Abstract
Objective
Vertical sleeve gastrectomy (VSG) results in weight loss, increased bile acids (BA) and fibroblast growth factor 19 (FGF19) levels. FGF21 shares essential co-factors with FGF19 but its physiology early post-VSG has not been assessed.
Methods
Ten adolescents (17.4 ± 0.5 years and BMI 51.5 ± 2.5 kg/m2) were enrolled. Fasting and post-meal (100 mL Ensure™) samples (0–120 min) were collected (Pre-VSG [V1], 1 [V2], & 3 months [V3] post-VSG) for analysis of BA, FGF19, and FGF21.
Results
Post-VSG subjects lost weight (V2 11.8 ± 0.8 kg; V3 21.9 ± 1.7 kg). BA and FGF19 increased by V2; 143.6% at 30 min and 74.9% at 90 min postmeal, respectively. BA hydrophobicity index also improved by V3; 21.1% at 30 min postmeal. Interestingly, fasting and 120 min postmeal FGF21 levels at V2 were increased by 135.7% and 253.9% respectively, but then returned to baseline at V3. BA levels correlated with FGF21 at V2 (P = 0.003, r = 0.89) and body weight lost post-VSG correlated with FGF21 levels (V2; P = 0.012, R = 0.82).
Conclusion
Expected changes were seen in BA and FGF19 biology after VSG in adolescents, but novel changes were seen in correlation between the early postsurgical increase in FGF21 and weight loss, suggesting that FGF21 may play a role in energy balance postoperatively and further investigation was warranted.
Introduction
Bariatric procedures such as Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) result in more and durable weight loss than lifestyle modifications and/or medications alone. The weight loss and improvement in glucose homeostasis is comparable following RYGB and VSG (1), in which approximately 80% of the stomach is removed along the greater curvature. Indeed, the improvement of glycemic control following bariatric surgery often occurs before significant weight reduction (2). To better understand how bariatric surgery achieves metabolic benefit, we and others have explored the mechanisms behind physiologic outcomes of surgery.
Amongst the many changes, the rise in serum circulating BA levels following RYGB and VSG (3–5) has significance given that BAs can behave as signaling molecules, serving as regulators of energy homeostasis and glucose metabolism. For example, BAs have been shown to lower triglyceride levels via a pathway involving the farnesoid X receptor (FXR) and sterol regulatory element-binding protein-1c (6). More recently, obeticholic acid, an FXR-specific agonist, has been shown to reverse NASH (7). FXR knock-out mice further demonstrate that weight loss and improved glucose tolerance following VSG depend on the FXR signaling pathway (8). Thus the data suggest that FXR is a key metabolic target of BAs, and may be responsible for important outcomes after VSG.
BAs also influence certain hormones of the fibroblast growth factor (FGF) family. Intraluminal BAs taken up by enterocytes bind to intranuclear FXR molecules acting as a heterodimeric transcription factor inducing intestinal secretion of FGF19 (FGF15 in rodents). In turn, FGF19 suppresses hepatic BA synthesis by repressing the rate limiting step in BA synthesis, cholesterol 7α-hydroxylase (CYP7A1) (9). Interestingly, after murine VSG, intestinal expression of FGF15—the murine ortholog of FGF19—is increased (8,10). Further, Tomlinson et al. noted that transgenic mice expressing human FGF19 had increased metabolic rates and were resistant to diet-induced obesity (DIO) (11). Recombinant FGF19 administered to both DIO mice and leptin-deficient mice also resulted in increased metabolic rate, reduced body weight, and reversal of diabetes (12).
Another hormone of the FGF family, FGF21, is predominantly expressed in the liver and adipose tissue. FGF21 increases adipocyte glucose uptake mainly due to the upregulation of glucose transporter GLUT-1 (13). FGF21 also improves glucose tolerance, insulin sensitivity, and decreases blood glucose levels (13). Unlike FGF19, FGF21 lacks a mitogenic function thus likely reducing carcinogenicity concerns. FGF21 is believed to be a main downstream effector of peroxisome proliferator-activated receptor (PPAR)-α function in the liver as FGF21 deficiency inhibits the biological actions of hepatic PPAR-α (14). Systemic administration of FGF21 in DIO mice reduced body weight, whole-body fat mass, blood glucose, insulin, lipid levels, and reversed hepatic steatosis (15,16).
