Abstract
Objective
In this study we investigate the association between the expression of inflammatory mediators measured in clots retrieved by mechanical thrombectomy, stroke etiology, and the susceptibility vessel sign (SVS) on gradient‐echo (GRE) MR imaging in acute ischemic stroke patients.
Methods
We performed molecular analysis of intracranial clots retrieved by mechanical thrombectomy from 82 patients with acute stroke. Seventy‐two of these patients underwent GRE imaging before endovascular therapy. We measured the relative expression of inflammatory mediators by performing the quantitative real‐time polymerase chain reaction on the retrieved clots and assessed associations between the expression of inflammatory mediators and stroke subtypes as well as with GRE SVS.
Results
Classifications of stroke etiology for the cohort were as follows: cardioembolism (51, 62.2%), large artery atherosclerosis (9, 11%), and undetermined etiology (22, 26.8%). Clots associated with large artery atherosclerosis showed significantly higher interleukin (IL)‐1β expression than clots from both cardioembolism and undetermined etiology (P = 0.008). A positive SVS was identified in 48 of 72 patients (66.7%) who had GRE imaging. IL‐1β, tumor necrosis factor‐α, and matrix metalloproteinase‐9 expressions were significantly higher in clots with a negative SVS than in those with a positive SVS (P = 0.010, 0.049, and 0.004, respectively).
Interpretation
Expression of inflammatory mediators in intracranial clots differs significantly based on stroke etiology or presence or the absence of SVS on GRE imaging. This study suggests that molecular analysis of inflammatory mediators in retrieved clots is a promising tool for determining stroke mechanism in acute ischemic stroke patients.
Introduction
Mechanical thrombectomy is accepted as standard therapy for selected acute anterior circulation stroke patients.1 Mechanical thrombectomy is notable because it allows for histologic evaluation of clots retrieved from human intracranial arteries, potentially broadening our insight into stroke etiology as well as our understanding of clot appearance on imaging studies. Several recent studies involving the histologic analysis of intracranial clots retrieved by mechanical thrombectomy in acute stroke patients have focused on blood cell composition in clots using either hematoxylin and eosin or immunohistochemical staining.2, 3, 4, 5, 6, 7
Large artery atherosclerosis is an important etiology of acute ischemic stroke and inflammation is well‐known to play a pivotal role in all stages of atherosclerosis, from lesion initiation to progression and destabilization.8 The pathogenesis of atherosclerosis is crucially influenced by inflammatory mediators such as proinflammatory cytokines and extracellular proteases secreted by smooth muscle cells, endothelial cells, and immune cells.8, 9, 10 We hypothesized that intracranial clots retrieved from patients with acute ischemic stroke attributable to atherosclerosis would show greater expression of inflammatory mediators than clots attributable to other stroke etiologies. Additionally, we hypothesized that differences in clot appearance observed on imaging may associate with differences in inflammatory mediator expression. On gradient‐echo (GRE) MR imaging, a dark blooming artifact in the occluded vessel due to magnetic susceptibility effect of deoxyhemoglobin within the clot has been described as susceptibility vessel sign (SVS) (Fig. 1).11, 12 Previous studies suggested that the presence of SVS may predict cardioembolic stroke and absence of SVS may be associated with large artery atherosclerosis.3, 12, 13 In this study we investigate the existence of an association between the expression of representative inflammatory mediators measured in intracranial clots and stroke etiology, as well as the SVS on T2*‐weighted GRE MR imaging in acute ischemic stroke patients.
Figure 1.

Gradient‐echo image from a 56‐year‐old man with acute ischemic stroke. Axial gradient‐echo image reveals a positive susceptibility vessel sign (arrows) in the M1 segment of the left middle cerebral artery.
Materials and Methods
Patients
We collected visible retrieved clots from 82 patients treated with mechanical thrombectomy at a regional comprehensive stroke center, including stent‐retriever and clot aspiration thrombectomy for acute ischemic stroke due to an intracranial large vessel occlusion. All patients underwent nonenhanced CT and/or multimodal MRI prior to the endovascular thrombectomy procedure. We postoperatively collected demographic features, cerebrovascular risk factors, National Institute Health Stroke Scale scores on admission and at discharge, use of intravenous thrombolysis, time to endovascular treatment, procedure time, time to reperfusion, reperfusion status,14 functional outcomes, and stroke subtype.
