Abstract
Objective
We aimed to evaluate the impact of underlying mechanism of basilar artery (BA) occlusion on the outcomes after endovascular therapy (EVT) for reperfusion and the outcome factors associated with each mechanism, and to identify radiologic parameters enabling to distinguish the underlying mechanism.
Methods
From a registry database, 194 consecutive patients with acute BA occlusion who underwent EVT were analyzed. Stroke mechanism, classified into in situ atherosclerotic thrombosis (ISAT) and embolism, clot sign location profiles in pre‐angiography magnetic resonance image (MRI), parameters of angiography and EVT, and reperfusion were assessed. Poor outcome was defined as a modified Rankin‐Scale score at 90 days of 3–6.
Results
The mean age was 68.8±11.8 years (range 21–92 years) and seventy‐eight (40.2%) were female patients. 102 (52.6%) patients were classified into an embolism mechanism and 92 (47.4%) into an ISAT mechanism. Overall, ISAT mechanism compared with embolism was significantly associated with poor outcomes (P = 0.002), along with the NIHSS scores, reperfusion status, and collateral status. In the embolism group, reperfusion (P = 0.001), NIHSS scores (P < 0.001), and onset‐to‐treatment time (P = 0.030) were significant outcome factors. However, in the ISAT group, baseline collateral status (P = 0.001) and NIHSS scores (P < 0.001) were significant outcome factors. A clot sign at the distal BA segment on pre‐angiography MRI was strongly associated with embolism mechanism (P < 0.001).
Interpretation
Stroke mechanism has a major influence on outcomes, and outcome predictors differ according to the underlying mechanism in acute BA occlusion with EVT. Clot sign profile on pre‐angiography MRI might be useful to determine the underlying mechanism.
Introduction
Stroke caused by an acute basilar artery (BA) occlusion is associated with poor clinical outcomes when treated conventionally.1, 2 Although the efficacy of endovascular therapies (EVT) over systemic thrombolysis has not been fully established,2, 3, 4, 5 achieving the reperfusion of BA territory might be crucial to improve the outcomes.6, 7, 8 Therefore, longer time windows are generally applied in the EVT for BA occlusion (up to 12–24 h from stroke onset), compared to the strokes in the anterior‐circulation.9 In this regard, investigating the factors that influence the outcomes after EVT is of fundamental value in demonstrating the clinical benefit of EVT and selecting the subjects who would benefit from EVT.
A recent study reported that the two main mechanisms of BA occlusion, in situ atherosclerotic thrombosis (ISAT) and embolism, may have a major influence on the response to EVT and long‐term outcomes.6, 10 Considering the distinct hemodynamic characteristic of BA compared to the anterior‐circulation arteries,9 the site of occlusion in BA segment may have a strong association with the underlying stroke mechanism and outcomes after EVT.11
In this study, we hypothesized that the clinical outcomes would be different between the two underlying mechanisms of BA occlusion. We aimed to evaluate the impact of underlying mechanism of BA occlusion on the outcomes after EVT and the outcome factors associated with each mechanism. Furthermore, we tried to identify a radiologic parameter representing the site of occlusion and might be useful in the early distinguishment of the mechanism of BA occlusion.
Materials and Methods
Study population
From a prospectively acquired registry database of all consecutive in‐patients with an acute ischemic stroke in two tertiary hospitals between January 2010 and March 2017, 2020 patients with a posterior‐circulation stroke were initially recruited. Among them, 287 patients were diagnosed with acute BA occlusion and 201 patients underwent EVT for reperfusion. An acute BA occlusion was defined as a stroke with time from onset to groin puncture less than 24 h,9 using the onset time of symptoms consistent with the diagnosis of acute BA syndrome,2, 8 or the last time that the patient appeared normal if the exact onset was unidentifiable.2, 4 This study was reviewed and approved by the institutional review board of Seoul National University Hospital. The requirement for informed consent was waived because the patients' medical information was de‐identified prior to use in our analysis.
Evaluation and treatment protocol
We obtained patients' premorbid information including age, sex, cerebrovascular risk factor profiles, and antithrombotic medication history. Stroke severity was assessed using the National Institute‐of‐Health Stroke‐Scale (NIHSS) scores at the initial visit.
For the 52 (26.8%) cases of hyper‐acute BA strokes (<4.5 h from the onset), intravenous tissue plasminogen activator (tPA, 0.6–0.9 mg/kg) was administered after obtaining the CT images. Due to the longer time windows applied for the EVT for BA occlusion (up to 12–24 h from stroke onset),9 MRI and MR angiography (MRA) images were obtained before the angiographic evaluation in most (191/194. 98.5%) cases, to evaluate the exact extent of ischemic involvement and the risk of stroke progression that could be prevented by the EVT.
All angiographic procedures were performed on a biplanar system (Integris Allura 12/12; Philips) via the transfemoral approach by two or more trained neuro‐interventionists. The devices used for mechanical thrombectomy were categorized as a stent retriever, including Solitaire (Covidien, Dublin, Ireland) and Trevo (Stryker, Mountain View, CA), and a non‐stent retriever such as MERCI (Stryker) and Penumbra (Penumbra, Alameda, CA).4 Intra‐arterial (IA) infusion of thrombolytic agents including pro‐urokinase, urokinase, tPA, or reteplace, balloon‐assisted angioplasty, or stent placement in the BA were performed complementary to the mechanical thrombectomy, based on the clinician's decision. We also recorded the use of anticoagulation agents or combined antithrombotic agents after EVT. Delayed reocclusion was designated as a deterioration of the neurological status associated with a documentation of reocclusion of BA on follow‐up evaluations such as CT angiography, MRA, or conventional angiography, after a successful reperfusion was decided at the end of the initial angiographic procedure.
