Abstract
The relationship between clinicopathologic diagnosis and 123I‐FP‐CIT SPECT in 18 patients with dementia (12 with Lewy body disease) from one center in the United States was assessed. The sensitivity and specificity of abnormal 123I‐FP‐CIT SPECT with reduced striatal uptake on visual inspection for predicting Lewy body disease were 91.7% and 83.3%, respectively. The mean calculated putamen to occipital ratio (mPOR) based on regions of interest was significantly reduced in Lewy body disease compared to non‐Lewy body disease cases (P = 0.002). In this study, abnormal 123I‐FP‐CIT SPECT was strongly associated with underlying Lewy body disease pathology, supporting the utility of 123I‐FP‐CIT SPECT in the clinical diagnosis of dementia with Lewy bodies.
Introduction
Dementia with Lewy bodies (DLB) is the second most common dementia type following Alzheimer's disease, accounting for 4.2% of all dementia cases in the population.1 In our population‐based study of residents of Olmsted County, Minnesota, the incidence rate of DLB was estimated at 3.5 per 100,000 person‐years, which significantly increased with age.2 Ante‐mortem diagnostic accuracy of DLB is important for clinical management and disease‐specific pharmacologic trials. The utility of 123I‐2β‐carbomethoxy‐3β‐(4‐iodophenyl)‐N‐(3‐fluoropropyl) nortropane (123I‐FP‐CIT) single‐photon emission computed tomography (SPECT) in differentiating DLB from other dementia types has been evaluated.3 Decreased striatal dopamine transporter uptake of 123I‐FP‐CIT in DLB is thought to reflect nigrostriatal degeneration in Lewy body disease (LBD).4 In a multicenter phase III trial, abnormal 123I‐FP‐CIT SPECT findings had 77.7% sensitivity and 90.4% specificity for distinguishing probable DLB from other dementias.3 Given the high sensitivity and specificity of 123I‐FP‐CIT SPECT for the diagnosis of DLB when combined with other clinical features, the revised consensus criteria for the clinical diagnosis of DLB include 123I‐FP‐CIT SPECT as an indicative biomarker of DLB.5
Limited studies have validated 123I‐FP‐CIT SPECT findings in DLB with neuropathologic confirmation.6, 7, 8 Decreased 123I‐FP‐CIT binding in putamen measured semiquantitatively showed 88% sensitivity and 100% specificity for predicting LBD pathology at autopsy.6 Abnormal 123I‐FP‐CIT SPECT on visual rating was associated with LBD pathology with 80% sensitivity and 92% specificity.8 Although high diagnostic accuracy of 123I‐FP‐CIT SPECT for DLB/LBD was seen in these studies, the findings were based only on European patients with dementia. Here, we assess the clinical, pathologic, and 123I‐FP‐CIT SPECT correlations in patients with dementia from one center in the United States.
Methods
Mayo Alzheimer's Disease Research Center (ADRC) participants who were diagnosed with a clinical dementia syndrome, underwent 123I‐FP‐CIT SPECT imaging (performed as part of an ongoing ADRC imaging biomarker study in neurodegenerative conditions), and had neuropathologic evaluation at autopsy were included in the study. These patients had been followed longitudinally until death or until they were no longer able to participate in the research program between August 2003 and May 2016. Consensus clinical diagnosis of a dementia type, including Alzheimer's disease dementia (ADem),9, 10 DLB,5, 11 primary progressive aphasia (PPA)/frontotemporal lobar degeneration (FTLD),12, 13 and corticobasal syndrome (CBS)14, 15 was made based on previously published criteria. Rapid eye movement (REM) sleep behavior disorder (RBD) was diagnosed based on clinical +/− polysomnographic features.16
123I‐FP‐CIT SPECT (DaTscan, GE Healthcare, Chicago, IL) was performed 1–59 months prior to death according to previously published protocol.17 123I‐FP‐CIT SPECT findings were interpreted by radiologists in a blinded manner, and were considered abnormal if there was unequivocally reduced uptake in one or both putamen based on visual inspection. A mean putamen to occipital ratio (mPOR) was calculated for each patient by placing regions of interest over the right and left putamen and ipsilateral occipital cortical tissue and averaging the right and left putamen to occipital ratios. The calculation of mPOR was blinded with respect to the visual rating results as well as the clinical and pathologic diagnoses.
