Abstract
Central aspects of emotional experiences are often well remembered at the expense of background details. Previous studies have focused on memory after brief delays, but little is known about how these components of emotional memories change over time. Here we investigated the evolution of negative scene memories across 30 minutes, 12 daytime hours spent awake, or 12 nighttime hours including sleep. Negative objects were well remembered at the expense of their backgrounds after 30min. Time spent awake led to forgetting of the entire negative scene, with both objects and their backgrounds decaying at similar rates. Sleep, on the other hand, led to a preservation of negative objects, but not their backgrounds, suggesting that the two components undergo differential processing during sleep. Negative scene memories develop differentially across time delays containing sleep and wake, with sleep selectively consolidating those aspects of a memory that are of greatest value to the organism.
Sleep plays an important role in memory consolidation. Although most studies to date have focused on procedural memory, emerging evidence suggests that sleep benefits episodic memory as well (Born, Rasch and Gais, 2006). In behavioral studies of word recognition and word pair association (Gais et al., 2006), sleep following learning has been shown to improve performance relative to waking control conditions, and to increase resistance to interfering information (Ellenbogen et al., 2006). Training on such episodic memory tasks has been shown to modify the architecture of subsequent sleep stages (Gais et al., 2002) and to promote the reactivation of neural ensembles during post-training sleep – effects which often correlate with memory improvement (Piegneux et al., 2004). Moreover, performance on hippocampally-dependent tasks is frequently impaired following post-training sleep deprivation (Smith & Rose, 1996), indicating that sleep may be necessary for these consolidation benefits. These studies strongly suggest that sleep contributes to the consolidation of episodic memories, perhaps through slow, “off-line” processes that stabilize memories.
Episodic memory performance can be enhanced by the presence of emotional arousal, and, interestingly, this enhancing effect of emotion on memory is often greater after longer (≥ 24hr) retention delays than after shorter ones (Kleinsmith & Kaplan, 1963; Walker & Tarte, 1963; Sharot & Yonelinas, 2007). These findings demonstrate that emotion can influence slow, off-line memory consolidation processes, and suggest that these processes may be sleep-dependent.
Despite the current interest in emotion and memory, we are still learning how emotional memories evolve over time, and only two studies have examined changes across periods of wake and sleep (Wagner, Gais & Born, 2001; Hu, Stylos-Allan, & Walker, 2006). As a consequence, little is known about how these memories are changed by time spent in different brain states.
In their study of sleep and emotional memory, Wagner, Gais and Born (2001) reported that memory for negative arousing narratives was facilitated after 3 hours of late night sleep, which is rich in REM (rapid-eye movement) sleep1. As REM sleep intensely activates the limbic system, particularly the amygdala, and is the stage of sleep in which most emotional dreaming occurs, this was the predicted result. Yet it remained to be determined whether this finding, which was obtained only after 3 hours of sleep late in the night, could be obtained after a full (7–8 hour) night of sleep.
Hu, Stylos-Allan and Walker (2006) demonstrated that sleep’s beneficial influence on emotional memory does indeed persist across an entire night. These authors examined the impact of a full night of sleep on negative arousing and neutral pictures, across both “Remember” and “Know” measures of recognition memory. A night of sleep improved memory accuracy for negative arousing pictures relevant to an equivalent period of daytime wakefulness, but only for Know judgments. Moreover, memory bias changed across a night of sleep relative to wake, such that participants became more conservative when making Remember judgments, especially for emotionally arousing pictures. These findings provide further evidence that the facilitation of memory for emotionally salient information may preferentially develop during sleep.
Both of these studies suggest a role for sleep in the processing and consolidation of memory for emotional experiences. We thought an important next step was to examine exactly which aspects of emotional events are influenced by sleep. This question is an important one to address, because memories of emotional events are not preserved as precise replicas of original experience. Rather, central, emotional information is often remembered at the expense of background details (Payne et al., 2004; Reisberg & Heuer, 2004). A real-world example of this trade-off is the “weapon focus effect”, where victims vividly remember an assailant’s weapon but have little memory for other important aspects of the scene (Stanny & Johnson, 2000). This divergence in memory for central and peripheral aspects of emotional events reflects, at least in part, differential encoding of the two components of the scene. But it is also possible that these elements undergo qualitatively different processing subsequent to encoding.