Determination of the stroke mechanism
We categorized the mechanism underlying BA occlusion into in situ atherosclerotic thrombosis (ISAT) and embolism, based on that this classification better discriminates the different clinical profiles and outcomes between the mechanisms than the traditionally used Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification.6 Using the Stop Stroke Study TOAST (SSS‐TOAST) algorithm12 and the method introduced by Kim et al.6 with modification, evidences of ISAT mechanism from embolism were designated as: (1) a detection of moderate to severe (≥50%) stenosis or a stenosis with significant distal flow disturbance at the target arterial lesion when successful reperfusion was achieved,6, 12, 13 and (2) transient visualization of eccentric plaque contour or a recurrent reocclusion tendency in cases wherein reperfusion was unsuccessful.12 When no evidence of ISAT was demonstrated, complete recanalization with no residual stenosis in BA, or an established source of embolism with or without reperfusion were categorized as embolism.6 Patients with a mild residual stenosis or an unsuccessful reperfusion, without an embolic source, were determined to have an undeterminable mechanism and excluded (Fig. 1).12 Determination of stroke mechanism was performed by two neurologists (KHJ, 16 years of experience and WJL, 5 years of experience) independently of each other, blinded to all clinical information and MRI data. Inter‐observer agreement of the stroke mechanism determination was evaluated using Cohen's weighted Kappa. In cases of discrepancy, stroke mechanism was decided by consensus.
Figure 1.
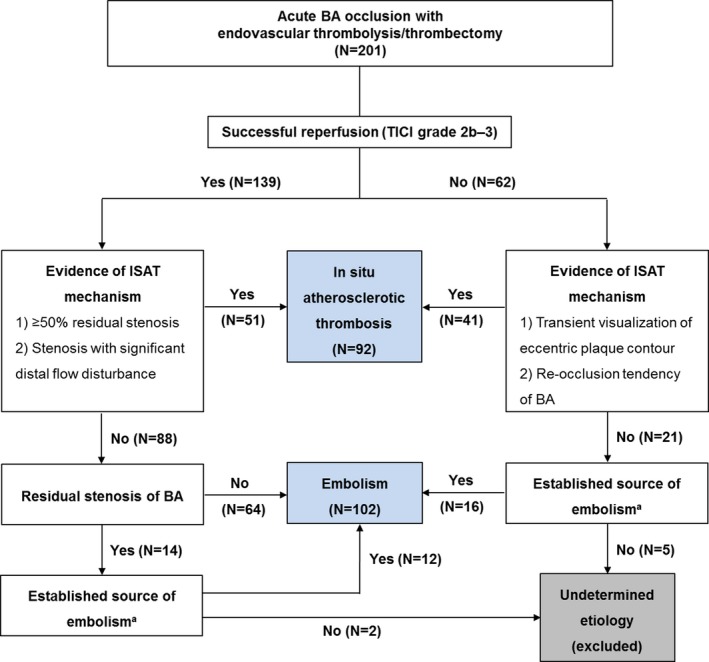
Algorithm used to determine stroke mechanism. BA: basilar artery, mTICI: modified‐Thrombolysis in Cerebral Infarction, ISAT: in situ atherosclerotic thrombosis.
aPresence of a cardiac source with high primary risk for ischemic stroke, listed in the SSS‐TOAST classification algorithm, or a ≥ 50% stenosis or a fresh thrombus on atherosclerotic plaque in proximal arteries, or a vertebral artery dissection, or a systemic coagulopathy associated with cancer or rheumatic disease.
The source of embolism included a cardiac source with high primary risk for ischemic stroke, listed in the SSS‐TOAST classification algorithm,12 a ≥ 50% stenosis or a fresh thrombus on atherosclerotic plaque in proximal arteries, a dissection in vertebral arteries,6, 11, 12 and a systemic coagulopathy associated with cancer or rheumatic disease. To establish the source of embolism, we investigated continuous electrocardiography monitoring during the acute stroke phase, transthoracic echocardiography (TTE), and, if available, 24‐h heart‐rate monitoring, aortic CT angiography, or transesophageal echocardiography (TEE) performed in‐hospital or during follow‐up after discharge.12 Conventional TOAST classification of the study population was also evaluated.13
Angiography data
The time from stroke onset to groin puncture (onset‐to‐treatment) were categorized into <3, 3–6, 6–9, and >9 h throughout the analyses, according to the previous studies.2, 4 The location of BA occlusion was classified into proximal, middle, and distal, according to the most inferior extent of the obstruction. A proximal, middle, and distal BA segments were separated by the levels of the ramifications of the anterior‐inferior cerebellar arteries (AICAs) and the superior cerebellar arteries (SCAs), respectively.4, 14
Baseline collateral status was evaluated by the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) collateral grading system using four‐vessel angiography.15 Collateral scores were categorized into ASITN/SIR scores 0–1, 2, and 3–4.4 Reperfusion status was assessed using the modified Thrombolysis in Cerebral Infarction (mTICI) scale.15 Successful reperfusion was defined as an mTICI score of 2b–3.4, 5, 15 The duration of EVT was defined as the time from groin puncture to the last angiographic series.4 ASITN/SIR and mTICI scores were evaluated by a radiologist (YJR, 6 years of experience) and a neurologist (WJL), independently of each other, blinded to all clinical information and MRI data. In cases of discrepancy, consensus was made by the two evaluators. Inter‐observer agreements were evaluated using Cohen's weighted Kappa.
Clot sign analysis
Pre‐angiography MRI was performed using 1.5‐T or 3‐T scanners (Intera Achieva; Philips Medical Systems, Best, the Netherlands;, Signa Excite; GE Medical Systems, Milwaukee, WI, USA; and Verio; Siemens Medical Solutions, Erlangen, Germany) with an eight‐channel head coil. MRI protocols included diffusion‐weighted imaging (DWI), fluid‐attenuated inversion recovery (FLAIR), gradient‐echo (GRE) or susceptibility‐weighted imaging (SWI), intracranial time‐of‐flight (TOF) angiography, and neck angiography. The MRI parameters were as follows: 3800–5000/45–60 msec (TR [repetition time]/TE [echo time]) for DWI; 690–1100/15–24 msec for GRE; 28/20 msec for SWI; 9000–9900/97–162.9 msec for FLAIR; and 20–25/3–7 msec for both the intracranial TOF and neck angiography. The other parameters were as follows: section thickness, 5 mm with a 1 mm gap and field‐of‐view, 240 × 240 mm.