The aim of our current study was to quantify changes in fasting and postprandial serum levels of BAs and related FGF signaling hormones (Refs. 19 and 21), in adolescent patients undergoing VSG.
Methods
Recruitment and enrollment
All study procedures for this single-center prospective study were approved by the Cincinnati Children’s Hospital Medical Center IRB before implementation. Recruitment for this study was focused on adolescent patients who were 19 years of age or less and had been approved and consented to have VSG at Cincinnati Children’s Hospital Medical Center.
Adolescents and their families were approached and offered participation in the study using the IRB approved study flyer and consent form. Enrollment was offered to consecutive patients that met inclusion criteria with an enrollment goal of 10 participants. The enrollment period was March 2014 to January 2015, during which time 10 participants consented and participated, and 21 declined participation. Of the 21 who were offered enrollment but did not participate, 52.4% (n = 11) stated they did not want to be bothered; follow-up too burdensome, 19.1% (n = 4) were unable to schedule baseline visit within study parameters, 19.1% (n = 4) had a general lack of interest in research, 4.8% (n = 1) did not want to participate in medical research, and 4.8% (n = 1) ultimately chose to not have bariatric surgery. Although 21 patients declined participation, the nonparticipants were demographically similar to those who chose participation. All recruitment was done and informed consents were administered by trained clinical research coordinators.
Study visits and data collection
Each participant was asked to complete three meal challenge visits (baseline, 1 month after VSG surgery, and 3 months after VSG surgery). In an effort to keep sample and data collection consistent, each visit was conducted within the following visit windows: baseline visit (V1): enrollment up to 7 days before bariatric surgery; 1-month postoperative visit (V2): ±10 days of the 1-month surgical anniversary; 3-month visit (V3): ±30 days of the 3-month surgical anniversary. All visit procedures and data collection were conducted identically.
All visits were conducted at the Clinical Translational Research Center and with participants who had fasted for 12 h. Anthropometric measurements were done and an IV was placed. Fasting baseline blood was drawn and a standardized liquid meal (total of 100 mL; Ensure, Abbott Laboratories, Abbott Park, IL) was administered. Phlebotomy was done at baseline and every 30 min thereafter for 120 min. This meal challenge test was repeated at the subsequent 1-and 3-month visits after surgery.
Demographics, laboratory data, medical history, weight, height, BMI, blood pressure were abstracted from the medical records.
Serum and plasma were isolated and stored at −80° until batched testing was conducted. BA quantity and composition, and C4 (biomarker of BA production rate) were determined by mass spectrometry as before (17). FGF21 and FGF19 levels were determined by ELISA (R&D Biosystems, Minneapolis, MN).
Statistical analysis
All values were expressed as mean ± SEM. Statistical significance was evaluated by one- or two-way ANOVA or by Student’s t-test in two groups across two time-points. Pearson’s coefficient was utilized to evaluate correlation between BA, FGF19, FGF21, and weight loss. P values less than 0.05 were considered significant.
Results
We enrolled 10 participants in the study. One was excluded due to inconsistent values and concern for sample degradation. All results were reported for the nine remaining participants (Table 1). All nine completed the presurgery visit while eight of nine (88.9%) completed the 1 month visit and seven of nine (77.8%) completed the 3 months post-VSG visit.
TABLE 1.
