Abstract
Background
Despite great progress in studies on Wolbachia infection in insects, the knowledge about its relations with beetle species, populations and individuals, and the effects of bacteria on these hosts, is still unsatisfactory. In this review we summarize the current state of knowledge about Wolbachia occurrence and interactions with Coleopteran hosts.
Methods
An intensive search of the available literature resulted in the selection of 86 publications that describe the relevant details about Wolbachia presence among beetles. These publications were then examined with respect to the distribution and taxonomy of infected hosts and diversity of Wolbachia found in beetles. Sequences of Wolbachia genes (16S rDNA, ftsZ) were used for the phylogenetic analyses.
Results
The collected publications revealed that Wolbachia has been confirmed in 204 beetle species and that the estimated average prevalence of this bacteria across beetle species is 38.3% and varies greatly across families and genera (0–88% infected members) and is much lower (c. 13%) in geographic studies. The majority of the examined and infected beetles were from Europe and East Asia. The most intensively studied have been two groups of herbivorous beetles: Curculionidae and Chrysomelidae. Coleoptera harbor Wolbachia belonging to three supergroups: F found in only three species, and A and B found in similar numbers of beetles (including some doubly infected); however the latter two were most prevalent in different families. A total of 59% of species with precise data were found to be totally infected. Single infections were found in 69% of species and others were doubly- or multiply-infected. Wolbachia caused numerous effects on its beetle hosts, including selective sweep with host mtDNA (found in 3% of species), cytoplasmic incompatibility (detected in c. 6% of beetles) and other effects related to reproduction or development (like male-killing, possible parthenogenesis or haplodiploidy induction, and egg development). Phylogenetic reconstructions for Wolbachia genes rejected cospeciation between these bacteria and Coleoptera, with minor exceptions found in some Hydraenidae, Curculionidae and Chrysomelidae. In contrast, horizontal transmission of bacteria has been suspected or proven in numerous cases (e.g., among beetles sharing habitats and/or host plants).
Discussion
The present knowledge about Wolbachia infection across beetle species and populations is very uneven. Even the basic data about infection status in species and frequency of infected species across genera and families is very superficial, as only c. 0.15% of all beetle species have been tested so far. Future studies on Wolbachia diversity in Coleoptera should still be based on the Multi-locus Sequence Typing system, and next-generation sequencing technologies will be important for uncovering Wolbachia relations with host evolution and ecology, as well as with other, co-occurring endosymbiotic bacteria.
Keywords: α-proteobacteria, Beetles, Ecology, Endosymbiont, Evolution, Interactions, Intracellular
Introduction
The relations between the intracellular α-proteobacterium Wolbachia pipientis Hertig 1936 (hereafter Wolbachia) and its hosts from various groups of arthropods and nematodes have been the object of much research and numerous publications (O’Neill et al., 1992; Werren, Windsor & Guo, 1995; Weinert et al., 2015). The majority of these studies have focused on verifying endosymbiotic bacteria occurrence and diversity in various hosts at different levels: (i) among selected species sharing a geographic area (e.g., O’Neill et al., 1992; Werren, Windsor & Guo, 1995; 2000), (ii) among species inhabiting the same environment or that are ecologically-associated (e.g., Stahlhut et al., 2010), (iii) among species from particular taxonomic groups (e.g., Czarnetzki & Tebbe, 2004; Lachowska, Kajtoch & Knutelski, 2010; Sontowski et al., 2015), and (iv) within populations of selected taxa (e.g., Stenberg & Lundmark, 2004; Mazur et al., 2016). Another branch of research on the relations between Wolbachia and its hosts has focused on host species phylogenetics or population genetics, which is in some cases related to population differentiation and speciation (e.g., Kubisz et al., 2012; Montagna et al., 2014). In this research, Wolbachia is sometimes treated as an additional “marker”—a source of genetic data about the eco-evolutionary relations of its hosts. A third type of Wolbachia studies has concerned the direct or indirect effects of the infection on host fitness, development or survival at the individual and population levels (e.g., Weeks, Reynolds & Hoffmann, 2002; O’Neill, 2007). Moreover, in a separate branch of research (or in conjunction with the abovementioned types of studies), Wolbachia is often examined directly, mainly with respect to strain diversity, distribution and relations with other strains or different co-existing bacteria (Baldo & Werren, 2007). All these branches of research have substantially extended the knowledge about the relations between the most widespread intracellular endosymbiont—Wolbachia and its various hosts. Moreover, these studies have been expanded to encompass other bacteria with similar biologies and effects on hosts (like Cardinium, Spiroplasma, Rickettsia) (Zchori-Fein & Perlman, 2004; Goto, Anbutsu & Fukatsu, 2006; Duron et al., 2008; Weinert et al., 2015); however, a great majority of studies are still conducted on Wolbachia (Zug & Hammerstein, 2012). Recently, the various Wolbachia supergroups have been proposed to belong to several “Candidatus Wolbachia” species (Ramirez-Puebla et al., 2015); however, this approach has been criticized (Lindsey et al., 2016). Due to the uncertain species status of the “Candidatus Wolbachia” and because all previous studies considered these presumed different species as distant supergroups, in this review we have followed the previous Wolbachia taxonomy.
In summary, Wolbachia has been detected in 10–70% of examined hosts (Hilgenboecker et al., 2008; Zug & Hammerstein, 2012), depending on the geographical, ecological or taxonomical association of the selected species. Moreover, more detailed studies, at the population level, have shown that infection is not as straightforward as was assumed in the early stages of Wolbachia research. More and more species have been found to be only partially infected, e.g., in only some parts of their ranges or infection was associated with only some phylogenetic lineages (usually correlated with the distribution of mitochondrial lineages) (Clark et al., 2001; Roehrdanz et al., 2006). Furthermore, examples of multiply infected species and individuals have been reported, which has important consequences for the understanding of some of the effects of Wolbachia infection (Malloch, Fenton & Butcher, 2000). Wolbachia is known to have numerous effects on its hosts, among which the most interesting and important are those that disturb host reproduction, such as cytoplasmic incompatibility, thelytokous parthenogenesis, feminization of genetic males, male-killing, increased mating success of infected males via sperm competition and the host’s complete dependence on bacteria for egg production (for reviews see Werren, 1997; Werren & O’Neill, 1997; Stouthamer, Breeuwer & Hurst, 1999). Some of these effects are responsible for diversification of host populations and consequently Wolbachia have probably been involved in speciation (e.g., by the selective sweep of mtDNA or the whole genome of the infected host with the genome of bacteria; Keller et al., 2004; Mazur et al., 2016). This could be another major factor, additional to those already known, responsible for radiation of insects and particularly beetles.
There are several reviews summarizing the state of knowledge on Wolbachia infection among various taxonomic groups of nematodes and arthropods. Over the last years, such reviews have been prepared for the following groups: filarial nematodes (Filarioidea) (Taylor & Hoerauf, 1999; Casiraghi et al., 2001), crustaceans (Crustacea) (Cordaux, Bouchon & Greve, 2011), spiders (Araneae) (Goodacre et al., 2006; Yun et al., 2011), mites (Acari) (Chaisiri et al., 2015), springtails (Collembola) (Czarnetzki & Tebbe, 2004), Heteropteran Bugs (Heteroptera) (Kikuchi & Fukatsu, 2003), ants (Formicidae) (Russell, 2012), wasps (Hymenoptera: Apocrita) (Shoemaker et al., 2002) and butterflies (Lepidoptera) (Tagami & Miura, 2004). Surprisingly, there is no such review for beetles (Coleoptera), which include large number of diversified taxa, known from various habitats, and whose members belong to all major trophic guilds of animals. Some groups of beetles have been examined with respect to Wolbachia infection, but usually only with a limited coverage of species (e.g., weevils, Curculionidae, Lachowska, Kajtoch & Knutelski, 2010; leaf beetles; Chrysomelidae, Clark et al., 2001; Jäckel, Mora & Dobler, 2013; jewel beetles; Buprestidae, Sontowski et al., 2015 and minute moss beetles, Hydraenidae, Sontowski et al., 2015).
In this review we have summarized the current state of knowledge on the relations between beetles and Wolbachia by referring to all the abovementioned aspects of research. Moreover, we have highlighted future research directions concerning Wolbachia relationships with their diverse Coleopteran hosts.
Survey Methodology
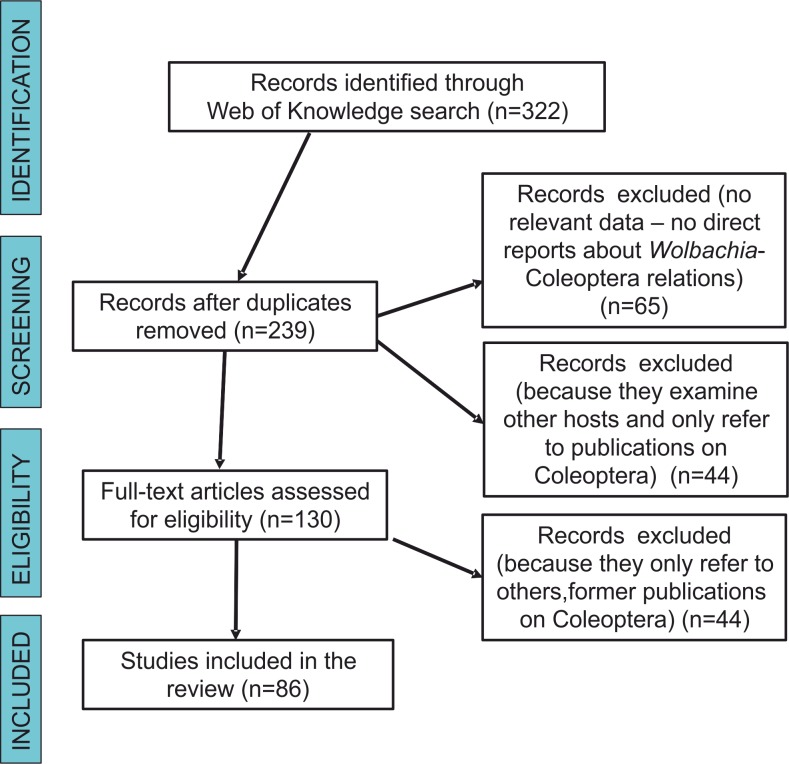
We searched the scientific literature with Web of Knowledge databases, using the following combination of keywords linked by AND (the Boolean search term to stipulate that the record should contain this AND the next term): “Wolbachia” AND “Coleoptera” and “Wolbachia” AND “beetles”. Our final literature search for this analysis was conducted on December 22, 2017. This produced 322 results. Each result was inspected to determine whether or not it contained information on the subject matter. Articles that had no relevance (e.g., any reports that were not about Wolbachia-Coleoptera relations, including those that only had some references to either beetles or bacteria in the citations) were excluded. After the removal of duplicates, 65 were excluded from the remaining articles (n = 239) for not being direct reports about Wolbachia-Coleoptera relations, 44 were excluded because they examined other hosts and only referred to publications on Coleoptera, and 44 others were excluded because they referred to data already presented in previous publications on Coleoptera. Each document was read critically for the information that it contained on Wolbachia-Coleoptera relations, with special reference to answering the study questions listed below. Figure 1 shows a flow diagram for the systematic review following Prisma guidelines (Moher et al., 2009). We intended to also use data from The National Center for Biotechnology Information database (GenBank) but the majority of hits (if “Wolbachia” AND “Coleoptera” or “beetle” were used) led to either studies not related with Wolbachia infection in beetles (which only included references to some other studies on either bacteria or beetles), or to Wolbachia sequences submitted to GenBank but without any references to published (and reviewed) articles. Searches in NCBI (GenBank) resulted only in the finding of some beetle hosts, which have been already described in papers found via Web of Science searches.
Figure 1. Prisma flow-diagram (see Moher et al., 2009) for literature on Wolbachia-Coleoptera relations included in this study.
We examined the collected data on various aspects of Wolbachia infection in Coleoptera with respect to the following: the (i) characteristics of the publications (to determine the scope and progress of studies on Wolbachia) (n = 86), (ii) geographic distribution of infected beetle species and populations (n = 84), (iii) sampling design (how many sites and individuals were examined) (n = 63), (iv) characteristics of the markers (genes) used for genotyping the bacteria (n = 82) and their hosts (n = 34), (v) numbers and frequencies of species found to be infected in particular beetle families and genera (n = 58), (vi) supergroup prevalence in examined taxonomic groups (n = 43), (vii) strain distribution and diversity in populations and individuals (n = 30), (vii) effects of Wolbachia on its beetle hosts (n = 39). Statistical analyses (Spearman correlation for number of publication across years and for the number of examined and number of infected species, Chi2 test for frequency of supergroups and infected taxa in particular taxonomic groups, Chi2 ANOVA for comparison of single/double/multiple infected taxa, Kruskal–Wallis Z test for infection frequency in Chrysomelidae and Curculionidae) were done in Statistica 11 (Statsoft).
