Abstract
Objective
Hematopoietic stem cell transplantation (HSCT) is the only treatment known to slow or halt inflammatory demyelination among boys with the cerebral form of X‐linked adrenoleukodystrophy (cALD), a devastating childhood condition affecting the central nervous system. HSCT can lead to a range of adverse outcomes including fatality. Previous studies have examined the potential predictors of post‐HSCT survival and neurologic functioning. However, little is known about patients' daily‐life adaptive functional outcomes (i.e., ability to communicate, maintain social relationships, and independently execute tasks of daily living). The purpose of this retrospective cohort study was to identify which patient characteristics and treatment‐related variables predict long‐term adaptive function among the survivors of HSCT for cALD.
Methods
We obtained caregiver ratings of adaptive functioning of 65 transplant survivors at an average of 4.6 years (range: 1.0–24.1 years) post‐HSCT. Using linear regression with penalized maximum likelihood estimation, we modeled the relative contribution of pre‐transplant neurocognitive test performance, MRI severity, transplant regimen, and length of time since transplant on patient adaptive functioning outcomes.
Results
Higher radiographic disease severity and poorer performance on baseline neurocognitive tests requiring fine motor skills and visual perception were associated with inferior adaptive functioning after HSCT. Use of radiation during the transplant preparative regimen also predicted poorer adaptive outcomes.
Interpretation
In addition to radiological disease severity, baseline neurocognitive test performance is associated with post‐transplant adaptive functional outcomes. Neurocognitive measures may play an important role in prognostic counseling and post‐transplant treatment planning for patients considering HSCT for cALD.
Introduction
Adrenoleukodystrophy (ALD) is an X‐linked disorder of very long‐chain fatty acid metabolism affecting 1 in 21,000 males.1 One‐third of boys with ALD will develop cerebral disease in childhood (cALD), with initial onset of symptoms around 3–10 years of age.2 The childhood cerebral phenotype is characterized by white matter signal changes on brain magnetic resonance imaging (MRI), and progressive inflammatory demyelination manifesting with problems in vision, auditory processing, motor function and cognition. If untreated, cALD is nearly always fatal within several years of initial symptoms.3
In the early 1990s, allogeneic hematopoietic stem cell transplantation (HSCT) emerged as an effective therapy for cALD.4, 5 Although HSCT does not restore brain myelination or lost cognitive function, arrest in further demyelination after transplant is usually observed. Patients transplanted in the early stages of cerebral disease (also termed “standard risk” cALD and characterized by an MRI severity score <10 on the Loes scale6) typically have more favorable survival and neurological outcomes, although selective neurocognitive deficits are observed in some patients.4, 7, 8, 9 Patients treated at a more advanced stage of demyelination (“higher risk”) frequently experience serious, irreversible neurological complications after transplantation that may include seizures, blindness, speech problems, loss of continence, and restricted gross motor function and mobility.10
Most published studies reporting the effects of HSCT have focused on outcomes such as engraftment, survival, neurological function, or neurocognitive ability.3 Although these metrics represent essential benchmarks for evaluating the success of intervention for cALD, recent publications have commented on the failure of these endpoints to fully characterize the experience of transplant survivors in terms of their quality of life or ability to actively and fully participate in their world (i.e., to independently handle life demands).11, 12 A more comprehensive understanding of the range of possible adaptive functional outcomes is needed to inform counseling for patients and families undergoing evaluation at transplant centers. Furthermore, although a robust association between pre‐HSCT MRI severity score and post‐HSCT endpoints has been observed across studies, little information is available as to whether other variables, such as pre‐HSCT neurocognitive function or treatment regimen, may predict a transplant survivor's capacity for independent functioning in the years following HSCT. Such information can play a vital role when weighing the risks and benefits of HSCT and conveying concrete prognostic information to families. To address these gaps, we characterized adaptive functioning outcomes among a large cohort of boys who survived HSCT for cALD, and modeled the factors that were most associated with these outcomes.
Methods
Participants
Records from the prospectively maintained program database indicated that 137 boys with cALD between ages 4 and 16 years underwent allogeneic HSCT at the University of Minnesota Blood and Marrow Transplant Center prior to April 2016. Following transplantation, 31 boys (23%) in this cohort died within the first year. Among the 106 surviving patients, a valid parent/caregiver adaptive function rating from ≥1 year post‐HSCT was available for 65 patients (61% of the surviving boys). These ratings were obtained either as part of routine neuropsychological follow‐up at our institution (n = 56) or as part of a clinical research study on HSCT outcomes (n = 9). Written informed consent to participate in this clinical outcomes research was obtained from parents/guardians. Adaptive outcomes for each patient were determined from the most recent assessment available. Outcomes data were collected from evaluations that occurred between August 1994 and April 2017.
