administrated.
These results illustrate the potential of the model as a diagnosis and prognosis numerical tool for assessment of the occult metastatic burden and post-surgery growth. In this, it could help to determine the extent of adjuvant therapy necessary to achieve a long-term control of the disease.
Impact of tumor size on postsurgical survival
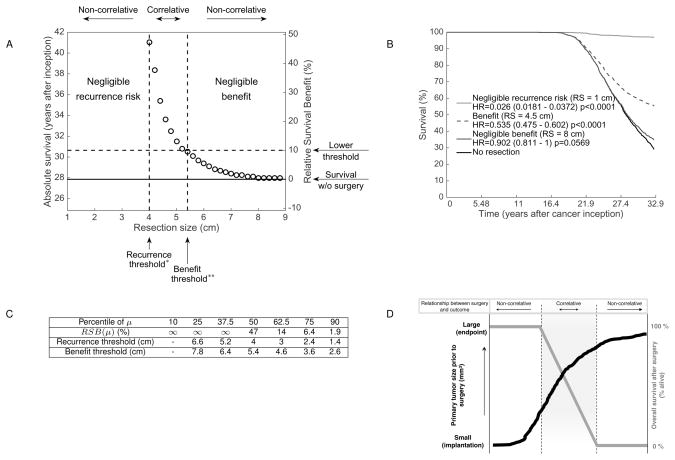
To further examine the relationship between the PT size at surgery and survival, we performed simulations for 1) an individual with fixed value of μ (the population median, see Figure 5A) or 2) an entire population (simulated survival curves in Figure 5B), for three PT sizes. Numerical survival was defined by the time to reach a lethal burden of 1 kg (≃ 1012 cells) (2) from the time of cancer inception. Interestingly, we observed a highly nonlinear relationship between the PT size and the survival, which suggested three size ranges delimited by two thresholds (Figure 5A). The lower threshold — termed ‘recurrence’ threshold (4 cm in Figure 5A) — was defined as the maximal limit whereupon no metastasis was present at surgery (number of metastases lower than 1). The upper size threshold — termed ‘benefit’ threshold (5.2 cm in Figure 5A) — was defined as the size above which surgery had a negligible (< 10%) impact on survival time. Above and below these ‘recurrence’ and ‘benefit’ thresholds, PT size had no important correlative value. Conversely, within the PT size range delimited by these two bounds, the relationship between presurgical PT and postsurgical MB/survival was highly correlative, with a large derivative and a sharp transition between the two extremes. The same qualitative PT size/survival relationship was obtained for any value of μ sampled within the population distribution (see supplementary Figure 9).
Figure 5.
In Figure 5C, we present quantitative estimates of the recurrence and benefit thresholds for various percentiles of μ within the population distribution (see also supplementary Figure 9). Our simulations predicted that for the first half of the population, surgery was almost always leading to negligible metastatic recurrence risk, with large values of the recurrence threshold (larger than the usual detection levels). On the other hand, the patients with large metastatic potential were predicted not to substantially benefit from the surgery, as far as reduction of future MB was concerned. For instance, a patient with μ at the level of the 90th percentile and a PT diagnosed at 4 cm would have an increase in absolute survival time of only 1.9% following surgery (Figure 5C).
Discussion
Using a formalism based on simple laws of metastatic development (including dissemination and proliferation), we derived mathematical models able to connect presurgical PT growth to postsurgical development of the MB in two ortho-surgical animal models (with two immune states) as well as one clinical data set. These quantitative models allowed identification of different metastatic growth patterns and characterization of the metastatic potential (and associated inter-animal/individual variability) as a critical parameter, μ. Our results also revealed a nonlinear quantitative relationship between the PT size at diagnosis and post-surgical survival improvement.
