Abstract
Purpose
This phase I portion of a prospective phase I/II study sought to establish the maximum tolerated dose of image-guided, intensity-modulated radiation therapy (IMRT) or proton therapy (IMPT), both with a simultaneous integrated boost (SIB), for patients with stage II–IIIB non-small cell lung cancer (NSCLC) receiving concurrent chemoradiation therapy.
Methods
Patients had pathologically proven NSCLC, either unresectable stage II–IIIB disease or recurrent disease after surgical resection, who could tolerate concurrent chemoradiation. Radiation doses were selectively escalated to the SIB volume (SIBV; internal gross tumor volume + 5 mm margin), and the dose to the planning target volume (PTV; internal gross target volume + 8 mm margin for CTV + 5 mm) was kept at 60 Gy (CGE) over 30 fractions. Patients were randomized between the IMRT and IMPT groups if slots were available on the treatment machines for both groups. Otherwise, patients were allocated to IMRT or IMPT, whichever had an open treatment slot on the machine without randomization.
Results
Fifteen patients (6 IMRT, 9 IMPT) were enrolled. The highest doses to the SIB were 72 Gy in the IMRT group and 78 Gy (CGE) in the IMPT group. Nine patients (6 IMRT, 3 IMPT) received an SIB dose of 72 Gy (CGE) (BED=89.3 Gy (CGE)) and 6 patients (IMPT) received an SIB dose of 78 Gy (CGE) (BED=98.3 Gy (CGE)). Dose-limiting (grade ≥3) toxicity (esophagitis) developed in 1 of the 9 patients given 72 Gy (CGE) SIB. Grade ≥3 pneumonitis developed in 2 of the 6 patients treated to 78 Gy (CGE) IMPT SIB; one (grade 3) at 3 months after treatment, and the other (grade 5, possibly related to treatment) at 2 months after treatment. Only 1 patient developed a marginal tumor recurrence with a median follow-up of 25 months (range 4.3–47.4 months).
Conclusion
We recommend that an SIB dose of 72 Gy (CGE) be used as the highest SIB dose for the planned randomized phase II study.
INTRODUCTION
Local failure is common after conventionally fractionated, standard-dose chemoradiation for non-small cell lung cancer (NSCLC), because the sensitivity of normal lung prevents the delivery of tumoricidal doses that are high enough to control clonogenic cells. Studies of stereotactic ablative radiation therapy (SABR) have shown a steep radiation dose response in the treatment of early-stage lung cancer, with a biologically effective dose (BED) of >100 Gy needed to control the tumor [1]. However, delivering such high doses to locally advanced lung cancer is difficult, especially when the disease involves the mediastinal lymph nodes, and thus are part of the target volume. Other constraints include the need to minimize the dose to surrounding critical structures such as the esophagus, lung, heart, and brachial plexus.
Previous results from phase II trials [2,3] suggested that high doses (up to 74 Gy) may confer a survival benefit in such patients, and theoretically, higher radiation doses would be expected to result in better control [4]. Thus far, phase III prospective studies of radiation dose escalation have not shown a dose response in terms of overall survival (OS) when radiation is given in conventional fractionation with concurrent chemotherapy for the treatment of locally advanced NSCLC. Findings from the phase III Radiation Therapy Oncology Group (RTOG) trial 0617 [5] indicated that OS was lower among patients who received the high (74 Gy) dose than among those who received the standard 60 Gy, with median OS times of 21.7 months and 27.8 months. Many secondary analyses have been undertaken in attempts to explain the apparent lack of a radiation dose response in this trial. One potential explanation is that the overall treatment time in the 74-Gy group was longer than that in the 60-Gy group; prolonging treatment time is known to be associated with decreased tumor control and survival, probably owing to repopulation of cancer stem cells. In NSCLC, prolonging treatment time by more than 4 or 5 days is associated with inferior OS among patients receiving radiation therapy alone or concurrent chemoradiation therapy [6,7]. The experience with SABR for NSCLC further suggests that the BED in RTOG 0617, even in the high-dose group, was not high enough to produce good tumor control, because the BED for 74 Gy delivered in 2-Gy fractions is 88.8 Gy, which is considerably less than 100 Gy. Therefore, escalating the dose per fraction is an attractive alternative, in that delivering higher physical doses without prolonging the overall treatment time would result in an increased BED to the tumor, which would be expected to increase local tumor control [8,9].
Another way to avoid acute and late complications associated with using large radiation doses per fraction without reducing the total dose (or even giving higher doses to the tumor) is to deliver different doses to the gross tumor volume (GTV) and the clinical target volume (CTV) during the same fraction. Use of intensity-modulation photon-based radiation therapy (IMRT) or intensity- and energy-modulated proton beam therapy (IMPT) techniques allows the delivery of non-uniform radiation doses to specific spatial volumes. The so-called “simultaneous integrated boost” (SIB) technique delivers a higher per-fraction dose and a higher total dose to the GTV while maintaining the per-fraction dose and total dose to the surrounding CTV at a dose level consistent with current practice [10–13]. The CTV is designed to cover areas of microscopic disease and subclinical involvement around the GTV and is generally considered to have a lower tumor burden than the GTV. Thus, the SIB technique has the theoretical advantage of giving higher doses selectively to volumes of high tumor burden (GTV) while maintaining minimum doses to subclinical disease (CTV). The SIB technique has been used successfully to treat cancer at several anatomic sites, including head and neck [11], gynecologic [14], and prostate [15], with acceptable acute and long-term toxicity and usually better tumor control than standard radiation treatment. A planning study that included an SIB to accelerate radiotherapy for stage III NSCLC [16] indicated that the SIB technique permits accelerated radiotherapy for such patients without increasing the expected risks of normal tissue toxicity. By reducing the overall treatment time, the SIB technique may improve local tumor control and survival.