Participant demographic and anthropometric characteristics
| Demographics and anthropometrics | |
|---|---|
| Mean age, yr ± SEM (range) | 17.4 ± 0.5 (15.1–19.8) |
| Gender, no. (%) | |
| Female | 7 (77.8%) |
| Male | 2 (22.2%) |
| Race, no. (%) | |
| Caucasian | 7 (77.8%) |
| African American | 1 (11.1%) |
| Not reported/unknown | 1 (11.1%) |
| Mean baseline weight, kg ± SEM (range) | 144.2 ± 6.2 (118.1–182.5) |
| Mean baseline BMI, BMI ± SEM (range) | 51.5 ± 2.5 (43.9–67.6) |
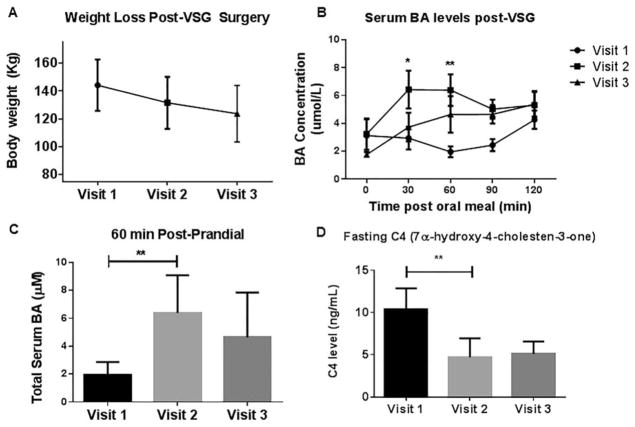
At V1, the participants were 17.4 ± 0.5 years of age, with mean weight of 144.2 kg ± 6.2 and BMI of 51.5 kg/m2 ± 2.5. All participants lost weight postoperatively, with a mean weight loss at V2 of 11.8 ± 0.8 kg and at V3 weight loss averaged 21.9 ± 1.7 kg (Figure 1A).
Figure 1.
(A) Body weight change after VSG: At the presurgery visit (V1) the participants’ mean weight was 144.2±6.2 kg which decreased by V2 to 131.5±6.6 kg; and by V3 to 123.7±7.7 kg. Mean weight loss after 1 month post-VSG 11.8±0.8 kg and at the 3 months post-VSG visit 21.9±1.7 kg. (B) Serum bile acid levels after VSG: serum concentrations of bile acids at baseline (before VSG) and at 1 and 3 months after surgery. All data are reported as mean±SEM. The 30-, 60-min postmeal challenge bile acid levels were elevated at the 1 month post- VSG visit (V2 vs. V1 30 min *P < 0.05; and V2 vs. V1 60 min **P < 0.01; two-way ANOVA with Bonferroni post-test). (C) 60-min postmeal challenge bile acid levels were elevated at the 1 month post-VSG visit (**P < 0.01; two-way ANOVA with Bonferroni post-test). (D) Circulating C4, was significantly lower at the 1 month post-VSG (V2) clinic visit compared with baseline (V1; **P=0.029; paired t-test).
Postprandial BA excursions were elevated at V2 as shown in Figure 1B, C (143.6% increase for V2 vs. V1 at 30-min postmeal challenge assay, P < 0.05; similar findings were noted at the 60-min test; P < 0.01) The surrogate marker for hepatic BA production, circulating C4, was 54.6% lower at V2 compared with V1 (P = 0.029; Figure 1D).
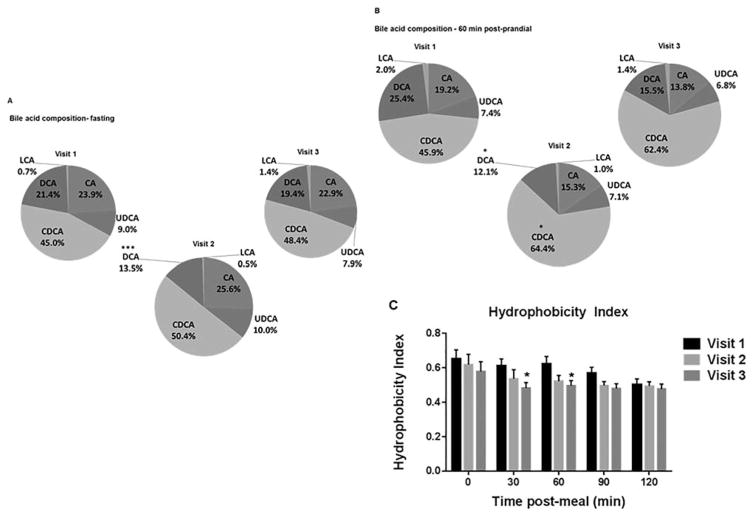
To further understand changes in BA physiology post-VSG, we assessed serum BA composition. There were significant changes in the proportion of various bile acids. For fasting BA measurements, deoxycholic acid (DCA) decreased from 21.4% at V1 to 13.5% at V2 (Figure 2A). The share of chenodeoxycholic acid (CDCA) increased from 45.9% at V1 to 64.4% at V2 (Figure 2B). On the other hand, both cholic acid (CA) and DCA had a smaller proportion of the 60 min postmeal challenge BA composition after VSG (Figure 2B). As a result of these specific BA composition changes, the composite hydrophobicity index, measuring overall hepatotoxicity of the bile acid pool, decreased from V1 to V2 by 12.5% and by V3 had decreased by 21.1% (as assessed in samples collected 30 min postmeal challenge). A similar improvement was seen in the 60 min postmeal challenge hydrophobicity index as well (Figure 2C).