Previous studies have utilized experimental data derived from mouse metastasis models to inform mathematical analysis. For instance, Hartung and colleagues used human MDA-MB-231 breast cancer cells implanted orthotopically in mice in order to validate a mathematical model for longitudinal data of metastatic burden growth (21). This animal model was non-surgical and utilized severe immunocompromised Nod SCID γ mice to improve the low metastatic potential observed in the MDA-MB-231, a phenomena recently reported elsewhere (47). In our studies, we utilized a variant of the MDA-MB-231 previously selected for increased metastatic potential by repeated orthotopic implantation and metastatic resection in SCID mice (36). Since the selection of cells and immune state could influence analysis, we also included an immunocompetent mouse kidney model to confirm (and compare) findings. While these and other modifications to the metastatic systems could significantly influence mathematical modeling (i.e., different mouse strain and cell line, different bioluminescence technique, etc…), the impact of surgery appears to be the most significant factor. In this regard, several technical discrepancies likely impair a relevant comparison between surgical and non-surgical models presented by Hartung, et al. (21) and the current study. For instance, in surgical models we found it unnecessary to assume different growth between the primary and secondary lesions in surgical models. Additionally, we considered a less complex dissemination rate (expression and was used in (21)). Notably, we could fit our data equally well with various values of γ and thus concluded that it cannot be identified from combined PT growth and MB dynamics data alone (supplementary Figure 10). Future studies would require more data, especially on the number and size distribution of the secondary lesions, to precisely determine the shape of the dissemination coefficient. When using the dissemination and growth terms from (21) and fitting the resulting model to our surgical data, we found a much larger metastatic potential μ and a significantly faster metastatic growth kinetics parameter than computed in the non-surgical model (21) (see supplementary text). While the former probably illustrates higher metastatic propensity due to a more permissive immune state, the latter possibly suggests post-surgery metastatic acceleration (48–50).
In this regard, this raises another critical consideration of the impact of surgery on metastatic potential in mathematical modeling. Preclinical and clinical works have suggested that removal of the PT might provoke acceleration of metastatic growth (50,52). There are various biological rationales that could explain this, including inhibition of secondary growth by the presence of a primary neoplasm as a result of nutrient availability, concomitant immunity, or even systemic inhibition of angiogenesis (53). Such a theory could conceivably be assessed within the context of our model by defining different pre- and post-surgical metastatic growth rates g(v) and comparing goodness of fit. However, this would add at least one degree of freedom (thus deteriorating the reliability of the estimation) and invalidate the convolution formula used for computation of the metastatic burden in a model with non-autonomous g(t,v) (instead of g(v)), and therefore was not considered here. Importantly, theoretical integration of higher order phenomena for the biological dynamics of metastatic development has been considered elsewhere (14,16,18,54) and recent findings in the organism-scale dynamics of metastases (such as the self-seeding phenomenon (5,6) or the influence of the (pre-) metastatic niche (55)) could be embedded within the general formalism developed in our model. This could lead to complex models, however, and given the amount of information contained in our present data, reliable identification of such dynamics was not realistic. Instead, we only considered metastatic dynamics as reduced to its most essential features: dissemination and proliferation. Future studies should examine the potential of metastases to metastasize, as has been extensively debated in the past (56–58), particularly with the recent demonstration that some metastases are able to re-seed the primary tumor (5,6). Although not included in this study, preliminary tests using our model suggest negligible differences in the simulations and no impact on our results, however a more extensive analysis is required.
Our modeling philosophy elaborates on Fisher’s theory (59) of cancer as a systemic disease and relates also to the parallel progression model (2). The dissemination rate d, characterized by parameter μ, quantifies the metastatic potential and allows for a continuum of possibilities between early and late dissemination. Our results seem to parallel clinical evidence of the impact (and importance) of early surgery – particularly in the case of breast cancer. For example, in a retrospective study of 2838 breast cancer patients, the post-surgical residual recurrence-free survival rate at 5 years for Stage I disease was 7% (60). Consistently, our quantitative analysis demonstrates that in this case, for most patients, metastases that could have been shed before diagnosis would not develop into overt clinical disease during the remaining life history of the patient. For Stage IV breast cancer (that would correspond, in our formalism, to a large value of μ), our analysis predicts only negligible benefit of the surgery (if only considering reduction of metastatic shedding), in accordance with preliminary results of a recent clinical trial (61). In order to use our model as a practical diagnosis and prognosis tool that could help to refine and individualize adjuvant therapy, the critical next step is to find a way to estimate the parameter μ, in a patient-specific manner. One of the main challenges will be to do so using data derived from the primary tumor only, since metastases are often undetectable at the time of diagnosis. While the value of μ might very likely depend on the combination of several phenomena (including some genetic alterations or the immune status of the patient which could be linked to different biomarkers (62)), recent successes of genetic signatures as prognosis factors for metastasis might allow for patient-specific estimation of μ (63).