At present, it is unclear how the components of emotional memories are processed and stored, whether they change over time or remain the same, and whether periods of sleep would affect their consolidation differently than periods spent awake. Emotional scenes could be stored as intact units, suffering some forgetting over time but retaining the same relative vividness for central and peripheral components. Alternatively, the components of the scene could undergo differential processing, perhaps with a selective emphasis on what is most salient and worthy of remembering. In this study, we asked how the consolidation process influences memories for negative emotional scenes, and whether the distinctive brain state of sleep leads to a unique pattern of memory retrieval.
We presented participants with neutral or negatively arousing objects (e.g., a dead body) on a neutral background (e.g., a sidewalk), and later tested their memory separately for the objects and backgrounds. This task reveals an “emotional trade-off” following brief (30min) delays (Kensinger et al., 2007). While negative emotional objects are better remembered than neutral ones, neutral backgrounds associated with these negatively arousing objects are remembered more poorly than similar backgrounds presented with neutral objects. Our goal was to investigate the development of memory for these two scene components across time delays of 30-minutes, 12 daytime hours spent awake, and 12 nighttime hours including a night of sleep.
Method
Participants
Eighty-eight college students from Boston College and Harvard University participated for payment. Participants were randomly assigned to one of four conditions: wake-delay condition (24 participants), sleep-delay condition (24 participants), morning 30-min condition (20 participants), or evening 30-min condition (20 participants). Participants in the wake-delay condition viewed the stimuli at 9AM and were tested 12 hours later at 9PM with no napping between sessions. Participants in the sleep-delay condition viewed the stimuli at 9PM and were tested 12 hours later after a full night (7–8 hours) of sleep at 9AM the following morning. The two baseline circadian control groups viewed the stimuli at 9AM or 9PM and were tested just 30 minutes later. All participants were native English speakers with normal or corrected-to-normal vision. No participant reported a history of psychiatric or sleep disorders or was taking medications that affect the central nervous system or sleep architecture.
Materials
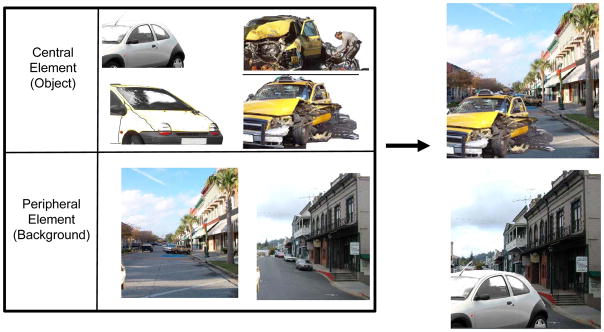
Scenes consisted of negative arousing or neutral objects placed on plausible neutral backgrounds. Eight versions of 96 scenes were created from similar pairs of neutral objects (e.g., two images of a car), negative objects (e.g., two images of a car accident), and neutral backgrounds (e.g., two images of a street), and then placing each of the four objects on each of the two backgrounds (Figure 1). Objects and backgrounds had previously been rated for valence and arousal using 7-point scales (Kensinger, Garoff-Eaton, & Schacter, 2006). All negative objects were given arousal ratings of 5–7 (with high scores signifying an exciting or arousing image) and valence ratings lower than 3 (with low scores signifying a negative image). All neutral items (objects and backgrounds) were rated as non-arousing (arousal values lower than 4) and neutral (valence ratings between 3 and 5).
Figure 1.
Each set of 8 scenes was created from two versions of a neutral object (e.g., two cars), two versions of a negative and arousing emotional object (e.g., two car accidents), and two versions of a neutral background on which these objects could plausibly be found (e.g., two streets). Objects and backgrounds were used to create eight versions of a scene, representing all possible combinations of an object and a background (only two of which are shown here).
Procedure
Participants studied a set of 64 scenes (32 with a neutral object and 32 with a negative object, all on neutral backgrounds) for 5sec each, and then indicated on a 7-point scale whether they would approach or move away from the scene if encountered in real life, which was used to maximize encoding. The scene version studied (of the 8 possible versions) was counterbalanced across participants.