Pre‐transplant MRI
Boys in the study cohort underwent brain MRI at an average of 1.1 months (SD, 1.3 months) prior to HSCT. Each baseline study was reviewed by a board‐certified neuroradiologist (D.N.) and characterized according to the extent of white matter signal abnormality and atrophy using a 0–34 point severity scale developed by Loes et al.6 Higher scores denote more extensive cALD, with a pre‐HSCT threshold of 10 or more points indicating “higher risk” disease status.8
Pre‐transplant neurocognitive evaluation
Following institutional protocol, patients underwent comprehensive neurocognitive evaluation at an average of 2.0 months (SD, 1.4 months) prior to transplant. Scores in six neurocognitive domains were widely available across the pre‐HSCT assessments. Four domains (verbal comprehension, perceptual reasoning, working memory, and processing speed) were assessed using the age‐appropriate version of the Wechsler Intelligence Scales.13, 14, 15, 16, 17 Using a method previously described,9 prorating procedures were used to standardize these four constructs across multiple versions of the Wechsler Scales. A fifth domain, fine motor dexterity, was evaluated using the average of scores obtained from three trials (dominant hand, non‐dominant hand and both hands) of the Purdue Pegboard Test.18 This task requires the examinee to grasp metal pegs and place them into a series of small holes as rapidly as possible. The Beery‐Buktenica Developmental Test of Visual‐Motor Integration (VMI), a task requiring the examinee to copy a series of increasingly complex geometric shapes with paper and pencil, was used to evaluate visual perception and visual‐motor function.19
Hematopoietic stem cell transplantation
All boys were treated on IRB‐approved HSCT protocols. Patients underwent preparative regimens comprising chemotherapy with or without irradiation prior to blood stem cell graft infusion. These regimens varied based upon institutional standards at the time of HSCT. Nine patients (14%) in the study cohort required second transplantation due to failed engraftment with first HSCT. Eight boys (12%) were only exposed to reduced intensity preparative regimens, while 57 (88%) had exposure to at least one myeloablative regimen. The majority of patients undergoing myeloablative regimens were conditioned with a busulfan‐based strategy. In an earlier transplant era, high‐dose total body irradiation (TBI) regimens were used in 14 (22%) patients. Regarding blood stem cell source, nearly all patients (97%) received either bone marrow or umbilical cord blood (UCB); 2 (3%) patients received blood stem cell grafts harvested via peripheral blood leukocytopheresis (PBSC). Patients with more advanced cALD tended to be more likely to receive UCB, a graft source more readily available than bone marrow or PBSC from volunteer donor registries.
Adaptive function outcome measure
The Vineland Adaptive Behavior Scales (VABS)20 is a widely used, norm‐referenced parent/caregiver report measure that evaluates an individual's degree of competence and independence in daily life within three domains: Communication, Daily Living Skills, and Socialization. A fourth domain, Motor Skills, was available for patients who were <7 years old when the VABS was administered (n = 3). These domain scores are combined to form an overall Adaptive Behavior Composite (VABS‐ABC) score. Domain and composite scores have a mean of 100 and a standard deviation of 15. There is empirical evidence of high reliability and validity of this instrument in the general population as well as among individuals with significant cognitive impairment.21 The VABS was first published in 1984; a second edition containing a normative update and revisions to scale items was published in 2005.22 Eleven patients (17%) in this study were assessed using the first edition. Fifty‐five patients (83%) were rated using the second edition, which can be administered as an interview or in a questionnaire format.
For descriptive purposes, the degree of impairment on the VABS was defined as follows: scores above or within 1 SD of the population mean (not impaired), between −1 SD and −2 SD (below average), and less than or equal to −2 SD (severely impaired).
Statistical analysis
Baseline MRI severity scores and neurocognitive evaluation results for all patients who received HSCT at the University of Minnesota (including those who died or were lost to follow‐up) are presented in Table 1. Patients who died within one year of HSCT presented with the highest MRI severity and lowest performance on neuropsychological testing at the pre‐HSCT evaluation. Among the patients who survived but did not participate in adaptive function evaluation at ≥1 year post‐HSCT, greater effects of cALD disease were evident on baseline evaluation than among the survivors who received follow‐up neurocognitive evaluations.
Table 1.
Baseline MRI severity and neurocognitive test scores for all patients with cALD (n = 137) who underwent HSCT at University of Minnesota prior to April 2016
| Patients who were deceased by 1 year post‐HSCT (n = 31) | HSCT survivors lost to follow‐up (n = 41) | HSCT survivors with 1+ year follow‐up (n = 65) | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| MRI Severity Score (0–34) | 11.88 (4.47) | 10.26 (4.65) | 7.52 (5.02) |
| Neurocognitive measures a | |||
| Wechsler scales | |||
| Verbal comprehension | 88.85 (17.82) | 89.63 (16.17) | 94.98 (14.01) |
| Perceptual reasoning | 72.88 (26.57) | 80.78 (18.03) | 91.65 (18.42) |
| Working memory | 82.04 (16.40) | 86.87 (11.43) | 88.53 (13.82) |
| Processing speed | 65.08 (16.33) | 72.71 (18.53) | 83.48 (19.33) |
| Purdue pegboard | |||
| Fine motor dexterity | 61.98 (25.02) | 66.10 (26.38) | 75.76 (24.54) |
| Beery‐Buktenica VMI | |||
| Visual‐motor integration | 78.65 (16.87) | 80.97 (17.12) | 85.45 (15.38) |
Neurocognitive measures are represented in Standard Scores, with normative population mean of 100 and SD of 15.