The clot sign was defined as a hypo‐intense signal within a vascular lumen at the level of occlusion with a diameter that exceeded that of the vessel proximal to the occlusion, on the GRE sequences in 161 patients.16 In 30 patients, SWI sequences were used for the clot sign analyses, based on that SWI images are more sensitive in detecting blood signals than GRE.17 The location profile of clots signs was classified into proximal, middle, and distal, according to the most distal part of the clot sign in the BA segment,14 by a radiologist (YJR) and a neurologist (WJL), blinded to all clinical information and angiography data. In cases of discrepancy, consensus was made by the two evaluators.
Outcome parameter
We designated the modified Rankin Scale at 90 days (mRS90) evaluated by the referring physician as the main outcome in this study. A favorable mRS90 was defined as a score of 0–2 and a poor mRS90 as a score of 3–6.
Statistical analysis
SPSS (version 22.0; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Data were reported as numbers (percentages), means ± standard deviations, or medians [interquartile ranges, IQR]. Student's t‐test was used to compare continuous variables. Categorical variables were compared using Pearson's chi‐square test. In every analysis, a two‐tailed P‐value <0.05 was regarded as statistically significant.
To evaluate the association of stroke mechanism with the clinical outcomes, a logistic regression analysis including the stroke mechanism and the previously recognized outcome parameters such as age, hypertension, NIHSS scores, location of occlusion, collateral status, onset‐to‐treatment time, and reperfusion/recanalization,2, 4, 5, 7, 10, 14, 18, 19, 20 as well as the parameters with a P < 0.20 in univariate analysis, was performed for the entire study population using a full model fit. Then, a full model fit logistic regression analyses for the outcome factors were performed again separately in each group of stroke mechanism.
To identify the pre‐angiographically available parameters which might be useful to discriminate the underlying stroke mechanism, logistic regression analyses were performed with a model‐building method.21 The clinical and MRI parameters with a potential significance (P < 0.20) were introduced into serial regression analyses in backward step‐down methods, deleting the variable with the largest P (if ≥0.05) from the next step.22 Using the likelihood ratio statistics, a model with the lowest model chi‐square was accepted. Pearson chi‐square, deviance, and Hosmer‐Lemeshow tests were evaluated to assess the goodness of fit.23 Model sensitivity and specificity to predict underlying stroke mechanism were also calculated.
Results
Among the 201 patients initially included in the study, seven patients with undetermined mechanism were excluded. The remaining 194 patients (mean age 68.8 ± 11.8, range 21–92 years) were included in the final analysis. According to the algorithm used, 92 (47.4%) patients were classified as the ISAT mechanism and 102 (52.6%) as the embolic mechanism (Fig. 1). To investigate the source of cardioembolism, TTE was performed in 177 (91.2%) s, 24‐h heart‐rate monitoring in 92 (47.4%), and TEE and/or aortic CT angiography in 30 (15.5%) patients. TEE and/or aortic CT angiography was performed more frequently in the embolism group and 24‐h heart‐rate monitoring was performed more frequently in the ISAT group (Table 1). The inter‐observer agreement of mechanism determination was excellent (0.92, 95% confidence interval [95% CI]:0.86–0.97). The profiles of embolic sources and TOAST classification in each stroke mechanism are summarized in Table 1.
Table 1.
Workup for cardioembolism, embolic sources, and TOAST classification profiles in each stroke mechanism
| In situ atherosclerotic thrombosis (N = 92) | Embolism (N = 102) | P | |
|---|---|---|---|
| Work up for an embolism source | |||
| Work up profiles | |||
| Diagnosed without additional workup | 18 (19.6) | 67 (65.7) | <0.001** |
| Transthoracic echocardiography | 81 (88.0) | 96 (94.1) | 0.143 |
| 24‐h heart‐rate monitoring | 58 (63.0) | 34 (33.3) | <0.001** |
| Transesophageal echocardiography/aortic CT angiography | 8 (8.7) | 22 (21.6) | 0.012* |
| Embolism source profile | |||
| Embolism source overall | 25 (27.2) | 96 (94.1) | <0.001** |
| Atrial fibrillation | 18 (19.6) | 70 (68.6) | <0.001** |
| Cardioembolic high‐risk sources other than atrial fibrillation1 | 2 (2.2) | 16 (15.7) | 0.001** |
| Embolic sources in proximal arteries2 | 7 (7.6) | 21 (20.6) | 0.009** |
| Systemic hypercoagulability3 | 1 (1.1) | 4 (3.9) | 0.203 |
| TOAST classification | |||
| Large‐artery atherosclerosis | 73 (79.3) | 21 (20.6) | <0.001** |
| Cardioembolism | 0 (0.0) | 71 (69.6) | <0.001** |
| Other determined | 0 (0.0) | 1 (1.0) | 0.344 |
| Undetermined (≥2 causes) | 19 (20.7) | 3 (2.9) | <0.001** |
| Undetermined (negative) | 0 (0.0) | 5 (4.9) | 0.025* |
| Undetermined (incomplete) | 0 (0.0) | 1 (1.0) | 0.344 |
Data are reported as number (percentage). *P < 0.05, **P < 0.01.
1 Seven with a mitral valve disease with a replacement surgery, four with dilated cardiomyopathy, four with a thrombus or a myxoma in left atrium, three with a large right‐to‐left shunt, and two with an akinetic left ventricular segment.
2 Twenty patients with a ≥ 50% stenosis, six with a dissection, and two with a thrombosed aneurysm in proximal arteries.
3 Three patients with cancer‐related hypercoagulability and two with hypercoagulability‐associated with rheumatic diseases.