Any mathematical modeling attempt is limited by the intrinsic measurement error of the experimental technique. For monitoring the dynamics of total metastatic burden, bioluminescence imaging represents one of the best methods so far (51). However, measurement variability is hard to assess due to inherent issues, such as the long half-life of luciferin that prevents immediate replication of the measurements. Comparison of bioluminescence with caliper measurements showed large variance (supplementary Figure 1B), which increased with tumor size. This justified our assumption of a proportional measurement error model. Standard deviation of the relative error could in turn be estimated from the fit procedure and yielded a value of 0.72. This high degree of uncertainty should be taken into account as an inevitable limitation for quantitative modeling studies of bioluminescence data. We therefore put a strong emphasis on using a minimal number of parameters and assessed the robustness of our results on various assumptions, such as the shape of d and the value of V0 (supplementary Figures 10 and 11).
Together, our mathematical methodology provides a quantitative in silico framework that could be of valuable help for preclinical and clinical aims. Indeed, validation of our modeling methodology allows us to address in future works the differential effects of systemic therapies on primary tumor growth and metastases (39,40). Clinically, our methodology could be used to refine/optimize therapeutic strategies for patients diagnosed with a localized cancer and inform on the timing of surgery, extent of occult metastatic disease and probability of recurrence. In turn, this may impact decisions on duration and intensity of presurgical neoadjuvant or postsurgical adjuvant treatments (64).
Supplementary Material
Primary tumor (PT) was assumed to be detected when reaching the size of 4.32 cm in diameter. Post-diagnosis PT growth and development of metastases in the case of no surgical intervention are indicated as dashed line in the left plot and white bars in the histogram on the right, respectively.
Primary tumor (PT) was assumed to be detected when reaching the size of 4.32 cm in diameter. Post-diagnosis PT growth and development of metastases in the case of no surgical intervention are indicated as dashed line in the left plot and white bars in the histogram on the right, respectively.
Acknowledgments
This study has been carried out within the frame of the LABEX TRAIL, ANR-10-LABX-0057 with financial support from the French State, managed by the French National Research Agency (ANR) in the frame of the « Investments for the future » Programme IdEx (ANR-10-IDEX-03-02). This work was also supported by Roswell Park Alliance Foundation (RPAF) and by the Department of Defense (DoD), through the Peer Reviewed Cancer Research Program, under Award No. W81XWH-14-1-0210 (both to JMLE).
Footnotes
Disclaimer
Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the RPAF or DoD.
References
- 1.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–12. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Comen E, Norton L, Massagué J. Clinical implications of cancer self-seeding. Nat Rev Clin Oncol. 2011;8:369–77. doi: 10.1038/nrclinonc.2011.64. [DOI] [PubMed] [Google Scholar]
- 6.Kim M-Y, Oskarsson T, Acharyya S, Nguyen DX, Zhang XHF, Norton L, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–26. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slack NH, Blumenson LE, Bross ID. Therapeutic implications from a mathematical model characterizing the course of breast cancer. Cancer. 1969;24:960–71. doi: 10.1002/1097-0142(196911)24:5<960::aid-cncr2820240515>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Koscielny S, Tubiana M, Valleron AJ. A simulation model of the natural history of human breast cancer. Br J Cancer. 1985;52:515–24. doi: 10.1038/bjc.1985.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liotta LA, Saidel GM, Kleinerman J. Stochastic model of metastases formation. Biometrics. 1976;32:535–50. [PubMed] [Google Scholar]