For the 65 surviving patients who comprised the study cohort, we examined pre‐HSCT neurocognitive measures, transplant characteristics, and adaptive behavior outcomes relative to baseline MRI severity score (standard vs. higher risk) and in aggregate (Table 2). A linear regression model to predict long‐term functioning as measured by the VABS‐ABC score was performed using R version 3.0.2.23 Eleven pre‐specified independent variables were considered and retained in the final model. One additional variable, VABS version (I or II), was not expected to be predictive, but we included it in a second model to verify that the two versions had similar mean scores, which was confirmed (partial R 2 = 0.01). Five of the pre‐transplant neurocognitive tests had some missing values (3–15%) which were assumed to be missing at random. These were imputed using predictive mean matching.24 The primary analysis was performed using multiple imputation with the R rms25 and Hmisc26 packages. Final estimates were pooled across five imputed data sets and standard errors adjusted for imputation.
Table 2.
Study cohort characteristics
| Full cohort (n = 65) | MRI severity <10 “standard risk” (n = 42) | MRI severity ≥ 10 “higher risk” (n = 23) | |
|---|---|---|---|
| Pre‐HSCT baseline variables | |||
| Mean (SD) | Mean (SD) | Mean (SD) | |
| MRI Severity Score (0–34) | 7.52 (5.02) | 4.36 (2.68) | 13.28 (2.44) |
| Neurocognitive measures a | |||
| Wechsler scales | |||
| Verbal comprehension | 94.98 (14.01) | 97.57 (14.04) | 89.81 (12.74) |
| Perceptual reasoning | 91.65 (18.42) | 98.93 (16.45) | 78.35 (14.02) |
| Working memory | 88.53 (13.82) | 91.27 (14.75) | 84.41 (11.39) |
| Processing speed | 83.48 (19.33) | 91.97 (17.11) | 67.71 (11.99) |
| Purdue pegboard | |||
| Fine motor dexterity | 75.76 (24.54) | 88.24 (16.83) | 53.08 (19.69) |
| Beery‐Buktenica VMI | |||
| Visual‐motor integration | 85.45 (15.38) | 90.23 (13.83) | 75.90 (14.07) |
| Treatment variables | |||
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age at transplant, years | 8.51 (2.51) | 7.96 (2.63) | 9.50 (1.95) |
| N (%) | N (%) | N (%) | |
| Graft source | |||
| Bone marrow | 37 (57%) | 26 (62%) | 11 (48%) |
| Umbilical cord blood | 27 (42%) | 15 (36%) | 12 (52%) |
| Peripheral blood | 1 (2%) | 1 (2%) | – |
| Total body irradiation | 14 (22%) | 9 (21%) | 5 (22%) |
| Post‐HSCT adaptive behavior assessment | |||
| N (%) | N (%) | N (%) | |
| VABS version I | 11 (17%) | 10 (24%) | 1 (4%) |
| VABS version II | 54 (83%) | 32 (76%) | 22 (96%) |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Years since transplant | 4.55 (4.44) | 4.59 (3.91) | 4.46 (5.36) |
| VABS sub‐domain scoresa | |||
| Communication | 73.83 (24.85) | 80.19 (23.19) | 62.22 (24.00) |
| Daily living skills | 76.30 (28.92) | 87.90 (26.29) | 55.61 (20.98) |
| Socialization | 77.85 (24.61) | 84.76 (22.26) | 65.22 (24.10) |
| VABS totala | |||
| Adaptive behavior composite | 74.59 (25.61) | 83.00 (23.54) | 59.22 (22.20) |
Neurocognitive and adaptive behavior measures are represented in Standard Scores, with normative population mean of 100 and SD of 15.
A linear regression model was fit for 11 predictors. Pre‐transplant MRI severity and years from HSCT to VABS assessment were allowed to have non‐linear effects, using three‐knot restricted cubic splines. However, the non‐linear effects were negligible. Because the number of variables was high relative to the number of observations, penalized maximum likelihood estimation was used to reduce overfitting. This algorithm shrinks (penalizes) large coefficients toward zero, resulting in estimates that are more likely to be close to the true parameter values.27 Coefficient estimates and predicted values for individual patients are presented based on this pre‐specified, penalized model.