Neuropathologic diagnosis was made using previously published neuropathologic criteria, including Braak stages,18, 19 Consortium to Establish a Registry for Alzheimer's Disease scores,19, 20 Thal Aβ phases,19, 21 LBD pathologic criteria,11 and FTLD classification22 by neuropathologists who were blinded to the 123I‐FP‐CIT SPECT findings.
Comparisons for semiquantitatively analyzed 123I‐FP‐CIT SPECT findings between pathologically confirmed LBD and non‐LBD cases were made using two‐sided Wilcoxon rank‐sum test due to non‐normal distribution of the variable with a P < 0.05 being considered statistically significant. Statistical analysis was performed using JMP, version 10.0 (SAS Institute Inc, Cary, NC).
This study was approved by the Mayo IRB. Informed consent was obtained from the patients and/or their proxies.
Results
Eighteen patients met the inclusion criteria, of whom 12 had pathologic confirmation of LBD. The demographic, clinical, pathologic, and 123I‐FP‐CIT SPECT characteristics of these patients are summarized in the Table 1. The median age of onset of cognitive decline was 65.5 years (interquartile range [IQR] 59.5–71.0). These patients were longitudinally followed up for a median duration of 5 years (IQR 2–8). The patients with clinical diagnosis of AD predominantly presented with amnestic features, with impairment in other nonmemory domains. All DLB patients met criteria for probable DLB based on the third and fourth consensus criteria5, 11 (i.e., all patients had at least two of the core clinical features, but 123I‐FP‐CIT SPECT was not used for diagnosis). Patients who were diagnosed with DLB exhibited visuospatial and executive dysfunction in addition to memory impairment. Most of the DLB patients (11 of 12) had concurrent or subsequent development of parkinsonism and/or visual hallucinations. All patients with DLB were also diagnosed with polysomnography‐confirmed RBD (10 of 12) or probable RBD (pRBD, 2 of 12) based on a strong history of dream enactment behavior. In most cases, RBD preceded the onset of cognitive decline in DLB by a median duration of 11 years (IQR 3.8–16.3). One patient with clinical diagnosis of ADem (Case 1) had a history consistent with probable RBD, although polysomnography demonstrated equivocal findings. Another patient with ADem (Case 4) had polysomnographic evidence of REM sleep without atonia in the absence of any clinical history of dream enactment behavior. Both of these patients were taking antidepressants at the time of polysomnography. Two patients (Cases 3 and 16) showed clinical features of both DLB and ADem, and were diagnosed as mixed DLB and ADem. Asymmetric limb apraxia, rigidity, and myoclonus were seen in the patient with CBS in addition to cognitive symptoms. One patient (Case 6) presented with prominent language dysfunction characterized by dysnomia, impaired object knowledge, and variable performance on repetition and comprehension measures, and he was diagnosed with PPA.13
Table 1.
Demographic, clinical, pathologic, and 123I‐FP‐CIT SPECT findings in 18 autopsied patients with dementia
| Case | Sex | AAO (years) | AAD (years) | Clinical course (years) | Clinical diagnosis | Pathologic diagnosis | Braak tangle stage | Thal Aβ phase | Fourth cDLB likelihood | Age at 123I‐FP‐CIT SPECT (years) | 123I‐FP‐CIT SPECT to death (months) | 123I‐FP‐CIT SPECT visual inspection | mPOR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 60 | 70 | 10 | ADem/pRBD | AD | 6 | 5 | None | 70 | 1 | Normal | 3.6 |