Figure 2.
(A) Fasting bile acid composition changes post-VSG. The proportion of fasting deoxycholic acid (DCA) decreased [V1 21.4% vs. V2 13.5%; ***P < 0.001 and V1 vs. V3 19.4%;*P=0.039; two-way ANOVA]. (B) 60-min postprandial serum BA composition changes post-VSG: Increased share of chenodeoxycholic acid (CDCA) 1 month post-VSG (V2) [V1 45.9% vs. V2 64.4%; *P=0.038] which remains elevated at the 3-month post-VSG visit (V3) [62.4%]. Cholic acid (CA) had a smaller proportion of the 60 min postprandial BA composition after VSG [V1 19.2% vs. V2 15.3% vs. V3 13.8%]. Postprandial deoxycholic acid (DCA) also decreased post-VSG [V1 25.4% vs. V2 12.1%; *P=0.012], [V3 15.5%; P=0.052 compared with V1] (two-way ANOVA). Lithocholic acid (LCA) and ursodeoxycholic acid (UDCA) were not different in fasting or postprandial. (C) The bile acid hydrophobicity index decreased post-VSG (V3 vs. V1; 30 min *P=0.03 and 60 min *P=0.03; t-test). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
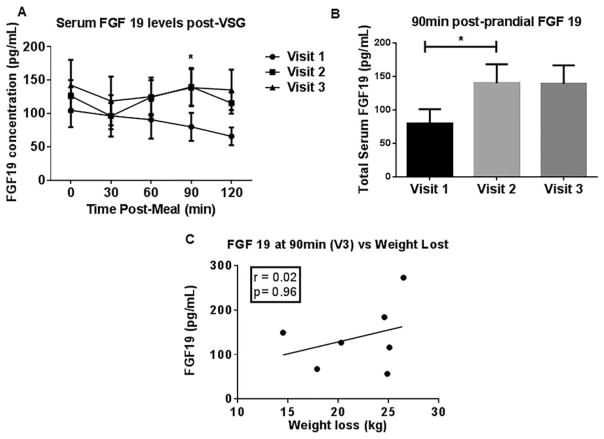
Next, to test the hypothesis that BA-stimulated FGF19 intestinal release would be increased post-VSG, we measured both fasting and postmeal challenge plasma FGF19 levels (Figure 3A). A 74.9% increase in FGF19 levels was found at V2 compared with presurgery (V1) levels (90 min postmeal challenge plasma; Figure 3B). This elevation in FGF19 remained unchanged through V3. We did not find any significant correlation between postprandial FGF19 levels and weight loss (Figure 3C). However, a positive correlation was observed between the percentage increase in postprandial BA levels and FGF19 at V3 (90-min sample; P = 0.041, Pearson r = 0.774).
Figure 3.
(A) FGF 19 changes during the 3-month period after VSG. Serum concentrations of FGF 19 at baseline (visit 1) and at 1 (visit 2) and 3 months (visit 3) after surgery. All data are reported as mean±SEM. (B) Plasma postprandial FGF 19 levels increased 1 month post-VSG (V2 90 min vs. V1; paired t-test; *P=0.026). (C) No significant correlation between postprandial FGF 19 levels and weight loss.