Results
Adaptive outcomes
Caregiver ratings of long‐term adaptive function were obtained at an average of 4.5 years (SD 4.4, range: 1.0–24.1 years) after transplant. Mean chronological age at this follow‐up was 13.1 years (SD 5.0). Among this cohort of HSCT survivors, 22 patients (34%) were rated as having no impairment in adaptive functioning based on the VABS‐ABC, 16 patients (25%) scored below average, and 27 patients (42%) were severely impaired.
Length of time of the VABS rating since transplant was similar across the standard risk and higher risk cALD subgroups (Table 2). Consistent with previous research,9 patients with standard risk cALD performed better on neurocognitive testing at the pre‐HSCT evaluation than higher risk patients. Individuals who had standard risk status before HSCT also demonstrated less impaired overall adaptive functioning in the years after transplant. When comparing across domains, patients in the higher risk group showed more impaired long‐term functioning in the Daily Living Skills domain, which reflects ability to complete tasks to care for self and others in the home and community settings, relative to Communication and Socialization domains. In contrast, the Daily Living Skills domain was relatively strong in the post‐HSCT period among those who underwent HCST with standard risk cALD.
In order to aid in prognostic counseling when considering HSCT for patients with higher risk cALD, outcomes with regard to post‐transplant function for several benchmark life activities are presented in Table 3. Outcomes for this subgroup were obtained at an average age of 14.0 years (SD, 5.6; range: 9.4–35.9 years). Approximately one‐third of patients with higher risk status who survived transplant had difficulty with basic communication (comprehending simple instructions and expressing themselves verbally), and almost two‐thirds were unable to complete basic reading or writing tasks. About half of these boys and young men had significant mobility limitations (i.e., were unable to walk unassisted), and many needed assistance for activities such as toileting and bathing/showering. The majority of survivors of HSCT with higher risk cALD were able to show affection toward others, but most individuals did not consistently engage in social activities such as regularly meeting with friends, making telephone calls, and initiating conversations about shared interests.
Table 3.
Patients with cALD in the “higher risk” category (pre‐transplant MRI severity score ≥10) who were able to independently complete each activity of daily living on a consistent basis at most recent follow‐up (n = 23; mean age 14.0 years, SD, 5.6 years)
| Activity | n (%) |
|---|---|
| Communication/literacy | |
| Tells about experiences in simple sentences | 16 (70) |
| Follows simple instructions (e.g., “bring me the book,” “close the door”) | 14 (61) |
| Reads at least 10 words | 9 (39) |
| Prints or writes own first and last name | 8 (35) |
| Daily living skills | |
| Uses toilet independently | 15 (65) |
| Drinks from cup or glass without spilling | 14 (61) |
| Walks without wheelchair or assistance | 11 (48) |
| Bathes/showers without assistance | 8 (35) |
| Makes telephone calls to others | 6 (26) |
| Socialization | |
| Shows affection toward familiar people | 19 (83) |
| Meets with friends regularly | 5 (22) |
| Initiates conversation on topics of interest to others | 2 (9) |
Predictors of adaptive function
Radiological, neuropsychological, and treatment‐related variables all demonstrated a reliable association with adaptive function outcomes of transplant survivors (Table 4). Performance on the Purdue Pegboard Test at pre‐transplant evaluation predicted the largest amount of variance in functional outcomes. Based on the penalized model, a decrease of four‐points on this measure of fine motor dexterity prior to transplant predicted a one‐point loss in overall adaptive function (VABS‐ABC) after transplant, keeping other variables constant. MRI severity scores were a similarly robust predictor of VABS outcomes. Each 1‐unit increase in pre‐transplant MRI severity score estimated a decline of one‐point on the VABS‐ABC. The combined influence of these two predictors on VABS outcomes is depicted in Figure 1. Notably, patients with MRI severity scores >11 or Purdue Pegboard Scores <70 invariably had below average or severely impaired long‐term adaptive function outcomes.
Table 4.
Linear regression model predicting post‐transplant parent/caregiver ratings on the Vineland Adaptive Behavior Composite among 65 surviving patients of HSCT for cALD
| Variable | Coefficient | Lower 95% CL | Upper 95% CL |
|---|---|---|---|
| Purdue pegboard test | 0.25 | 0.05 | 0.44 |
| Pre‐transplant MRI severity score | −1.00 | −1.95 | −0.06 |
| Total body irradiation (0 = no; 1 = yes) | −9.41 | −18.67 | −0.15 |
| Graft source (0 = marrow; 1 = UCB) | 6.25 | −1.70 | 14.20 |
| Perceptual reasoning index (Wechsler) | 0.18 | −0.09 | 0.44 |
| Beery VMI | 0.18 | −0.14 | 0.49 |
| Age at transplant (years) | −0.52 | −2.15 | 1.12 |
| Working memory (Wechsler) | −0.10 | −0.41 | 0.22 |
| Processing speed (Wechsler) | 0.03 | −0.22 | 0.29 |
| Years since transplant | 0.02 | −0.93 | 0.97 |
| Verbal comprehension index (Wechsler) | 0.02 | −0.28 | 0.32 |
Coefficients represent the expected absolute change in long‐term Vineland composite score associated with a one unit increase in the given variable. Variables are sorted by decreasing partial R 2, that is, the proportion of outcome variance explained by each variable, accounting for all other variables in the model. The intercept of this model is 40, with the reference values being 0 for all continuous variables and no TBI or UCB. Adjusted R 2 is 0.48, meaning this set of variables explains approximately half the outcome variance. CL, confidence limit.