- 10.Iwata K, Kawasaki K, Shigesada N. A Dynamical Model for the Growth and Size Distribution of Multiple Metastatic Tumors. J Theor Biol. 2000;203:177–86. doi: 10.1006/jtbi.2000.1075. [DOI] [PubMed] [Google Scholar]
- 11.Hahnfeldt P, Panigrahy D, Folkman J, Hlatky L. Tumor development under angiogenic signaling: a dynamical theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res. 1999;59:4770–5. [PubMed] [Google Scholar]
- 12.Michor F. Mathematical models of cancer stem cells. J Clin Oncol. 2008;26:2854–61. doi: 10.1200/JCO.2007.15.2421. [DOI] [PubMed] [Google Scholar]
- 13.Wilkie KP, Hahnfeldt P. Tumor-immune dynamics regulated in the microenvironment inform the transient nature of immune-induced tumor dormancy. Cancer Res. 2013;73:3534–44. doi: 10.1158/0008-5472.CAN-12-4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y, Boushaba K. Regulation of tumor dormancy and role of microenvironment: a mathematical model. Advances Exp Med Biol. 2013;734:237–59. doi: 10.1007/978-1-4614-1445-2_11. [DOI] [PubMed] [Google Scholar]
- 15.Michor F, Nowak MA, Iwasa Y. Stochastic dynamics of metastasis formation. J Theor Biol. 2006;240:521–30. doi: 10.1016/j.jtbi.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Diego D, Calvo GF, Pérez-García VM. Modeling the connection between primary and metastatic tumors. J Math Biol. 2013;67:657–92. doi: 10.1007/s00285-012-0565-2. [DOI] [PubMed] [Google Scholar]
- 17.Bartoszyński R. A modeling approach to metastatic progression of cancer. In: Thompson JR, Brown BW, editors. Cancer Modeling. 1987. pp. 237–67. [Google Scholar]
- 18.Hanin L. Seeing the invisible: how mathematical models uncover tumor dormancy, reconstruct the natural history of cancer, and assess the effects of treatment. Advances Exp Med Biol. 2013;734:261–82. doi: 10.1007/978-1-4614-1445-2_12. [DOI] [PubMed] [Google Scholar]
- 19.Retsky MW, Demicheli R, Swartzendruber DE, Bame PD, Wardwell RH, Bonadonna G, et al. Computer simulation of a breast cancer metastasis model. Breast Cancer Res Treat. 1997;45:193–202. doi: 10.1023/a:1005849301420. [DOI] [PubMed] [Google Scholar]
- 20.Yorke ED, Fuks Z, Norton L, Whitmore W, Ling CC. Modeling the development of metastases from primary and locally recurrent tumors: comparison with a clinical data base for prostatic cancer. Cancer Res. 1993;53:2987–93. [PubMed] [Google Scholar]
- 21.Hartung N, Mollard S, Barbolosi D, Benabdallah A, Chapuisat G, Henry G, et al. Mathematical modeling of tumor growth and metastatic spreading: validation in tumor-bearing mice. Cancer Res. 2014;74:6397–407. doi: 10.1158/0008-5472.CAN-14-0721. [DOI] [PubMed] [Google Scholar]
- 22.Haeno H, Gonen M, Davis MB, Herman JM, Iacobuzio-Donahue CA, Michor F. Computational Modeling of Pancreatic Cancer Reveals Kinetics of Metastasis Suggesting Optimum Treatment Strategies. Cell. 2012;148:362–75. doi: 10.1016/j.cell.2011.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer. 2011;11:135–41. doi: 10.1038/nrc3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007;170:793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coumans FAW, Siesling S, Terstappen LWMM. Detection of cancer before distant metastasis. BMC Cancer. 2013;13:283. doi: 10.1186/1471-2407-13-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koscielny S, Tubiana M, Le MG, Valleron A, Mouriesse H, Contesso G, et al. Breast cancer: relationship between the size of the primary tumour and the probability of metastatic dissemination. Br J Cancer. 1984;49:709–15. doi: 10.1038/bjc.1984.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMillin DW, Negri JM, Mitsiades CS. The role of tumour-stromal interactions in modifying drug response: challenges and opportunities. Nat Rev Drug Discov. 2013;12:217–28. doi: 10.1038/nrd3870. [DOI] [PubMed] [Google Scholar]