A retrospective study [17] identified 71 patients who are not candidates for surgery or conventional chemoradiation with NSCLC, delivered IMRT+SIB in 15 fractions to ≥ 52.5 Gy. It showed that hypofractionated IMRT+SIB is a viable approach for selective patients with NSCLC, with tolerable toxicity. A Phase II study [18] evaluated the efficacy of synchronous three-dimensional (3D) conformal boost with 60 Gy to the GTV and 45 Gy to the PTV in 25 fractions, which presented favorable outcome with acceptable toxicity. We hypothesized that a higher-than-standard BED of radiation, delivered in fractions larger than 2 Gy (CGE) to the GTV, while maintaining biologically effective normal tissue doses at the currently accepted levels, will increase local control in patients with locally advanced NSCLC.
METHODS
Trial design
We undertook a prospective phase I/II trial designed to address whether dose escalation, without prolonging overall treatment time, would be beneficial in terms of local progression-free survival for patients with stage IIB to IIIB NSCLC scheduled to undergo concurrent chemoradiation therapy as definitive therapy. All patients were to receive image-guided IMRT or IMPT, both with an escalated dose to the SIB planning volume (SIBV; defined as the iGTV with a 5 mm margin). The phase I portion of the study, the subject of this report, was done to establish the maximum tolerated SIBV dose to inform the phase II portion of the study. This dose was defined as the highest dose at which no more than 30% of patients have acute dose-limiting toxicity (defined as grade 3 or higher on the Common Terminology Criteria for Adverse Effects v.4.0) during the 90 days starting from day 1 of radiation treatment in at-risk regions (including but not limited to lung, esophagus, brachial plexus, or heart) that is definitely or probably related to radiation treatment. Cardiotoxicities prospectively evaluated included dysrhythmias as well as pericarditis. Our long-term goal was to use the results for a randomized phase II comparison of SIB IMPT with SIB IMRT.
Three dose levels (1.0, 2.0, and 3.0) were used in the SIBV dose escalation. If the MTD was exceeded at one of the planned dose levels and the maximum of 30 patients planned for the phase I part of the study had not been reached, we would treat cohorts of 3 patients at the level midway between the unacceptable SIBV dose level and the previous acceptable SIBV dose level. As an example, if the rate of dose-limiting toxicity exceeds 30% at 84 Gy (CGE) (SIBV Level 3), then a cohort of 3 patients would be treated at 81 Gy (CGE), which would be the midway between SIBV Level 2 and SIBV Level 3. As another example, if the rate of dose-limiting toxicity exceeded 30% at 78 Gy (CGE) (SIBV Level 2), a cohort of 3 patients will be enrolled at 75 Gy (CGE), which would be midway between SIBV Level 1 and SIBV Level 2. After a 90-day observation period (beginning on day 1 of radiation treatment), the dose per fraction and the total dose to the SIBV were increased if no dose-limiting toxicity was observed.
We used a modified version of the continual reassessment method of O’Quigley et al. (1990) to run the trial for each arm. Patients were randomized to either IMRT + SIBV or IMPT + SIBV, when time slots were available on the treatment machines in both arms. Patients were assigned to the particular treatment when the only available time slot was available in that arm. Once at least 9 patients have been treated at a given SIBV Dose Level and the next patient is assigned to the same SIBV Dose Level, then the study will be stopped for that arm. The MTD for an arm will be that SIBV Dose Level with posterior probability of toxicity closest to the target toxicity probability of 0.30.
As an added measure of safety, the trial would be stopped early for an arm if the lowest SIBV Dose Level for that arm was unacceptably toxic. That is, if there was more than a 90% chance that the probability of DLT at the lowest SIBV Dose Level for an arm was greater than 30%, then we would stop the study for that arm. We would stop at SIBVi dose level 3.0 even if the MTD was not reached. These operating characteristics were based on 1000 simulations of the trial.
Patient eligibility
Inclusion criteria included pathologically proven diagnosis of unresectable stage II–IIIB NSCLC, and recurrent disease after surgical resection. Patients must have been deemed suitable for concurrent chemoradiation therapy, have a Karnofsky Performance Status of ≥70, age >18 years, and have weight loss of <15% during the 3 months before diagnosis. Adequate lung function (indicated by forced expiratory volume in 1 second [FEV1] ≥1 L) and adequate bone marrow function were also required.