Finally, we downloaded from GenBank (https://www.ncbi.nlm.nih.gov/genbank/) and the Wolbachia MLST database (https://pubmlst.org/wolbachia/) all available sequences of Wolbachia genes found in any species of beetle. We restricted further analyses to the most widely used bacteria genes, i.e., 16S rDNA and cell division protein gene ftsZ. Because of the different lengths and spans of available sequences, the long parts of the 3′ and 5′ ends of each gene were trimmed, which resulted in alignments of length 663 bp for 16S rDNA and 241 bp for ftsZ. The length of the ftsZ alignment was particularly short as two different sets of primers have been used for its amplification, and its amplicons only overlapped across a relatively short part of the gene. Phylogenetic trees were only reconstructed for unique gene variants found in particular host taxa. Trees were inferred using Maximum Likelihood (ML) implemented in the IQ-TREE web server (http://www.iqtree.org/) (Trifinopoulos et al., 2016) under the following settings Auto selection of substitution model, ultrafast bootstrap approximation (UFBoot) (Minh, Nguyen & Von Haeseler, 2013) with 10,000 iterations, maximum correlation coefficient = 0.99, single branch test with use of the approximate Likelihood-Ratio Test (SH-aLRT) (Anisimova & Gascuel, 2006; Guindon et al., 2010) and other default options.
The nomenclature of host taxa and their systematic positions throughout the paper follow the articles from which the data was derived.
Characterization of Wolbachia Infection Among Coleoptera
Publications
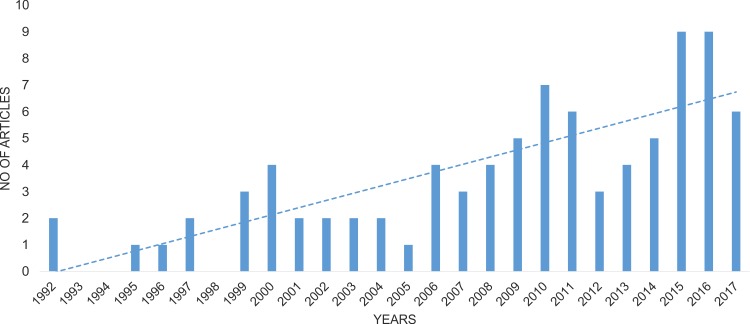
The final list of publications concerning data about Wolbachia infection in Coleoptera comprised 86 papers (Table S1). The oldest articles with relevant information about Wolbachia infection in beetles were published in 1992 (Campbell, Bragg & Turner, 1992; O’Neill et al., 1992), and the number of articles since then has increased significantly year by year (Spearman correlation = 0.841; Fig. 2). The majority of these articles (60%) concerned infection in only single beetle species, whereas 19% discussed infection in multiple species belonging to the same genus, 6%—multiple species from the same family, 6%—various species of Coleoptera et al., and a further 9%—studies on geographic groups of insects that included some, usually random species of beetles (O’Neill et al., 1992; Werren, Windsor & Guo, 1995; Weinert et al., 2015.
Figure 2. Change in the number of publications considering Wolbachia infection among Coleoptera.
Most studies were done on Curculionidae (34) and Chrysomelidae (34), following Coccinellidae (10), Tenebrionidae (9), and Sylvanidae (3) (Table S1). The members of all other families were investigated in only 1–2 studies. Consequently, 2.5 and 1.6 Curculionidae and Chrysomelidae species were respectively examined per article. All species of Hydraenidae and Buprestidae were included in only single article (Sontowski et al., 2015), whereas limited numbers of species of Coccinellidae and Tenebrionidae were examined in several articles (Hurst et al., 19991; Hurst et al., 1999b; Fialho & Stevens, 1996; Fialho & Stevens, 1997; Fialho & Stevens, 2000; Majerus & Majerus, 2000; Weinert et al., 2007; Elnagdy et al., 2013; Ming et al., 2015; Goodacre, Fricke & Martin, 2015; Kageyama et al., 2010; Li et al., 2015; Li et al., 2016b; Dudek et al., 2017). Wolbachia infection was only studied more than once in 20 species.
Sampling design
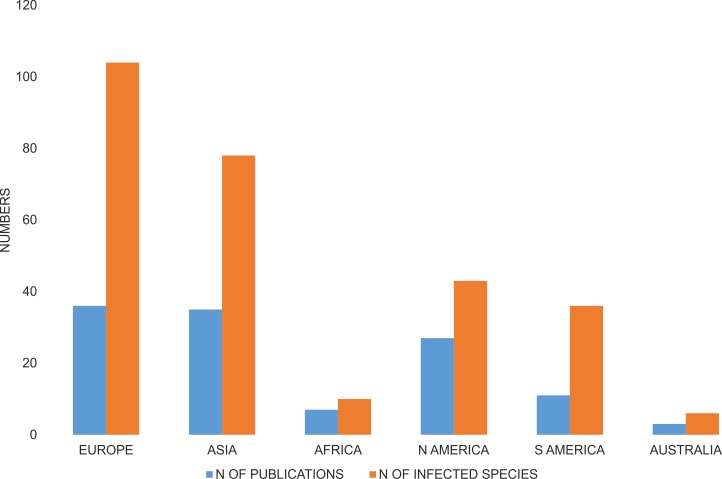
The majority of species investigated with respect to Wolbachia infection were from Europe, and a relatively high number of species were from Asia and both Americas, whereas only ten infected species were from Africa, and three from Australia-Oceania (Fig. 3). A number of publications describing Wolbachia infection in Coleoptera had similar geographic coverages (Fig. 3).
Figure 3. Number of publications that described Wolbachia infection among Coleoptera and number of infected beetle species.
Both are shown with respect to the zoogeography of the examined hosts (from which continent the host was collected).
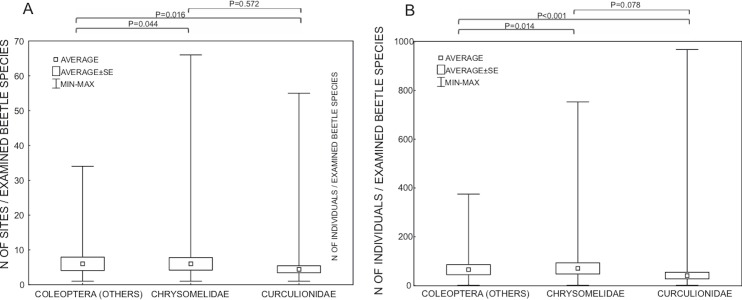
Studies were done on samples collected from an average of 5.2 sites and concerned on average 53.0 specimens, or if excluding the most widely studied families Curculionidae and Chrysomelidae, 6.0 sites and 65.1 individuals (Fig. 4). For Curculionidae and Chrysomelidae, these numbers were on average 4.4 and 6.0 sites, respectively, and 40.7 and 70.2 individuals, respectively (Fig. 4). The numbers of sites and individuals examined in particular groups were insignificantly different, with the exception of the numbers of examined individuals in Curculionidae and Chrysomelidae (Fig. 4).
Figure 4. Number of sites (A) and number of individuals (B) of beetles examined with respect to Wolbachia infection.
P—Mann-Whitney test p-values.
Examined genetic markers
The most often used Wolbachia gene for studies on Coleoptera was ftsZ, followed by hcpA, wsp and 16S rDNA (Fig. 5). Most studies using hcpA also used other MLST genes, including ftsZ. On the other hand, many species were only investigated with either 16S rDNA or wsp or ftsZ alone. Single studies used groEL (Monochamus alternatus, Aikawa et al., 2009; Tribolium madens, Fialho & Stevens, 2000) or ITS genes (Tribolium madens, Fialho & Stevens, 2000). So far, only five studies have used next-generation sequencing technology (Illumina or 454) to detect Wolbachia; two used 16S rDNA for metabarcoding of microbiota (Sitona obsoletus, Steriphus variabilis, White et al., 2015; Aleochara bilineata and Aleochara bipustulata, Bili et al., 2016; Hylobius abietis, Berasategui et al., 2016; Brontispa longissimi, Takano et al., 2017; Harmonia axyridis, Dudek et al., 2017) and one used shotgun genomic sequencing (Amara alpine, Heintzman et al., 2014). For genotyping of hosts, 52.4% of studies utilized fragments of COI from mtDNA (usually a barcode fragment of this gene). Fewer studies (23.1%) analyzed rDNA (usually ITS1 and/or ITS2 spacers), EF1α (14.0%), Wingless (2.2%), Histone H3 (2.2%) and microsatellites (6.1%). In Wolbachia-related studies, host genes have been used for several purposes like (i) using host DNA as a control for genetic material quality, (ii) barcoding for host species identification, (iii) phylogenetics, phylogeography and population genetics, (iv) estimating co-evolutionary relations between the bacteria and host, and (v) detecting some of the effects of Wolbachia on its hosts (like linkage disequilibrium, selective sweep, cytoplasmic incompatibility).
Figure 5. Shares of Wolbachia genes used in studies on Wolbachia infection among Coleoptera.
Taxonomic coverage
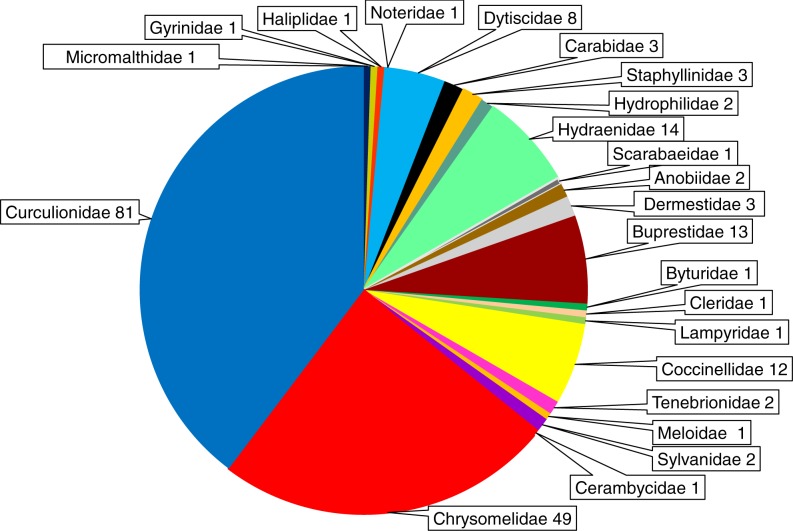
The beetles examined with respect to Wolbachia infection belong to 23 families (Micromalthidae, Gyrinidae, Haliplidae, Noteridae, Dytiscidae, Carabidae, Staphyllinidae, Hydrophilidae, Hydraenidae, Anobiidae, Dermestidae, Buprestidae, Byturidae, Cleridae, Lampyridae, Coccinellidae, Tenebrionidae, Scarabeidae, Meloidae, Sylvanidae, Cerambycidae, Chrysomelidae, Curculionidae). In total 204 beetle species were found to harbor Wolbachia infection; however, the distribution of infected species among families varied markedly. The highest numbers of infected beetle species were found for the Curculionidae (81 species), Chrysomelidae (49 species), Hydraenidae (14 species), Buprestidae (13 species), Coccinellidae (12 species) and Dytiscidae (8 species) (Fig. 6). In all other families only 1–3 species were reported to harbor Wolbachia (Table S1). However, these numbers are biased by the low number of articles (studies) dealing with members of particular beetle families (see above).
Figure 6. Shares of Wolbachia infected beetle species across the examined families of Coleoptera.
The numbers presented after the family names indicate the number of infected species.
Considering infection across beetle genera, the most richly infected genera were Altica (Chrysomelidae, 17 species), Naupactus (Curculionidae, 11 species), Hydraena (Hydraenidae, eight species) and Agrilus (Buprestidae, 6 species) (Table S1). In total, 49 genera were found to have infected members (Table S1, Table 1). The infection in Coleoptera was estimated at 38.3% of examined species; however, the proportion of infected species varied greatly between families and genera. At the family level the infection frequency was from 10.5% (Tenebrionidae) to 100% (Noteridae) (Goodacre, Fricke & Martin, 2015; Sontowski et al., 2015); however when considering only families for which more than 30 species were investigated (e.g., Clark et al., 2001; Lachowska, Kajtoch & Knutelski, 2010; Rodriguero et al., 2010a; Kondo et al., 2011; Jäckel, Mora & Dobler, 2013; Sontowski et al., 2015; Kawasaki et al., 2016), infection was found in up to 63% of species (Hydraenidae) (Table 1). At lower taxonomic levels, Wolbachia was found in 25% of Diabroticite (Chrysomelidae; Clark et al., 2001), 14.3–16.7% of Bruchina (Chrysomelidae; Kondo et al., 2011), 34.8% of Scolytinae (Curculionidae, Kawasaki et al., 2016) and 16.7% of Curculioninii (Toju et al., 2013). Among 54 genera in which Wolbachia infection was examined for at least two species, 12 genera were completely uninfected, while six genera were completely infected (Table 1). If considering only genera with at least five verified species, Wolbachia was found in 0% (Acmaeodera; Buprestidae; Sontowski et al., 2015) to 88% of species (Altica, Chrysomelidae; Jäckel, Mora & Dobler, 2013). There was only a marginally negative and insignificant correlation between the number of examined and number of infected species (R = − 0.078). If considering only the most widely examined families, Chrysomelidae and Curculionidae, the difference in infection frequency between these two groups was insignificant (Z = − 1.656, P = 0.098). Geographic studies on Wolbachia prevalence in insects have found much lower frequencies of infection in Coleoptera species: the bacterium was found in only 10.5% of beetles from Panama and 13.5% of beetles from North America (Werren, Windsor & Guo, 1995).