- 28.Benzekry S, André N, Benabdallah A, Ciccolini J, Faivre C, Hubert F, et al. Modelling the impact of anticancer agents on metastatic spreading. Math Model Nat Phenom. 2012;7:306–36. [Google Scholar]
- 29.Benzekry S, Hahnfeldt P. Maximum tolerated dose versus metronomic scheduling in the treatment of metastatic cancers. J Theor Biol. 2013;335:235–44. doi: 10.1016/j.jtbi.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 30.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–69. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott JG, Gerlee P, Basanta D, Fletcher AG. Mathematical modeling of the metastatic process. In: Malek A, editor. Experimental Metastasis: Modeling and Analysis. 2013. [Google Scholar]
- 32.Wheldon TE. Mathematical models in cancer research. 1988. [Google Scholar]
- 33.Benzekry S, Lamont C, Beheshti A, Tracz A, Ebos JML, Hlatky L, et al. Classical mathematical models for description and prediction of experimental tumor growth. PLoS Comput Biol. 2014;10:e1003800. doi: 10.1371/journal.pcbi.1003800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steel GG, Lamerton LF. The growth rate of human tumours. Br J Cancer. 1966;20:74–86. doi: 10.1038/bjc.1966.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartung N. Efficient resolution of metastatic tumor growth models by reformulation into integral equations. Discrete Contin Dyn Syst Ser B. 2015;20:445–67. [Google Scholar]
- 36.Munoz R, Man S, Shaked Y, Lee CR, Wong J, Francia G, et al. Highly efficacious nontoxic preclinical treatment for advanced metastatic breast cancer using combination oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66:3386–91. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- 37.Ebos JML, Lee CR, Bogdanovic E, Alami J, Van Slyke P, Francia G, et al. Vascular endothelial growth factor-mediated decrease in plasma soluble vascular endothelial growth factor receptor-2 levels as a surrogate biomarker for tumor growth. Cancer Res. 2008;68:521–9. doi: 10.1158/0008-5472.CAN-07-3217. [DOI] [PubMed] [Google Scholar]
- 38.Tracz A, Mastri M, Lee CR, Pili R, Ebos JML. Modeling spontaneous metastatic renal cell carcinoma (mRCC) in mice following nephrectomy. J Vis Exp. 2014:e51485–5. doi: 10.3791/51485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebos JML, Mastri M, Lee CR, Tracz A, Hudson JM, Attwood K, et al. Neoadjuvant antiangiogenic therapy reveals contrasts in primary and metastatic tumor efficacy. EMBO Mol Med. 2014;6:1561–76. doi: 10.15252/emmm.201403989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebos JML, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–9. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavielle M. Mixed Effects Models for the Population Approach. 2014. [Google Scholar]
- 42.Norton L. A Gompertzian model of human breast cancer growth. Cancer Res. 1988;48:7067–71. [PubMed] [Google Scholar]
- 43.Mathworks T. Matlab with statistics and optimization toolboxes. 2013. [Google Scholar]
- 44.Barbolosi D, Verga F, You B, Benabdallah A, Hubert F, Mercier C, et al. Modélisation du risque d’évolution métastatique chez les patients supposés avoir une maladie localisée. Oncologie. 2011;13:528–33. [Google Scholar]
- 45.Spratt JS, Meyer JS, Spratt JA. Rates of growth of human solid neoplasms: Part I. J Surg Oncol. 1995;60:137–46. doi: 10.1002/jso.2930600216. [DOI] [PubMed] [Google Scholar]
- 46.Shan L, Wang S, Korotcov A, Sridhar R, Wang PC. Bioluminescent animal models of human breast cancer for tumor biomass evaluation and metastasis detection. Ethn Dis. 2008;18:S2–65–S2–69. [PubMed] [Google Scholar]
- 47.Milsom CC, Lee CR, Hackl C, Man S, Kerbel RS. Differential post-surgical metastasis and survival in SCID, NOD-SCID and NOD-SCID-IL-2Rγ(null) mice with parental and subline variants of human breast cancer: implications for host defense mechanisms regulating metastasis. PLoS ONE. 2013;8:e71270. doi: 10.1371/journal.pone.0071270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demicheli R, Retsky MW, Hrushesky WJM, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. 2008;19:1821–8. doi: 10.1093/annonc/mdn386. [DOI] [PubMed] [Google Scholar]