Exclusion criteria included prior receipt of radiation to the mediastinum or to any other region that would result in overlap of radiation dose distribution to critical structures (esophagus, heart, spinal cord, or brachial plexus); T4 tumors with direct invasion of the esophagus, spinal cord, major blood vessels, or heart; GTV motion >5 mm on 4D computed tomography (CT) with or without respiratory management using gating or breath-holding; or pregnancy.
The protocol for this trial was approved by the appropriate institutional review board, and all patients provided written informed consent to participate before any treatment was begun.
Treatment
All patients underwent CT-based treatment simulation while supine and immobilized in an upper body cradle with their arms overhead. Four-dimensional simulation was used for all patients to account for respiratory motion. All patients were to undergo image-guided IMRT or IMPT. The internal GTV (iGTV) was contoured such that it encompassed the position of the tumor throughout the respiratory cycle. The SIBV was defined as the iGTV plus a 5 mm margin. The CTV was created by expanding the iGTV by 8 mm, with an additional 5 mm for the planning target volume (PTV).
For all SIBV dose levels, the dose to the PTV was kept constant at 60 Gy (CGE) in 30 fractions. We started the SIB at the lowest dose range level (Table 1). Three patients were to be treated at the first dose range level in the IMRT SIB arm and in the IMPT SIB arm. Normal tissue constraints: lung minus GTV, V20 ≤ 37%, MLD ≤ 20 Gy (CGE); esophagus, max dose 74 Gy (CGE) ≤ 1cc of partial circumference; heart, V30 ≤ 50% V45 ≤ 35%; spinal cord, V50 < 0.03 cc. Plans called for ≥99% of the PTV and the SIBV to be covered by 95% of the prescribed doses; 95% of the prescribed dose to the PTV and SIBV was considered acceptable if 100% could not be reached.
Table 1.
Dose escalation schedule
| Total Dose, Gy(CGE) |
No. of Fractions |
Biologically Effective Dose or BED, Gy(CGE)a |
Biologically Equivalent Total Dose in 2-Gy Fractions [EQD2], Gy(CGE)b |
|
|---|---|---|---|---|
| Dose to planning target volume | 60 | 30 | 72 | 60 |
| Simultaneous dose to SIBVc | ||||
| Level 1 | 66–72 | 30 | 80.5–89.3 | 67.1–74.4 |
| Level 2 | 75–78 | 30 | 93.8–98.3 | 78.2–81.9 |
| Level 3 | 81–84 | 30 | 102.9–107.5 | 85.6–89.6 |
BED = no. of fractions × dose per fraction × [1 + (dose per fraction/[α/β])], where no. of fractions is 30 and α/β is 10
EQD2 = BED / {1 + [2/(α/β)]}
SIBV, simultaneous integrated boost volume = gross tumor volume + 5-mm margin
All patients received standard concurrent chemotherapy as prescribed by the treating medical oncologist.
RESULTS
Seventeen patients provided informed consent to participate; 1 patient withdrew consent and another was denied insurance coverage, for a total of 15 patients treated according to protocol specifications. Patient characteristics are shown in Table 2. Among those 15 patients, 1 patient who was to receive IMPT to 78 Gy (CGE) voluntarily discontinued treatment after having received 22 of 30 planned fractions (GTV 57Gy, PTV 44Gy). The highest SIB dose levels reached were 72 Gy in the IMRT arm and 78 Gy (CGE) in the IMPT arm. Nine patients (6 IMRT, 3 IMPT) received an SIBV dose of 72 Gy (CGE) (BED=89.3 Gy (CGE)) and 6 patients (all IMPT) received an SIBV dose of 78 Gy (CGE) (BED=98.3 Gy (CGE)).
Table 2.
Patient, disease, and treatment characteristics
| Characteristics | Value or No. (%) |
|---|---|
| Number of Patients | |
| SIBV Dose Level 1 (72 Gy or CGE) | 9 |
| SIBV Dose Level 2 (78 Gy or CGE) | 6 |
| Age, years | |
| Median (range) | 67 (49–82) |
| Sex | |
| Male | 7 (47) |
| Female | 8 (53) |
| Disease Stage | |
| IIA | 2 (13) |
| IIB | 1 (7) |
| IIIA | 5 (33) |
| IIIB | 6 (40) |
| Recurrence | 1 (7) |
| Tumor Histology | |
| Adenocarcinoma | 8 (53) |
| Squamous cell carcinoma | 5 (33) |
| NSCLC, NOS | 2 (13) |
| Gross Tumor Volume, cm3 | |
| Median (range) | 72.45 (18–392.8) |
| Planning Target Volume, cm3 | |
| Mean (range) | 511.1 (56.4–1390.3) |
Toxicity
Individual patient survival, mean lung dose, and mean heart dose are shown in Table 3. At a median follow-up of 25 months (range 4.3–47.4 months), the rates of dose-limiting toxicity (grade ≥3) were 11.1% in the SIBV dose level 1 (72 Gy (CGE)) group and 33.3% in the SIBV dose level 2 (78 Gy (CGE)) group (Table 4). Because the 33.3% for dose level 2 exceeded the pre-specified limit of 30%, the maximum tolerated dose (72 Gy (CGE)) was reached before the third SIBV dose level (81–84 Gy (CGE)).