The median initial NIHSS score was 16 [7–25]. Pre‐angiographic stage MRI was performed at 326.2 ± 377.4 min from onset and the clot sign was detected in 181 (93.3%) patients. Angiography was performed at 371.0 ± 271.0 min from onset. Baseline collateral status was evaluable in 184 (94.8%) patients and the median ASITN/SIR grade was 2 [1–3]. For EVT, a stent retriever was used in 142 (73.2%) patients and 35 (18.0%) patients underwent IA thrombolysis. After 74.8 ± 61.3 min duration of EVT, the median mTICI score was 2b [2a–3], and successful reperfusion was achieved in 137 (70.6%) patients. The inter‐observer agreements of ASITN/SIR and mTICI scores were good (0.69; 95% CI: 0.61–0.79 and 0.72; 95% CI: 0.65–0.80, respectively). The median mRS90 was 3 [2–5], and 127 (65.5%) patients had poor outcomes.
In the logistic regression analysis for the outcome predictors, a poor functional outcome was significantly associated with the ISAT mechanism compared to embolism (Odds ratio [OR]: 4.10, 95% CI: 1.67–9.92, P = 0.002), along with the NIHSS scores (P < 0.001), reperfusion status (P = 0.005), and ASITN/SIR grades (P = 0.006, Table 2, see Table 3 for the univariate analysis).
Table 2.
Multivariate analyses for a poor outcome
| Odds ratio | 95% CI | P | |
|---|---|---|---|
| Stroke mechanism (ISAT to embolism) | 4.10 | 1.67–9.92 | 0.002** |
| NIHSS scores | 1.14 | 1.08–1.20 | <0.001** |
| Successful reperfusion | 0.28 | 0.11–0.68 | 0.005** |
| Collateral status grade (categorized) | 0.50 | 0.31–0.82 | 0.006** |
ISAT: In situ atherosclerotic thrombosis, NIHSS: NIH Stroke‐Scale, CI: confidence interval. **P < 0.01. Incorporated parameters are: stroke mechanism, age, male sex, hypertension, NIHSS scores, location of occlusion, collateral status grade, onset‐to‐treatment time, and reperfusion. Recanalization, use of combined modality and anticoagulation were not included because they are largely influenced by the reperfusion status.
Table 3.
Intergroup comparisons of demographic, clinical, radiologic, treatment and outcome profiles
| Good outcomes mRS90 0–2 (N = 87) | Poor outcomes mRS90 3–6 (N = 107) | P | |
|---|---|---|---|
| Demographic and clinical profiles | |||
| Age | 65.7 ± 11.1 | 71.2 ± 11.9 | 0.001* |
| Male sex | 56 (64.4) | 60 (56.1) | 0.168 |
| Previous stroke history | 22 (25.3) | 33 (30.8) | 0.392 |
| Hypertension | 53 (60.9) | 79 (73.8) | 0.055 |
| Diabetes mellitus | 25 (28.7) | 34 (31.8) | 0.647 |
| Smoking in past 5 years | 21 (24.1) | 19 (17.8) | 0.276 |
| Hyperlipidemia | 14 (16.1) | 15 (14.0) | 0.688 |
| Antithrombotic use prior to attack | 37 (42.5) | 44 (41.1) | 0.843 |
| NIHSS scores (IQR) | 8 (5–16) | 23 (14–28) | <0.001** |
| Stroke mechanism | 0.017* | ||
| In situ atherosclerotic thrombosis | 33 (37.9) | 59 (55.1) | |
| Embolism | 54 (62.1) | 48 (44.9) | |
| MRI and angiographic profiles | |||
| Onset to MRI (minutes)a | 337.8 ± 334.0 | 318.2 ± 310.5 | 0.714 |
| Clot signa | 79 (90.8) | 102 (95.3) | 0.317 |
| Location of occlusion | 0.008* | ||
| Proximal | 31 (35.6) | 49 (45.8) | |
| Middle | 22 (25.3) | 35 (32.7) | |
| Distal | 34 (39.1) | 23 (21.5) | |
| Collateral status gradeb | <0.001** | ||
| ASITN/SIR grade 0–1 | 15 (17.2) | 45 (42.1) | |
| ASITN/SIR grade 2 | 21 (24.1) | 33 (30.8) | |
| ASITN/SIR grade 3–4 | 47 (54.0) | 23 (21.5) | |
| Treatment profiles | |||
| Onset to treatment (minutes) | 0.254 | ||
| <3 h | 27 (31.0) | 22 (20.6) | |
| 3–6 h | 28 (32.2) | 46 (43.0) | |
| 6–9 h | 15 (17.2) | 15 (14.0) | |
| >9 h | 17 (19.5) | 24 (22.4) | |
| Treatment modality | |||
| Intravenous thrombolysis | 25 (28.7) | 27 (25.2) | 0.584 |
| IA thrombolysis | 20 (23.0) | 15 (14.0) | 0.133 |
| Stent retriever | 61 (70.1) | 81 (75.7) | 0.383 |
| Angioplasty/stent | 14 (16.1) | 20 (18.7) | 0.635 |
| Combined modality1 | 54 (62.1) | 76 (71.0) | 0.187 |
| Reperfusion status | <0.001** | ||
| mTICI 0–2a | 11 (12.6) | 46 (43.0) | |
| mTICI 2b–3 | 76 (87.4) | 61 (57.0) | |
| Recanalization status | 0.023* | ||
| AOL 0–2 | 42 (48.3) | 69 (64.5) | |
| AOL 3 | 45 (51.7) | 38 (45.5) | |
| Duration of angiography (minutes) | 55.2 ± 40.2 | 90.7 ± 70.5 | <0.001** |
| Anticoagulation | 35 (40.2) | 32 (29.9) | 0.133 |
| Combined antithrombotic agents | 57 (65.5) | 65 (60.7) | 0.494 |
| Delayed reocclusion | 4 (4.6) | 11 (10.3) | 0.131 |
Data are reported as number (percentage), as mean ± standard deviation, or as median (interquartile range, IQR). NIHSS: NIH Stroke‐Scale, MRI: magnetic resonance image, ASITN/SIR: American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology, mTICI: modified Thrombolysis in Cerebral Infarction Scale, AOL: arterial occlusive lesion scale, mRS90: modified Rankin Scale score at 90 days. *P < 0.05. **P < 0.01. Datasets available: a n = 191, b n = 184.
