Abstract
Purpose
Due to the complex pharmacokinetic profiles of phenytoin (PHT) and fosphenytoin (FOS), achieving sustained, targeted serum PHT levels in the first day of use is challenging.
Methods
A population based approach was used to analyze total serum PHT (tPHT) level within 2–24 hours of PHT/FOS loading with or without supplementary maintenance or additional loading doses among PHT-naïve patients in the acute hospital setting. Adequate tPHT serum level was defined as ≥ 20 μg/mL.
Results
Among 494 patients with 545 tPHT serum levels obtained in the first 2–24 hours after the loading dose (LD), tPHT serum levels of either < or ≥ 20 μg/mL were observed along wide and overlapping cumulative dose ranges. Among those receiving 15–20 mg/kg and 20–55 mg/kg weight-based loading dose, 63 % and 51 % respectively did not attain tPHT serum level of ≥ 20 μg/mL even within the first 6 hours of treatment. For the 393 available concomitant free and total serum PHT levels, correlation was weak, r=0.36.
Conclusion
Close laboratory surveillance and PHT/FOS dose adjustments are recommended to ensure adequate and sustained tPHT serum levels early in treatment. Free serum PHT level is the preferred method of drug monitoring.
Keywords: Fosphenytoin, Serum Phenytoin Level, Status Epilepticus
Introduction
Phenytoin (PHT) is currently approved for treatment of generalized and focal seizures and to prevent seizures following neurosurgical procedures1. Fosphenytoin (FOS) is a phosphate ester prodrug developed as an alternative to parenteral PHT2. Rapid intravenous (IV) administration is convenient for the management of acute seizures, status epilepticus and seizure prophylaxis. Following benzodiazepines PHT/FOS are listed as second line agents for management of status epilepticus by the European Federation of Neurological Societies (EFNS) 3, Neurocritical Care Society (NCS) 4 and the American Epilepsy Society (AES) 5 with an initial recommended loading dose (LD) of PHT/FOS ranging between 15 and 30 mg/kg or phenytoin equivalents (PE) 6,7,8,9 with the targeted serum PHT level being ≥ 20 μg/mL. Despite that recommendation, the latest guideline also recommends a single dose of FOS to be capped at 1,500 mg PE5. Moreover, in spite of widespread PHT/FOS use, there are no comprehensive guidelines on the timing of maintenance dosing (MD) initiation. Cranford et al. (1978) state that “IV administration of a single 18 mg/kg PHT dose was safe and effective in maintaining serum PHT levels above 10 μg/mL for 24 hours in most participants after which an oral or IV maintenance PHT dose can be started when convenient, approximately 24 hours after the infusion.” Published references suggest 5 mg/kg/day dose6 or 100 mg every six to eight hours10.
We compared different approaches to PHT/FOS administration in the acute hospital setting by evaluating the total PHT (tPHT) serum levels obtained 2–24 hours post LD. We sought to know whether in this time period different medication dosing regimens resulted in sustained tPHT serum levels ≥20 μg/mL during the first day of use. A level greater than 20 μg/mL was chosen, as it is typically desired in urgent hospital situations.
Methods
The study was approved by the University of Rochester Research Subjects Review Board. A retrospective automated electronic medical record (Epic) search identified 1,361 PHT-naïve patients admitted between March of 2011 and August of 2015 who received a PHT/FOS dose of 500 mg or more and subsequently had a tPHT serum level drawn. Five hundred milligram cut off dose was used to include children into the analysis. The laboratory analysis of tPHT serum levels was not performed at predetermined time points but was obtained as clinically indicated pending availability of a laboratory technician. Patients were excluded if they did not have tPHT serum levels within 24 hours of dosing, were on PHT prior to admission, or had tPHT serum levels measured within the first two hours of medication administration. Patients with extreme outlying laboratory values or those with clear documentation errors were also excluded (tPHT levels > 70 μg/mL or undetectable levels, multiple simultaneously recorded LDs, PHT LD not recorded, or clinically obvious errors such as an adult patient with a weight listed as < 4 kg). When a patient had more than one tPHTa serum level drawn within a 24 hour period, the laboratory values were included for analysis only if maintenance or a second loading dose was given prior to the second tPHT serum level draw. There were a total of 494 patients with 545 available tPHT serum level measurements for the primary end point analysis. The laboratory data were divided into three groups based on the PHT/FOS dosing regimens. The first group consisted of patients with tPHT serum levels drawn following a single Loading Dose (LD only, N=355). The second group consisted of patients with tPHT serum levels drawn following a LD and supplemented by a Maintenance Dose(s) [LD+MD(s)], (N=137). The last group consisted of patients with tPHT serum levels obtained after an additional LD (defined as at least 350 mg of PHT/FOS) (N=53).
We hypothesized there would be a strong relationship between the cumulative weight-adjusted PHT/FOS dose and the tPHT serum level. To test this hypothesis we further subdivided each group into [3–15) mg/kg, [15–20) mg/kg, or [20–55) mg/kg subgroups based on the cumulative weight-based PHT/FOS dose administered prior to the blood draw. Three hundred and ninety-three concomitant free and total serum PHT levels were available from 344 patients for an additional analysis.
The weight on admission was converted to kilograms (kg). Timing of obtaining serum tPHT level is presented in hours (h) following the first LD administration. The medication loading dose was calculated based on recorded weight and presented in mg/kg for PHT and mg/kg PE for FOS. The serum PHT levels were reported in micrograms per milliliter (μg/mL). ANOVA supplemented by the Tukey’s test for differences and correlation analysis were applied for statistical assessment of numerical data. The Kruskal-Wallis Rank Sum test was performed to analyze the association of ordinal variables. P-values less than 0.05 were considered significant for all statistical tests. See Table 1 for additional demographic data.