Stroke neurologists used the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification15 to determine ischemic stroke subtype at the time of discharge. Cardioembolism was defined as having at least one of the following predisposing factors: atrial fibrillation or flutter, left atrial thrombus, prosthetic valve, severe mitral stenosis, patent foramen ovale, concomitant acute myocardial infarction, congestive heart failure, infective endocarditis, and/or sick‐sinus syndrome in the absence of ipsilateral arterial stenosis on imaging studies. In the absence of evidence of potential sources of cardioembolism in other diagnostic studies, large artery atherosclerosis was defined by catheter angiographic findings showing ≥50% luminal stenosis or occlusion of the ipsilateral extracranial or intracranial carotid artery proximal to the occlusion site. Underlying intracranial atherosclerosis causing ≥50% luminal stenosis at the occlusion site was also regarded as large artery atherosclerosis. Undetermined etiology was defined by the absence of evidence of ipsilateral extracranial or intracranial atherosclerosis causing ≥50% luminal stenosis, major risk sources of cardioembolism as mentioned above, or any other specific cause of stroke. This study was approved by the Institutional Ethics Committee, and written informed consent for endovascular therapy and molecular analysis of retrieved thrombi was obtained from a family member for each patient.
Quantitative real‐time polymerase chain reaction for expression of inflammatory mediators
Retrieved clots were washed with normal saline to eliminate contamination from circulating blood and kept in RNAlater (Thermo Scientific, Waltham, MA). We used quantitative real‐time polymerase chain reaction (qRT‐PCR) according to a previously published methods to measure mRNA expression levels for interleukin (IL)‐1β, IL‐6, IL‐8, IL‐18, tumor necrosis factor (TNF)‐α, monocyte chemoattractant protein‐1 (MCP‐1), matrix metalloproteinase (MMP)‐2, and MMP‐9 in retrieved clots.16 Briefly, RNA was obtained from the total clot using TRIzol™ (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Complementary DNA was synthesized using GoScript™ (Promega, Madison, WI, USA), following the manufacturer's instructions. qRT‐PCR was carried out on 25 ng equivalents in triplicate on an QuantStudio 3™ Sequence Detection System (Applied Biosystems Inc., Foster City, CA, USA), using SYBR Green Assays for inflammatory mediators. We combined threshold amplification cycle number data from multiple plates using AB Relative Quantitation software (Applied Biosystems Inc., Foster City, CA, USA). Glyceraldehydes 3‐phosphate dehydrogenase was used for internal control. The reliability of the qRT‐PCR results was determined by solubility curve. The Ct (cycle threshold) values were taken to calculate the relative expression of the target gene (2−▵CT) using the following equation: ▵CT = Ct (target gene) − Ct (internal control). The primers used in this study can be found in Table S1.
MR imaging analysis
We used a 1.5‐T unit (Signa HDxt; GE Medical Systems, Milwaukee, WI) to perform MRI examinations. Patients underwent MRI including diffusion‐weighted imaging, GRE imaging, FLAIR sequence, three‐dimensional time‐of‐flight MR angiography, and perfusion imaging before the endovascular procedure. GRE sequence parameters included a TR of 750–800 msec, TE of 14 msec, flip angle of 20°, slice thickness of 4 mm, interslice gap of 0 mm, and field of view of 230 × 230 mm. Two neuroradiologists who were blinded to the findings from catheter angiography retrospectively reviewed a GRE images. Conclusions regarding the presence or absence of an SVS on GRE imaging were reached by consensus. A positive SVS was defined as a hypointense signal change on GRE imaging within the occluded artery, and in cases of occlusion in the middle cerebral artery (MCA) or internal carotid‐T bifurcation, in which the diameter of the hypointense signals exceed that of the contralateral artery.11, 12 In cases of basilar artery occlusion, a positive SVS was defined as a hypointense signal within the prepontine cistern, in which the diameter of the hypointense signals exceeds the diameter of the parent basilar artery.17 A negative SVS was defined as the absence of a positive SVS in the occluded artery.13
Statistical analysis
First, the relative expressions of each inflammatory mediator were compared among patients classified with large artery atherosclerosis, cardioembolism, and undetermined etiology, followed by a comparison of the relative expressions of each inflammatory mediator between patients with a positive SVS and a negative SVS on GRE imaging. We used a Kolmogorov–Smirnov test to check the normality of the data. If the data were non‐normally distributed, Kruskal–Wallis test or Mann–Whitney U test were used for each comparison. Post hoc pairwise comparisons among patients classified with large artery atherosclerosis, cardioembolism, and undetermined etiology for inflammatory mediators were done using Dunn's test to adjust for multiple comparisons. All statistical analyses were performed with SPSS software (version 23.0; IBM SPSS, Chicago, IL), and P < 0.05 was considered statistically significant.