Figure 1.
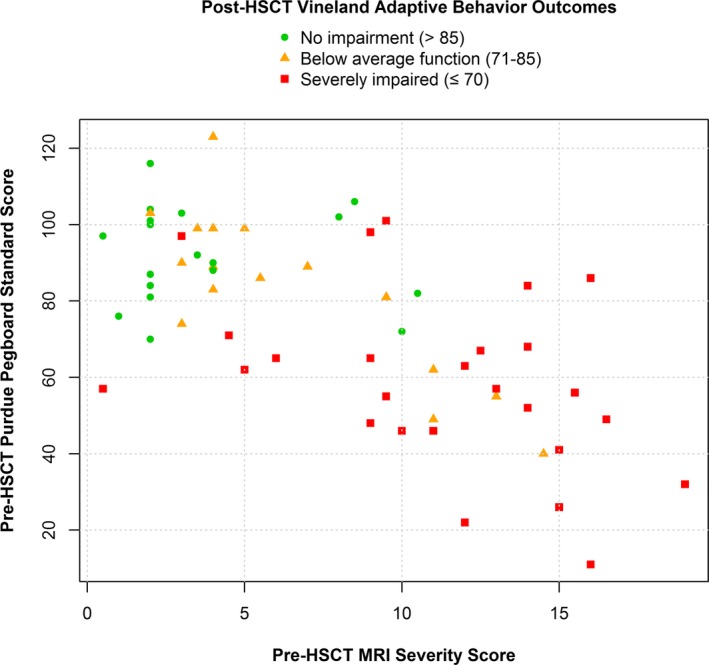
Adaptive behavior outcomes in survivors of hematopoietic stem cell transplantation for cALD, plotted according to pre‐transplant MRI severity and pre‐transplant evaluation of fine motor speed and dexterity.
Additional variables predicted small amounts of variability within the model. The binary treatment‐related variables had a reliable association with outcomes, with use of TBI predicting a 9.41 point drop in VABS‐ABC scores and umbilical cord blood being associated with a 6.25 point increase in VABS outcomes, holding other variables constant. Other pre‐HSCT neurocognitive measures, including tasks of visual reasoning and visual‐motor integration (VMI) predicted small amounts of variability in the functional outcomes of surviving patients.
Table 5 presents predicted adaptive functioning scores based on several pre‐specified hypothetical combinations of the two strongest predictors, MRI severity score and Purdue Pegboard Test scores.
Table 5.
Predicted adaptive functioning outcomes for a set of hypothetical patients with a pre‐selected series of pre‐transplant MRI severity and Purdue Pegboard Test scores
| Pre‐HSCT MRI severity score | Pre‐HSCT purdue pegboard test score | Predicted Post‐HSCT vineland score | Lower 80% PL | Upper 80% PL |
|---|---|---|---|---|
| 2 | 100 | 87 | 62 | 112 |
| 2 | 70 | 80 | 55 | 104 |
| 2 | 55 | 76a | 51 | 101 |
| 5 | 100 | 84 | 59 | 108 |
| 5 | 70 | 76 | 52 | 101 |
| 5 | 55 | 73a | 48 | 98 |
| 10 | 100 | 78 | 54 | 103 |
| 10 | 70 | 71 | 46 | 96 |
| 10 | 55 | 67 | 43 | 92 |
| 15 | 100 | 73a | 47 | 98 |
| 15 | 70 | 65 | 40 | 90 |
| 15 | 55 | 62 | 36 | 87 |
The table shows model‐estimated predicted values for a pre‐selected series of scores. Other variables were set to their median value, and no TBI or UCB. PL, prediction limit.
These combinations of MRI severity and purdue pegboard test scores were not common; therefore model fit is unknown for these predicted outcomes.