| 2 | M | 51 | 60 | 9 | CBS | AD | 6 | 5 | None | 58 | 15 | Normal | 3.1 |
| 3 | M | 52 | 57 | 5 | DLB/ADem/RBD | AD | 5 | 3 | None | 55 | 24 | Normal | 3.1 |
| 4 | M | 62 | 73 | 11 | ADem/RSWA | AD/ALB | 5 | 3 | Low | 69 | 48 | Normal | 2.1 |
| 5 | M | 56 | 62 | 6 | ADem | ADa | 4 | 5 | None | 59 | 36 | Abnormal | 3.2 |
| 6 | M | 66 | 76 | 10 | PPA/bvFTD | FTLD‐TDP | 2 | 1 | None | 74 | 25 | Normal | 3.0 |
| 7 | M | 76 | 86 | 12 | DLB/RBD | DLBD/PA | 4 | 3 | High | 86 | 6 | Abnormal | 1.0 |
| 8 | M | 70 | 75 | 5 | DLB/RBD | DLBD/PA | 3 | 2 | High | 74 | 17 | Abnormal | 1.8 |
| 9 | M | 58 | 61 | 3 | DLB/RBD | DLBD | 2 | 3 | High | 61 | 1 | Abnormal | 1.4 |
| 10 | F | 60 | 68 | 8 | DLB/pRBD | DLBD/AD | 4 | 4 | High | 64 | 45 | Abnormal | 2.1 |
| 11 | F | 70 | 78 | 8 | ADem | DLBD/AD | 6 | 4 | Intermediate | 75 | 27 | Abnormal | 1.3 |
| 12 | M | 65 | 80 | 15 | DLB/RBD | DLBD/PA | 1 | 3 | High | 75 | 59 | Abnormal | 1.3 |
| 13 | M | 70 | 81 | 11 | DLB/RBD | DLBD/PA | 3 | 1 | High | 77 | 48 | Abnormal | 1.2 |
| 14 | M | 65 | 75 | 10 | DLB/RBD | DLBD/PA | 2 | 2 | High | 72 | 43 | Abnormal | 1.0 |
| 15 | M | 77 | 87 | 10 | DLB/RBD | DLBD/AD | 5 | 4 | Intermediate | 83 | 46 | Abnormal | 1.2 |
| 16 | M | 74 | 81 | 7 | DLB/ADem/RBD | DLBD/PA | 2 | 4 | High | 77 | 48 | Abnormal | 2.0 |
| 17 | M | 70 | 75 | 5 | DLB/pRBD | DLBD/AD | 4 | 4 | High | 72 | 32 | Abnormal | 2.3 |
| 18 | M | 77 | 85 | 8 | DLB/RBD | DLBD/PA | 2 | 3 | High | 81 | 42 | Normal | 2.1 |
All autopsied cases were Caucasian, except Case 18 who was an African American.
Fourth cDLB, fourth consortium's recommendation for likelihood of clinical dementia with Lewy bodies based on neuropathologic severity; AAD, age at death; AAO, age at onset; AD, Alzheimer's disease; ADem, Alzheimer's disease dementia; ALB, amygdala Lewy bodies; bvFTD, behavioral variant frontotemporal dementia; CBS, corticobasal syndrome; DLB, dementia with Lewy bodies; DLBD, diffuse Lewy body disease; F, female; FTLD‐TDP, frontotemporal lobar degeneration with TDP‐43 positive inclusions; 123I‐FP‐CIT, 123I‐2β‐carbomethoxy‐3β‐(4‐iodophenyl)‐N‐(3‐fluoropropyl) nortropane; M, male; mPOR, mean putamen to occipital ratio; PA, pathologic aging; PPA, primary progressive aphasia; pRBD, probable rapid eye movement sleep behavior disorder; RBD, polysomnography‐confirmed rapid eye movement sleep behavior disorder; SPECT, single‐photon emission computed tomography; TDP, TAR DNA‐binding protein 43.
Case 5 was found to have multiple metastatic melanoma nodules, predominantly found in frontal cortex and medial temporal lobe structures.
The patients came to autopsy at a median age of 75.0 years (IQR 66.5–81.0) after 8.5 years (IQR 5.8–10.0) of clinical course. Neuropathologic diagnoses were mostly concordant with clinical diagnoses. Of 12 patients with neuropathologic features consistent with LBD, 11 were diagnosed with DLB. One patient (Case 11) diagnosed as ADem due to the absence of parkinsonism, fluctuating cognitive symptoms, visual hallucinations, and RBD was found to have mixed LBD and AD pathologies. Of six patients with non‐LBD pathology, only one patient (Case 3) carried a diagnosis of DLB, although he was clinically thought to have mixed dementia with DLB plus ADem features. The sensitivity and specificity of the clinical diagnosis for detecting underlying LBD pathology were 91.7% and 83.3%, respectively. Clinical diagnostic accuracy was 88.9%.