Results
We analyzed data from 82 patients (40 men and 42 women; median age, 75.5 years; age range, 38–89 years). The baseline characteristics and clinical outcomes of the patients are summarized in Table 1. 67.1% (55/82) of patients had hypertension, 61% (50/82) had atrial fibrillation, 28% (23/82) had diabetes mellitus, 30.5% (25/82) had dyslipidemia, 22.9% (19/82) had a history of prior ischemic stroke, 26.8% (22/82) had smoking history, 13.4% (11/82) had a history of previous coronary artery disease, and 9% (11/82) had a congestive heart failure. Forty‐six patients had an occlusion in the MCA, 24 in the internal carotid artery (ICA)‐T bifurcation, and 12 in the basilar artery.
Table 1.
Patient baseline characteristics and clinical data
| Numbers (n = 82) | |
|---|---|
| Age, years, median (IQR) | 75.5 (64–81) |
| Sex, male | 40 (48.8%) |
| Risk factors | |
| Hypertension | 55 (67.1%) |
| Atrial fibrillation | 50 (61.0%) |
| Diabetes mellitus | 23 (28.0%) |
| Dyslipidemia | 25 (30.5%) |
| History of stroke or TIA | 19 (22.9%) |
| Smoking | 22 (26.8%) |
| Coronary artery disease | 11 (13.4%) |
| Congestive heart failure | 9 (11.0%) |
| Occlusion sites | |
| MCA | 46 (56.1%) |
| ICA‐T bifurcation | 24 (29.3%) |
| Basilar artery | 12 (14.6%) |
| Intravenous thrombolysis | 40 (48.8%) |
| Time to procedure, min, median (IQR) | 190 (140–260.75) |
| Procedure time, min, median (IQR) | 28.5 (18–38.75) |
| Time to revascularization, min, median (IQR) | 221.5 (180.75–291.75) |
| Baseline NIHSS, median (IQR) | 13 (10–17) |
| Stroke subtypes | |
| Cardioembolism | 51 (62.2%) |
| Large artery atherosclerosis | 9 (11.0%) |
| Undetermined | 22 (26.8%) |
| m‐TICI 2b or 3 | 72 (87.8%) |
| mRS 0–2 at 90‐day | 42 (51.2%) |
| Mortality | 6 (7.3%) |
IQR, interquartile range; TIA, transient ischemic attack; MCA, middle cerebral artery; ICA indicates internal carotid artery; NIHSS, National Institute Health Stroke Scale; m‐TICI, modified treatment in cerebral infarction; mRS, modified Rankin Scale.
Association between expression of inflammatory mediators and stroke subtypes
We classified stroke subtypes as cardioembolism (n = 51, 62.2%), large artery atherosclerosis (n = 9, 11%), and undetermined (n = 22, 26.8%). IL‐1β expression was significantly higher in clots retrieved from patients with large artery atherosclerosis than in those with cardioembolism or undetermined etiology (Kruskal–Wallis test, P = 0.008) (Fig. 2). Pairwise comparisons of IL‐1β expression show that the clots retrieved from patients with large artery atherosclerosis had a higher IL‐1β expression than in those with a cardioembolism (post hoc Dunn's test, P = 0.006). There was no difference in IL‐1β expression between the clots from patients with cardioembolism and those with undetermined etiology (post hoc Dunn's test, P = 1.000). IL‐6, IL‐8, IL‐18, TNF‐ α, MCP‐1, MMP‐2, and MMP‐9 expression was not significantly different among the three groups.
Figure 2.

IL‐1β expression is significantly higher in clots retrieved from patients with large artery atherosclerosis than those with other etiologies. IL, interleukin.