After the delay period, participants performed an unexpected, self-paced recognition task. Participants viewed objects and backgrounds presented separately and one at a time. Some of these objects and backgrounds were identical to the scene components that had been studied (same), others shared the same verbal label but differed in the specific visual details (i.e. the alternate version of the object or background; similar), and others had not been studied (new). Participants saw either the same or the similar version of an item at test (never both versions). Each object or background was presented with a question (e.g., “Did you see a monkey?”). If the answer to the question was “yes”, participants pressed a button to indicate “same” if the object or background was an exact match to a studied component, or a second button to indicate “similar” if it was not an exact match to the one presented at study. If the answer to the question was “no.”, they pressed a third button.2
The recognition task included 32 same objects (16 negative, 16 neutral), 32 similar objects (16 negative, 16 neutral), 32 new objects (16 negative, 16 neutral), 32 same backgrounds (16 previously shown with a negative object, 16 previously shown with a neutral object), 32 similar backgrounds (16 previously shown with a negative object, 16 previously shown with a neutral object), and 32 new backgrounds.
Data Analysis
To permit a direct replication of Kensinger et al (2007), we calculated an “overall recognition” score as “same” + “similar” responses to same items. This measure was used to determine whether we could replicate the trade-off at 30-minutes and extend it to12-hours. For all other analyses, however, we separate this measure into “specific” and “general” recognition memory for scene components. This was key, because previous studies suggest that sleep preferentially promotes memory for general over detailed information (Hu, Stylos-Allen, & Walker, 2006; Payne et al., 2007). Consistent with previous studies asking participants to make a “same” or “similar” distinction at retrieval (e.g. Garoff et al., 2005; Kensinger et al., 2007), we scored a response as “specific recognition” of visual details when a subject correctly responded “same” to same items, but as “general recognition” without specific details when subjects gave “similar” responses to same items. Because “similar” responses are constrained by the number of “same” responses (i.e., subjects give “similar” responses only when they do not remember the visual details), we computed the general recognition score as the proportion of “similar” responses after exclusion of “same” responses (similar/[1-same]). This calculation parallels that used for “independent know” calculations in the “remember/know” procedure (Tulving, 1985; Rajaram, 1993; Yonelinas and Jacoby, 1995), and accounts for the fact that the two response types are mutually exclusive.
Specific and general recognition scores were computed separately for the “central” object (negative or neutral) and for the “peripheral” neutral background (studied with either a negative or neutral object). By comparing memory after a short (30-min) delay to a 12-hour waking delay and a 12-hour sleeping delay, we could examine how the passage of time, with and without sleep, influenced memory.
Because false alarms (“same” or “similar” responses to new items) were extremely low (less than 5% for “same” responses to new items and less than 20% for “similar” responses to new items), and did not differ between groups (all ps>.16), we report uncorrected recognition scores in the main body of the results section. Corrected values and statistics are presented in the “Other Analyses” section below.
Results
Circadian Effects
We first examined whether circadian effects influenced memory performance on this task, but we found no evidence of such influences. Memory performance did not differ between the morning and evening 30-minute delay groups on any measure (Table 1). We thus present data from a single, collapsed 30-minute group in the analyses below. Standard measures of subjective alertness, acquired using the Stanford Sleepiness Scale (Hoddes et al., 1973), also were not significantly different between the AM and PM 30-minute control groups (3.0±.30 vs. 3.3±.28; p=.60). These findings strongly suggest that diurnal differences in cognitive performance, or general levels of alertness, do not account for memory differences seen between the Sleep and Wake groups.
Table 1.