Table 3.
Patient overall survival, treatment dose arm, mean heart dose, mean lung dose
| Patient | Overall Survival (months) |
Treatment Dose Arm |
Mean Heart Dose |
Mean lung dose |
|---|---|---|---|---|
| 1 | 47.4 | IMPT 72 CGE | 2.5 CGE | 0.3 CGE |
| 2 | 38.6 | IMPT 72 CGE | 13.9 CGE | 1.3 CGE |
| 3 | 30.0 | IMPT 72 CGE | 15.2 CGE | 10.1 CGE |
| 4 | 38.3 | IMPT 78 CGE | 17.1 CGE | 7.5 CGE |
| 5 | 31.9 | IMPT 78 CGE | 8.5 CGE | 4.3 CGE |
| 6 | 25.0 | IMPT 78 CGE | 12.7 CGE | 4.0 CGE |
| 7 | 32.2 | IMPT 78 CGE | 9.6 CGE | 2.4 CGE |
| 8 | 4.3 | IMPT 78 CGE | 15.0 CGE | 2.5 CGE |
| 9 | 13.0 | IMPT 78 CGE | 19.0 CGE | 9.8 CGE |
| 10 | 24.1 | IMRT 72 Gy | 15.7 Gy | 10.1 Gy |
| 11 | 25.3 | IMRT 72 Gy | 7.3 Gy | 4.2 Gy |
| 12 | 24.3 | IMRT 72 Gy | 9.8 Gy | 0.7 Gy |
| 13 | 24.7 | IMRT 72 Gy | 4.8 Gy | 0.3 Gy |
| 14 | 9.6 | IMRT 72 Gy | 20.8 Gy | 11.6 Gy |
| 15 | 7.4 | IMRT 72 Gy | 16.1 Gy | 10.1 Gy |
Table 4.
Dose-limiting (grade ≥3) toxicity
| SIBV Dose Level | Toxicity Type | Toxicity Grade | Rates of Severe (Grade ≥3) Treatment-Related Toxicity |
||
|---|---|---|---|---|---|
| 3 | 4 | 5 | |||
| 72 Gy(CGE) (n=9) | Esophagitis | 1 | 0 | 0 | 11.1% (1/9) |
| Pneumonitis | 0 | 0 | 0 | ||
| Cardiotoxity | 0 | 0 | 0 | ||
| Total | 1 | 0 | 0 | ||
| 78 Gy(CGE) (n=6) | Esophagitis | 0 | 0 | 0 | 33.3% (2/6) |
| Pneumonitis | 1 | 0 | 1 | ||
| Cardiotoxicity | 0 | 0 | 0 | ||
| Total | 1 | 0 | 1 | ||
Treatment-related toxicity outcomes are shown in Table 5. Esophagitis developed in a total of 8 patients: 3 patients given SIB IMRT at 72 Gy (one grade 3 and two grade 2); 2 patients given SIB IMPT at 72 Gy (CGE) (one grade 1 and one grade 2); and 3 patients given SIB IMPT at 78 Gy (CGE) (three grade 2). No dysrhythmias or evidence of pericarditis was seen.
Table 5.
Toxicity definitely, probably, or possibly related to treatment
| Toxicity | IMRT (72 Gy) (n=6) |
MPT (72 CGE) (n=3) |
IMPT (78 CGE) (n=6) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
|
| Nausea/vomiting | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Fatigue | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Pneumonitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Esophagitis | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Dermatitis | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anemia | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| Neutropenia | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
| Lymphocytopenia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Dysrhythmia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pericarditis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Values shown are numbers of patients.
Severe pneumonitis (grade ≥3) developed in 2 patients, both of whom had been treated with SIB IMPT 78 Gy (CGE); one patient developed grade 3 pneumonitis at 3 months after completing treatment, and the other a grade 5 pulmonary event at 2 months after completing treatment. The patient who died had a right upper lobe stage III T3N2M0 squamous cell carcinoma and significant comorbidity, including breast cancer that had been treated with combined modality therapy; moderate pulmonary hypertension; atrial fibrillation; and mitral and tricuspid valve prolapses with replacement in 2001. That patient developed acute dyspnea and failure to thrive and was found to have lung infiltration, mainly outside the radiation fields, and a broken mitral valve ring. Dosimetric variables for this patient were total lung volume 2455 cm3, mean lung dose 15 Gy, V5 35%, V10 31%, V20 26%, and V40 18%. This patient’s death was considered to be possibly related to treatment. Two patients had grade 2 radiation-induced dermatitis (one with SIBV IMRT to 72 Gy and the other with SIBV IMPT to 72 Gy (CGE)). Thus, one of nine patients developed grade ≥3 esophagitis at an SIBV dose of 72 Gy (CGE), and two of six patients developed grade ≥3 toxicity at an SIBV dose of 78 Gy (CGE).