Association between expression of inflammatory mediators and SVS
Seventy‐two of the 82 patients underwent GRE imaging before endovascular therapy. A positive SVS was identified in 48 of 72 patients (66.7%) at GRE imaging (Fig. 1). The SVS was identified in 27 MCAs, 14 ICA‐T bifurcations, and 7 basilar arteries. Thirty‐four of 45 (76%) of patients with cardioembolic etiology and 13 of 18 (72.2%) of those with undetermined etiology had an SVS, whereas only one of nine (11%) of those with large‐artery atherosclerosis showed an SVS (Table 2). Figures 3, 4, 5 show the association between mRNA expression of inflammatory mediators and an SVS on GRE imaging. IL‐1β expression was higher in clots with a negative SVS than in those with a positive SVS (Mann–Whitney U test, P = 0.010) (Fig. 3). Similarly, clots from patients with a negative SVS had a higher TNF‐α and MMP‐9 expression than those with a positive SVS (Mann–Whitney U test, P = 0.049 and 0.004, respectively) (Figs. 4, 5). There were no differences in IL‐6, IL‐8, IL‐18, MCP‐1, or MMP‐2 expression between patients with and without SVS on GRE imaging.
Table 2.
Incidence of susceptibility vessel sign on gradient‐echo MRI according to stroke etiology
| Positive SVS (n = 48) | Negative SVS (n = 24) | |
|---|---|---|
| Cardioembolism (n = 45) | 34 (70.8%) | 11 (45.9%) |
| Large artery atherosclerosis (n = 9) | 1 (2.1%) | 8 (33.3%) |
| Undetermined (n = 18) | 13 (27.1%) | 5 (20.8%) |
SVS indicates susceptibility vessel sign.
Figure 3.
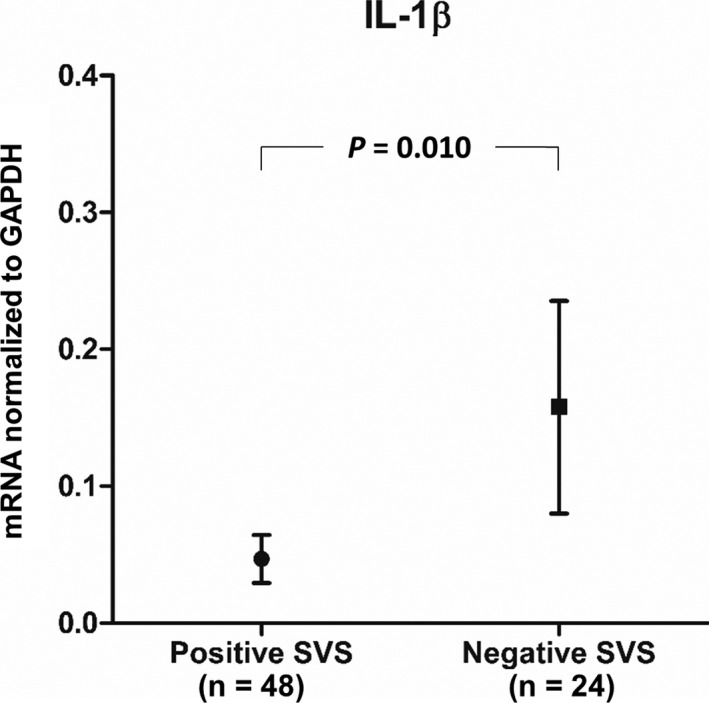
IL‐1β expression is significantly higher in clots with a negative SVS on GRE imaging relative to those with a positive SVS. IL, interleukin; SVS, susceptibility vessel sign; GRE, gradient‐echo.
Figure 4.

TNF‐α expression is significantly higher in clots with a negative SVS on GRE imaging relative to those with a positive SVS. TNF, tumor necrosis factor; SVS, susceptibility vessel sign; GRE, gradient‐echo.
Figure 5.

MMP‐9 expression is significantly higher in clots with a negative SVS on GRE imaging relative to those with a positive SVS. MMP‐9, matrix metalloproteinase; SVS, susceptibility vessel sign; GRE, gradient‐echo.