| Memory Type | Condition | Negative objects | Negative backgrounds | Neutral objects | Neutral backgrounds | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | t (p) | Mean ± SEM | t (p) | Mean ± SEM | t (p) | Mean ± SEM | t (p) | ||
| Overall | AM | .83 ± .03 | .81 (.42) | .69 ± .03 | .92 (.36) | .74 ± .03 | .52 (.61) | .76 ± .03 | .20 (.84) |
| PM | .86 ± .02 | .65 ± .03 | .76 ± .03 | .75 ± .03 | |||||
| General | AM | .57 ± .07 | .71 (.49) | .53 ± .05 | 1.8 (.08) | .49 ± .04 | 1.1 (.26) | .48 ± .05 | .20 (.84) |
| PM | .63 ± .06 | .40 ± .06 | .57 ± .05 | .49 ± .06 | |||||
| Specific | AM | .72 ± .04 | .34 (.72) | .42 ± .03 | .18 (.85) | .60 ± .04 | 1.0 (.31) | .58 ± .04 | .25 (.80) |
| PM | .71 ± .03 | .43 ± .04 | .55 ± .04 | .57 ± .03 | |||||
The Emotional Memory Trade-off and its Persistence over Time
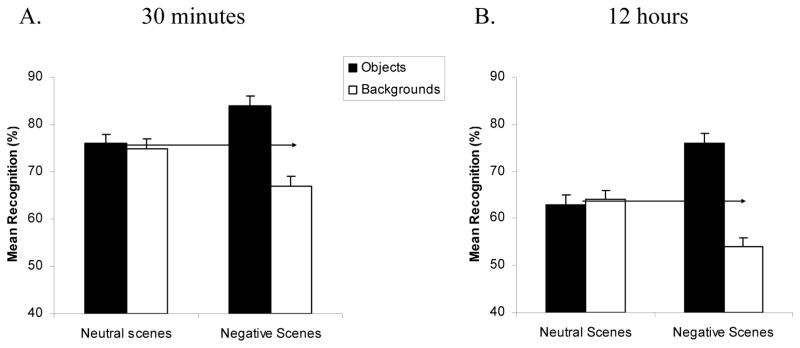
Our first objective was to confirm the existence of the emotional memory trade-off at 30 minutes, and more critically, determine whether it would remain pronounced after 12 hours (across the combined sleep and wake conditions). We thus conducted a 2 (Object valence: Negative, Neutral) × 2 (Scene component: Object, Background) × 2 (Delay: 30min, 12hr combined) mixed ANOVA on overall recognition (“same” + “similar” responses), after Kensinger et al. (2007). The analysis revealed a main effect of Delay, with memories for both scene components better at 30min than 12hr, F(1, 86)=27.8, p<.0001, ηp2=.25. Critically, there was a significant interaction between Valence and Scene component, F(1,86)=68.7, p<.0001, ηp2=.44, which confirms the existence of the trade-off. These factors did not interact with the delay variable, however, indicating that the trade-off was present at both 30min and 12hr (Fig 2A,B). Although objects and backgrounds were recognized at similar rates within neutral scenes (Fig 2A,B, left), objects were significantly better recognized than backgrounds within negative scenes [30min: t(39)=6.3, p<.0001; Fig 2A, right; 12hr: t(47)=7.7, p<.0001; Fig 2B, right]. Moreover, while memory was significantly better for negative than neutral objects [30min: t(39) = 3.9, p<.0001; 12hr: t(47) = 5.0, p<.0001], memory for backgrounds that had contained these negative objects was impaired relative to backgrounds that had contained neutral objects [30min: t(39)=3.9, p<.0001; 12hr: t(47) = 4.5, p<.0001].
Figure 2.
Mean overall recognition memory for objects (black bars), and backgrounds (white bars) for neutral (left) and negative emotional (right) scenes after 30 minutes and 12 hours (collapsed across wake and sleep groups). Negative emotional objects were well remembered at the expense of their (neutral) backgrounds (note the difference in height between the black and white bars for the negative scenes), which reflects the predicted central/peripheral trade-off in emotional memory for scenes. Arrows represent the average recall of objects and backgrounds in the neutral condition, and emphasize that negative objects are better recognized than neutral objects, while backgrounds containing emotional objects are more poorly recognized than backgrounds containing neutral objects. Overall recognition = same + similar responses to old stimuli – false alarms, after Kensinger et al. (2007). Note that because the Y-axes reflect overall recognition scores, they not directly comparable with Figure 3 (which splits memory into general and specific recognition).
The Valence by Scene component interaction also emerged in specific recognition (same responses), F(1,86)=150.3, p<.0001, ηp2=.64, and in general recognition (similar/[1-same responses]), F(1,86)=10.6, p=.002, ηp2=.11. These findings confirm and extend the well-documented trade-off for the central and peripheral components of emotional scenes after brief time delays (Kensinger et al., 2007).