1 Combined modality: using two or more modalities during endovascular (intra‐arterial) treatment.
The two underlying mechanisms had a substantial difference on the outcome parameters. In the embolism group, reperfusion (P = 0.001), NIHSS scores (P < 0.001), and a longer onset‐to‐treatment time (P = 0.030) were significant outcome factors. However, in the ISAT group, the collateral status grades (P = 0.001) and NIHSS scores (P < 0.001) were significantly associated with a poor mRS90, while the reperfusion status or the onset‐to‐treatment time were not significantly associated with outcomes (Table 4 and Fig. S1, see Table S1 for the univariate analyses).
Table 4.
Multivariate analyses for outcome, separated by underlying mechanism
| mRS90 3–6 | Odds ratio | 95% CI | P |
|---|---|---|---|
| Embolism (N = 102) | |||
| NIHSS scores | 1.16 | 1.09–1.24 | <0.001** |
| Onset to treatment (categorized) | 2.04 | 1.07–3.89 | 0.030* |
| Successful reperfusion | 0.03 | 0.00–0.24 | 0.001** |
| In situ atherosclerotic thrombosis (N = 92) | |||
| NIHSS scores | 1.21 | 1.10–1.34 | <0.001** |
| Collateral status grade (categorized) | 0.20 | 0.08–0.54 | 0.001** |
mRS90: modified Rankin‐Scale score at 90 days, NIHSS: NIH Stroke‐Scale, CI: confidence interval. *P < 0.05, **P < 0.01. Incorporated parameters in each analysis are: stroke mechanism, age, male sex, hypertension, NIHSS scores, location of occlusion, collateral status grade, onset‐to‐treatment time, and reperfusion. Recanalization, use of combined modality and anticoagulation were not included because they are largely influenced by the reperfusion status.
In the study population, 12 patients with a stenosis of <50% and a coexisting embolic source were categorized into the embolism group.12, 13 When outcome analyses were re‐performed after excluding those patients, the remaining 182 patients had the same profiles of outcome parameters with the original population. (Tables S2 and S3). Additionally, the outcome factors in the embolism group (90 patients) were also same with those of the original population (Table S3).
The two underlying mechanisms also exhibited a substantial difference on the clinical, radiologic, and outcome profiles. Compared to embolism, ISAT was associated with a lower initial NIHSS scores, longer onset‐to‐treatment time, less frequent use of intravenous thrombolysis, more frequent use of angioplasty/stent, longer duration of angiography, and less successful reperfusion (Table 5). Especially, ISAT was associated with more proximal location of occlusion on angiography and more proximal involvement of clot signs, compared to embolism (all, P < 0.001).
Table 5.
Intergroup comparisons of demographic, clinical, radiologic, treatment and outcome profiles
| In situ atherosclerotic thrombosis (N = 92) | Embolism (N = 102) | P | |
|---|---|---|---|
| Demographic and clinical profiles | |||
| Age | 70.2 ± 10.6 | 67.4 ± 12.7 | 0.088 |
| Male sex | 49 (53.3) | 67 (65.7) | 0.078 |
| Previous stroke history | 30 (32.6) | 25 (24.5) | 0.211 |
| Hypertension | 62 (67.4) | 70 (68.6) | 0.722 |
| Diabetes mellitus | 30 (32.6) | 29 (28.4) | 0.528 |
| Smoking in past 5 years | 18 (19.6) | 22 (21.6) | 0.730 |
| Hyperlipidemia | 15 (16.3) | 14 (13.7) | 0.511 |
| NIHSS scores (IQR) | 12 (6–24.75) | 19.5 (9.75–26.25) | 0.007* |
| MRI and angiographic profiles | |||
| Clot signsa | 85 (92.4) | 96 (94.1) | 0.161 |
| Clot sign present atb | <0.001** | ||
| Distal BA segment | 5 (5.4) | 86 (84.3) | |
| Middle BA segment | 50 (54.3) | 10 (9.8) | |
| Proximal BA segment | 30 (32.6) | 0 (0.0) | |
| Onset to treatment (minutes) | 466.5 ± 291.2 | 277.0 ± 204.7 | <0.001** |
| Location of occlusion | <0.001** | ||
| Proximal | 64 (69.6) | 16 (15.7) | |
| Middle | 26 (28.3) | 31 (30.4) | |
| Distal | 2 (2.2) | 55 (53.9) | |
| Collateral status gradec | 0.316 | ||
| ASITN/SIR grade 0–1 | 23 (25.0) | 37 (36.3) | |
| ASITN/SIR grade 2 | 29 (31.5) | 25 (4.5) | |
| ASITN/SIR grade 3–4 | 34 (37.0) | 36 (35.3) | |
| Treatment/outcome profiles | |||
| Treatment | |||
| Intravenous thrombolysis | 12 (13.0 | 40 (39.2) | <0.001** |
| Stent retriever | 65 (70.7) | 77 (75.5) | 0.448 |
| IA thrombolysis | 16 (17.4) | 19 (18.6) | 0.823 |
| Angioplasty/stent | 29 (31.5) | 5 (4.9) | 0.001** |
| Combined modality1 | 67 (72.8) | 63 (61.8) | 0.101 |
| Successful reperfusion | 51 (55.4) | 86 (84.3) | <0.001** |
| Duration of angiography (minutes) | 87.4 ± 67.6 | 63.2 ± 52.1 | 0.006** |
Data are reported as number (percentage), as mean ± standard deviation, or as median [interquartile range, IQR]. NIHSS: NIH Stroke‐Scale, ASITN/SIR: American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology. *P < 0.05, **P < 0.01. Datasets available: a n = 191, b n = 181, c n = 184.