Table 1. Share of Wolbachia infected species among families and genera of examined beetles.
Only taxonomic groups for which at least two species were tested are presented.
| N of examined | % of infected | Genus | N of examined | % of infected | genus | N of examined | % of infected | |
|---|---|---|---|---|---|---|---|---|
| Family | ||||||||
| Buprestidae | 61 | 23.0 | Barypeithes | 9 | 11.0 | Julodis | 2 | 0.0 |
| Chrysomelidae | 84 | 45.2 | Brachysomus | 4 | 0.0 | Koreoculio | 2 | 50.0 |
| Curculionidae | 137 | 41.6 | Brumoides | ‘2 | 0.0 | Laccophilus | 2 | 0.0 |
| Dytiscidae | 36 | 16.7 | Buprestis | 3 | 0.0 | Limnebius | 7 | 28.6 |
| Gyrinidae | 3 | 33.3 | Byturus | 3 | 33.0 | Longitarsus | 3 | 100.0 |
| Haliplidae | 2 | 50.0 | Callosbruchus | 3 | 33.3 | Meliboeus | 2 | 0.0 |
| Hydraenidae | 27 | 63.0 | Callosobruchus | 7 | 33.0 | Micraspis | 2 | 0.0 |
| Hydrophilidae | 12 | 16.7 | Capnodis | 3 | 33.3 | Naupactus | 16 | 69.0 |
| Noteridae | 2 | 100.0 | Charidotella | 2 | 50.0 | Neoglanis | 2 | 0.0 |
| Tenebrionidae | 11 | 9.1 | Chlaenius | 7 | 14.3 | Ochthebius | 12 | 41.7 |
| Subfamily | Chrysobothris | 3 | 33.3 | Ophionea | 3 | 0.0 | ||
| Bruchinae | 24 | 16.7 | Coccinella | 2 | 50.0 | Oreina | 5 | 80.0 |
| Galerucinae | 12 | 25.0 | Crioceris | 5 | 40.0 | Otiorhynchus | 4 | 50.0 |
| Curculionidae | 36 | 16.7 | Curculio | 23 | 17.4 | Paederus | 3 | 0.0 |
| Scolytinae | 23 | 34.8 | Cyanapion | 6 | 50.0 | Pantomorus | 3 | 100.0 |
| Genus | Deronectes | 11 | 45.4 | Polydrosus | 4 | 75.0 | ||
| Acalymma | 2 | 100.0 | Diabrotica | 12 | 25.0 | Rhantus | 2 | 0.0 |
| Acmaeodera | 5 | 0.0 | Dorytomus | 3 | 67.0 | Rhinusa | 3 | 33.3 |
| Acmaeoderella | 4 | 0.0 | Epilachna | 2 | 0.0 | Sciaphobus | 2 | 50.0 |
| Agabus | 6 | 16.7 | Eurymetopus | 2 | 100.0 | Sitophilus | 3 | 100.0 |
| Agrilus | 34 | 17.6 | Gyrinus | 3 | 33.0 | Sphenoptera | 11 | 9.1 |
| Altica | 16 | 88.0 | Haliplus | 3 | 33.0 | Strophosoma | 3 | 67.0 |
| Anthaxia | 6 | 16.7 | Helophorus | 3 | 0.0 | Trachypteris | 2 | 0.0 |
| Aramigus | 3 | 100.0 | Hydraena | 24 | 33.3 | Trachys | 6 | 16.7 |
| Archarius | 6 | 16.7 | Hydroporus | 5 | 0.0 | Tribolium | 8 | 12.5 |
| Atrichonotus | 2 | 50.0 | Hygrotus | 5 | 20.0 | |||
| Aulacophora | 3 | 0.0 | Ilybius | 2 | 0.0 |
Wolbachia diversity
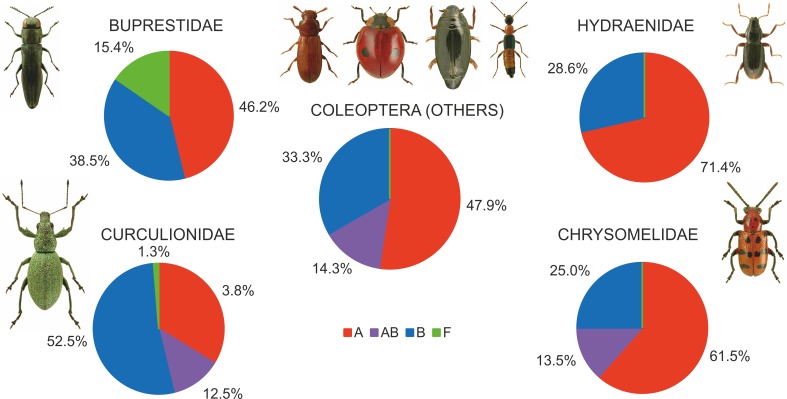
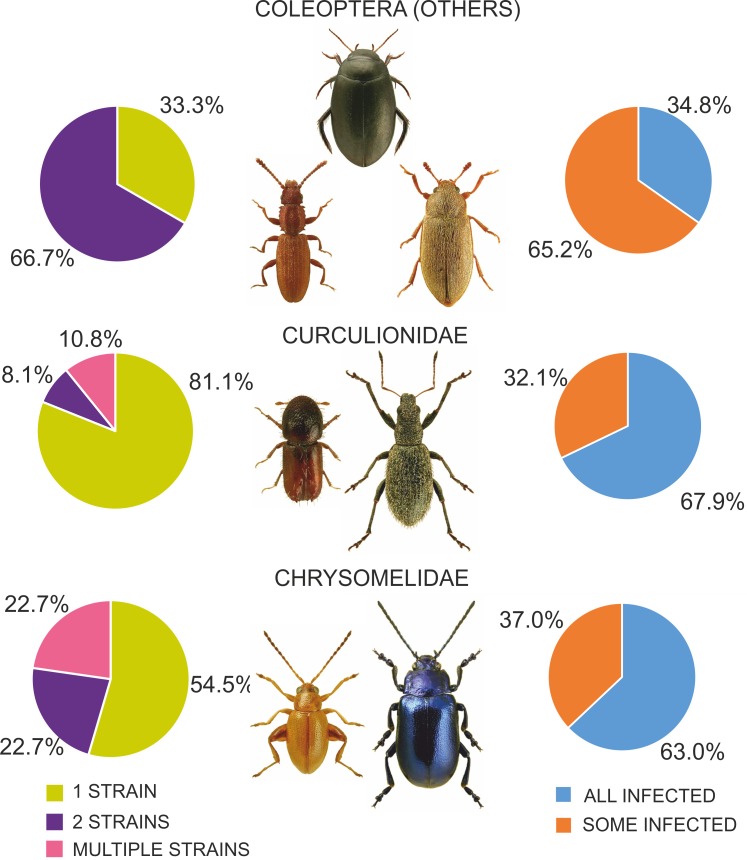
Among the various beetle species, Wolbachia strains belonged to three supergroups (A, B and F). However, they occurred at very different proportions in different groups of beetles, and these differences were significant (Chi2 = 98.78, P = 0.000). Overall, the proportion of beetle species found to be infected with Wolbachia strains belonging to supergroups A or B was similar, with approximately 12% of all species harboring either supergroup (either as single infections in different species or populations or as multiple infections within individuals) (Fig. 7), whereas supergroup F was found in only three beetle species: Agrilus araxenus and Lamprodila mirifica (both Buprestidae; Sontowski et al., 2015) and Rhinocyllus conicus (Curculionidae; Campbell, Bragg & Turner, 1992). In the four groups of beetles with the highest numbers of examined and infected species, the distributions of supergroups varied: in Buprestidae, a similar numbers of species were infected by supergroups A and B (all singly infected), with a relatively high proportion of F infected species (Sontowski et al., 2015). In contrast, in Hydraenida, supergroup A dominated over supergroup B (Sontowski et al., 2015). This was also the case in Chrysomelidae, with some species infected by both strains (Kondo et al., 2011; Jäckel, Mora & Dobler, 2013; Kolasa et al., 2017). The most varied infections were observed in Curculionidae, with supergroup B dominating, a presence of taxa infected by both A and B supergroups, and a single species infected by F supergroup (Lachowska, Kajtoch & Knutelski, 2010; Rodriguero et al., 2010a; Kawasaki et al., 2016) (Fig. 7). Considering the frequency of infected specimens in the examined beetle species represented by the available data (n = 106), 63 species were reported to be totally infected (all individuals possessed Wolbachia), whereas 43 species had this bacterium in only some individuals (if exclude Chrysomelidae and Curculionidae: 8 and 15 species, respectively) (Fig. 8). The same calculated for Chrysomelidae resulted in 17 and 10 species, respectively, and for Curculionidae in 38 and 18 species, respectively (Fig. 8). These differences between these values (between these groups of species) were significant (Ch2 = 72.03, P = 0.000). A single Wolbachia strain was observed in 43 species (species with available data n = 62), whereas two strains were reported in 10 species (Byturus tomentosus, Malloch, Fenton & Butcher, 2000; Altica quercetorum, Jäckel, Mora & Dobler, 2013; Callosobruchus chinensis, Okayama et al., 2016; Chelymorpha alternans, Keller et al., 2004; Crioceris quaterdecimpunctata and Crioceris quinquepunctata, Kolasa et al., 2017; Adalia bipunctata, Majerus & Majerus, 2000; Polydrusus inustus, Kajtoch, Korotyaev & Lachowska-Cierlik, 2012; Cyanapion afer and C. spencii, Kajtoch, Montagna & Wanat, 2018) and multiple infection in a further nine species (Callosobruchus chinensis, Kondo et al., 2002; Diabrotica barberi, Roehrdanz & Levine, 2007; Conotrachelus nenuphar, Zhang et al., 2010; Pityogenes chalcographus, Arthofer et al., 2009; Xyleborus dispar and Xylosandrus germanus, Kawasaki et al., 2016) (Fig. 8). In Chrysomelidae (n = 22) these numbers were 12, 5 and five, respectively and in Curculionidae (n = 37), 30, 3 and four, respectively (Fig. 8). The numbers of single, double and multiple infected individuals in these groups of beetles differed insignificantly (Chi2 ANOVA = 2.364, P = 0.307).
Figure 7. Shares of beetles infected by Wolbachia supergroups (A, B, F).
(Beetle photographs are from ICONOGRAPHIA COLEOPTERORUM POLONIAE (©Copyright by Prof. Lech Borowiec).
Figure 8. Diversity of Wolbachia infection in Coleoptera with respect to shares of infected individuals within species and numbers of strains found in beetles.
(Beetle photographs are from ICONOGRAPHIA COLEOPTERORUM POLONIAE (©Copyright by Prof. Lech Borowiec).