Sleep vs. wake
Given the growing literature on sleep and memory consolidation, our main goal was to determine whether a period of sleep would affect the consolidation of these scenes differently than a period of wake. Because Hu, Stylos-Allen, & Walker (2006) found that sleep benefited emotional memory for “Know” but not “Remember” responses, and because other work in our laboratory suggests that sleep preferentially promotes memory for general information over detailed information (Payne et al., 2007), we began with an analysis of general recognition memory (similar/[1-same responses]).
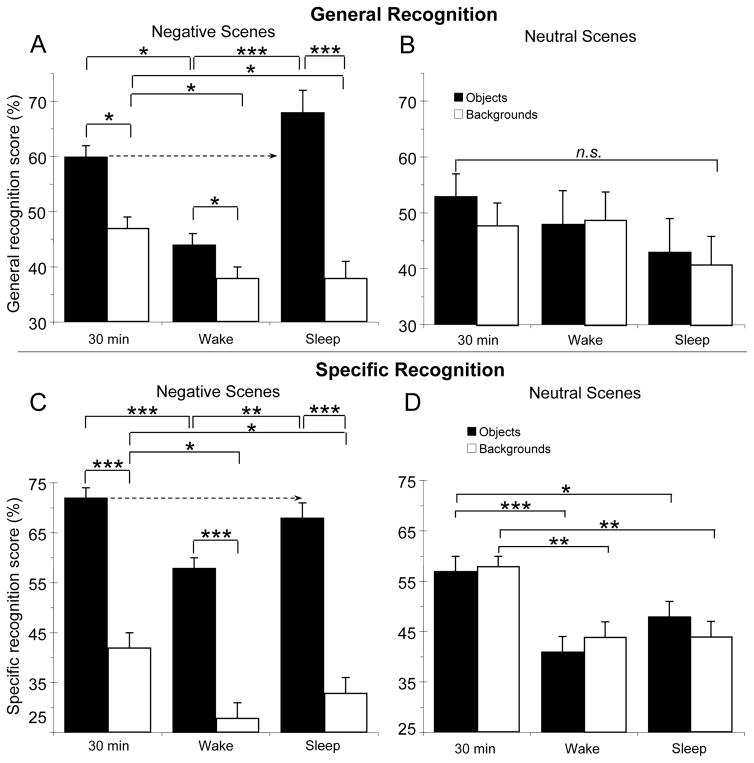
A 2 (Delay: Sleep, Wake) × 2 (Object valence: Negative, Neutral) × 2 (Scene component: Object, Background) mixed ANOVA revealed interactions between Delay and Valence, F(1, 46)=13.1, p=.001, ηp2=.20, between Delay and Scene component, F(1, 46)=7.6, p=.008, ηp2=.14, and, most importantly, among the three factors, F(1, 46)=4.7, p=.03, ηp2=.10; negative, but not neutral, objects were better remembered after sleep than after wake, F(1, 46)=11.5, p=.001, ηp2=.20 (Fig 3A,B, Sleep vs. Wake, black bars). In contrast, sleep offered no benefit for backgrounds, regardless of whether they were presented with negative or neutral objects (Valence × Delay interaction, p>.10, Fig 3A,B, Sleep vs. Wake, white bars). Thus, while negative object memory was enhanced by sleep relative to wake (68% vs. 44%), memory for backgrounds was unchanged by sleep (38% vs. 38%). The same pattern emerged in analyses of specific recognition memory, but the 3-way interaction did not reach significance (p>.1, ns; Fig 3C,D).
Figure 3.
Mean recognition memory for objects (black bars), and backgrounds (white bars) for the three delay conditions – 30 minutes, wake, and sleep. (A) General recognition for negative scenes: memory for emotional objects is maintained and even slightly enhanced across sleep relative to 30 minutes (arrow), resulting in different patterns of negative scene memory components in the wake and sleep conditions; (B) General recognition for neutral scenes: both sleep and wake lead to roughly equivalent reductions in memory for objects and backgrounds, to values of 45±4%. Note that there are no significant differences between the sleep and wake groups, nor are there differences between objects and backgrounds at any of the three time delays; (C) Specific recognition for negative scenes: as with general recognition, negative objects are selectively maintained in the sleep condition; (D) Specific recognition for neutral scenes: as with general recognition, there are no significant differences between object and background recall at any delay, and none between the wake and sleep condition. But both wake and sleep delays show significantly poorer recall than at 30 min. Significant effects are denoted by asterisks (* p <.05, ** p ≤ .01, *** p ≤ .001).