- 49.Demicheli R, Retsky MW, Hrushesky WJM, Baum M. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: learning from failures. Nat Clin Rev Oncol. 2007;4:699–710. doi: 10.1038/ncponc0999. [DOI] [PubMed] [Google Scholar]
- 50.Coffey JC, Wang JH, Smith MJF, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncology. 2003;4:760–8. doi: 10.1016/s1470-2045(03)01282-8. [DOI] [PubMed] [Google Scholar]
- 51.Neill KO, Lyons SK, Gallagher WM, Curran KM, Byrne AT. Bioluminescent imaging: a critical tool in pre-clinical oncology research. J Pathol. 2010;220:317–27. doi: 10.1002/path.2656. [DOI] [PubMed] [Google Scholar]
- 52.Retsky M, Demicheli R, Hrushesky W, Baum M, Gukas I. Surgery triggers outgrowth of latent distant disease in breast cancer: an inconvenient truth? Cancers. 2010;2:305–37. doi: 10.3390/cancers2020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiarella P, Bruzzo J, Meiss RP, Ruggiero RA. Concomitant tumor resistance. Cancer Lett. 2012;324:133–41. doi: 10.1016/j.canlet.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 54.Benzekry S, Gandolfi A, Hahnfeldt P. Global Dormancy of Metastases Due to Systemic Inhibition of Angiogenesis. PLoS ONE. 2014;9:e84249–11. doi: 10.1371/journal.pone.0084249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21:139–46. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Tait CR, Dodwell D, Horgan K. Do metastases metastasize? J Pathol. 2004;203:515–8. doi: 10.1002/path.1544. [DOI] [PubMed] [Google Scholar]
- 57.Sugarbaker EV, Cohen AM, Ketcham AS. Do metastases metastasize? Annals of surgery. 1971;174:161–6. doi: 10.1097/00000658-197108000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bethge A, Schumacher U, Wree A, Wedemann G. Are metastases from metastases clinical relevant? Computer modelling of cancer spread in a case of hepatocellular carcinoma. PLoS ONE. 2012;7:e35689–9. doi: 10.1371/journal.pone.0035689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fisher B. Biological and clinical considerations regarding the use of surgery and chemotherapy in the treatment of primary breast cancer. Cancer. 1977;40:574–87. doi: 10.1002/1097-0142(197707)40:1+<574::aid-cncr2820400724>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 60.Brewster AM, Hortobagyi GN, Broglio KR, Kau S-W, Santa-Maria CA, Arun B, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. JNCI Journal of the National Cancer Institute. 2008;100:1179–83. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Badwe R, Parmar V, Hawaldar R, Nair N, Kaushik R, Siddique S, et al. Surgical removal of primary tumor and axillary lymph nodes in women with metastatic breast cancer at first presentation: A randomized controlled trial. San Antonio Breast Cancer Symposium; 2013; p. S2-02. [Google Scholar]
- 62.Di Gioia D, Stieber P, Schmidt GP, Nagel D, Heinemann V, Baur-Melnyk A. Early detection of metastatic disease in asymptomatic breast cancer patients with whole-body imaging and defined tumour marker increase. Br J Cancer. 2015;112:809–18. doi: 10.1038/bjc.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reis-Filho JS, Pusztai L. Gene expression profiling in breast cancer: classification, prognostication, and prediction. Lancet. 2011;378:1812–23. doi: 10.1016/S0140-6736(11)61539-0. [DOI] [PubMed] [Google Scholar]
- 64.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary tumor (PT) was assumed to be detected when reaching the size of 4.32 cm in diameter. Post-diagnosis PT growth and development of metastases in the case of no surgical intervention are indicated as dashed line in the left plot and white bars in the histogram on the right, respectively.
Primary tumor (PT) was assumed to be detected when reaching the size of 4.32 cm in diameter. Post-diagnosis PT growth and development of metastases in the case of no surgical intervention are indicated as dashed line in the left plot and white bars in the histogram on the right, respectively.