Effects on hosts
Wolbachia affected beetle hosts in several ways. Linkage disequilibrium and/or selective sweep between bacteria and host genomes (usually with host mtDNA) were detected in six species (3% or 9% if excluding Chrysomelidae and Curculionidae): two (4%) Chrysomelidae (Altica lythri, Jäckel, Mora & Dobler, 2013; Aphthona nigriscutis, Roehrdanz et al., 2006) and four (5%) Curculionidae (Eusomus ovulum, Mazur et al., 2016; Naupactus cervinus, Rodriguero, Lanteri & Confalonieri, 2010b, Polydrusus inustus, Polydrusus pilifer, Kajtoch, Korotyaev & Lachowska-Cierlik, 2012). Cytoplasmic incompatibility was detected or suspected but unconfirmed in 12 (6% or 18% if excluding Chrysomelidae and Curculionidae) Coleoptera: six (13%) Chrysomelidae (Chelymorpha alternans, Keller et al., 2004, Diabrotica barberi, Roehrdanz & Levine, 2007, et al., Diabrotica virgifera virgifera, Giordano, Jackson & Robertson, 1997; Callosobruchus chinensis, Kondo et al., 2002; Callosobruchus analis, Numajiri, Kondo & Toquenaga, 2017; Brontispa longissimi, Takano et al., 2017), three (4%) of Curculionidae (Cossomus sp., Zhang et al., 2010; Hypothenemus hampei, Mariño, Verle Rodrigues & Bayman, 2017, Xylosandrus germanus, Kawasaki et al., 2016), one of Sylvanidae (Oryzaephilus surinamensis, Sharaf et al., 2010) and one of Tenebrionidae (Tribolium confusum, Li et al., 2016b; Ming et al., 2015). Horizontal transfer of Wolbachia was detected or suspected in 26 species of Coleoptera (13% or 39% if excluding Chrysomelidae and Curculionidae)—16 (33%) species of Chrysomelidae (several species of Altica, Jäckel, Mora & Dobler, 2013, Crioceris quaterdecimpunctata and Crioceris quinquepunctata, Kolasa et al., 2017) and 10 (14%) species of Curculionidae (members of Euwallacea, Xyleborus, Xylosandrus, Xyleborinus schaufussi and Taphrorychus bicolor, Kawasaki et al., 2016, Polydrusus and Parafoucartia squamulata, Kajtoch, Korotyaev & Lachowska-Cierlik, 2012; Sitophilus oryzae and S. zaemais, Carvalho et al., 2014). Other effects of Wolbachia on beetles included the following: (i) transfer of bacteria genes to the autosomes of the host (so far detected only for Monochamus alternatus, Cerambycidae, Aikawa et al., 2009 and Callosobruchus chinensis, Chrysomelidae, Nikoh et al., 2008); (ii) coexistence of Wolbachia with Rickettsia (Calvia quattuordecimguttata, Coccidula rufa, Coccinella septempunctata, Halyzia sedecimguttata, Rhizobius litura, Weinert et al., 2007; Sitona obsoletus, White et al., 2015; Micromalthus debilis, Perotti, Young & Braig, 2016) in the host or with Spiroplasma (Chilocorus bipustulatus, Weinert et al., 2007; Aleochara bipustulata, Bili et al., 2016) or with both (Adalia bipunctata, Majerus & Majerus, 2000, Harmonia axyridis, Dudek et al., 2017; Curculio sikkimensis, Toju & Fukatsu, 2011; Aleochara bilineata, Bili et al., 2016); (iii) induction and reinforcement of parthenogenesis, however this effect had weak support and had other possible alternative explanations (numerous species of Naupactini, Rodriguero et al., 2010a and Eusomus ovulum, Mazur et al., 2016; all Curculionidae; Micromalthus debilis, Perotti, Young & Braig, 2016); (iv) possible induction of haplodiploidy (Euwallacea interjectus, Euwallacea validus, Curculionidae, Kawasaki et al., 2016); (v) male-killing (Tribolium madens, Tenebrionidae, Fialho & Stevens, 2000); (vi) necessity of infection for egg development (Otiorhynchus sulcatus, Curculionidae, Son et al., 2008; Coccotrypes dactyliperda, Zchori-Fein, Borad & Harari, 2006); (vii) populations evolving towards endosymbiont loss and repeated intraspecific horizontal transfer of Wolbachia (Pityogenes chalcographus, Curculionidae, Arthofer et al., 2009), (viii) fitness decline in infected beetles (Callosobruchus analis, Numajiri, Kondo & Toquenaga, 2017), (ix) modification of sperm (Chelymorpha alternans, Clark et al., 2008), (x) down-regulation of defense genes in host plants (Diabrotica virgifera virgifera on maize, Barr et al., 2010).
Phylogenetic relations
The tree reconstructed for 16S rDNA included 52 sequences from bacteria found in 45 host beetle species. This tree included three major lineages, with separate clusters of Wolbachia sequences belonging to A, B and F supergroups (Fig. S1). F supergroup was represented by a single sequence from Rhinocyllus conicus (Curculionidae) (Fig. S1). Sequences assigned to supergroup A (based on information available in the articles) were found to be polyphyletic. Some 16S sequences from Xylosandrus spp. and Curculio spp. (Curculionidae), or Oreina cacaliae and Galeruca tanaceti (Chrysomelidae) clustered as a sister lineage to all other A and B sequences (Fig. S1). Overall, the diversity of 16S sequences assigned to supergroup B was much greater than those assigned to supergroup A (Fig. S1).
The tree reconstructed for ftsZ included 131 sequences found in 114 host beetle species. The ftsZ phylogenetic tree resulted in a topology similar to that of 16S rDNA—it included groups of sequences belonging to A, B and F supergroups (Fig. S2). Supergroup F was represented by Agrilus araxenus and Sphaerobothris aghababiani (both Buprestidae). Moreover, the supergroup B clade was divided into two clusters, among which one included a small group of sequences found in four beetle hosts: Chelymorpha alternans (Chrysomelidae), Eurymetopus fallax, Sitophilus oryzae and Conotrachelus nenuphar (all three Curculionidae) (Fig. S2). Also in this gene, the genetic variation of sequences belonging to supergroup A was much lower, and only a few sequences were highly diverged (e.g., strains of Callosobruchus chinensis, Chrysomelidae; Tribolium confusum, Tenebrionidae or Polydrosus pilosus, Curculionidae) (Fig. S2). There was also one slightly distinct clade that mainly consisted of bacteria sequences found in some Hydraenidae, Curculionidae and Chrysomelidae (Fig. S2).
The abovementioned phylogenetic reconstructions of the relations among Wolbachia strains identified on the basis of polymorphism of two genes show that there is no strict correlation between host phylogeny and bacterial strain relationships. Even in studies that covered multiple related species (e.g., those belonging to the same genus), evidence for direct inheritance of Wolbachia strains from common ancestors is restricted to Hydraenidae (Sontowski et al., 2015) and some species of Oreina (Montagna et al., 2014) or Curculio (Toju et al., 2013). In the case of Altica, the data show that cospeciation was rare and restricted to a few recently diverged species (Jäckel, Mora & Dobler, 2013). In contrast, there are numerous examples of phylogenetically related beetle species possessing different Wolbachia strains (e.g., Lachowska, Kajtoch & Knutelski, 2010). It is also often the case among related species that some are infected, whereas others not (Crioceris, Kubisz et al., 2012; Oreina, Montagna et al., 2014; Cyanapion, Kajtoch, Montagna & Wanat, 2018); so any assumption that the bacteria were inherited from a common ancestor would also need to consider multiple losses of infection. The latter phenomenon is probable; however, there is no direct evidence from natural populations, at least in studies on beetles, of Wolbachia disappearing over time. Some exemplary studies that found Wolbachia present in related species, after detailed examination, rejected the idea that bacteria was inherited from a common ancestor. This was because different host species harbored unrelated stains (e.g., among weevils, Lachowska, Kajtoch & Knutelski, 2010) or in cases where strains were identical or similar, the hosts were not phylogenetically close to each other (e.g., Crioceris, Kubisz et al., 2012). Finally, there is evermore proof of horizontal Wolbachia transmission via different mechanisms, such as via predators, parasitoids, common habitat or foraging on the same host plants (Huigens et al., 2004; Stahlhut et al., 2010; Caspi-Fluger et al., 2012; Ahmed et al., 2015; Kolasa et al., 2017). Studies on beetles have mainly provided indirect evidence of such transmissions. There are known groups of species that inhabit the same environments and share the same or very similar Wolbachia strains, e.g., steppic weevils from East-central Europe (Mazur, Kubisz & Kajtoch, 2014) and bark beetles in Japan (Kawasaki et al., 2016). Recently, evidence for has also appeared for the role of host plants in bacteria spread—Wolbachia DNA was detected in two species of Crioceris leaf beetles and in their host plant—Asparagus spp. (Kolasa et al., 2017).
Finally, in light of the proposed “Candidatus Wolbachia” species, the summarized phylogenetic relations among Wolbachia strains infecting various beetles indicate that the taxonomic distinctiveness of supergroups is inconclusive (Ramirez-Puebla et al., 2015; Lindsey et al., 2016). First, beetles generally harbor members of supergroups A and B, and only occasionally members of supergroup F. Therefore, it is not possible to make any conclusions about broader Wolbachia taxonomy based only on Wolbachia strains found in Coleoptera. However, there are numerous examples of beetle hosts harboring both supergroups, including beetles in which some Wolbachia genes are of supergroup A origin, while others are of supergroup B origin; this indicates that recombination between strains belonging to different supergroups is quite frequent. This is evidence against the designation of the “Candidatus Wolbachia” species, at least with respect to members of supergroup A and B.
Current Gaps and Future Endeavors
The present knowledge on Wolbachia infection across beetle species and populations is very uneven. Even the basic data about infection statuses in species and frequencies of infected species across genera and families is superficial, as there are only c. 200 beetle species known to be infected. This means that if 38% is the average frequency of infection among beetle species, then only c. 530 species have been tested so far. This is merely c. 0.15% of the total number of beetles, which is estimated to be around 360,000 species (Farrell, 1998; Bouchard et al., 2009). We know even less at the population level, as the majority of beetle species have only had single individuals tested for Wolbachia infection (e.g., Lachowska, Kajtoch & Knutelski, 2010; Sontowski et al., 2015). These very basic screens have probably underestimated the number of infected species because of false-negative results obtained for species with low or local infection in populations. There is also another and important cause that should be mentioned—low titer infections that are under the detection limit of conventional PCR (e.g., Arthofer et al., 2009; Schneider et al., 2013). On the other hand, these preliminary estimates could have overestimated the real number infected beetles, as sampling in these studies was rarely random and most often focused on specific groups, e.g., on genera for which preliminary data suggested the presence of Wolbachia infection. Indeed, an intensive search of Wolbachia infection across hundreds of beetle species from Europe suggested a lower infection rate—c. 27% to be infected (Ł Kajtoch et al., 2018, unpublished data). Also, knowledge about infection at the geographic scale is very uneven, and only Europe and Asia (basically China and Japan) have been relatively well investigated. There is a huge gap in the knowledge for African, Australian and Oceanian beetles, where a high diversity of beetles exists and probably a similar diversity of Wolbachia could be expected (e.g., compared to preliminary data available from Central and South America (Werren, Windsor & Guo, 1995; Rodriguero et al., 2010a).
Little is known about Wolbachia diversity in beetle hosts, as the majority of studies used only single genetic markers, and often different genes were sequenced for different taxa. This precludes complex analysis of Wolbachia diversity across all tested beetle hosts. This has changed since 2006, since Baldo et al. (2006) proposed Multilocus Sequence Typing (MLST), which is based on the genotyping of five housekeeping genes, usually in conjunction with wsp sequencing. MLST is and should remain a sufficient way to understand basic Wolbachia diversity. On the other hand, to fully understand Wolbachia relations among strains and supergroups (or presumed species), between Wolbachia and its hosts and especially between Wolbachia and other microorganisms, amplicon-sequencing (e.g., 16S rDNA) or genome-sequencing are needed. This could be achieved thanks to the development of next-generation sequencing technologies (NGS). Surprisingly, despite fast development of NGS in the last years, very few studies have used this technology for studying Wolbachia in beetle populations. For example, five studies sequenced 16S amplicons generated from microbiota and detected Wolbachia (White et al., 2015; Bili et al., 2016; Berasategui et al., 2016; Takano et al., 2017; Dudek et al., 2017). The only study that utilized shotgun sequencing was executed for other purposes and only accidentally showed Wolbachia genes in examined species (Heintzman et al., 2014). NGS is probably the best prospect for studies on Wolbachia infection and diversity, and will help to answer most current riddles and issues.
The big challenge is to understand the impact of infection on beetle biology, physiology and ecology. It is known that Wolbachia has several effects on host reproduction, but relatively few studies prove or suggest e.g., cytoplasmic incompatibility, male-killing or other effects on the development of selected beetles (Clark et al., 2001; Keller et al., 2004; Roehrdanz et al., 2006; Roehrdanz & Levine, 2007; Sharaf et al., 2010; Zhang et al., 2010; Jäckel, Mora & Dobler, 2013; Ming et al., 2015; Kawasaki et al., 2016; Li et al., 2016b; Mariño, Verle Rodrigues & Bayman, 2017; Numajiri, Kondo & Toquenaga, 2017; Takano et al., 2017). It is very probable that this bacteria has large and frequent effects on beetle reproduction and is consequently partially responsible for beetle radiation, at least in some taxonomic groups, geographic areas or habitats. Also, very few studies have shown data on linkage disequilibrium and selective sweep between bacteriium and host genomes (Roehrdanz et al., 2006; Rodriguero, Lanteri & Confalonieri, 2010b; Kajtoch, Korotyaev & Lachowska-Cierlik, 2012; Jäckel, Mora & Dobler, 2013; Mazur et al., 2016). These effects could also have probably been involved in speciation of numerous beetles. Moreover, this phenomenon could have serious implications for beetle barcoding, as selective sweep is known to reduce mitochondrial diversity in its hosts and therefore could decrease the number of identified species (Hurst & Jiggins, 2005). On the other hand, cytoplasmic incompatibility can lead to the origin of highly diverged phylogenetic mitochondrial lineages within species, which would increase the number of identified taxa (Smith et al., 2012). Also here, NGS technologies will enable more sophisticated analyses of these genetic relations and their effects (e.g., by the sequencing of transcriptomes for physiological studies or by genotyping-by-sequencing for phylogenetic studies). Genotyping with NGS should also verify whether the recent assumption that different supergroups are indeed “Candidatus Wolbachia” species is correct or not (Ramirez-Puebla et al., 2015; Lindsey et al., 2016).