1 Combined modality: using two or more modalities during endovascular (intra‐arterial) treatment.
The multivariate logistic regression to identify the pre‐angiographically available parameters associated with the underlying stroke mechanism resulted in a model with a single variable, a clot sign at the distal BA segment (OR: 115.11, 95% CI: 39.34–336.79, P < 0.001, Table 6), that best predicted an embolism mechanism. Parameters for the goodness–of‐fit tests were not significant; 86/91 (94.5%) patients with a clot sign at distal basilar segment had an embolic mechanism, whereas 80/90 (88.9%) patients without a clot sign at distal basilar segment were associated with an ISAT (both, P < 0.001, Fig. 2). The inter‐reader agreement of the analyses of clot sign location was excellent (0.82, 95% CI 0.78–0.88). When the regression analyses were performed separately for the subpopulations that used GRE or SWI sequences, the same results were deduced (OR: 58.21, 95% CI: 20.00–169.44, P < 0.001 for GRE [n = 161] and OR: 76.92, 95% CI: 6.13–965.19, P < 0.001 for SWI [n = 30]).
Table 6.
Multivariate analysis for clinical and MRI parameters associated with underlying mechanism
| Embolism to in situ atherosclerosis | Odds ratio | 95% CI | P |
|---|---|---|---|
| Clot sign (+) at distal BA segment | 115.11 | 39.34–336.79 | <0.001** |
BA, basilar artery, CI; confidence interval. **P < 0.01.
Figure 2.
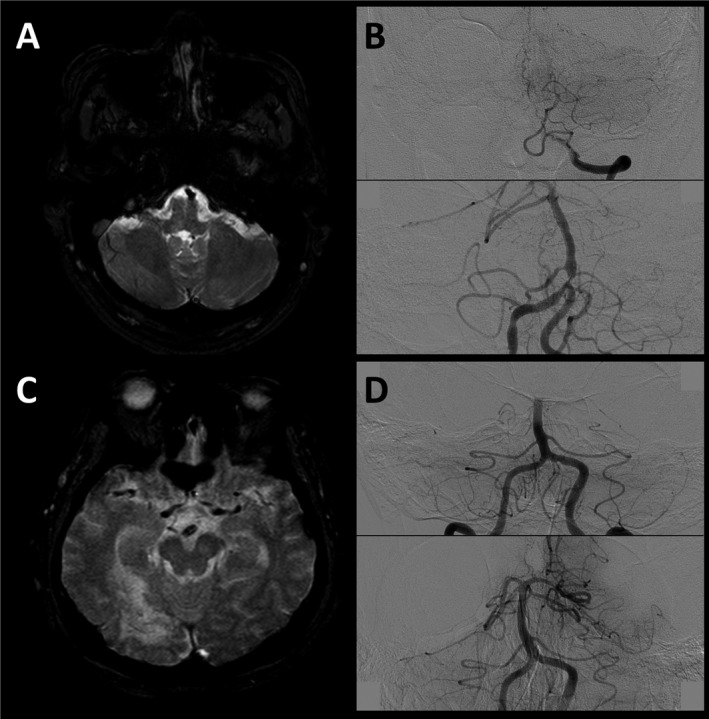
Representative cases. A 60‐year‐old male patient with initial NIH Stroke‐Scale (NIHSS) score of 18. In axial gradient‐echo images (GRE) of initial MRI, a clot in the proximal basilar segment is observed (panel A). Angiography reveals a basilar artery (BA) occlusion at the vertebra‐basilar junction level (panel B, upper). Immediate follow‐up angiography after achieving a successful reperfusion by endovascular treatment revealed a tight residual stenosis in the proximal BA, suggesting an in situ atherosclerotic stenosis mechanism (panel B, lower). In GRE images of an 85‐year‐old female patient with initial NIHSS score of 21, clot sign was detected in the distal basilar segment (panel C). BA was occluded at the middle segment (panel D, upper), and no residual stenosis was observed after a complete reperfusion, suggesting an embolic mechanism (panel D, lower).
Discussion
This study demonstrated that underlying mechanism has a substantial influence on the clinical profiles of BA occlusion and the outcomes after EVT. Furthermore, each stroke mechanism had distinct outcome factors, with outcomes of ISAT being associated with baseline collateral status, and outcomes of embolism with onset‐to‐treatment time and reperfusion status. Additionally, the presence of clot signs in the distal BA segment on axial GRE or SWI images of pre‐angiography MRI was strongly associated with an embolism mechanism.
Numerous studies have assessed the outcome predictors of BA occlusion after thrombolysis. While initial stroke severity has been repeatedly recognized as an outcome parameter, other factors such as atrial fibrillation,5 location of BA obstruction,19 onset‐to‐treatment time,4, 5, 24 and reperfusion4, 8, 18, 19, 25 were only inconsistently associated with outcomes. While the importance of collateral status and successful reperfusion is well established in anterior circulation,26 their implications have remained inconclusive in BA occlusive strokes.4 This might be attributable to the substantial impact of underlying stroke mechanism on the clinical behaviors and outcomes of BA occlusion, as ISAT mechanism is associated with milder initial severity, a longer onset‐to‐treatment time, lower response to endovascular treatment, and subsequent poorer long‐term outcomes, compared to the embolism, as observed in this study and previous reports.6, 24
Previous studies using the TOAST classification reported that stroke mechanism was not significantly associated with the outcomes.4, 5, 7 A considerable difference in the classification result between the TOAST and the algorithm used in this study might be a possible explanation. Twenty‐one out of ninety‐four (22.3%) of patients classified as a large‐artery atherosclerosis in TOAST classification were classified as embolism in this study, as they had a source of artery‐to‐artery embolism in the proximal arteries with atheromatous plaque. Twenty‐nine patients with undetermined or other‐determined mechanisms according to the TOAST criteria were also classified as either ISAT or embolism. This might suggest that the classification used in this study might appropriately reflect the underlying pathophysiology and clinical behaviors of the BA occlusive strokes.