Discussion
This study demonstrates that inflammatory mediators, such as proinflammatory cytokines and matrix‐degrading proteases, can be detected in clots retrieved from human intracranial arteries, and that expression levels vary based on stroke subtype and imaging appearance. This observation suggests that inflammatory mediators expressed in retrieved clots could be used as novel biomarkers for determining etiologic mechanisms in patients with acute ischemic stroke. Our study also advances the understanding of the pathophysiology of the SVS on GRE imaging. The few previous studies performing histopathologic analysis on human intracranial clots retrieved by mechanical thrombectomy in acute stroke have looked only at analysis of blood cell composition using hematoxylin and eosin2, 3, 4 or immunohistochemical staining.5, 6, 7 This is the first report of molecular analysis including mRNA expression of inflammatory mediators in retrieved clots. Previous histologic studies have shown that intracranial clots have very small white blood cell compositions, which could act as a source of the inflammatory mediators described in this study.3
In this study, we hypothesized that intracranial clots associated with atherosclerosis would have higher inflammatory mediator expression than clots associated with other stroke etiologies. We selected several proinflammatory molecules considered to be representative inflammatory mediators involved in atherogenesis to test this hypothesis.8, 9, 10 Of these selected mediators, we observed that IL‐1β expression was significantly higher in clots from patients with large artery atherosclerosis than in those with either cardioembolism or undetermined etiology. IL‐1 is a prototypic proinflammatory cytokine that leads to the recruitment of inflammatory cells by inducing the production of cytokines and chemokines and increasing the expression of adhesion molecules on endothelial cells.18 The term IL‐1 refers to two distinct but related proteins, IL‐1α and IL‐1β. IL‐1β is not produced unless the cell receives an inflammatory signal, unlike IL‐1α.19, 20 The ability of IL‐1 to modulate a number of key events involved in the complex inflammatory process of atherogenesis including vessel wall inflammation, leukocyte chemotaxis and adhesion or plaque rupture is the likely source of its proatherogenic effect.20
This study demonstrates that IL‐1β, TNF‐α, and MMP‐9 expression differs based on the status of SVS on GRE imaging. Similar to IL‐1β, TNF‐α is a key cytokine for the recruitment and activation of inflammatory cells.9 In addition to its known ability to alter endothelial functions and increase the extent of necrosis in advanced atherosclerotic lesions,9, 21, 22 TNF‐α is also a potent stimulator of MMP.23 MMP‐9 is a member of the gelatinase class of the metalloproteinase family and is known to play an important role in several stages of atherosclerosis.24 MMP‐9 activity contributes to the dysregulation of the extracellular matrix that leads to plaque rupture during atherothrombosis complication.25 In this study, clots with a GRE SVS showed significantly lower IL‐1β, TNF‐α and MMP‐9 expression compared to clots without an SVS. These results support the idea that a GRE SVS is more frequently observed in patients with cardiogenic embolic stroke than in those with other stroke subtypes3, 12 and clots with a negative SVS could be associated with atherosclerosis.3, 13 The T2‐shortening effect of the intracellular deoxyhemoglobin component in red blood cells within the clot is the pathophysiologic basis of an SVS.3 Paramagnetic intracellular deoxyhemoglobin leads to a nonuniform magnetic field causing marked signal loss on the GRE sequence because of spin dephasing. It was previously established that the presence of an SVS on GRE imaging was markedly associated with a higher red blood cell count in the clot, whereas fibrin‐dominant clots were associated with the absence of an SVS.3, 12 Fibrin‐dominant clots are typically formed because of endothelium injury overlies the complicated plaque in the setting of advanced atherosclerosis.13 Therefore, fibrin‐dominant clots related to advanced atherosclerosis would not appear as an SVS on GRE imaging due to the lack of deoxyhemoglobin, and could have increased expression of cytokines, matrix‐degrading proteases, or other inflammatory mediators. Our results confirm this hypothesis, indicating that intracranial clots with a negative SVS showed significantly higher expression of IL‐1β, TNF‐α, and MMP‐9.
This study has several limitations, including the limited number of patients. In particular, the number of patients with large artery atherosclerosis was relatively small compared to those with cardioembolism. We did not investigate other IL family members, interferons, colony‐stimulating factors, transforming growth factors, chemokines, acute phase reactants, and adhesion molecules or inflammatory mediators known to be expressed in human atherosclerotic plaques. Further studies are needed to evaluate the expression of these inflammatory mediators in human intracranial clots and their associations with stroke etiology, imaging appearance, and treatment response.
Conclusions
This study showed that one of the key cytokines involved in the pathogenesis of atherosclerosis, IL‐1β, is expressed differently according to stroke subtype within intracranial clots in patients with acute ischemic stroke. IL‐1β expression was significantly higher in clots from patients with large artery atherosclerosis relative to those with cardioembolism or undetermined etiology. In addition, clots with a negative GRE SVS showed increased IL‐1β, TNF‐α, and MMP‐9 expression compared with clots with a positive GRE SVS, indirectly indicating that clots with a negative GRE SVS are fibrin‐dominant clots related to advanced atherosclerosis. This study suggests that the molecular analysis of inflammatory mediators in retrieved clots from patients with acute ischemic stroke can be a useful tool for elucidating stroke mechanism and for understanding the pathophysiology of clot signs on imaging.