Discussion
A magnitude of evidence points to the success of HSCT as a life‐saving procedure that can prevent rapid cognitive deterioration among boys with cALD in the early stages of cerebral disease. By comparison, clinical decision‐making and prognostic counseling for boys in more advanced stages of disease remains a greater challenge, as the relative benefits and risks of transplantation can be less clear. This study identifies potential parameters that can be used to predict long‐term functional outcomes among transplant survivors. Previous studies found that post‐transplant neurological and neurocognitive outcomes in boys with cALD were associated with key variables evaluated in the pre‐transplant assessment. In particular, the association between MRI severity with disease progression and neurological/neurocognitive outcomes is well‐established.10, 28, 29 In addition, previous studies reported that boys scoring <80 on the Wechsler Performance IQ scale (a subscale available on older versions of this measure, which assessed visual reasoning and processing speed) at the time of HSCT were at greater risk for worsening neurological status after transplant.7, 8 These reports underscored the importance of initiating intervention as early as possible upon detection of active cerebral disease.
This study builds on previous research by examining the relative influence of several different neurocognitive tests, as well as MRI severity and treatment variables, in terms of predicting long‐term adaptive functional outcomes. Relative to earlier studies, the current analysis found that subdomains of the Wechsler scales predicted only a small amount of variance in long‐term functional (VABS‐ABC) outcomes when controlling for other neurocognitive and treatment variables. In our model, the strongest predictors of post‐HSCT adaptive functioning were a boy's MRI severity score prior to transplant and his performance on a test of fine motor dexterity (Purdue Pegboard Test). These two predictors had sizeable independent associations with likelihood of impaired long‐term adaptive functioning (Fig 1). The Purdue Pegboard Test requires a combination of visual acuity, rapid processing, and fine motor accuracy. Thus, individuals who are experiencing progressive cortical vision loss, slowing of cognitive functioning, and/or significant motor impairment would be expected to score poorly on this test. Given that these capacities are susceptible to cALD disease progression, this test may therefore be a sensitive marker of neural integrity in untreated boys with cALD. Additional neuropsychological measures with visual or motor components, including visual‐spatial reasoning tests from the Wechsler scales and visual‐motor integration (VMI) abilities, also predicted small amounts of variance in the model. Thus, our results highlight the potentially important predictive role of baseline neurocognitive testing to help guide transplant centers in estimating likely adaptive functioning outcomes for patients with cALD.
Consistent with studies showing an association between the use of radiation within the conditioning regimen (a procedure which is not commonly used in modern transplant protocols for nonmalignant disease indications), and neurocognitive outcome,9, 30, 31 our study found that the use of TBI also predicted greater impairments in adaptive functioning after transplant. A small association between UCB grafts and higher VABS‐ABC scores was observed. The use of cryopreserved UCB, an immediately‐available graft source, has grown in modern HSCT. Thus, this association may reflect the effects of more current and improved transplant care and regimens, as well as more rapid time to transplantation among individuals receiving UCB grafts.
Research investigating “real‐world” functioning and life quality among transplant survivors with cALD has been notably lacking.11, 12 Measures of adaptive functioning, such as the VABS, quantify key attributes of how a person interacts with their world and have demonstrated associations with life satisfaction in a variety of clinical populations.32, 33 Adaptive function is also an important correlate of academic achievement and employment/income independence.34, 35 Given this tangible significance for patients and families, adaptive functioning is of great importance to medical teams and caregivers facing difficult treatment‐related decisions. These considerations are often especially difficult for boys in the “higher risk” cALD category, as these patients are over‐represented among the patients who die of HSCT complications and those who are unlikely to participate in follow‐up evaluation (Table 1). Our data indicate that boys with higher risk cALD who do survive HSCT are at great risk for severe, permanent impairments that will impact quality of life. Analysis of the VABS outcomes for the 23 boys with higher risk cALD indicates that at an average age of 14 years (and, for most patients, several years after HSCT), over half of transplant survivors were unable to ambulate independently, read 10 words, write their name, make phone calls, or bathe without assistance. Few were meeting with friends regularly or initiating reciprocal conversations, thus limiting their capacity for meaningful relationships beyond family members. Finally, even for patients with standard risk disease, our model predicts greater long‐term functional impairment with increasing radiographic disease and declining neuromotor ability (Table 5).
Limitations of this study include the lack of data pertaining to the perspectives of the patients themselves. We employed a well‐validated parent/caregiver rating scale to assess function, since self‐report measures can have limited feasibility and validity when assessing patients with significant cognitive and sensory impairments. Sensory impairments will also impact scores on caregiver report measures of daily function, as adapting to loss of vision or auditory processing abilities takes time (i.e., a patient may eventually learn new modes of adaptation such as braille or sign, but this process can take many years). Furthermore, although several predictors were reliably associated with long‐term functional outcomes, about half of the variance in these outcomes remains unexplained by this model. Due to the wide interval of years during which our data were collected (1994–2017), a variety of advances in medical treatment for patients undergoing HSCT (e.g., better antimicrobials; better donor options; improved prevention, recognition and treatment of graft‐versus‐host disease; increased ability to precisely deliver chemotherapy with individual‐patient pharmacokinetic monitoring and dose alteration) may have contributed to variation in outcomes among patients in our cohort. Finally, even with improved ability to predict long‐term outcomes, the acceptability of various levels of adaptive impairment to different families is likely to be wide‐ranging.