Only very preliminary results suggest Wolbachia was not only transmitted vertically, but that it could also have spread horizontally (Jäckel, Mora & Dobler, 2013; Carvalho et al., 2014; Kawasaki et al., 2016; Kolasa et al., 2017; Mazur et al., 2016). Horizontal transmission was considered as an event that happens in evolutionary timescales. Only recently, Schuler et al. (2013) showed that such a transfer can happen within a few years after arrival of a new strain. In light of the general lack of cospeciation between bacteria and beetles, horizontal transmission must be a highly underestimated phenomenon. Horizontal transmission of Wolbachia among beetles cannot be confirmed without considering other coexisting insects that can mediate transmission, such as predators, parasitoids or beetle prey. Moreover, other arthropods that share habitats with beetles, e.g., phoretic ticks (Hartelt et al., 2004) and nematodes (Casiraghi et al., 2001), need to be examined. Finally, host plants are promising objects of studies on Wolbachia transmission across beetle populations (Kolasa et al., 2017), as phloem is probably an important mediator of this bacteria’s spread across insect populations (DeLay et al., 2012; Li et al., 2016a). Concerning transmission—another very interesting topic is the transfer of Wolbachia genes into host genomes (Dunning Hotopp et al., 2007; Koutsovoulos et al., 2014; Funkhouser-Jones et al., 2015). This issue has only been reported twice for beetle hosts so far (Nikoh et al., 2008; Aikawa et al., 2009). This problem could be important as if such transfers are frequent, simple testing of Wolbachia presence in a host based on single or even several gene sequencing could overestimate the number of truly infected species, populations or individuals.
Finally, a very interesting topic for future studies is the examination of the presence of other intracellular and symbiotic bacteria (like Cardinium, Spiroplasma, Rickettsia) in Coleoptera and their relations, both with the host and Wolbachia. So far, only seven studies have found Wolbachia with Rickettsia and/or Spiroplasma together in beetle hosts (Majerus & Majerus, 2000; Weinert et al., 2007; Toju & Fukatsu, 2011; White et al., 2015; Perotti, Young & Braig, 2016; Bili et al., 2016; Dudek et al., 2017). Preliminary results suggest that there is some balance in the number of these bacteria, probably caused by competition within host cells (Goto, Anbutsu & Fukatsu, 2006). A recent summary of the presence of these bacteria in insects showed that Rickettsia has been found in single species of Micromalthidae, Staphylinidae, Buprestidae, Coccinellidae and Curculionidae (Werren et al., 1994; Lawson et al., 2001; Weinert et al., 2007; Toju & Fukatsu, 2011; White et al., 2015; Perotti, Young & Braig, 2016; Bili et al., 2016), Spiroplasma in some species of Staphylinidae, Coccinellidae and Curculionidae (Majerus et al., 1998; Hurst et al., 1999a; Hurst et al., 1999b; Tinsley & Majerus, 2006; Weinert et al., 2007; Toju & Fukatsu, 2011; Bili et al., 2016), and Cardinium has not been detected so far in any beetle species (Zchori-Fein & Perlman, 2004). The coexistence of different endosymbiotic bacteria and their effects on hosts should also be investigated with NGS technologies, which are able to detect bacteria in numerous hosts (e.g., individuals) at once and estimate prevalence of bacteria in various hosts or different tissues. NGS has already been proven to be a powerful tool for detecting undescribed bacteria (e.g., it allowed the identification of new Alphaproteobacteria in Brontispa longissimi; Takano et al., 2017). Different endosymbiotic bacteria could have either similar or contrasting effects on beetle species, populations and individuals and could be the greatest overlooked phenomenon in the evolution and ecology of Coleoptera.
In our opinion, beetles are still an insufficiently examined group of Wolbachia hosts, especially considering their systematic and ecological diversity. All issues in studies on Wolbachia in Coleoptera are generally the same as in other hosts of these bacteria, or vice versa; there is no issue that has been or is being studied on Wolbachia infection in other (non-beetle) hosts that could not also be examined in beetle hosts. Also, the extraordinary diversity of beetles (with respect to their diverse systematics at various taxonomic levels, complex phylogenetic relations and extensive ecological relations with each other and numerous other species) makes this group an excellent target for Wolbachia studies. The presented summary about Wolbachia infection in beetles shows that despite numerous studies, there are still many issues that need to be investigated. We hope that this systematic review will facilitate various future studies on Wolbachia infection among beetles.
Supplemental Information
Maximum likelihood phylogenetic tree reconstructed based on Wolbachia 16S rDNA gene sequences obtained from hosts belonging to various species of Coleoptera (data downloaded from NCBI GenBank https://www.ncbi.nlm.nih.gov/genbank/). Statistical supports (bootstrap values) are presented above the branches (shown only if >0.50).
Maximum likelihood phylogenetic tree reconstructed based on Wolbachia ftsZ gene sequences obtained from hosts belonging to various species of Coleoptera (data downloaded from NCBI GenBank https://www.ncbi.nlm.nih.gov/genbank/). Statistical supports (bootstrap values) are presented above the branches (shown only if >0.50).
Acknowledgments
We kindly thank Prof. Lech Borowiec for providing the pictures of beetles from his ICONOGRAPHIA COLEOPTERORUM POLONIAE (©Copyright by Prof. Lech Borowiec, Wrocław 2007–2014, Department of Biodiversity and Evolutionary Taxonomy, University of Wroclaw, Poland), which were used for preparation of the graphics. We are grateful to anonymous reviewers for all their comments and suggestions, which allowed for a great improvement of the manuscript.
Funding Statement
This work was supported by grant DEC-2013/11/D/NZ8/00583 from the National Science Centre, Poland (to Kajtoch Ł.) and by Institutional Research Support grants (SGS15/PřF/2017) from the University of Ostrava (to Kotásková N.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Łukasz Kajtoch conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Nela Kotásková performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The database of published articles used for systematic review is presented as Table S1 in the manuscript.
References
- Ahmed et al. (2015).Ahmed MZ, Li SJ, Xue X, Yin XJ, Ren SX, Jiggins FM, Greeff JM, Qiu BL. The Intracellular bacterium Wolbachia uses parasitoid wasps as phoretic vectors for efficient horizontal transmission. PLOS Pathogens. 2015;11:19. doi: 10.1371/journal.ppat.1004672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa et al. (2009).Aikawa T, Anbutsu H, Nikoh N, Kikuchi T, Shibata F, Fukatsu T. Longicorn beetle that vectors pinewood nematode carries many Wolbachia genes on an autosome. Proceedings of the Royal Society B: Biological Sciences. 2009;276:3791–3798. doi: 10.1098/rspb.2009.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimova & Gascuel (2006).Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Systematic Biology. 2006;55:539–552. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
- Arthofer et al. (2009).Arthofer W, Riegler M, Avtzis D, Stauffer C. Evidence for low-titre infections in insect symbiosis: Wolbachia in the bark beetle Pityogenes chalcographus (Coleoptera, Scolytinae) Environmental Microbiology. 2009;11:1923–1933. doi: 10.1111/j.1462-2920.2009.01914.x. [DOI] [PubMed] [Google Scholar]
- Baldo et al. (2006).Baldo L, Hotopp JCD, Jolley KA, Bordenstein SR, Biber SA, Choudhury RR, Hayashi C, Maiden MCJ, Tettelin H, Werren JH. Multilocus sequence typing system for the Endosymbiont Wolbachia pipientis. Applied and Environmental Microbiology. 2006;72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo & Werren (2007).Baldo L, Werren JH. Revisiting Wolbachia supergroup typing based on WSP: spurious lineages and discordance with MLST. Current Microbiology. 2007;55:81–87. doi: 10.1007/s00284-007-0055-8. [DOI] [PubMed] [Google Scholar]
- Barr et al. (2010).Barr KL, Hearne LB, Briesacher S, Clark TL, Davis GE. Microbial symbionts in insects influence down-regulation of defense genes in maize. PLOS ONE. 2010;5:e11339. doi: 10.1371/journal.pone.0011339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berasategui et al. (2016).Berasategui A, Axelsson K, Nordlander G, Schmidt A, Borg-Karlson A-K, Gershenzon J, Terenius O, Kaltenpoth M, Schmidt A. The gut microbiota of the pine weevil is similar across Europe and resembles that of other 649 conifer beetles. Molecular Ecology. 2016;25:4014–4031. doi: 10.1111/mec.14186. [DOI] [PubMed] [Google Scholar]
- Bili et al. (2016).Bili M, Cortesero AM, Mougel C, Gauthier JP, Ermel G, Simon JC, Outreman Y, Terrat S, Mahéo F, Poinsot D. Bacterial community diversity harboured by interacting species. PLOS ONE. 2016;11:e0155392. doi: 10.1371/journal.pone.0155392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard et al. (2009).Bouchard P, Grebennikov VV, Smith ABT, Douglas H. Biodiversity of coleoptera. In: Foottit RG, Adler PH, editors. Insect biodiversity: science and society. Blackwell Publishing; Oxford: 2009. pp. 265–301. [Google Scholar]
- Campbell, Bragg & Turner (1992).Campbell BC, Bragg TS, Turner CE. Phylogeny of symbiotic bacteria of four weevil species (Coleoptera: Curculionidae) based on analysis of 16s ribosomal DNA. Insect Biochemistry and Molecular Biology. 1992;22:415–421. doi: 10.1016/0965-1748(92)90136-3. [DOI] [Google Scholar]
- Carvalho et al. (2014).Carvalho GA, Correa AS, Oliveira LO, Guedes RNC. Evidence of horizontal transmission of primary and secondary endosymbionts between the maize and rice weevils (Sitophilus zeamais and S. oryzae) and the parasitoid Theocolax elegans. Journal of Stored Products Research. 2014;59:61–65. doi: 10.1016/j.jspr.2014.05.004. [DOI] [Google Scholar]
- Casiraghi et al. (2001).Casiraghi M, Anderson TJ, Bandi C, Bazzocchi C, Genchi C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122:93–103. doi: 10.1017/S0031182000007149. [DOI] [PubMed] [Google Scholar]
- Caspi-Fluger et al. (2012).Caspi-Fluger A, Inbar M, Mozes-Daube N, Katzir N, Portnoy V, Belausov E, Hunter MS, Zchori-Fein E. Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proceedings of the Royal Society B: Biological Sciences. 2012;279:1791–1796. doi: 10.1098/rspb.2011.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisiri et al. (2015).Chaisiri K, McGarry JW, Morand S, Makepeace B. Symbiosis in an overlooked microcosm: a systematic review of the bacterial flora of mites. Parasitology FirstView. 2015;142:1152–1162. doi: 10.1017/S0031182015000530. [DOI] [PubMed] [Google Scholar]
- Clark et al. (2008).Clark ME, Bailey-Jourdain C, Ferree PM, England SJ, Sullivan W, Windsor DM, Werren JH. Wolbachia modification of sperm does not always require residence within developing sperm. Heredity. 2008;101:420–428. doi: 10.1038/hdy.2008.71. [DOI] [PubMed] [Google Scholar]
- Clark et al. (2001).Clark TL, Meinke LJ, Skoda SR, Foster JE. Occurrence of Wolbachia in Selected Diabroticite (Coleoptera: Chrysomelidae) Beetles. Annals of the Entomological Society of America. 2001;94:877–885. [Google Scholar]
- Cordaux, Bouchon & Greve (2011).Cordaux R, Bouchon D, Greve P. The impact of endosymbionts on the evolution of host sex-determination mechanisms. Trends in Genetics. 2011;27:332–341. doi: 10.1016/j.tig.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Czarnetzki & Tebbe (2004).Czarnetzki AB, Tebbe CC. Detection and phylogenetic analysis of Wolbachia in Collembola. Environmental Microbiology. 2004;6:35–44. doi: 10.1046/j.1462-2920.2003.00537.x. [DOI] [PubMed] [Google Scholar]