Recently, a similar study regarding acute BA occlusion with endovascular treatment found no differences in recanalization rate and outcome between patients with and without underlying ISAT.27 However, the criterion for an ISAT used in that study was a severe (≥70%) stenosis of BA on the initial diagnostic angiography or on the follow‐up angiography after a reperfusion procedure. Therefore, at least transient recanalization of the occluded BA was required to determine an ICAS mechanism, unless the patient was diagnosed as a severe BA stenosis previously to the stroke event. In this regard, only 15/62 (24.2%) patients were classified as ISAT mechanism and all of them (15/15, 100%) achieved a successful reperfusion. As unsuccessful reperfusion is frequently associated with underlying ISAT mechanism, criteria of determining ISAT in the cases of unsuccessful reperfusion are necessary. In this study, transient visualization of eccentric plaque contour or recurrent reocclusion tendency was used as the criteria for ISAT. However, a careful discrimination of mechanism is warranted especially for the cases with unsuccessful reperfusion, although the inter‐observer agreement of mechanism determination was excellent.
In this study, onset‐to‐treatment time, collateral grade, and reperfusion statuses had discriminative impacts on outcomes according to stroke mechanism. In ISAT, collateral flow of the BA may have developed during the progression of atherosclerosis to compensate diminishing flow in the BA, resulting in BA territory perfusion that is not dependent on the patency of the BA.28, 29 Thus, the integrity of the backup collateral system, which might be related to the instability and the speed of plaque progression, would have more dominant influence on outcomes than the recanalization of BA itself. In this regard, onset‐to‐treatment time for a reperfusion therapy might not significantly influence the outcomes in ISAT. Meanwhile, in embolism, sudden deterioration of flow does not provide adequate time for the development of collateral flow. Therefore, fast and complete reperfusion of the BA might have a fundamental value, which explains that onset‐to‐treatment time and successfulness of reperfusion were recognized as significant factors for outcomes in this study.5, 8, 18, 19, 24, 25
Different strategies and treatment goals should be set according to the underlying mechanisms. For ISAT, angiography should include an evaluation of baseline collateral circulation, multiple modalities should be prepared to raise the possibility of successful reperfusion, and acute management after thrombolysis should focus on hemodynamic issues. However, for embolic strokes, minimizing the delay and optimizing the modalities to achieve a prompt and successful reperfusion might be the primary goal.
In this regard, analyzing the location profile of the BA occlusion is useful in early discrimination of stroke mechanism. As turbulent flow is mainly formed at vertebra‐basilar junctions,30 this site is the most frequent location of atherosclerotic plaque development. In contrast, as the BA has vertebral arteries in its proximity and posterior cerebral arteries (PCAs) distally of which luminal diameters were smaller than BA,31 an embolus drifting from the proximal vessels usually get stuck in the branching site between the BA and PCAs. Moreover, as the BA system has abundant collateral system including the retrograde flow from posterior communicating arteries, clot growth due to the obstruction‐related blood stasis might dominantly develop to caudal directions.9 Therefore, the location of the distal portion of clot sign might more properly represent the primary occlusion site than the proximal portion of clot, which explains how the presence of clot signs in the distal BA segment well correlates with an embolism mechanism.6, 11
This study possesses some limitations. First, the criteria used to determine stroke mechanism were not fully validated. Especially for the cases with unsuccessful reperfusion, an embolus with high fibrin components might mimic an eccentric atheromatous plaque, warranting a careful discrimination of mechanism. Second, different completeness of the embolic source evaluations between the groups might also influence the reliability of stroke mechanism determination. However, continuous electrocardiography monitoring was performed on every patient during the acute stroke phase and most of the patients received TTE as the basic evaluation. Additionally, 24‐h heart‐rate monitoring was performed more frequently in the ISAT group, which indicates that more thorough investigations were exerted to exclude an atrial fibrillation before determining and ISAT mechanism. Third, as EVT was performed according to the clinician's decision without a certain criteria being satisfied but a long time window, a selection bias might have been introduced in the study population. Fourth, the modalities and devices used in EVT were not standardized, which might have influenced the success of reperfusion. However, the types or numbers of modalities used in EVT showed no significant association with outcomes. A prospective study with homogenized treatment modalities and a predefined algorithm of mechanism classification is warranted to strengthen the findings of this study.
In conclusion, stroke mechanism has a significant impact on the clinical features and the outcomes in BA occlusion with endovascular reperfusion treatment. Analyzing the clot sign location on pre‐angiography MRI might be useful in the early determination of stroke mechanism.
Conflict of Interest
None.
Supporting information
Figure S1. Impact of successful reperfusion and baseline collateral status on stroke outcomes in each stroke mechanism.
Table S1. Univariate analyses for an outcome parameter, separated by underlying stroke mechanism.
Table S2. Intergroup comparisons of demographic, clinical, radiologic, treatment, and outcome profiles, after excluding 12 patients with stenosis of less than 50% and coexisting embolic sources.
Table S3. Multivariate analyses for a poor outcome, after excluding 12 patients with stenosis of less than 50% and coexisting embolic sources.
Acknowledgments
This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2016M3C7A1914002).
Funding Information
This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2016M3C7A1914002). Jung KH was supported by research grant from Korea United Pharma.Inc. (0620170340).
Funding Statement
This work was funded by Ministry of Science, ICT & Future Planning grant 2016M3C7A1914002; Korea United Pharma.Inc grant 0620170340.
Contributor Information
Keun‐Hwa Jung, Email: jungkh@gmail.com.
Chul‐Ho Sohn, Email: neurorad63@gmail.com.