123I‐FP‐CIT SPECT was performed 34.0 months (IQR 16.5–46.5) prior to death. Representative 123I‐FP‐CIT SPECT images are shown in Figure 1. The median disease duration at the time of 123I‐FP‐CIT SPECT was 5.5 years (IQR 3.0–7.3). Eleven of 12 patients with LBD pathology, including mixed LBD and AD, had abnormal 123I‐FP‐CIT SPECT upon visual inspection (false negative Case 18). 123I‐FP‐CIT SPECT was considered normal in five of six patients with non‐LBD pathology (false positive Case 5). Reduced striatal uptake of 123I‐FP‐CIT on visual rating had sensitivity of 91.7% and specificity of 83.3% for detecting underlying LBD pathology. Diagnostic accuracy of 123I‐FP‐CIT SPECT using visual inspection was 88.9%. Semiquantitatively analyzed 123I‐FP‐CIT SPECT imaging demonstrated that mPOR in LBD cases was significantly reduced with a median mPOR of 1.4 (IQR 1.2–2.1), compared to a median mPOR of 3.1 (IQR 2.8–3.3) in non‐LBD cases (P = 0.002).
Figure 1.
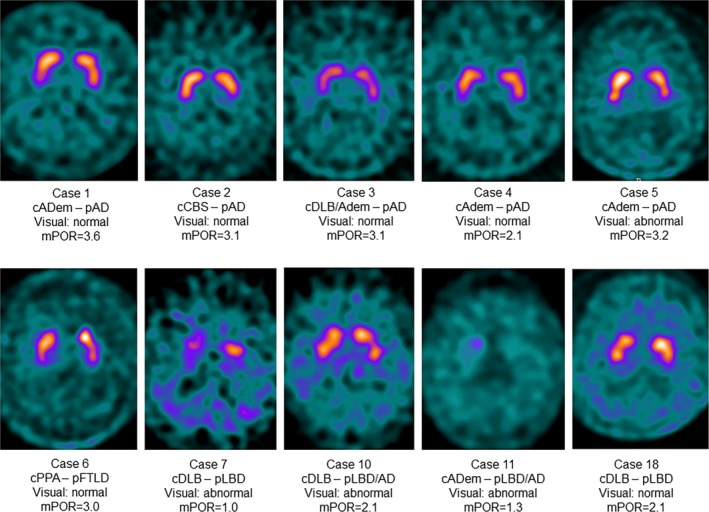
123I‐FP‐CIT SPECT demonstrates reduced striatal uptake in LBD cases compared to non‐LBD cases. Representative images are shown. AD, Alzheimer's disease; ADem, Alzheimer's disease dementia; c, clinical diagnosis; CBS, corticobasal syndrome; DLB, dementia with Lewy bodies; FTLD, frontotemporal lobar degeneration; LBD, Lewy body disease; mPOR, mean putamen to occipital ratio; p, pathologic diagnosis; PPA, primary progressive aphasia
Discussion
In this study, reduced striatal uptake of 123I‐FP‐CIT on visual rating was highly predictive of underlying LBD pathology in patients with dementia. Furthermore, semiquantitative analysis of 123I‐FP‐CIT SPECT revealed that a reduction in mPOR was strongly associated with LBD. These findings are consistent with the results of previous European studies (Table 2),6, 7, 8 and support the utility of 123I‐FP‐CIT SPECT in ante‐mortem clinical diagnosis of DLB.
Table 2.
Sensitivity, specificity, and accuracy of clinical diagnosis and 123I‐FP‐CIT SPECT for diagnosing dementia with Lewy bodies
| Clinical diagnosis | 123I‐FP‐CIT SPECT | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | FP | TN | FN | SN (%) | SP (%) | Accuracy (%) | TP | FP | TN | FN | SN (%) | SP (%) | Accuracy (%) | ||
| Walker et al.6 | 6 | 7 | 5 | 2 | 75 | 42 | 55 | Semiquantitative | 7 | 0 | 12 | 1 | 88 | 100 | 95 |
| Visual rating | 7 | 2 | 10 | 1 | 88 | 83 | 85 | ||||||||
| Walker and Walker7 | 8 | 7 | 6 | 2 | 80 | 46 | 61 | Semiquantitative | 10 | 1 | 12 | 0 | 100 | 92 | 96 |
| Thomas et al.8 | 26 | 7 | 18 | 4 | 87 | 72 | 80 | Visual rating | 24 | 2 | 23 | 6 | 80 | 92 | 85 |
| Present study | 11 | 1 | 5 | 1 | 92 | 83 | 89 | Visual rating | 11 | 1 | 5 | 1 | 92 | 83 | 89 |
| Combined | 51 | 22 | 34 | 9 | 85 | 61 | 73 | 59 | 6 | 62 | 9 | 87 | 91 | 89 | |
TP, true positive; FP, false positive; TN, true negative; FN, false negative; SN, sensitivity; SP, specificity.