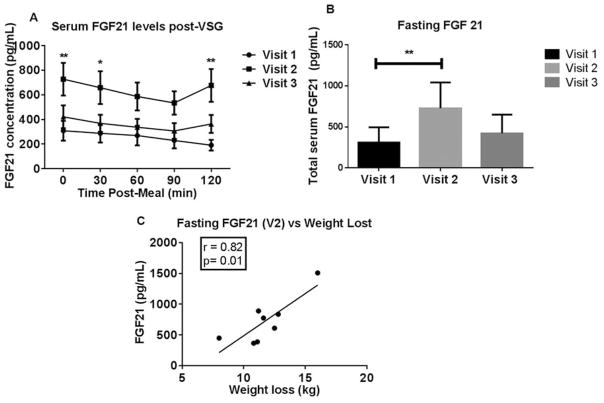
Finally, both fasting and postprandial FGF21 levels were interrogated. Fasting plasma FGF21 levels increased by 136% at V2 compared with V1 but returned to pre-surgery levels by V3 (Figure 4A, B). Interestingly, there was a positive correlation between fasting FGF21 plasma levels and body weight loss at V2 (Pearson r = 0.82; P = 0.012; Figure 4C). Postmeal challenge FGF21 levels and weight loss also positively correlated at V2 (30 min r = 0.7781; P =0.0230; 90 min r =0.7828; P =0.0216 and 120 min r = 0.7276; P =0.0408). Further, there was a positive correlation between the rise in both fasting BA and fasting FGF21 at V2 (Pearson r = 0.894; P = 0.003). A positive correlation was found between the percentage rise in postprandial FGF19 and 21 levels at V2 (30 min r = 0.7727; P = 0.0246; 60 min r = 0.7118; P = 0.0476; 90 min r = 0.9202; P = 0.0012).
Figure 4.
(A) FGF 21 changes during the 3-month period after VSG. Serum concentrations of FGF 21 at baseline (visit 1) and at 1 (visit 2) and 3 months (visit 3) after surgery. All data are reported as mean±SEM. **P < 0.01; *P < 0.05 V2 vs. V1; two-way ANOVA. (B) Fasting serum FGF 21 levels were increased at the 1 month post-VSG visit (V2; fasting **P < 0.01; two-way ANOVA) but returned back to presurgery levels at the 3-month post-VSG visit (V3). (C) A positive correlation between fasting serum FGF 21 and body weight lost at 1 month (visit 2) post- VSG visit (Pearson’s R=0.82; P=0.012).
Discussion
In our study, we observed that BA, FGF19, and FGF21 physiology was altered after VSG surgery. The novel identification of an early change in plasma FGF21 levels suggests that this signaling molecule may play an important role in the response to VSG in humans. We found that plasma FGF21 levels increased early after surgery and the increase in FGF21 plasma levels strongly correlated with surgical weight loss. We found that circulating postprandial BA levels increased 1 month postoperatively, and suppression of BA synthesis was deduced by virtue of the reduction in C4. Postprandial plasma FGF19 levels were increased at 1 and 3 months post-VSG surgery. Further, we report changes in BA composition post-VSG, which together with changes in BA hydrophobicity index indicate a less hepatotoxic pool of circulating BA.
The changes in BA physiology post-VSG seen in our study are consistent with previous reports including experimental murine VSG (10) and human studies (18,19). At baseline, our subjects had a blunted postprandial BA response which improved post-VSG surgery, a finding which has also been noted after Roux-en-Y gastric bypass (20). This “normalization” of postprandial BA excursion may be related to the weight loss and metabolic benefits of VSG surgery. The decrease observed in C4 serum levels, a surrogate measure of BA synthesis, is also consistent with prior work (19).
The decrease in the hydrophobicity index post-VSG surgery is also an important observation from our study. BA composition changes impact the degree of binding of BA to receptors and ionic channels as this depends on a ratio between their hydrophobic and hydrophilic surfaces. For example, 7α-methylated ursodeoxycholic acid (UDCA) has a significantly higher affinity for and ability to activate the G-protein-coupled receptor TGR5 (21) which plays a key role in glucose and energy homeostasis. Conversely, FXR activation ability of 7α-methylated UDCA is low compared with that of UDCA, while CDCA is the most potent activating ligand of FXR. The G protein coupled receptor sphingosine-1-phosphate receptor 2 (S1PR2) is highly expressed in hepatocytes and has an affinity for conjugated BA. In primary rodent hepatocytes, this S1PR2 activation leads to downstream activation of the extracellular regulated protein kinase (ERK) 1/2 and (protein kinase B) AKT signaling pathways which play important roles in the regulation of hepatic glucose and lipid metabolism (22). Thus, overall the improved hydrophobicity index may explain some of the hepatoprotective effects seen post-VSG surgery. The pathways noted above may be important to dissect in future mechanistic studies of VSG impact on liver pathology, especially with respect to treatment of nonalcoholic fatty liver disease.