References
- 1. Mattle HP, Arnold M, Lindsberg PJ, et al. Basilar artery occlusion. Lancet Neurol 2011;10:1002–1014. [DOI] [PubMed] [Google Scholar]
- 2. Schonewille WJ, Wijman CA, Michel P, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol 2009;8:724–730. [DOI] [PubMed] [Google Scholar]
- 3. Kumar G, Shahripour RB, Alexandrov AV. Recanalization of acute basilar artery occlusion improves outcomes: a meta‐analysis. J NeuroIntervent Surg 2015;7:868–874. [DOI] [PubMed] [Google Scholar]
- 4. Singer OC, Berkefeld J, Nolte CH, et al. Mechanical recanalization in basilar artery occlusion: the ENDOSTROKE study. Ann Neurol 2015;77:415–424. [DOI] [PubMed] [Google Scholar]
- 5. Strbian D, Sairanen T, Silvennoinen H, et al. Thrombolysis of basilar artery occlusion: impact of baseline ischemia and time. Ann Neurol 2013;73:688–694. [DOI] [PubMed] [Google Scholar]
- 6. Kim Y, Hong J, Park D, et al. Effect of intracranial atherosclerotic disease on endovascular treatment for patients with acute vertebrobasilar occlusion. Am J Neuroradiol 2016;37:2072–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jung S, Mono M‐L, Fischer U, et al. Three‐month and long‐term outcomes and their predictors in acute basilar artery occlusion treated with intra‐arterial thrombolysis. Stroke 2011;42:1946–1951. [DOI] [PubMed] [Google Scholar]
- 8. Nagel S, Schellinger PD, Hartmann M, et al. Therapy of acute basilar artery occlusion intraarterial thrombolysis alone vs bridging therapy. Stroke 2009;40:140–146. [DOI] [PubMed] [Google Scholar]
- 9. Lindsberg PJ, Pekkola J, Strbian D, et al. Time window for recanalization in basilar artery occlusion Speculative synthesis. Neurology 2015;85:1806–1815. [DOI] [PubMed] [Google Scholar]
- 10. Hwang Y‐H, Kim Y‐W, Kang D‐H, et al. Impact of target arterial residual stenosis on outcome after endovascular revascularization. Stroke 2016;47:1850–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baek J‐H, Kim BM, Kim DJ, et al. Importance of truncal‐type occlusion in stentriever‐based thrombectomy for acute stroke. Neurology 2016;87:1542–1550. [DOI] [PubMed] [Google Scholar]
- 12. Ay H, Furie KL, Singhal A, et al. An evidence‐based causative classification system for acute ischemic stroke. Ann Neurol 2005;58:688–697. [DOI] [PubMed] [Google Scholar]
- 13. Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 14. Voetsch B, DeWitt LD, Pessin MS, Caplan LR. Basilar artery occlusive disease in the New England Medical Center posterior circulation registry. Arch Neurol 2004;61:496–504. [DOI] [PubMed] [Google Scholar]
- 15. Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke a consensus statement. Stroke 2013;44:2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soize S, Batista A, Rodriguez Regent C, et al. Susceptibility vessel sign on T2* magnetic resonance imaging and recanalization results of mechanical thrombectomy with stent retrievers: a multicentre cohort study. Eur J Neurol 2015;22:967–972. [DOI] [PubMed] [Google Scholar]
- 17. Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility‐weighted imaging: technical aspects and clinical applications, part 2. Am J Neuroradiol 2009;30:232–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sairanen T, Strbian D, Soinne L, et al. Intravenous thrombolysis of basilar artery occlusion predictors of recanalization and outcome. Stroke 2011;42:2175–2179. [DOI] [PubMed] [Google Scholar]
- 19. Cross D, Moran CJ, Akins PT, et al. Relationship between clot location and outcome after basilar artery thrombolysis. Am J Neuroradiol 1997;18:1221–1228. [PMC free article] [PubMed] [Google Scholar]
- 20. Lee W‐J, Jung K‐H, Ryu YJ, et al. Acute symptomatic basilar artery stenosis: MR imaging predictors of early neurologic deterioration and long‐term outcomes. Radiology 2016;280:193–201. [DOI] [PubMed] [Google Scholar]
- 21. Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 22. Sun G‐W, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J Clin Epidemiol 1996;49:907–916. [DOI] [PubMed] [Google Scholar]
- 23. Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness‐of‐fit tests for the logistic regression model. Stat Med 1997;16:965–980. [DOI] [PubMed] [Google Scholar]
- 24. Greving JP, Schonewille WJ, Wijman CA, et al. Predicting outcome after acute basilar artery occlusion based on admission characteristics. Neurology 2012;78:1058–1063. [DOI] [PubMed] [Google Scholar]
- 25. Arnold M, Nedeltchev K, Schroth G, et al. Clinical and radiological predictors of recanalisation and outcome of 40 patients with acute basilar artery occlusion treated with intra‐arterial thrombolysis. J Neurol Neurosurg Psychiatry 2004;75:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bang OY, Saver JL, Kim SJ, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke 2011;42:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee Y, Yoon W, Kim S, et al. Acute basilar artery occlusion: differences in characteristics and outcomes after endovascular therapy between patients with and without underlying severe atherosclerotic stenosis. Am J Neuroradiol 2017;38:1600–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liebeskind DS, Cotsonis GA, Saver JL, et al. Collateral circulation in symptomatic intracranial atherosclerosis. J Cereb Blood Flow Metab 2011;31:1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liebeskind DS, Cotsonis GA, Saver JL, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol 2011;69:963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akins JFC, Hutson D, Chandler AB. Localization of atherosclerotic lesions in the human basilar artery. Atherosclerosis 1980;35:77–86. [DOI] [PubMed] [Google Scholar]
- 31. Kamath S. Observations on the length and diameter of vessels forming the circle of Willis. J Anat 1981;133(Pt 3):419. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Impact of successful reperfusion and baseline collateral status on stroke outcomes in each stroke mechanism.
Table S1. Univariate analyses for an outcome parameter, separated by underlying stroke mechanism.
Table S2. Intergroup comparisons of demographic, clinical, radiologic, treatment, and outcome profiles, after excluding 12 patients with stenosis of less than 50% and coexisting embolic sources.
Table S3. Multivariate analyses for a poor outcome, after excluding 12 patients with stenosis of less than 50% and coexisting embolic sources.