Notably, the length of time between HSCT and the post‐HSCT assessment was not predictive of outcomes when accounting for the baseline assessments and treatment‐related variables. This finding suggests that although some patients' abilities may improve over time as they explore new modes of adaptation, other patients may experience a worsening of function relative to the general population. Rather than reflecting a true “loss” of skills, this worsening of functioning may indicate a failure to gain new skills at a similar rate as peers. Generally, our data suggest that the most dramatic changes in adaptive skills may be occurring prior to transplantation or within the first year after HSCT, and are highly related to the severity of cALD disease, as measured based on both observable white matter changes (MRI) and functional changes (neurocognitive testing). Given that we were only able to measure adaptive outcomes among the engrafted survivors of HSCT, combined with established literature showing better survival rates for patients with less advanced disease, our estimates of the impact of these variables may underestimate the influence of the pre‐HSCT variables on patient adaptive function. Indeed, data from our baseline evaluations confirm that the patients who died were more likely to have presented for treatment with more advanced cALD. Scores on the relevant neurocognitive scales also tended to reflect worse pre‐HSCT functioning in surviving patients for whom follow‐up adaptive outcomes were unavailable.
Conclusion
As a previous analysis has associated superior longitudinal cognition with transplantation that is performed in the very early stages of cALD,9 so does this study predict incrementally better long‐term adaptive functioning with treatment in an earlier disease state. Recently, ALD was added to the recommended uniform screening panel for public newborn screening by the US Secretary of Health and Human Services, and a growing number of states are participating. This study's findings reinforce the importance of close monitoring of all boys with identified ALD, with urgent and expeditious HSCT upon detection of cALD. For regions of the world where newborn screening is not yet available, boys will continue to present for evaluation in later stages of disease. The current data have implications for practitioners seeking to estimate risk for functional impairment after transplant. They indicate that in addition to MRI severity, neurocognitive tests involving fine motor function, visual perception, and/or visual reasoning may aid in prognostic counseling regarding daily life functioning after transplant.
Author Contributions
E.I.P., E.M., and W.M. conceptualized the study and developed the study design with R.S. All authors participated in the acquisition and analysis of data. The initial draft of the manuscript and figures were completed by E.I.P., R.S. and W.M., and was critically reviewed and edited by all authors.
Conflicts of Interest
Nothing to report.
Acknowledgments
The authors are grateful to the patients and their families who participated in this research. Research reported in this publication was supported by NIH grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Statement
This work was funded by NIH grants P30 CA77598 and UL1TR000114.
References
- 1. Bezman L, Moser AB, Raymond GV, et al. Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann Neurol 2001;49:512–517. [PubMed] [Google Scholar]
- 2. Moser HW, Loes DJ, Melhem ER, et al. X‐Linked adrenoleukodystrophy: overview and prognosis as a function of age and brain magnetic resonance imaging abnormality. A study involving 372 patients. Neuropediatrics. 2000;31:227–239. [DOI] [PubMed] [Google Scholar]
- 3. Moser HW, Mahmood A, Raymond GV. X‐linked adrenoleukodystrophy. Nat Clin Pract Neurol 2007;3:140–151. [DOI] [PubMed] [Google Scholar]
- 4. Shapiro E, Krivit W, Lockman L, et al. Long‐term effect of bone‐marrow transplantation for childhood‐onset cerebral X‐linked adrenoleukodystrophy. Lancet 2000;356:713–718. [DOI] [PubMed] [Google Scholar]
- 5. Aubourg P, Blanche S, Jambaqué I, et al. Reversal of early neurologic and neuroradiologic manifestations of X‐linked adrenoleukodystrophy by bone marrow transplantation. N Engl J Med 1990;322:1860–1866. [DOI] [PubMed] [Google Scholar]
- 6. Loes DJ, Hite S, Moser H, et al. Adrenoleukodystrophy: a scoring method for brain MR observations. AJNR Am J Neuroradiol 1994;15:1761–1766. [PMC free article] [PubMed] [Google Scholar]
- 7. Peters C, Charnas LR, Tan Y, et al. Cerebral X‐linked adrenoleukodystrophy: the international hematopoietic cell transplantation experience from 1982 to 1999. Blood 2004;104:881–888. [DOI] [PubMed] [Google Scholar]