- DeLay et al. (2012).DeLay B, Mamidala P, Wijeratne A, Wijeratne S, Mittapalli O, Wang J, Lamp W. Transcriptome analysis of the salivary glands of potato leafhopper, Empoasca fabae. Journal of Insect Physiology. 2012;58:1626–1634. doi: 10.1016/j.jinsphys.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Dudek et al. (2017).Dudek K, Humińska K, Wojciechowicz J, Tryjanowski P. Metagenomic survey of bacteria associated with the invasive ladybird Harmonia axyridis (Coleoptera: Coccinellidae) European Journal of Entomology. 2017;114:312–316. doi: 10.14411/eje.2017.038. [DOI] [Google Scholar]
- Dunning Hotopp et al. (2007).Dunning Hotopp JC, Clark ME, Oliveira DCSG, Foster JM, Fischer P, Muño Torres MC, Giebel JD, Kumar N, Ishmael N, Wang S, Ingram J, Nene RV, Shepard J, Tomkins J, Richards S, Spiro DJ, Ghedin E, Slatko BE, Tettelin H, Werren JH. Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science. 2007;317:1753–1756. doi: 10.1126/science.1142490. [DOI] [PubMed] [Google Scholar]
- Duron et al. (2008).Duron O, Bouchon D, Boutin S, Bellamy L, Zhou LQ, Engelstadter J, Hurst GD. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biology. 2008;6:27. doi: 10.1186/1741-7007-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnagdy et al. (2013).Elnagdy S, Majerus MEN, Gardener M, Lawson-Handley L. The direct effects of male-killer infection on fitness of ladybird hosts (Coleoptera: Coccinellidae) Journal of Evolutionary Biology. 2013;26:1816–1825. doi: 10.1111/jeb.12186. [DOI] [PubMed] [Google Scholar]
- Farrell (1998).Farrell BD. “Inordinate fondness” explained: why are there so many beetles? Science. 1998;281:555–559. doi: 10.1126/science.281.5376.555. [DOI] [PubMed] [Google Scholar]
- Fialho & Stevens (1996).Fialho RF, Stevens L. Wolbachia infections in the flour beetle Tribolium confusum: evidence for a common incompatibility type across strains. Journal of Invertebrate Pathology. 1996;67:195–197. doi: 10.1006/jipa.1996.0032. [DOI] [Google Scholar]
- Fialho & Stevens (1997).Fialho RF, Stevens L. Molecular evidence for single Wolbachia infections among geographic strains of the flour beetle Tribolium confusum. Proceedings of the Royal Society B: Biological Sciences. 1997;264:1065–1068. doi: 10.1098/rspb.1997.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialho & Stevens (2000).Fialho RF, Stevens L. Male-killing Wolbachia in a flour beetle. Proceedings of the Royal Society B: Biological Sciences. 2000;267:1469–1474. doi: 10.1098/rspb.2000.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkhouser-Jones et al. (2015).Funkhouser-Jones LJ, Sehnert SR, Martínez-Rodríguez P, Toribio-Fernández R, Pita M, Bella JL, Bordenstein SR. Wolbachia co-infection in a hybrid zone: discovery of horizontal gene transfers from two Wolbachia supergroups into an animal genome. PeerJ. 2015;3:e1479. doi: 10.7717/peerj.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano, Jackson & Robertson (1997).Giordano R, Jackson JJ, Robertson HM. The role of Wolbachia bacteria in reproductive incompatibilities and hybrid zones of Diabrotica beetles and Gryllus crickets. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:11439–11444. doi: 10.1073/pnas.94.21.11439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodacre, Fricke & Martin (2015).Goodacre SL, Fricke C, Martin OY. A screen for bacterial endosymbionts in the model organisms Tribolium castaneum, T.confusum, Callosobruchus maculatus, and related species. Insect Science. 2015;22:165–177. doi: 10.1111/1744-7917.12096. [DOI] [PubMed] [Google Scholar]
- Goodacre et al. (2006).Goodacre SL, Martin OY, Thomas CFG, Hewitt GM. Wolbachia and other endosymbiont infections in spiders. Molecular Ecology. 2006;15:517–527. doi: 10.1111/j.1365-294X.2005.02802.x. [DOI] [PubMed] [Google Scholar]
- Goto, Anbutsu & Fukatsu (2006).Goto S, Anbutsu H, Fukatsu T. Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Applied and Environmental Microbiology. 2006;72:4805–4810. doi: 10.1128/AEM.00416-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon et al. (2010).Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Hartelt et al. (2004).Hartelt K, Oehme R, Frank H, Brockmann SO, Hassler D, Kimmig P. Pathogens and symbionts in ticks: prevalence of Anaplasma phagocytophilum (Ehrlichia sp.), Wolbachia sp., Rickettsia sp., and Babesia sp. in Southern Germany. International Journal of Medical Microbiology Supplements. 2004;293:86–92. doi: 10.1016/S1433-1128(04)80013-5. [DOI] [PubMed] [Google Scholar]
- Heintzman et al. (2014).Heintzman PD, Elias SA, Moore K, Paszkiewicz K, Barnes I. Characterizing DNA preservation in degraded specimens of Amara alpina (Carabidae: Coleoptera) Molecular Ecology Resources. 2014;14:606–615. doi: 10.1111/1755-0998.12205. [DOI] [PubMed] [Google Scholar]
- Hilgenboecker et al. (2008).Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia? A statistical analysis of current data. FEMS Microbiology Letters. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huigens et al. (2004).Huigens ME, Almeida RP de, Boons PAH, Luck RF, Stouthamer R. Natural interspecific and intraspecific horizontal transfer of parthenogenesis–inducing Wolbachia in Trichogramma wasps. Proceedings of the Royal Society B: Biological Sciences. 2004;271:509–515. doi: 10.1098/rspb.2003.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst et al. (1999a).Hurst GDD, Bandi C, Sacchi L, Cochrane A, Bertr D, Karaca I, Majerus MEN. Adonia variegata (Coleoptera: Coccinellidae) bears maternally inherited Flavobacteria that kill males only. Parasitology. 1999a;118:125–134. doi: 10.1017/s0031182098003655. [DOI] [PubMed] [Google Scholar]
- Hurst & Jiggins (2005).Hurst GDD, Jiggins FM. Problems with mitochondrial DNA as a marker in population, phylogeographic and phylogenetic studies: the effects of inherited symbionts. Proceedings of the National Academy of Sciences of the United States of America. 2005;272:1525–1534. doi: 10.1098/rspb.2005.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst et al. (1999b).Hurst GDD, Jiggins FM, Von der Schulenburg JHG, Bertr D, West SA, Goriacheva II, Zakharov IA, Werren JH, Stouthamer R, Majerus MEN. Male-killing Wolbachia in two species of insect. Proceedings of the Royal Society B: Biological Sciences. 1999b;266:735–740. doi: 10.1098/rspb.1999.0698. [DOI] [Google Scholar]
- Jäckel, Mora & Dobler (2013).Jäckel R, Mora D, Dobler S. Evidence for selective sweeps by Wolbachia infections: phylogeny of Altica leaf beetles and their reproductive parasites. Molecular Ecology. 2013;22:4241–4255. doi: 10.1111/mec.12389. [DOI] [PubMed] [Google Scholar]
- Kageyama et al. (2010).Kageyama D, Narita S, Imamura T, Miyanoshita A. Detection and identification of Wolbachia endosymbionts from laboratory stocks of stored-product insect pests and their parasitoids. Journal of Stored Products Research. 2010;46:13–19. doi: 10.1016/j.jspr.2009.07.003. [DOI] [Google Scholar]
- Kajtoch, Korotyaev & Lachowska-Cierlik (2012).Kajtoch Ł, Korotyaev B, Lachowska-Cierlik D. Genetic distinctness of parthenogenetic forms of European Polydrusus weevils of the subgenus Scythodrusus. Insect Science. 2012;19:183–194. doi: 10.1111/j.1744-7917.2011.01448.x. [DOI] [Google Scholar]
- Kajtoch, Montagna & Wanat (2018).Kajtoch Ł, Montagna M, Wanat M. Species delimitation within the Bothryorrhynchapion weevils: multiple evidence from genetics, morphology and ecological associations. Molecular Phylogenetics and Evolution. 2018;120:354–363. doi: 10.1016/j.ympev.2017.12.022. [DOI] [PubMed] [Google Scholar]
- Kawasaki et al. (2016).Kawasaki Y, Schuler H, Stauffer C, Lakatos F, Kajimura H. Wolbachia endosymbionts in haplodiploid and diploid scolytine beetles (Coleoptera: Curculionidae: Scolytinae) Environmental Microbiology Reports. 2016;8:680–688. doi: 10.1111/1758-2229.12425. [DOI] [PubMed] [Google Scholar]
- Keller et al. (2004).Keller GP, Windsor DM, Scucedo JM, Werren JH. Reproductive effects and geographical distributions of two Wolbachia strains infecting the Neotropical beetle, Chelymorpha alternans Boh. (Chrysomelidae, Cassidinae) Molecular Ecology. 2004;13:2405–2420. doi: 10.1111/j.1365-294X.2004.02213.x. [DOI] [PubMed] [Google Scholar]
- Kikuchi & Fukatsu (2003).Kikuchi Y, Fukatsu T. Diversity of Wolbachia endosymbionts in heteropteran bugs. Applied and Environmental Microbiology. 2003;69:6082–6090. doi: 10.1128/AEM.69.10.6082-6090.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolasa et al. (2017).Kolasa M, Montagna M, Mereghetti V, Kubisz D, Mazur MA, Kajtoch Ł. Preliminary evidence of Wolbachia horizontal transmission between Crioceris leaf beetles and Asparagus host plants. European Journal of Entomology. 2017;114:446–454. doi: 10.14411/eje.2017.057. [DOI] [Google Scholar]
- Kondo et al. (2002).Kondo N, Ijichi N, Shimada M, Fukatsu T. Prevailing triple infection with Wolbachia in Callosobruchus chinensis (Coleoptera: Bruchidae) Molecular Ecology. 2002;11:167–180. doi: 10.1046/j.0962-1083.2001.01432.x. [DOI] [PubMed] [Google Scholar]
- Kondo et al. (2011).Kondo N, Tuda M, Toquenaga Y, Lan YC, Buranapanichpan S, Horng SB, Shimada M, Fukatsu T. Wolbachia infections in world populations of bean beetles (Coleoptera: Chrysomelidae: Bruchinae) infesting cultivated and wild legumes. Zoological Science. 2011;28:501–508. doi: 10.2108/zsj.28.501. [DOI] [PubMed] [Google Scholar]
- Koutsovoulos et al. (2014).Koutsovoulos G, Makepeace B, Tanya VN, Blaxter M. Palaeosymbiosis revealed by genomic fossils of wolbachia in a strongyloidean nematode. PLOS Genetics. 2014;10(6):e1004397. doi: 10.1371/journal.pgen.1004397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubisz et al. (2012).Kubisz D, Kajtoch Ł, Mazur MA, Lis A, Holecová M. Conservation genetics of highly isolated populations of xerothermic Crioceris quatuordecimpunctata (Coleoptera: Chrysomelidae) Invertebrate Biology. 2012;131:333–344. doi: 10.1111/j.1744-7410.2012.00276.x. [DOI] [Google Scholar]
- Lachowska, Kajtoch & Knutelski (2010).Lachowska D, Kajtoch Ł, Knutelski S. Occurrence of Wolbachia in central European weevils: correlations with host systematics, ecology and biology. Entomologia Experimentalis et Applicata. 2010;14:105–118. doi: 10.1111/j.1570-7458.2010.00974.x. [DOI] [Google Scholar]
- Lawson et al. (2001).Lawson ET, Mousseau TA, Klaper R, Hunter MD, Werren JH. Rickettsia associated with male-killing in a buprestid beetle. Heredity. 2001;86:497–505. doi: 10.1046/j.1365-2540.2001.00848.x. [DOI] [PubMed] [Google Scholar]
- Li et al. (2016a).Li SJ, Ahmed MZ, Lv N, Shi PQ, Wang XM, Huang JL, Qiu BL. Plant-mediated horizontal transmission of Wolbachia between whiteflies. The ISME Journal. 2016a;11:1019–1028. doi: 10.1038/ismej.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2015).Li YY, Fields PG, Pang B, Coghlin PC, Floate KD. Prevalence and diversity of Wolbachia bacteria infecting insect pests of stored products. Journal of Stored Products Research. 2015;62:93–100. doi: 10.1016/j.jspr.2009.07.003. [DOI] [Google Scholar]
- Li et al. (2016b).Li YY, Fields PG, Pang BP, Floate KD. Effects of tetracycline and rifampicin treatmens on the fecundity of the Wolbachia-infected host, Tribolium confusum (Coleoptera: Tenebrionidae) Journal of Economic Entomology. 2016b;109:1458–1464. doi: 10.1093/jee/tow067. [DOI] [PubMed] [Google Scholar]