Disease outcome
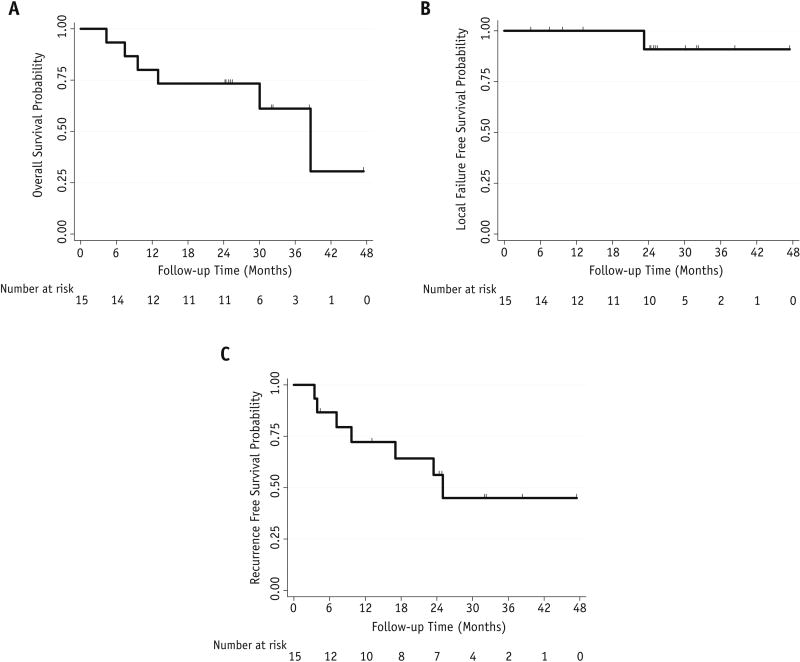
Only one of the 15 patients developed marginal recurrence, which appeared at 23 months after completing the treatment; another patient developed an intrathoracic recurrence, 4 developed distant metastasis (2 brain, 1 liver, 1 in both brain and bone), and 5 had died at the time of this analysis. Cumulative probabilities of local failure, disease-free survival, and OS are shown in Figure 1. The median OS time for all patients was 25.3 months with a Kaplan-Meier estimated median overall survival of 38.6 months.
Figure 1.
DISCUSSION
The prognosis of locally advanced NSCLC remains poor during past decades of years, local failure after standard dose radiotherapy is inadequate, and potentially serve as a source for metastatic. Improved local control should lead to improved overall survival [20]. Theoretically, higher radiation doses is a potential strategy to improve the local control [1,4]; however, the optimal dose and fractionation is unknown. It’s imperative to pursue new strategies to escalate dose to tumor while sparing normal tissue.
Hypofractionated RT regimens have been adopted in some studies. A study reported by Zhu et al. [21] delivered accelerated HypoRT (initially 50Gy/20 fractions, then a fraction dose of 3Gy) to a total dose of 65–68Gy in 34 NSCLC patients with stage III at diagnosis. The 1-, 2-, and 3-year LR-PFS were 69.6%, 60.9% and 60.9%, respectively. The 3-year OS, PFS were 32.1%, 29.8%, respectively. Hyperfractionated accelerated RT is another dose escalation approach. In a multicentric randomized CHARTWEL trial [22], 406 patients were randomized to receive 60Gy/40 fractions/2.5weeks or 66Gy/33 fractions/6.5weeks. The overall survival, local tumor control rates and distant metastases did not significantly differ. But in advanced stages and after chemotherapy there was higher efficacy in the hyperfractionation arm. Another option is to deliver stereotactic body radiation therapy (SBRT) to residual primary disease. A prospective study [23] evaluated the feasibility of conventional chemoradiation followed by SBRT. After a median follow-up of 13 months, the local control rate was 82.9%, only 1 patient (2.9%) developed persistent grade 3 radiation pneumonitis (RP), and one developed grade 4 or 5 RP.
Adaptive dose escalation has also been used in the past. Kong, et al. [29] recently described a phase II clinical trial involved Stage II/III NSCLC patients demonstrating that adaptive radiotherapy-escalated radiation dose to the 18F-fludeoxyglucose (FDG)-avid region detected by midtreatment positron emission tomography (PET) achieved 82% local tumor control at 2 years, with a reasonable rate of radiotherapy-induced toxicity. Conformal RT was individualized to a fixed risk of RILT (grade >2) and adaptively escalated to the residual tumor defined on midtreatment FDG-PET up to a total dose of 86 Gy in 30 daily fractions. This hypothesis is now being tested in a randomized phase 2 multicenter trial (RTOG 1106).