- 8. Miller WP, Rothman SM, Nascene D, et al. Outcomes after allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: the largest single‐institution cohort report. Blood 2011;118:1971–1978. [DOI] [PubMed] [Google Scholar]
- 9. Pierpont EI, Eisengart JB, Shanley R, et al. Neurocognitive trajectory of boys who received a hematopoietic stem cell transplant at an early stage of childhood cerebral adrenoleukodystrophy. JAMA Neurol. 2017;74:710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller WP. Stem cell‐transplantation therapy for adrenoleukodystrophy: current perspectives. J Neurorestoratol 2017;5:5–19. [Google Scholar]
- 11. Gassas A, Raiman J, White L, et al. Long‐term adaptive functioning outcomes of children with inherited metabolic and genetic diseases treated with hematopoietic stem cell transplantation in a single large pediatric center: parents' perspective. J Pediatr Hematol Oncol 2011;33:216–220. [DOI] [PubMed] [Google Scholar]
- 12. Van Haren K, Engelen M. Decision making in adrenoleukodystrophy: when is a good outcome really a good outcome? JAMA Neurol 2017;74:641–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wechsler D. Wechsler intelligence scale for children, revised. New York: Psychological Corporation, 1974. [Google Scholar]
- 14. Wechsler D. Wechsler intelligence scale for children, 3rd ed San Antonio, TX: The Psychological Corporation, 1991. [Google Scholar]
- 15. Wechsler D. Wechsler intelligence scale for children, 4th ed San Antonio, TX: Psychological Corporation, 2003. [Google Scholar]
- 16. Wechsler D. Wechsler preschool and primary scale of intelligence, 3rd ed San Antonio, TX: The Psychological Corporation, 2002. [Google Scholar]
- 17. Wechsler D. Wechsler preschool and primary scale of intelligence, revised. San Antonio, TX: The Psychological Corporation, 1989. [Google Scholar]
- 18. Tiffen J. Purdue pegboard test. Chicago: Science Research Associates, 1968. [Google Scholar]
- 19. Beery K, Buktenica N, Beery N. The beery‐buktenica developmental test of visual‐motor integration: administration, scoring and teaching manual. (6th Ed.)Minneapolis, MN: Pearson, 2010. [Google Scholar]
- 20. Sparrow S, Balla D, Cicchetti D. Vineland adaptive behavior scales. Circle Pines, MN: American Guidance Service, 1984. [Google Scholar]
- 21. de Bildt A, Kraijer D, Sytema S, Minderaa R. The psychometric properties of the Vineland Adaptive Behavior Scales in children and adolescents with mental retardation. J Autism Dev Disord 2005;35:53–62. [DOI] [PubMed] [Google Scholar]
- 22. Sparrow S, Balla D, Cicchetti D. Vineland adaptive behavior scales, 2nd ed Circle Pines, MN: American Guidance Services, Inc., 2005. [Google Scholar]
- 23. R Development Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2015. [Google Scholar]
- 24. Van Buuren S. Flexible imputation of missing data. Boca Raton: CRC Press, 2012. [Google Scholar]
- 25. Harrell FE Jr.. RMS: Regression Modeling Strategies. R package version 5.1‐0.2017.
- 26. Harrell FE Jr.with contributions from Charles Dupont and many others. Hmisc: Harrell Miscellaneous. R package version 4.0‐2.2016.
- 27. Harrell FE. Regression modeling strategies. New York: Springer‐Verlag, 2001. [Google Scholar]
- 28. Loes DJ, Fatemi A, Melhem ER, et al. Analysis of MRI patterns aids prediction of progression in X‐linked adrenoleukodystrophy. Neurology 2003;61:369–374. [DOI] [PubMed] [Google Scholar]
- 29. Beam D, Poe MD, Provenzale JM, et al. Outcomes of unrelated umbilical cord blood transplantation for X‐linked adrenoleukodystrophy. Biol Blood Marrow Transplant 2007;13:665–674. [DOI] [PubMed] [Google Scholar]
- 30. Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude lifetime cohort study. J Clin Oncol 2013;31:4407–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Willard VW, Leung W, Huang Q, Zhang H, Phipps S. Cognitive outcome after pediatric stem‐cell transplantation: impact of age and total‐body irradiation. J Clin Oncol 2014;32:3982–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hurvitz E, Warschausky S, Berg M, Tsai S. Long‐term functional outcome of pediatric stroke survivors. Top Stroke Rehabil 2004;11:51–59. [DOI] [PubMed] [Google Scholar]
- 33. Majnemer A, Shevell M, Rosenbaum P, Law M, Poulin C. Determinants of life quality in school‐age children with cerebral palsy. J Pediatr 2007;151:470–475. 5e1‐3. [DOI] [PubMed] [Google Scholar]
- 34. Butcher NJ, Chow EW, Costain G, Karas D, Ho A, Bassett AS. Functional outcomes of adults with 22q11.2 deletion syndrome. Genet Med 2012;14:836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Venter A, Lord C, Schopler E. A follow‐up study of high‐functioning autistic children. J Child Psychol Psychiatry 1992;33:489–507. [DOI] [PubMed] [Google Scholar]