Intraduodenal infusion of the specific BA, CDCA increases FGF19 secretion (23). This prior observation may explain the positive correlation in our study between the percentage increase in postmeal challenge BA and FGF19 levels. We observed an increase in both total BA level and the proportion of CDCA in the circulating BA pool at 60 min postmeal challenge. Interestingly, the peak in plasma FGF19 levels was observed at the 90 min postmeal challenge time point. This sequential rise of BA and FGF19 lends support to the concept that postprandial elevation of BAs (such as CDCA) trigger FGF19 release. This is consistent with the existing literature (19,24,25). The positive metabolic effects of FGF19 have been reported in murine studies including increased metabolic rate (11,12), improved weight loss and glucose metabolism (12), and resistance to diet induced obesity (11). These findings suggest that alterations in the BA-FGF19 axis may contribute to the beneficial metabolic changes following metabolic procedures such as VSG and RYGB.
Similarly, Haluzikova et al. (24) demonstrated an initial rise in FGF21 levels 6 months after VSG, followed by declining FGF21 levels 12 and 24 months after surgery. Our results extend these findings and further indicate a positive correlation between fasting and postprandial FGF21 plasma levels and body weight loss as early as 1 month after VSG. Individuals who are obese have been found to have higher FGF21 levels than lean controls (26) and 3 weeks of a very low calorie diet is associated with a further increase in FGF21 levels (27). Thus, it has been suggested that obesity may represent an FGF21-resistant state (28). One could hypothesize that hypocaloric intake post-VSG stimulates an initial rise in FGF21 followed by a weight loss induced decrease in FGF21 resistance and subsequent decline in circulating FGF21. Conversely, Hale et al. (29) demonstrated that FGF21 related whole-body metabolic responses were preserved in ob/ob and DIO mice, with models of murine obesity being more sensitive and responsive than lean mice to the glucose-lowering and weight-loss effects of recombinant human FGF21 (rhFGF21). Endogenous FGF21 levels, although elevated in obese mice, were below the half-maximal effective concentrations of rhFGF21, suggesting a state of relative deficiency. The authors speculate that supra-physiological levels of FGF21 or pharmacological intervention may be required to alleviate diabetes and obesity.
Adipose tissue has a central role in the regulation of energy balance and homeostasis. While white adipose tissue (WAT) serves as the primary site for energy storage, brown adipose tissue (BAT) is highly metabolically active and is known to protective against a body temperature drop via increased energy consumption due to thermogenesis. FGF21 activates BAT in response to cold exposure and β-adrenergic stimulation. Interestingly, FGF21 stimulates the production of brown-like or “beige” cells in WAT after cold exposure that become capable of adaptive thermogenesis and energy expenditure similar to classical BAT. While we did not measure energy homeostasis in this study, it is tempting to speculate that the thermogenic effects of FGF21 on adipose tissue may be linked with energy expenditure and contribute to the correlation we found between weight loss and FGF21. FGF21 did increase energy expenditure but without changes in substrate utilization. We speculate therefore that FGF21 may influence energy expenditure particularly in the first 30 days post-VSG.
Our study had certain limitations, including its small sample size (only seven of nine patients completed the 3 months visit) leading to imbalances in race, sex, and socioeconomic status. We also did not have a control group thus we were not able to study the influence of diet post-VSG either. A larger scale study that may include different cohorts, including appropriate dietary controls may help to further clarify these factors. Longer-term studies would also be helpful to understand the relevance of early elevated FGF21 on long-term weight loss.
Conclusion
Our data suggest a potentially important role for FGF21 in metabolism and weight loss post-VSG in adolescents. An improved understanding of the underlying biological mechanisms of the action of FGF21 may explain heterogeneity in the response to surgery in human populations, and may ultimately reveal a therapeutic potential of FGF21 on energy expenditure and obesity related comorbidities.