Changes in Memory Relative to the 30min Baseline
To determine how memories changed over time, we next analyzed general memory performance across wake and sleep relative to performance at 30 minutes by subtracting performance at 30min from performance at 12hr. A 2 (Delay: Sleep, Wake) × 2 (Object valence: Negative, Neutral) × 2 (Scene component: Object, Background) mixed ANOVA revealed significant 2-way interactions between Delay and Valence, F(1, 46)=11.6, p=.001, ηp2=.20 and Delay and Scene component, F(1, 46)=7.6, p=.008, ηp2=.14, as well as a significant 3-way interaction, F(1, 46)=4.7, p=.035, ηp2=.09. Looking first at neutral scenes for general recognition (Fig 3B), objects and backgrounds were similarly recognized in each of the three groups (30 min, Wake, Sleep, all ps>0.1, ns), and there was little change in memory for neutral scenes over time, whether spent awake or asleep. Changes in specific recognition memory were similar, except both object and background recognition were significantly reduced at 12hr relative to 30min (both ps<.05; Fig. 3D)
By contrast, memory for negative scene components was distinctly different across periods of wake and sleep (Fig 3A). Time spent awake led to a clear deterioration in memory relative to 30min, and this decline was present both for objects and their backgrounds (16% and 9% deterioration; Fig 3A, Wake vs. 30min). Sleep, however, produced a divergence in memory for objects and backgrounds within negative scenes. Memory for backgrounds containing negative objects was reduced by 9% across Sleep relative to 30-min, a reduction similar to that seen across wake, while memory for negative objects showed a nonsignificant 8% increase after sleep (Fig. 3A, Sleep vs. 30min). Critically, negative object memory was significantly better after sleep than after wake [68% vs. 44%, t(46) = 3.4, p=.001 for general recognition; 68% vs. 58%, t(46) = 2.7, p=.01 for specific recognition] These results suggest that, rather than preserving memory for the entire negative scene, sleep selectively preserved memory for the scene’s negative emotional center.
Other Analyses
As expected, given the low rates of false alarms in all conditions, correcting for false alarms did not change the pattern of findings for overall, general, or specific recognition. For example, when general recognition scores were corrected for false alarms (similar responses to new stimuli]/(1-[same responses to new stimuli]), the critical 2 (Delay: Sleep, Wake) × 2 (Object valence: Negative, Neutral) × 2 (Scene Component: Object, Background) ANOVA still revealed significant interactions between Delay and Valence, F(1, 46) = 13.0, p=.001, η2=.19, Delay and Scene component, F(1, 46) = 8.2, p=.006, η2=.14, and among the three factors, F(1, 46)=4.6, p=.04, η2=.10.
Discussion
Emotional episodic memories are often complex, with multiple components. This study provides important insights into how such memories develop over time. First, we replicated the emotional memory trade-off after 30 minutes (Kensinger et al., 2007). Negatively arousing objects are better remembered than neutral objects, while their backgrounds suffer relative to those associated with neutral objects. But what happens to these memories with further processing, as they begin the process of long-term consolidation? We have demonstrated that the disparity between negative objects and their backgrounds persists or even grows across 12 hours, and that these memory components develop differently across sleep and wakefulness.
While 12hr of wake produced similar forgetting of both components of negative scenes relative to 30min, sleep led to a divergence of the two memory components. Rather than conferring a general benefit on memory for negative scenes in their entirety, sleep only promoted memory for central emotional objects; sleep’s benefit did not extend to the backgrounds in which the emotional objects were embedded. As a result, the disparity in recognition between emotional objects and their backgrounds more than doubled compared to either the 30min or the 12hr wake condition – and increase that was entirely due to maintenance of emotional object memory. This is consistent with the possibility that the individual components of the scene become “unbound” during sleep, allowing sleep to selectively preserve only what is calculated to be most salient and perhaps most worthy of remembering.