The strategy we’ve adopted in our study is IMRT or IMPT with a simultaneous integrated boost for tumor dose escalation while limiting normal tissue dose and total treatment duration. A dosimetric analysis [24] demonstrated that a target’s median dose escalation of approximately 15 Gy (64.8 –79.2 Gy) via IMRT using SIB was deemed achievable with critical structures not significantly changed. Another study [25] tested the planning feasibility of simultaneous integrated volume-adapted boost which delivered to the shrunken primary tumor with dose escalation from 66 Gy to 82 Gy. The 82-Gy level was achieved with an increased EQD2 and tumor control probability (TCP). However, only 10–20% patients achieved the 82-Gy level, implying a need to modify the strategy before clinical implementation. The efficacy of SIB-IMRT on medically inoperable patients with stage II (T2b-3N0M0) NSCLC has been analyzed in a clinical trial [26]. The 1-, 2-, and 3-year overall survivals (OS) were 93, 85, and 61%, respectively, with a median survival of 46.5 months. 28.6% (8/28) were with Grade 1or 2 radiation pneumonitis (RP), and 7.1% (2/28) with Grade 1 esophagitis, 17.9% (5/28) developed Grade 1 radiation pulmonary fibrosis (RPF). Another phase II study [27] of SIB proton beam therapy (PBT) for locally advanced NSCLC was reported to present the preliminary results. The prescribed doses were 74Gy (CGE) for the primary tumor and 66Gy (CGE) for the lymph nodes. The mean survival time was 26.7 months. Acute pneumonitis was observed in 3(3/15) patients (Grade 1 in one, and Grade 3 in two), but Grade 3 pneumonitis was considered to be non-proton-related. Grade 3 acute esophagitis and dermatitis were observed in 1(1/15) and 2(2/15) patients, respectively.
However, to the best of our knowledge, the optimal strategy of SIB is still unknown. The maximum tolerated dose for SIB dose escalation for the definitive treatment of locally advanced NSCLC had not been clear, even though this technique has been used successfully for tumors at other anatomic sites [28]. Thus, we investigated the maximum tolerated dose for image-guided IMRT or IMPT, both delivered with an escalated dose to the SIBV, for patients with stage II–IIIB NSCLC. Our study showed that a dose to the SIBV of up to 72 Gy (CGE), given at 2.4 Gy (CGE) per fraction with concurrent chemotherapy, was safe for patients with locally advanced NSCLC. In our trial, the maximum tolerated dose (to the SIBV) was met before we reached the third (highest) dose level of 81–84 Gy (CGE), leading us to halt the trial at the SIBV dose level 2 (78 Gy (CGE)). The most common radiation-induced complication was esophagitis. Because IMPT seems to have been advantageous in terms of sparing the esophagus, we recommend using an SIBV that spares the esophagus (which we termed a “simultaneous integrated avoidance” volume), which we will test in the subsequent phase II portion of this study. As we know, most esophagitis is temporary and could be well managed by supportive treatment (including analgesics) to maintain nutrition and isohydria, and no patients needed a treatment break, but esophagitis should be minimized as much as possible to avoid complications like perforation and stenosis.
Among patients who received SIB IMPT 78 Gy (CGE), two experienced lung toxicity, one grade 3 and the other grade 5. As noted in the Results, the patient who died had a complex medical history, and that patient’s death was considered possibly related to the treatment. The inclusion of this patient as having had grade 5 toxicity led us to stop the trial at SIBV level 2 (78 Gy (CGE)). Other episodes of severe (grade ≥3) acute hematologic toxicity (myelosuppression considered strongly related to chemotherapy) were well managed and resolved.
RTOG 0617 [5] set new benchmarks for patients with inoperable stage III NSCLC given chemoradiotherapy, with median OS times 28.7 for the standard-dose group and 20.3 months for the high-dose group. In our study, the median OS time for all patients was 25.3 months with a Kaplan-Meier estimated median overall survival of 38.6 months, and the estimated 1-year OS rate was 82.4%. The local-regional recurrence rate in the current study (1 of 15, or 7%) compares favorably with those in RTOG 0617 (16.3% in the standard-dose and 24.8% in the high-dose groups). Although our results must be considered preliminary, this prospective test of the tolerability of a boost dose of 72 Gy (CGE) to the SIBV with IMRT or IMPT. Our follow-up time is too short as yet to assess late toxicity; this endpoint will be evaluated in the phase II portion of this study.
In conclusion, our results from this prospective phase I trial indicate that an SIBV dose of 72 Gy (CGE) is the maximum tolerated dose to be given with image-guided IMRT or IMPT. We recommend that this dose level be tested in the subsequent randomized phase II portion of this study, in which efficacy, late toxicity, local progression-free survival, and toxicity-free survival are included as endpoints.
Summary.
Local failure is common after conventionally fractionated, standard-dose chemoradiation for NSCLC. Theoretically, higher doses could confer a survival benefit, but this has not been confirmed in a phase III trial using conventional techniques. Dose escalation by new strategies seems promising. Our study is trying to find the optimal dose and fractionation by image-guided IMRT or IMPT with a simultaneous integrated boost. This article presents our preliminary results of a phase I study.