Conflict of Interest
The authors have no conflicts of interest to disclose.
Supporting information
Table S1. Primers used for measurement of each inflammatory mediator.
Funding Statement
This work was funded by Chonnam National University Hospital Biomedical Research Institute grant CRI16023‐1; National Research Foundation of Korea grant NRF‐2016R1A2B4008316.
References
- 1. Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:3020–3035. [DOI] [PubMed] [Google Scholar]
- 2. Liebeskind DS, Sanossian N, Yong WH, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 2011;42:1237–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim SK, Yoon W, Kim TS, et al. Histologic analysis of retrieved clots in acute ischemic stroke: correlation with stroke etiology and gradient‐echo MRI. AJNR Am J Neuroradiol 2015;36:1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boeckh‐Behrens T, Kleine JF, Zimmer C, et al. Thrombus histology suggests cardioembolic cause in cryptogenic stroke. Stroke 2016;47:1864–1871. [DOI] [PubMed] [Google Scholar]
- 5. Niesten JM, van der Schaaf IC, van Dam L, et al. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS One 2014;9:e88882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dargazanli C, Rigau V, Eker O, et al. High CD3+ cells in intracranial thrombi represent a biomarker of atherothrombotic stroke. PLoS One 2016;11:e0154945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schuhmann MK, Gunreben I, Kleinschnitz C, Kraft P. Immunohistochemical analysis of cerebral thrombi retrieved by mechanical thrombectomy from patients with acute ischemic stroke. Int J Mol Sci 2016;17:298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem 2008;54:24–38. [DOI] [PubMed] [Google Scholar]
- 9. Ait‐Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol 2011;31:969–979. [DOI] [PubMed] [Google Scholar]
- 10. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32:2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rovira A, Orellana P, Alvarez‐Sabin J, et al. Hyperacute ischemic stroke: middle cerebral artery susceptibility sign at echo‐planar gradient‐echo MR imaging. Radiology 2004;232:466–473. [DOI] [PubMed] [Google Scholar]
- 12. Cho KH, Kim JS, Kwon SU, et al. Significance of susceptibility vessel sign on T2*‐weighted gradient echo imaging for identification of stroke subtypes. Stroke 2005;36:2379–2383. [DOI] [PubMed] [Google Scholar]
- 13. Kim SK, Yoon W, Heo TW, et al. Negative susceptibility vessel sign and underlying intracranial atherosclerotic stenosis in acute middle cerebral artery occlusion. AJNR Am J Neuroradiol 2015;36:1266–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013;44:2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 16. Choi KH, Kim HS, Park MS, et al. Regulation of caveolin‐1 expression determines early brain edema after experimental focal cerebral ischemia. Stroke 2016;47:1336–1343. [DOI] [PubMed] [Google Scholar]
- 17. Song M, Hu Q, Wang Y, et al. Susceptibility vessel sign in isolated brainstem infarction with large artery occlusion. Eur Neurol 2016;75:251–256. [DOI] [PubMed] [Google Scholar]
- 18. Merhi‐Soussi F, Kwak BR, Magne D, et al. Interleukin‐1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein E‐knockout mice. Cardiovasc Res 2005;66:583–593. [DOI] [PubMed] [Google Scholar]
- 19. Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin‐1 in a broad spectrum of diseases. Nat Rev Drug Discov 2012;11:633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vicenová B, Vopálenský V, Burýsek L, Pospísek M. Emerging role of interleukin‐1 in cardiovascular diseases. Physiol Res 2009;58:481–498. [DOI] [PubMed] [Google Scholar]
- 21. Brånén L, Hovgaard L, Nitulescu M, et al. Inhibition of tumor necrosis factor‐alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 2004;24:2137–2142. [DOI] [PubMed] [Google Scholar]
- 22. Boesten LS, Zadelaar AS, van Nieuwkoop A, et al. Tumor necrosis factor‐alpha promotes atherosclerotic lesion progression in APOE*3‐Leiden transgenic mice. Cardiovasc Res 2005;66:179–185. [DOI] [PubMed] [Google Scholar]
- 23. Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol 2008;28:2108–2114. [DOI] [PubMed] [Google Scholar]
- 24. Bäck M, Ketelhuth DF, Agewall S. Matrix metalloproteinases in atherothrombosis. Prog Cardiovasc Dis 2010;52:410–428. [DOI] [PubMed] [Google Scholar]
- 25. Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994;94:2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers used for measurement of each inflammatory mediator.