These results add to a growing literature demonstrating that sleep benefits the consolidation of emotional over neutral information. Wagner, Gais & Born (2001) demonstrated a benefit of sleep for emotional, but not neutral, narratives, and showed that this benefit lasts for years (Wagner et al., 2006). Hu, Stylos-Allan, & Walker (2006) showed a similar sleep benefit for emotional vs. neutral photographs (without backgrounds). Together, these findings suggest that sleep plays a role in emotional memory consolidation that exceeds any benefit for neutral memories.
Intriguingly, sleep did not benefit neutral memory in any of these studies, which is curious given the literature on sleep-based consolidation of neutral episodic memories (see Payne et al., in press for review). It may be that mixed presentation of neutral and emotional stimuli biases processing toward enhancing only the emotional information, whereas blocked presentation produces benefits for both neutral and emotional information. Although this remains to be tested, we note that mixed vs. pure presentation of stimuli leads to very different patterns of memory retrieval in other paradigms (e.g. Hadley & McKay, 2006; Schmidt, 1994). Moreover, sleep exerted its strongest effects on general, rather than specific, memory. This is consistent with Hu et al. (2006) and Payne et al. (2007), and suggests that sleep may preferentially promote memory for gist over detail.
While we believe that sleep itself produced these unique patterns of memory consolidation, two alternative explanations deserve consideration. The first is that memory was simply better in the morning than in the evening. However, if this were the case, differences in negative memory should have emerged between the two 30 minute (AM and PM) groups, and the same circadian influence should have operated equally on negative and neutral memories. Neither pattern was observed. Moreover, the discrepancy between negative objects and their backgrounds was not only greater after a period of sleep than wakefulness, but also than after a much shorter 30-minute delay in either the morning or the evening (both Fs > 3, both ps < .05). Finally, there were no differences between morning and evening subjective ratings of alertness. Together, these points suggest that circadian influences cannot account for our findings.
Our findings also could reflect a lack of interference during sleep (Wixted, 2004). But in this case, sleep should provide a global consolidation benefit to memories for both objects and backgrounds and for both negative and neutral stimuli. Yet only negative objects benefited from sleep. One could argue that interference continued unabated across sleep for memory of backgrounds and neutral objects, while being completely absent for negative objects. But interference did not show such effects during wake. Furthermore, an interference argument must also predict better negative object memory after 30min (a time interval allowing very little interference) than in the 12hr sleep condition, given that participants in the sleep condition were awake for 2.5 hrs on average after their training at 9PM, and for at least 30min prior to their test at 9AM the following morning. These subjects thus had 3 hours of waking interference between training and test, yet memory for negative objects was actually non-significantly better after 12hr with sleep than after just 30min awake. We thus feel that interference alone cannot satisfactorily explain our findings.
Although interference likely does contribute to deterioration in the wake condition, we believe our results are most parsimoniously explained by an active role for sleep in the consolidation of emotional memories (Ellenbogen, Payne & Stickgold, 2006). By “active”, we mean sleep-specific neural processes that directly contribute to memory consolidation. These could include neurophysiological processes, such as 1–4Hz slow waves, <1Hz slow oscillations, or 12–16Hz sleep spindles, and neurochemical processes related to the fluctuations in aminergic and cholinergic neurotransmitters seen across the wake-sleep cycle (Payne et al., in press).
To our knowledge, these are the first findings to demonstrate (1) that the emotional memory trade-off persists over time, and (2) that the individual components of emotional scene memories evolve differently across time spent asleep and awake. As such, these findings provide important insights into the evolution of emotional memories over time, and suggest a unique role for sleep in their consolidation.
Acknowledgments
This research was supported by a Harvard Mind, Brain, and Behavior postdoctoral fellowship to J.D.P, by the National Science Foundation to E.K., and National Institute of Mental Health to R.S. We thank Yuliya Nikolova for her assistance running participants, and Jill Lany and Dan Schacter for their helpful comments on the manuscript.
Footnotes
The majority of non-REM slow-wave sleep (SWS) occurs in the first half of the night, whereas the majority of REM sleep occurs in the second half.
Text questions were included to limit the scope of “similar” responses. For example, remembering a church seen during encoding might lead a subject to score a mosque shown at test as similar. Asking “Did you see a mosque?” would force a negative response.