Acknowledgments
Supported in part by Cancer Center Support (Core) Grant CA016672, Program Project grant CA021239 from the National Cancer Institute to The University of Texas MD Anderson Cancer Center, China Hunan Provincial Science & Technology Fund (NO.2013SK2017)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare no conflicts of interest
References
- 1.Onimaru R, Fujino M, Yamazaki K, Onodera Y, Taguchi H, Katoh N, Hommura F, Oizumi S, Nishimura M, Shirato H. Steep dose-response relationship for stage i non-small-cell lung cancer using hypofractionated high-dose irradiation by real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:374–381. doi: 10.1016/j.ijrobp.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 2.Bradley JD, Bae K, Graham MV, Byhardt R, Govindan R, Fowler J, Purdy JA, Michalski JM, Gore E, Choy H. Primary analysis of the phase ii component of a phase i/ii dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: Rtog 0117. J Clin Oncol. 2010;28:2475–2480. doi: 10.1200/JCO.2009.27.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Socinski MA, Blackstock AW, Bogart JA, Wang X, Munley M, Rosenman J, Gu L, Masters GA, Ungaro P, Sleeper A, Green M, Miller AA, Vokes EE. Randomized phase ii trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 gy) in stage iii non-small-cell lung cancer: Calgb 30105. J Clin Oncol. 2008;26:2457–2463. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]
- 4.Machtay M, Bae K, Movsas B, Paulus R, Gore EM, Komaki R, Albain K, Sause WT, Curran WJ. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: An analysis of the radiation therapy oncology group. Int J Radiat Oncol Biol Phys. 2012;82:425–434. doi: 10.1016/j.ijrobp.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A, Kavadi V, Garces YI, Narayan S, Iyengar P, Robinson C, Wynn RB, Koprowski C, Meng J, Beitler J, Gaur R, Curran W, Jr, Choy H. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage iiia or iiib non-small-cell lung cancer (rtog 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox JD, Pajak TF, Asbell S, Russell AH, Pederson J, Byhardt RW, Emami B, Roach M., 3rd Interruptions of high-dose radiation therapy decrease long-term survival of favorable patients with unresectable non-small cell carcinoma of the lung: Analysis of 1244 cases from 3 radiation therapy oncology group (rtog) trials. Int J Radiat Oncol Biol Phys. 1993;27:493–498. doi: 10.1016/0360-3016(93)90371-2. [DOI] [PubMed] [Google Scholar]
- 7.Machtay M, Hsu C, Komaki R, Sause WT, Swann RS, Langer CJ, Byhardt RW, Curran WJ. Effect of overall treatment time on outcomes after concurrent chemoradiation for locally advanced non-small-cell lung carcinoma: Analysis of the radiation therapy oncology group (rtog) experience. Int J Radiat Oncol Biol Phys. 2005;63:667–671. doi: 10.1016/j.ijrobp.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 8.Abratt RP, Bogart JA, Hunter A. Hypofractionated irradiation for non-small cell lung cancer. Lung Cancer. 2002;36:225–233. doi: 10.1016/s0169-5002(02)00020-x. [DOI] [PubMed] [Google Scholar]
- 9.Baumann M, Appold S, Petersen C, Zips D, Herrmann T. Dose and fractionation concepts in the primary radiotherapy of non-small cell lung cancer. Lung Cancer. 2001;33(Suppl 1):S35–45. doi: 10.1016/s0169-5002(01)00301-4. [DOI] [PubMed] [Google Scholar]
- 10.Fiorino C, Dell'Oca I, Pierelli A, Broggi S, Cattaneo GM, Chiara A, De Martin E, Di Muzio N, Fazio F, Calandrino R. Simultaneous integrated boost (sib) for nasopharynx cancer with helical tomotherapy. A planning study. Strahlenther Onkol. 2007;183:497–505. doi: 10.1007/s00066-007-1698-x. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz M, Vuong T, Ballivy O, Parker W, Patrocinio H. Accelerated radiotherapy with simultaneous integrated boost fractionation and intensity-modulated radiotherapy for advanced head and neck cancer. Otolaryngol Head Neck Surg. 2007;136:549–555. doi: 10.1016/j.otohns.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 12.Yu WW, Zhu ZF, Fu XL, Zhao KL, Mao JF, Wu KL, Yang HJ, Fan M, Zhao S, Welsh J. Simultaneous integrated boost intensity-modulated radiotherapy in esophageal carcinoma: Early results of a phase ii study. Strahlenther Onkol. 2014;190:979–986. doi: 10.1007/s00066-014-0636-y. [DOI] [PubMed] [Google Scholar]
- 13.Franchin G, Vaccher E, Talamini R, Politi D, Gobitti C, Minatel E, Lleshi A, Sartor G, Mascarin M, Rumeileh IA, Trovo MG, Barzan L. Intensity-modulated radiotherapy (imrt)/tomotherapy following neoadjuvant chemotherapy in stage iib–iva/b undifferentiated nasopharyngeal carcinomas (ucnt): A mono-institutional experience. Oral Oncol. 2011;47:905–909. doi: 10.1016/j.oraloncology.2011.06.503. [DOI] [PubMed] [Google Scholar]