- Lindsey et al. (2016).Lindsey AR, Bordenstein SR, Newton IL, Rasgon JL. Wolbachia pipientis should not be split into multiple species: a response to Ramírez-Puebla et al., Species in Wolbachia? Proposal for the designation of ‘Candidatus Wolbachia bourtzisii’, ‘Candidatus Wolbachia onchocercicola’, ‘Candidatus Wolbachia blaxteri’, ‘Candidatus Wolbachia brugii’, ‘Candidatus Wolbachia taylori’ ‘Candidatus Wolbachia collembolicola’ and ‘Candidatus Wolbachia multihospitum’ for the different species within Wolbachia supergroups. Systematic and Applied Microbiology. 2016;39:220–222. doi: 10.1016/j.syapm.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus & Majerus (2000).Majerus MEN, Majerus TMO. Multiple causes of male-killing in a single sample of the 2-spot ladybird, Adalia bipunctata (Coleoptera:Coccinellidae) from Moscow. Heredity. 2000;84:605–609. doi: 10.1046/j.1365-2540.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- Malloch, Fenton & Butcher (2000).Malloch G, Fenton B, Butcher RD. Molecular evidence for multiple infections of a new subgroup of Wolbachia in the European raspberry beetle Byturus tomentosus. Molecular Ecology. 2000;9:77–90. doi: 10.1046/j.1365-294x.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- Majerus et al. (1998).Majerus TMO, Majerus MEN, Knowles B, Wheeler J, Bertr D, Kuznetsov VN, Ueno H, Hurst GDD. Extreme variation in the prevalence of inherited male-killing microorganisms between three populations of Harmonia axyridis (Coleoptera: Coccinellidae) Heredity. 1998;81:683–691. doi: 10.1046/j.1365-2540.1998.00438.x. [DOI] [Google Scholar]
- Mariño, Verle Rodrigues & Bayman (2017).Mariño Y, Verle Rodrigues JC, Bayman P. Wolbachia affects reproduction and population dynamics of the coffee berry borer (Hypothenemus hampei): implications for biological control. Insects. 2017;8(1):8. doi: 10.3390/insects8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur et al. (2016).Mazur MA, Holecová M, Lachowska-Cierlik D, Lis A, Kubisz D, Kajtoch Ł. Selective sweep of Wolbachia and parthenogenetic host genomes on the example of the weevil Eusomus ovulum. Insect Molecular Biology. 2016;25:701–711. doi: 10.1111/imb.1225. [DOI] [PubMed] [Google Scholar]
- Mazur, Kubisz & Kajtoch (2014).Mazur MA, Kubisz D, Kajtoch Ł. Restricted geographic distribution and low genetic distinctiveness of steppic Crioceris quinquepunctata (Coleoptera: Chrysomelidae) populations in central-east Europe. Entomologica Fennica. 2014;25:103–111. [Google Scholar]
- Ming et al. (2015).Ming QL, Shen JF, Cheng C, Liu CM, Feng ZJ. Wolbachia infection dynamics in Tribolium confusum (Coleoptera: Tenebrionidae) and their effects on host mating behavior and reproduction. Journal of Economic Entomology. 2015;108:1408–1415. doi: 10.1093/jee/tov053. [DOI] [PubMed] [Google Scholar]
- Minh, Nguyen & Von Haeseler (2013).Minh BQ, Nguyen NAT, Von Haeseler A. Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution. 2013;30:1188–1195. doi: 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher et al. (2009).Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Montagna et al. (2014).Montagna M, Chouaia B, Sacchi L, Porretta D, Martin E, Giorgi A, Lozzia GC, Epis SA. New strain of Wolbachia in an alpine population of the viviparous Oreina cacaliae (Coleoptera: Chrysomelidae) Environmental Entomology. 2014;43:913–922. doi: 10.1603/EN13228. [DOI] [PubMed] [Google Scholar]
- Nikoh et al. (2008).Nikoh N, Tanaka K, Shibata F, Kondo N, Hizume M, Shimada M, Fukatsu T. Wolbachia genome integrated in an insect chromosome: evolution and fate of laterally transferred endosymbiont genes. Genome Research. 2008;18:272–280. doi: 10.1101/gr.7144908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numajiri, Kondo & Toquenaga (2017).Numajiri Y, Kondo NI, Toquenaga Y. Melanic mutation causes a fitness decline in bean beetles infected by Wolbachia. Entomologia Experimentalis Et Applicata. 2017;164:54–65. doi: 10.1111/eea.12588. [DOI] [Google Scholar]
- Okayama et al. (2016).Okayama K, Katsuki M, Sumida Y, Okada K. Costs and benefits of symbiosis between a bean beetle and Wolbachia. Animal Behaviour. 2016;119:19–26. doi: 10.1016/j.anbehav.2016.07.004. [DOI] [Google Scholar]
- O’Neill (2007).O’Neill SL. Wolbachia—host interactions: connecting phenotype to genotype. Current Opinion in Microbiology. 2007;10:221–224. doi: 10.1016/j.mib.2007.05.002. [DOI] [PubMed] [Google Scholar]
- O’Neill et al. (1992).O’Neill SL, Giordano R, Colbert AM, Karr TL, Robertson HM. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibity in insects. Proceedings of National Academy of Sciences of the United States of America. 1992;89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perotti, Young & Braig (2016).Perotti MA, Young DK, Braig HR. The ghost sex-life of the paedogenetic beetle Micromalthus debilis. Scientific Reports. 2016;6:27364. doi: 10.1038/srep27364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Puebla et al. (2015).Ramirez-Puebla ST, Servin-Garciduenas LE, Ormeno-Orrillo E, Vera-Ponce de Leon A, Rosenblueth M, Delaye L, Martinez J, Martinez-Romero E. Species in Wolbachia? Proposal for the designation of ‘Candidatus Wolbachia bourtzisii’, ‘Candidatus Wolbachia onchocercicola’, ‘Candidatus Wolbachia blaxteri’, ‘Candidatus Wolbachia brugii’, ‘Candidatus Wolbachia taylori’ ‘Candidatus Wolbachia collembolicola’ and ‘Candidatus Wolbachia multihospitum’ for the different species within Wolbachia supergroups. Systematic and Applied Microbiology. 2015;38:390–399. doi: 10.1016/j.syapm.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Rodriguero et al. (2010a).Rodriguero MS, Confalonieri VA, Guedes JVC, Lanteri AA. Wolbachia infection in the tribe Naupactini (Coleoptera, Curculionidae): association between thelytokous parthenogenesis and infection status. Insect Molecular Biology. 2010a;19:631–640. doi: 10.1111/j.1365-2583.2010.01018.x. [DOI] [PubMed] [Google Scholar]
- Rodriguero, Lanteri & Confalonieri (2010b).Rodriguero MS, Lanteri AA, Confalonieri VA. Mito-nuclear genetic comparison in a Wolbachia infected weevil: insights on reproductive mode, infection age and evolutionary forces shaping genetic variation. BMC Evolutionary Biology. 2010b;10:340. doi: 10.1186/1471-2148-10-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrdanz & Levine (2007).Roehrdanz R, Levine E. Wolbachia bacterial infections linked to mitochondrial DNA reproductive isolation among populations of northern corn rootworm (Coleoptera: Chrysomelidae) Annals of the Entomological Society of America. 2007;100:522–531. doi: 10.1603/0013-8746(2007)100[522:WBILTM]2.0.CO;2. [DOI] [Google Scholar]
- Roehrdanz et al. (2006).Roehrdanz R, Olson D, Bourchier R, Sears S, Cortilet A, Fauske G. Mitochondrial DNA diversity and Wolbachia infection in the flea beetle Aphthona nigriscutis (Coleoptera: Chrysomelidae): an introduced biocontrol agent for leafy spurge. Biological Control. 2006;37:1–8. doi: 10.1016/j.biocontrol.2005.12.004. [DOI] [Google Scholar]
- Russell (2012).Russell JA. The ants (Hymenoptera: formicidae) are unique and enigmatic hosts of prevalent Wolbachi a (Alphaproteobacteria) symbionts. Myrmecological News. 2012;16:7–23. [Google Scholar]
- Schneider et al. (2013).Schneider DI, Riegler M, Arthofer W, Merçot H, Stauffer C, Miller WJ. Uncovering Wolbachia diversity upon artificial host transfer. PLOS ONE. 2013;8:e82402. doi: 10.1371/journal.pone.0082402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler et al. (2013).Schuler H, Bertheau C, Egan SP, Feder JL, Riegler M, Schlick-Steiner BC, Steiner FM, Johannesen J, Kern P, Tuba K, Lakatos F, Köppler K, Arthofer W, Stauffer C. Evidence for a recent horizontal transmission and spatial spread of Wolbachia from endemic Rhagoletis cerasi (Diptera: Tephritidae) to invasive Rhagoletis cingulata in Europe. Molecular Ecology. 2013;22:4101–4111. doi: 10.1111/mec.12362. [DOI] [PubMed] [Google Scholar]
- Sharaf et al. (2010).Sharaf K, Horovă L, Pavliček T, Nevo E, Bureš P. Genome size and base composition in Oryzaephilius surinamensis (Coleoptera: Sylvanidae) (Cikeiotera: Sylvanidae) and differences between native (feral) and silo pest populations in Israel. Journal of Stored Products Research. 2010;46:34–37. [Google Scholar]
- Shoemaker et al. (2002).Shoemaker DD, Machado CA, Molbo D, Werren JH, Windsor DM, Herre EA. The distribution of Wolbachia in fig wasps: correlations with host phylogeny, ecology and population structure. Proceedings of the Royal Society of London B: Biological Sciences. 2002;269:2257–2267. doi: 10.1098/rspb.2002.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith et al. (2012).Smith MA, Bertrand C, Crosby K, Eveleigh ES, Fernandez-Triana J, Fisher BL, Gibbs J, Hajibabaei M, Hallwachs W, Hind K, Hrcek J, Huang DW, Janda M, Janzen DH, Li Y, Miller SE, Packer L, Quicke D, Ratnasingham S, Rodriguez J, Rougerie R, Shaw MR, Sheffield C, Stahlhut JK, Steinke D, Whitfield J, Wood M, Zhou X. Wolbachia and DNA barcoding insects: patterns, potential, and problems. PLOS ONE. 2012;7:e36514. doi: 10.1371/journal.pone.0036514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son et al. (2008).Son Y, Luckhart S, Zhang X, Lieber MJ, Lewis EE. Effects and implications of antibiotic treatment on Wolbachia—infected vine weevil (Coleoptera: Curculionidae) Agricultural and Forest Entomology. 2008;10:147–155. doi: 10.1111/j.1461-9563.2008.00369.x. [DOI] [Google Scholar]
- Sontowski et al. (2015).Sontowski R, Bernhard D, Bleidorn C, Schlegel M, Gerth M. Wolbachia distribution in selected beetle taxa characterized by PCR screens and MLST data. Ecology and Evolution. 2015;5:4345–4353. doi: 10.1002/ece3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlhut et al. (2010).Stahlhut JK, Desjardins CA, Clark ME, Baldo L, Russell JA, Werren JH, Jaenike J. The mushroom habitat as an ecological arena for global exchange of Wolbachia. Molecular Ecology. 2010;19:1940–1952. doi: 10.1111/j.1365-294X.2010.04572.x. [DOI] [PubMed] [Google Scholar]
- Stenberg & Lundmark (2004).Stenberg P, Lundmark M. Distribution, mechanisms and evolutionary significance of clonality and polyploidy in weevils. Agricultural and Forest Entomology. 2004;6:259–266. doi: 10.1111/j.1461-9555.2004.00231.x. [DOI] [Google Scholar]
- Stouthamer, Breeuwer & Hurst (1999).Stouthamer R, Breeuwer JAJ, Hurst GDD. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annual Review of Microbiology. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. [DOI] [PubMed] [Google Scholar]
- Tagami & Miura (2004).Tagami Y, Miura K. Distribution and prevalence of Wolbachia in Japanese populations of Lepidoptera. Insect Molecular Biology. 2004;13:359–364. doi: 10.1111/j.0962-1075.2004.00492.x. [DOI] [PubMed] [Google Scholar]
- Takano et al. (2017).Takano SI, Tuda M, Takasu K, Furuya N, Imamura Y, Kim S, Tashiro K, Iiyama K, Tavares M, Amaral AC. Unique clade of alphaproteobacterial endosymbionts induces complete cytoplasmic incompatibility in the coconut beetle. Proceedings of National Academy of Sciences United States of America. 2017;114:6110–6115. doi: 10.1073/pnas.1618094114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor & Hoerauf (1999).Taylor MJ, Hoerauf A. Wolbachia bacteria of filarial nematodes. Parasitology Today. 1999;15:437–442. doi: 10.1016/S0169-4758(99)01533-1. [DOI] [PubMed] [Google Scholar]
- Tinsley & Majerus (2006).Tinsley MC, Majerus MEN. A new male-killing parasitism: Spiroplasma bacteria infect the ladybird beetle Anisosticta novemdecimpunctata (Coleoptera: Coccinellidae) Parasitology. 2006;132:757–765. doi: 10.1017/S0031182005009789. [DOI] [PubMed] [Google Scholar]
- Toju & Fukatsu (2011).Toju H, Fukatsu T. Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: relevance of local climate and host plants. Molecular Ecology. 2011;20:853–868. doi: 10.1111/j.1365-294X.2010.04980.x. [DOI] [PubMed] [Google Scholar]