In earlier studies, clinical diagnosis of DLB showed decreased diagnostic accuracy in comparison to 123I‐FP‐CIT SPECT as the clinical diagnosis was made during the first clinical encounter by a single clinician without the input of an expert panel.6, 7 In the present study, clinical evaluation and 123I‐FP‐CIT SPECT overall yielded the same diagnostic accuracy in the group of 18 patients with dementia. However, 123I‐FP‐CIT SPECT was useful for predicting LBD pathology in a patient (Case 11) who lacked the core features of DLB, making the clinical diagnosis of DLB difficult. Similarly, 123I‐FP‐CIT SPECT predicted absence of LBD pathology in a patient (Case 3) whose diagnosis between pure clinical DLB and ADem was challenging as he met the clinical criteria of ADem, but also had RBD and characteristic cognitive impairment of DLB without other core DLB features. As such, 123I‐FP‐CIT SPECT may be particularly helpful when atypical clinical features or diagnostic ambiguities are present. However, 123I‐FP‐CIT SPECT alone is not sufficient to make a diagnosis of DLB, and should be used in conjunction with clinical features for the diagnosis of DLB.5
In this series, the results from visual inspection of 123I‐FP‐CIT SPECT were mostly in agreement with mPOR. When discrepancy between visual rating and mPOR was present (Cases 5 and 18), mPOR appeared to be more consistent with the neuropathological findings. A potential limitation of visual rating in this study was the lack of assessment of interrater reliability as each 123I‐FP‐CIT SPECT was read by a single radiologist. However, evaluating the diagnostic utility of 123I‐FP‐CIT SPECT based on single radiologist interpretation is still meaningful as it mimics common clinical practice. Determining the superiority of semiquantitative analysis of 123I‐FP‐CIT SPECT over visual rating was not possible in this study as the lack of mPOR data in a sufficient number of neurologically normal controls made the determination of abnormal mPOR cut‐off difficult. A study comparing the qualitative and quantitative analyses of 123I‐FP‐CIT SPECT is underway.
Another potential limitation of the study was a relatively small sample size. However, autopsy validation of 123I‐FP‐CIT SPECT for predicting LBD strengthens the study findings. In addition, the results of the study were consistent with the findings of larger European studies, including ~10% of cases with LBD pathology with normal findings on 123I‐FP‐CIT SPECT.6, 7, 8 One would also predict neurodegenerative disorders associated with dementia plus striatonigral pathology such as progressive supranuclear palsy and corticobasal degeneration would have reduced uptake on 123I‐FP‐CIT SPECT, but since none of our patients had such pathologies, the generalizability to the full spectrum of patients with dementia cannot be addressed in this series. Another limitation was that the patients were evaluated by behavioral neurologists and their 123I‐FP‐CIT SPECT findings were interpreted by radiologists at a large tertiary referral center, which likely increased the clinical and imaging diagnostic accuracy. A larger prospective multicenter study assessing the diagnostic utility of 123I‐FP‐CIT SPECT in DLB with neuropathologic validation would increase the generalizability of the present findings.
Author Contributions
All authors were involved in the concept and design of the study, data acquisition and/or analysis for the study, and drafting and/or editing the manuscript and the figures.