- 14.Guerrero M, Li XA, Ma L, Linder J, Deyoung C, Erickson B. Simultaneous integrated intensity-modulated radiotherapy boost for locally advanced gynecological cancer: Radiobiological and dosimetric considerations. Int J Radiat Oncol Biol Phys. 2005;62:933–939. doi: 10.1016/j.ijrobp.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 15.Di Muzio N, Fiorino C, Cozzarini C, Alongi F, Broggi S, Mangili P, Guazzoni G, Valdagni R, Calandrino R, Fazio F. Phase i–ii study of hypofractionated simultaneous integrated boost with tomotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:392–398. doi: 10.1016/j.ijrobp.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 16.Dirkx ML, van Sornsen De Koste JR, Senan S. A treatment planning study evaluating a 'simultaneous integrated boost' technique for accelerated radiotherapy of stage iii non-small cell lung cancer. Lung Cancer. 2004;45:57–65. doi: 10.1016/j.lungcan.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Swanick CW, Lin SH, Sutton J, Naik NS, Allen PK, Levy LB, Liao Z, Welsh JW, Komaki R, Chang JY, Gomez DR. Use of simultaneous radiation boost achieves high control rates in patients with non-small-cell lung cancer who are not candidates for surgery or conventional chemoradiation. Clin Lung Cancer. 2015;16:156–163. doi: 10.1016/j.cllc.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Cho KH, Ahn SJ, Pyo HR, Kim KS, Kim YC, Moon SH, Han JY, Kim HT, Koom WS, Lee JS. A phase ii study of synchronous three-dimensional conformal boost to the gross tumor volume for patients with unresectable stage iii non-small-cell lung cancer: Results of korean radiation oncology group 0301 study. Int J Radiat Oncol Biol Phys. 2009;74:1397–1404. doi: 10.1016/j.ijrobp.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 19.O'Quigley J, Pepe M, Fisher L. Continual reassessment method: A practical design for phase 1 clinical trials in cancer. Biometrics. 1990;46:33–48. [PubMed] [Google Scholar]
- 20.Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus R, Yamanaka T, Bozonnat MC, Uitterhoeve A, Wang X, Stewart L, Arriagada R, Burdett S, Pignon JP. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 21.Zhu ZF, Fan M, Wu KL, Zhao KL, Yang HJ, Chen GY, Jiang GL, Wang LJ, Zhao S, Fu XL. A phase ii trial of accelerated hypofractionated three-dimensional conformal radiation therapy in locally advanced non-small cell lung cancer. Radiother Oncol. 2011;98:304–308. doi: 10.1016/j.radonc.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Baumann M, Herrmann T, Koch R, Matthiessen W, Appold S, Wahlers B, Kepka L, Marschke G, Feltl D, Fietkau R, Budach V, Dunst J, Dziadziuszko R, Krause M, Zips D studygroup CH-B. Final results of the randomized phase iii chartwel-trial (aro 97-1) comparing hyperfractionated-accelerated versus conventionally fractionated radiotherapy in non-small cell lung cancer (nsclc) Radiother Oncol. 2011;100:76–85. doi: 10.1016/j.radonc.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Feddock J, Arnold SM, Shelton BJ, Sinha P, Conrad G, Chen L, Rinehart J, McGarry RC. Stereotactic body radiation therapy can be used safely to boost residual disease in locally advanced non-small cell lung cancer: A prospective study. Int J Radiat Oncol Biol Phys. 2013;85:1325–1331. doi: 10.1016/j.ijrobp.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Turner LM, Howard JA, Dehghanpour P, Barrett RD, Rebueno N, Palmer M, Vedam S, Klopp A, Komaki R, Welsh JW. Exploring the feasibility of dose escalation positron emission tomography-positive disease with intensity-modulated radiation therapy and the effects on normal tissue structures for thoracic malignancies. Med Dosim. 2011;36:383–388. doi: 10.1016/j.meddos.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Weiss E, Fatyga M, Wu Y, Dogan N, Balik S, Sleeman Wt, Hugo G. Dose escalation for locally advanced lung cancer using adaptive radiation therapy with simultaneous integrated volume-adapted boost. Int J Radiat Oncol Biol Phys. 2013;86:414–419. doi: 10.1016/j.ijrobp.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Liu C, Lin H, Song Y, Huang W, Li H, Li M, Li B. Prospective study of special stage ii (t2b-3n0m0) non-small-cell lung cancer treated with hypofractionated-simultaneous integrated boost-intensity modulated radiation therapy. J Cancer Res Ther. 2015;11:381–387. doi: 10.4103/0973-1482.157332. [DOI] [PubMed] [Google Scholar]
- 27.Oshiro Y, Okumura T, Kurishima K, Homma S, Mizumoto M, Ishikawa H, Onizuka M, Sakai M, Goto Y, Hizawa N, Sato Y, Sakurai H. High-dose concurrent chemo-proton therapy for stage iii nsclc: Preliminary results of a phase ii study. J Radiat Res. 2014;55:959–965. doi: 10.1093/jrr/rru034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welsh J, Palmer MB, Ajani JA, Liao Z, Swisher SG, Hofstetter WL, Allen PK, Settle SH, Gomez D, Likhacheva A, Cox JD, Komaki R. Esophageal cancer dose escalation using a simultaneous integrated boost technique. Int J Radiat Oncol Biol Phys. 2012;82:468–474. doi: 10.1016/j.ijrobp.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong FM, Haken R, Schipper M, Frey K, Hayman J, Gross M, Ramnath N, Hassan K, Matuszak M, Ritter T, Bi N, Wang W, Orringer M, Cease K, Lawrence T, Kalemkerian G. Jama Oncol. 2017 doi: 10.1001/jamaoncol.2017.0982. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]