References
- Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- Ellenbogen JM, Hulbert JC, et al. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Current Biology. 2006;16:1290–4. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Gais S, Lucas B, et al. Sleep after learning aids memory recall. Learning & Memory. 2006;13:259–62. doi: 10.1101/lm.132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Molle M, et al. Learning-dependent increases in sleep spindle density. Journal of Neuroscience. 2002;22:6830–4. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff RJ, Slotnick SD, Schacter DL. The neural origins of specific and general memory: The role of the fusiform cortex. Neuropsychologia. 2005;43:847–859. doi: 10.1016/j.neuropsychologia.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Hadley CB, MacKay DG. Does emotion help or hinder immediate memory? Arousing vs. priority-binding mechanisms. Journal of Experimental Psychology: Learning, Memory and Cognition. 2006;32:79–88. doi: 10.1037/0278-7393.32.1.79. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: A new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Hu P, Stylos-Allan M, Walker M. Sleep facilitates consolidation of emotional declarative memory. Psychological Science. 2006;17:891–898. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Memory for specific visual details can be enhanced by negative arousing content. Journal of Memory and Language. 2006;54:99–112. [Google Scholar]
- Kensinger E, Garoff-Eaton R, Schacter D. Effects of emotion on memory specificity: Emotion-induced memory trade-offs. In press, Journal of Memory and Language. 2007;56:575–591. [Google Scholar]
- Kleinsmith LJ, Kaplan S. Paired-associate learning as a function of arousal and interpolated interval. Journal of Exprimental Psychology. 1963;65:190–193. doi: 10.1037/h0040288. [DOI] [PubMed] [Google Scholar]
- Payne JD, Ellenbogen JM, Walker MP, Stickgold R. Learning and Memory: A Comprehensive Reference. Elsevier; The role of sleep in memory consolidation. In Press. [Google Scholar]
- Payne JD, Nadel L, Britton WB, Jacobs WJ. The biopsychology of trauma and memory. In: Reisberg D, Hertel P, editors. Emotion and Memory. Oxford, UK: Oxford University Press; 2004. pp. 76–128. [Google Scholar]
- Peigneux P, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rajaram S. Remembering and knowing: Two means of access to the personal past. Memory & Cognition. 1993;21:89–102. doi: 10.3758/bf03211168. [DOI] [PubMed] [Google Scholar]
- Reisberg D, Heuer F. Memory for emotional events. In: Reisberg D, Hertel P, editors. Emotion and Memory. Oxford, UK: Oxford University Press; 2004. pp. 3–41. [Google Scholar]
- Schmidt SR. Effects of humor on sentence memory. Journal of Experimental Psychology: Learning, Memory and Cognition. 1994;20:953–967. doi: 10.1037//0278-7393.20.4.953. [DOI] [PubMed] [Google Scholar]
- Smith C, Rose GM. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiology and Behavior. 1996;59:93–97. doi: 10.1016/0031-9384(95)02054-3. [DOI] [PubMed] [Google Scholar]
- Sharot T, Yonelinas AP. Differential time-dependent effects of emotion on recollective experience and memory for contextual information. Cognition. 2007 doi: 10.1016/j.cognition.2007.03.002. in press. [DOI] [PubMed] [Google Scholar]
- Stanny CJ, Johnson TC. Effects of stress induced by a simulated shooting on recall by police and citizen witnesses. American Journal of Psychology. 2000;113:359–386. [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychologist. 1985;26:1–12. [Google Scholar]
- Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learning and Memory. 2001;8:112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Hallschmid M, Rasch B, Born J. Brief sleep after learning keeps emotional memories alive for years. Biological Psychiatry. 2006;60:788–790. doi: 10.1016/j.biopsych.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Walker EL, Tarte RD. Memory storage as a function of arousal and time with homogeneous and heterogeneous lists. Journal of Verbal Learning and Verbal Behavior. 1963;2:113–119. [Google Scholar]
- Wixted JT. The psychology and neuroscience of forgetting. Annual Review of Psychology. 2004;55:235–69. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Jacoby LL. The relation between remembering and knowing as bases for recognition: Effects of size congruency. Journal of Memory and Language. 1995;34:622–643. [Google Scholar]