Acknowledgments
Funding agencies: This study was supported by NASPGHAN Foundation and 5R01DK100314 (to RK) and 5UM1DK072493 (to THI, TJ, and LS).
Footnotes
Disclosure: The authors declared no conflict of interest.
Author contribution: Concept & Design of study, study implementation and assay performance/supervision, data collection and analysis, manuscript writing (RK, LS, THI, KDRS, TJ); Concept & Study implementation and assay performance/supervision, data collection and analysis, manuscript writing (WZ, RMSG, SM, MO, XZ).
References
- 1.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mingrone G, DeGaetano A, Greco AV, et al. Reversibility of insulin resistance in obese diabetic patients: role of plasma lipids. Diabetologia. 1997;40:599–605. doi: 10.1007/s001250050721. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs C, Claudel T, Trauner M. Bile acid-mediated control of liver triglycerides. Semin Liver Dis. 2013;33:330–342. doi: 10.1055/s-0033-1358520. [DOI] [PubMed] [Google Scholar]
- 4.Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity. 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–1407. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cicione C, Degirolamo C, Moschetta A. Emerging role of fibroblast growth factors 15/19 and 21 as metabolic integrators in the liver. Hepatology. 2012;56:2404–2411. doi: 10.1002/hep.25929. [DOI] [PubMed] [Google Scholar]
- 10.Myronovych A, Kirby M, Ryan KK, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity. 2013 doi: 10.1002/oby.20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomlinson E, Fu L, John L, et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741–1747. doi: 10.1210/endo.143.5.8850. [DOI] [PubMed] [Google Scholar]
- 12.Fu L, John LM, Adams SH, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 13.Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inagaki T, Dutchak P, Zhao G, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Lloyd DJ, Hale C, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coskun T, Bina HA, Schneider MA, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 17.Myronovych A, Salazar-Gonzalez RM, Ryan KK, et al. The role of small heterodimer partner in nonalcoholic fatty liver disease improvement after sleeve gastrectomy in mice. Obesity. 2014;22:2301–2311. doi: 10.1002/oby.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinert RE, Peterli R, Keller S, et al. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity. 2013;21:E660–E668. doi: 10.1002/oby.20522. [DOI] [PubMed] [Google Scholar]
- 19.Escalona A, Munoz R, Irribarra V, Solari S, Allende F, Francisco Miquel J. Bile acids synthesis decreases after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2016;12:763–769. doi: 10.1016/j.soard.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (Lond) 2013;37:1553–1559. doi: 10.1038/ijo.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iguchi Y, Nishimaki-Mogami T, Yamaguchi M, Teraoka F, Kaneko T, Une M. Effects of chemical modification of ursodeoxycholic acid on TGR5 activation. Biol Pharm Bull. 2011;34:1–7. doi: 10.1248/bpb.34.1. [DOI] [PubMed] [Google Scholar]
- 22.Studer E, Zhou X, Zhao R, et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012;55:267–276. doi: 10.1002/hep.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer-Gerspach AC, Steinert RE, Keller S, Malarski A, Schulte FH, Beglinger C. Effects of chenodeoxycholic acid on the secretion of gut peptides and fibroblast growth factors in healthy humans. J Clin Endocrinol Metab. 2013;98:3351–3358. doi: 10.1210/jc.2012-4109. [DOI] [PubMed] [Google Scholar]
- 24.Haluzikova D, Lacinova Z, Kavalkova P, et al. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity. 2013;21:1335–1342. doi: 10.1002/oby.20208. [DOI] [PubMed] [Google Scholar]
- 25.Belgaumkar AP, Vincent RP, Carswell KA, et al. Changes in bile acid profile after laparoscopic sleeve gastrectomy are associated with improvements in metabolic profile and fatty liver disease. Obes Surg. 2016;26:1195–1202. doi: 10.1007/s11695-015-1878-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Yeung DC, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 27.Mraz M, Bartlova M, Lacinova Z, et al. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf) 2009;71:369–375. doi: 10.1111/j.1365-2265.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- 28.Fisher FM, Chui PC, Antonellis PJ, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59:2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hale C, Chen MM, Stanislaus S, et al. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology. 2012;153:69–80. doi: 10.1210/en.2010-1262. [DOI] [PubMed] [Google Scholar]