Conflicts of Interest
Youngsin Jung, Lennon G. Jordan III, Joseph E. Parisi, Dennis W. Dickson, Melissa E. Murray, Ross R. Reichard, Tanis J. Ferman, David T. Jones, Jonathan Graff‐Radford, Rodolfo Savica, Mary M. Machulda, Julie A. Fields, Laura A. Allen, Daniel A. Drubach and Michael H. Silber had nothing to disclose. Val J. Lowe serves on scientific advisory boards for Bayer Schering Pharma, Piramal Life Sciences which hold patents for and sells PET radiopharmaceuticals; receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, and the NIH. Kejal Kantarci serves on the data safety monitoring board for Takeda Global Research & Development Center, Inc., and receives research support from the NIH. Erik K. St. Louis serves as consultant for Axovant, Inc.; receives research support from Mayo Clinic CCaTS, NIH/NINDS, NIH/NHLBI, Axovant, Inc., and Sunovion, Inc.; and receives book royalties from Wiley‐Blackwell. Clifford R. Jack, Jr. serves on scientific advisory board for Eli Lilly & Company; receives research support from the NIH/NIA, and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation; and holds stock in Johnson & Johnson. David S. Knopman serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by TauRX Pharmaceuticals, Lilly Pharmaceuticals, Biogen, and the Alzheimer's Disease Cooperative Study; and receives research support from the NIH. Ronald C. Petersen serves on data monitoring committees for Pfizer, Inc., Janssen Alzheimer Immunotherapy, and is a consultant for Biogen, Roche, Inc., Merck, Inc., and Genentech, Inc.; receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003), and receives research support from the NIH. Bradley F. Boeve is investigator for clinical trials sponsored by GE Healthcare and Axovant; receives royalties from the publication of a book entitled Behavioral Neurology Of Dementia (Cambridge Medicine, 2009, 2016); serves on the Scientific Advisory Board of the Tau Consortium; receives research support from the NIH, Mangurian Foundation, and the Little Family Foundation. Role of Sponsor: The staff at GE Healthcare was neither involved in the analysis and interpretation of the data nor in the preparation of this manuscript.
Acknowledgments
This study was supported by GE Healthcare, NIH grants AG015866, AG016574, and UL1 TR000135, the Mangurian Foundation, an anonymous donor, and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program of the Mayo Foundation. We thank the staff of Mayo Alzheimer's Disease Research Center and especially the subjects and family members involved in the study.
Funding Statement
This work was funded by GE Healthcare grant ; NIH grants AG015866, AG016574, and UL1 TR000135; Mangurian Foundation grant ; Mayo Foundation grant .
References
- 1. Vann Jones SA, O'Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med. 2014;44:673–683. [DOI] [PubMed] [Google Scholar]
- 2. Savica R, Grossardt BR, Bower JH, et al. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol 2013;70:1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McKeith I, O'Brien J, Walker Z, et al. Sensitivity and specificity of dopamine transporter imaging with 123I‐FP‐CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol 2007;6:305–313. [DOI] [PubMed] [Google Scholar]
- 4. Colloby SJ, McParland S, O'Brien JT, et al. Neuropathological correlates of dopaminergic imaging in Alzheimer's disease and Lewy body dementias. Brain 2012;135:2798–2808. [DOI] [PubMed] [Google Scholar]
- 5. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walker Z, Jaros E, Walker RW, et al. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP‐CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry 2007;78:1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker RW, Walker Z. Dopamine transporter single photon emission computerized tomography in the diagnosis of dementia with Lewy bodies. Mov Disord 2009;24(Suppl 2):S754–S759. [DOI] [PubMed] [Google Scholar]
- 8. Thomas AJ, Attems J, Colloby SJ, et al. Autopsy validation of 123I‐FP‐CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology 2017;88:276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 10. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 12. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 13. Gorno‐Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol 2003;54(Suppl 5):S15–S19. [DOI] [PubMed] [Google Scholar]
- 15. Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. International Classification of Sleep Disorders. 3rd ed Darien, IL: American Academy of Sleep Medicine, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DaTscan Protocol Manual . Available http://us.datscan.com/wp-content/uploads/2016/07/JB39854US-US-DaTscan-Protocol-Manual-digital-secure.pdf (Accessed December 3, 2017)
- 18. Braak H, Braak E. Neuropathological staging of Alzheimer‐related changes. Acta Neuropathol 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 19. Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging‐Alzheimer's Association guideline for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 2012;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 21. Thal DR, Rub U, Orantes M, Braak H. Phases of A beta‐deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–1800. [DOI] [PubMed] [Google Scholar]
- 22. Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 2010;119:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