- Toju et al. (2013).Toju H, Tanabe AS, Notsu Y, Sota T, Fukatsu T. Diversification of endosymbiosis: replacements, co-speciation and promiscuity of bacteriocyte symbionts in weevils. The ISME Journal. 2013;7:1378–1390. doi: 10.1038/ismej.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifinopoulos et al. (2016).Trifinopoulos J, Nguyen LT, Von Haeseler A, Minh BQ. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research. 2016;44:W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks, Reynolds & Hoffmann (2002).Weeks AR, Reynolds KT, Hoffmann AA. Wolbachia dynamics and host effects: what has (and has not) been demonstrated? Trends of Ecology & Evolution. 2002;17:257–262. doi: 10.1016/S0169-5347(02)02480-1. [DOI] [Google Scholar]
- Weinert et al. (2015).Weinert LA, Araujo-Jnr EV, Ahmed MZ, Welch JJ. The incidence of bacterial endosymbionts in terrestrial. arthropods. Proceedings of the Royal Society B: Biological Science. 2015;282:20150249. doi: 10.1098/rspb.2015.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert et al. (2007).Weinert LA, Tinsley MC, Temperley M, Jiggins FM. Are we underestimating the diversity and incidence of insect bacterial symbionts? A case study in ladybird beetles. Biology Letters. 2007;3:678–681. doi: 10.1098/rsbl.2007.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren (1997).Werren JH. Biology of Wolbachia. Annual Review of Entomology. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- Werren et al. (1994).Werren JH, Hurst GDD, Zhang W, Breeuwer JA, Stouthamer R, Majerus ME. Rickettsial relative associated with male killing in the ladybird beetle (Adalia bipunctata) Journal Bacteriology. 1994;176:388–394. doi: 10.1128/jb.176.2.388-394.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren & O’Neill (1997).Werren JH, O’Neill SL. The evolution of heritable symbionts. In: O’Neill SL, Hoffmann AA, Werren JH, editors. Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press; Oxford: 1997. pp. 1–41. [Google Scholar]
- Werren, Windsor & Guo (1995).Werren JH, Windsor D, Guo L. Distribution of Wolbachia among neotropical arthropods. Proceedings of the Royal Society B: Biological Sciences. 1995;262:197–204. doi: 10.1098/rspb.1995.0196. [DOI] [Google Scholar]
- White et al. (2015).White JA, Richards NK, Laugraud A, Saeed A, Curry MM, McNeill MR. Endosymbiotic candidates for parasitoid defense in exotic and native New Zealand weevils. Microbial Ecology. 2015;70:274–286. doi: 10.1007/s00248-014-0561-8. [DOI] [PubMed] [Google Scholar]
- Yun et al. (2011).Yun Y, Peng Y, Liu FX, Lei C. Wolbachia screening in spiders and assessment of horizontal transmission between predator and prey. Neotropical Entomology. 2011;40:164–169. doi: 10.1590/S1519-566X2011000200002. [DOI] [PubMed] [Google Scholar]
- Zchori-Fein, Borad & Harari (2006).Zchori-Fein E, Borad C, Harari AR. Oogenesis in the date stone beetle, Coccotrypesdactyliperda, depends on symbiotic bacteria. Physiological Entomology. 2006;31:164–169. doi: 10.1111/j.1365-3032.2006.00504.x. [DOI] [Google Scholar]
- Zchori-Fein & Perlman (2004).Zchori-Fein E, Perlman SJ. Distribution of the bacterial symbiont Cardinium in arthropods. Molecular Ecology. 2004;13:2009–2016. doi: 10.1111/j.1365-294X.2004.02203.x. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2010).Zhang X, Luckhart S, Tu J, Pfeiffer DG. Analysis of Wolbachia strains associated with Conotrachelus nenuphar (Coleoptera: Curculionidae) in the eastern United States. Environmental Entomology. 2010;39:396–405. doi: 10.1603/EN09276. [DOI] [PubMed] [Google Scholar]
- Zug & Hammerstein (2012).Zug R, Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLOS ONE. 2012;7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- Chafee et al. (2010).Chafee ME, Funk DJ, Harrison RG, Bordenstein SR. Lateral phage transfer in obligate intracellular bacteria (Wolbachia): verification from natural populations. Molecular Biology and Evolution. 2010;27:501–505. doi: 10.1093/molbev/msp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2012).Chen SJ, Lu F, Cheng JA, Jiang MX, Way MO. Identification and biological role of the endosymbionts Wolbachia in rice water weevil (Coleoptera: Curculionidae) Environmental Entomology. 2012;41:469–477. doi: 10.1603/EN11195. [DOI] [PubMed] [Google Scholar]
- Floate, Coghlin & Dosdall (2011).Floate KD, Coghlin PC, Dosdall L. A test using Wolbachia bacteria to identify eurasian source populations of cabbage seedpod weevil, Ceutorhynchus obstrictus (Marsham), in North America. Environmental Entomology. 2011;40:818–823. doi: 10.1603/EN10315. [DOI] [PubMed] [Google Scholar]
- Frank, Erwin & Hemenway (2009).Frank JH, Erwin T, Hemenway RC. Economically Beneficial Ground Beetles. The specialized predators Pheropsophus aequinoctialis (L.) and Stenaptinus jessoensis (Morawitz): their laboratory behavior and descriptions of immature stages (Coleoptera, Carabidae, Brachininae) Zookeys. 2009;14:1–36. doi: 10.3897/zookeys.14.188. [DOI] [Google Scholar]
- García-Vázquez & Ribera (2016).García-Vázquez D, Ribera I. The origin of widespread species in a poor dispersing lineage (diving beetle genus Deronectes) PeerJ. 2016;4:e2514. doi: 10.7717/peerj.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goryachevaa et al. (2015).Goryachevaa II, Blekhmanb AV, Andrianova BV, Gorelovaa TV, Zakharova IA. Genotypic diversity of Wolbachia pipientis in native and invasive Harmonia axyridis Pall. 1773 (Coleoptera, Coccinellidae) Populations. Russian Journal of Genetics. 2015;8:731–736. [PubMed] [Google Scholar]
- Heddi et al. (1999).Heddi A, Grenier AM, Khatchadourian CH, Charles H, Nardon P. Four intracellular genomes direct weevil biology: nuclear, mitochondrial, principal endosymbiont, and Wolbachia. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6814–6819. doi: 10.1073/pnas.96.12.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase et al. (2015).Iwase S, Tani S, Saeki Y, Tuda M, Haran J, Skuhrovec J, Takagi M. Dynamics of infection with Wolbachia in Hyperapostica (Coleoptera: Curculionidae) during invasion and establishment. Biological Invasions. 2015;17:3639–3648. doi: 10.1007/s10530-015-0985-1. [DOI] [Google Scholar]
- Jeong et al. (2009).Jeong G, Kang T, Park H, Choi J, Hwang S, Kim W, Choi Y, Lee K, Park I, Sim H. Wolbachia infection in the Korean endemic firefly, Luciola unmunsana (Coleoptera: Lampyridae) Journal of Asia-Pacific Entomology. 2009;12:33–36. doi: 10.1016/j.aspen.2008.11.001. [DOI] [Google Scholar]
- Jeyaprakash & Hoy (2000).Jeyaprakash A, Hoy MA. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of 63 arthropod species. Insect Molecular Biology. 2000;9:393–405. doi: 10.1046/j.1365-2583.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki et al. (2010).Kawasaki Y, Ito M, Miura K, Kajimura H. Superinfection of five Wolbachia in the alnus ambrosia beetle, Xylosandrus germanus (Blandford) (Coleoptera: Curuculionidae) Bullettin of Entomological Research. 2010;100:231–239. doi: 10.1017/S000748530999023X. [DOI] [PubMed] [Google Scholar]
- Kittayapong et al. (2003).Kittayapong P, Jamnongluk W, Thipaksorn A, Milne JR, Sindhusake C. Wolbachia infection complexity among insects in the tropical rice-field community. Molecular Ecology. 2003;12:1049–1060. doi: 10.1046/j.1365-294X.2003.01793.x. [DOI] [PubMed] [Google Scholar]
- Kondo, Shimada & Fukatsu (1999).Kondo N, Shimada M, Fukatsu T. High prevalence of Wolbachia in the azuki bean beetle Callosobruchus chinensis (Coleoptera, Bruchidae) Zoological Science. 1999;16:955–962. doi: 10.2108/zsj.16.955. [DOI] [Google Scholar]
- Kondo, Shimada & Fukatsu (2005).Kondo N, Shimada M, Fukatsu T. Infection density of Wolbachia endosymbiont affected by co-infection and host genotype. Biology Letters. 2005;1:488–491. doi: 10.1098/rsbl.2005.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al. (2014).Lu F, Kang XY, Lorenz G, Espino L, Jiang MX, Way MO. Culture-independent analysis of bacterial communities in the gut of rice water weevil (Coleoptera: Curculionidae) Annals of the Entomological Society of America. 2014;10:592–600. doi: 10.1603/AN13145. [DOI] [Google Scholar]
- Nirgianaki et al. (2003).Nirgianaki A, Banks GK, Frohlich DR, Veneti Z, Braig HR, Miller TA, Bedford ID, Markham PG, Savakis C, Bourtzis K. Wolbachia Infections of the Whitefly Bemisia tabaci. Current Microbiology. 2003;47:0093–0101. doi: 10.1007/s00284-002-3969-1. [DOI] [PubMed] [Google Scholar]
- Pankewitz et al. (2007).Pankewitz F, Zöllmer A, Hilker M, Gräser Y. Presence of Wolbachia in insect eggs containing antimicrobially active anthraquinones. Microbial Ecology. 2007;54:713–721. doi: 10.1007/s00248-007-9230-5. [DOI] [PubMed] [Google Scholar]
- Piper et al. (2001).Piper RW, Compton SG, Rasplus JY, Piry S. The species status of Cathormiocerus britannicus, an endemic, endangered British weevil. Biological Conservation. 2001;101:9–13. doi: 10.1016/S0006-3207(01)00048-9. [DOI] [Google Scholar]
- Pourali et al. (2009).Pourali P, Roayaei Ardakani M, Jolodar A, Razi Jalali MH. PCR screening of the Wolbachia in some arthropods and nematodes in Khuzestan province. Iranian Journal of Veterinary Research. 2009;10:216–222. [Google Scholar]
- Prakash & Puttaraju (2006).Prakash BM, Puttaraju HP. Wolbachia endosymbiont in some insect pests of sericulture. Current Science. 2006;90:1671–1674. [Google Scholar]
- Roehrdanz & Wichmann (2013).Roehrdanz RL, Wichmann SGS. Wolbachia wsp gene clones detect the distribution of Wolbachia variants and wsp hypervariable regions among 160 individuals of a multistrain infected population of Diabrotica barberi (Coleoptera: Chrysomelidae) Annals of the Entomological Society of America. 2013;106:329–338. doi: 10.1603/AN12118. [DOI] [Google Scholar]
- Sharaf et al. (2013).Sharaf K, Hadid Y, Pavliček T, Nevo E. Local genetic population divergence in a saw-toothed grain beetle, Oryzaephilus surinamensis (L.) (Coleoptera, Cucujidae) Journal of Stored Products Research. 2013;53:72–76. doi: 10.1016/j.jspr.2013.03.002. [DOI] [Google Scholar]
- Sintupachee et al. (2006).Sintupachee S, Milne JR, Poonchaisri S, Baimai V, Kittayapong P. Closely related wolbachia strains within the pumpkin arthropod community and the potential for horizontal transmission via the plant. Microbial Ecology. 2006;51:294–301. doi: 10.1007/s00248-006-9036-x. [DOI] [PubMed] [Google Scholar]
- Toševski et al. (2015).Toševski I, Caldara R, Jović J, Hernández-Vera G, Baviera C, Gassman A, Emerson BC. Host associated genetic divergence and taxonomy in the Rhinusa pilosa Gyllenhal species complex: an integrative approach. Systematic Entomology. 2015;40:268–287. doi: 10.1111/syen.12109. [DOI] [Google Scholar]
- Vega et al. (2002).Vega FE, Benavides P, Stuart J, O’Neill SL. Wolbachia infection in the coffee berry borer (Coleoptera: Scolytidae) Annals of the Entomological Society of America. 2002;95:374–378. doi: 10.1603/0013-8746(2002)095[0374:WIITCB]2.0.CO;2. [DOI] [Google Scholar]
- Xue et al. (2011).Xue H-J, Li W-Z, Nie R-E, Yang X-K. Recent speciation in three closely related sympatric specialists: inferences using multi-locus sequence, post-mating isolation and endosymbiont data. PLOS ONE. 2011;6:e27834. doi: 10.1371/journal.pone.0027834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Maximum likelihood phylogenetic tree reconstructed based on Wolbachia 16S rDNA gene sequences obtained from hosts belonging to various species of Coleoptera (data downloaded from NCBI GenBank https://www.ncbi.nlm.nih.gov/genbank/). Statistical supports (bootstrap values) are presented above the branches (shown only if >0.50).
Maximum likelihood phylogenetic tree reconstructed based on Wolbachia ftsZ gene sequences obtained from hosts belonging to various species of Coleoptera (data downloaded from NCBI GenBank https://www.ncbi.nlm.nih.gov/genbank/). Statistical supports (bootstrap values) are presented above the branches (shown only if >0.50).
Data Availability Statement
The following information was supplied regarding data availability:
The database of published articles used for systematic review is presented as Table S1 in the manuscript.