Table 1.
Demographics
| Sex – Female (%) | 50% |
| Age (years): Median (IQR; min/max) | 58 (29; 6/96) |
| Weight (kg) Median (IQR; min/max) | 75 (27; 22/159) |
| PHT vs. FOS used for loading dose | 41(8%)/453(92%) |
| Loading dose (mg) | |
| Median (IQR; min/max) | 1378 (629; 500/2614) |
| Mean | 1356 |
| Loading dose (mg/kg) | |
| Median (IQR; min/max) | 19 (5; 4/31) |
| Mean | 18 |
| Maintenance dose (MD), Mean (mg) | 112 |
| Weight adjusted maintenance dose, Mean (mg/kg) | 1.5 |
| Additional Loading dose, Mean (mg) | 931 |
| Weight adjusted additional Loading dose, Mean (mg/kg) | 11 |
| Timing in hours of the serum lab draw following a loading dose, Mean (Min/Max) | |
| LD Only | 7 (2/24) |
| LD + MD(s) | 16 (5/24) |
| Multiple LDs | 13 (2/24) |
| Timing in hours of the serum lab draw following last PHT dose, Mean (Min/Max) | |
| LD + MD(s) | 5 (2/13) |
| Multiple LDs | 7 (2/21) |
| Diagnoses on admission | |
| Known seizure disorder/Epilepsy | 158 |
| Altered mental status | 42 |
| Traumatic brain injury/Trauma | 35 |
| Intracerebral hemorrhage | 34 |
| Subdural hematoma | 24 |
| Brain mass (primary or secondary) | 24 |
| Acute stroke | 19 |
| Subarachnoid hemorrhage | 15 |
| Infectious meningitis/Encephalitis | 8 |
| Cardiac arrest | 5 |
| Epidural hematoma | 4 |
| Other medical conditions (*) | 93 |
(*) Other conditions registered as the primary diagnosis included urinary tract infections, asphyxiation, long bone fractures, pseudo-seizure, gunshot to the abdomen, etc. See Supplemental data for further details and the list of secondary and tertiary diagnoses.
Results
Relationship between the cumulative PHT/FOS dose and subsequent tPHT serum level obtained between 2 and 24 hours post-LD
Total PHT serum levels ≥ 20 μg/mL were observed in patients receiving LDsb between 6 and 49 mg/kg, whereas tPHT serum levels < 20 μg/mL occurred in patients receiving LDs from 4 to 51 mg/kg. Figure 1 demonstrates the relationship between the cumulative weight-based PHT/FOS doses and the tPHT serum levels obtained 2 to 24 hours after LD infusion.
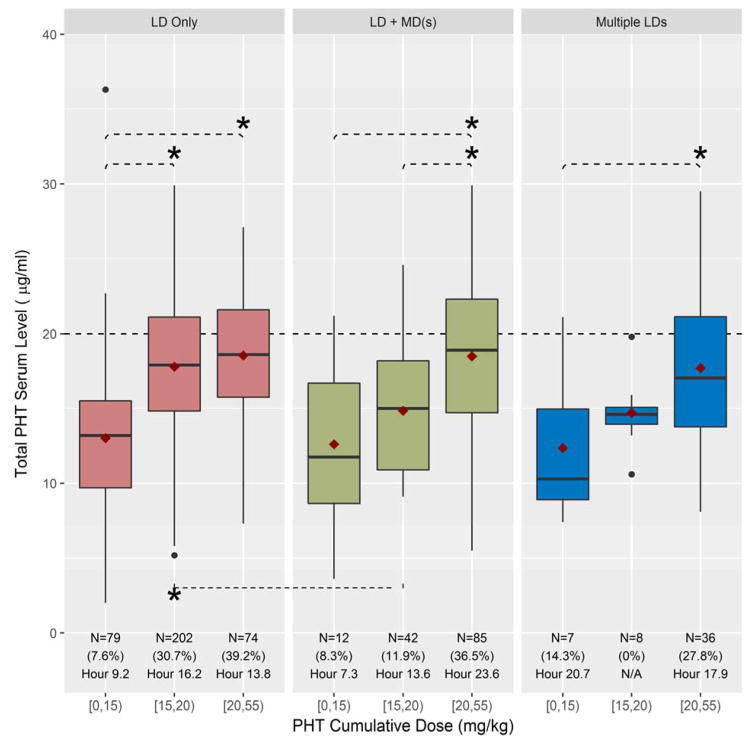
Figure 1. Cumulative weight adjusted Phenytoin (PHT)/Fosphenytoin (FOS) dose vs. Total PHT (tPHT) serum level obtained between 2 and 24 hours post initial loading dose.
Please use color in publication. Boxplots demonstrate the relationship between cumulative weight-based PHT/FOS dose and tPHT serum for each of the 3 medication regimen groups. X axis: weight-based ranges of cumulative dose of PHT/FOS in mg/kg ([3–15), [15–20), or [20–55)) prior to level drawn. Y axis: total PHT serum level in μg/mL. Red boxes: group receiving a single loading dose (LD Only). Green boxes: group receiving a single loading dose followed by at least one maintenance dose (LD+MD(s)). Blue boxes: group receiving multiple loading doses (Multiple LDs). The red diamond is the mean and black bar is the median tPHT serum level for each subgroup. Below each boxplot is the total number of serum tPHT levels (N) for each subgroup, the percent of levels ≥ 20 μg/mL (%) and the last hour post the loading dose administration when tPHT level ≥20 μg/mL was present. Asterisks (*) indicate p<0.05 for Tukey’s test of differences.
Within each dosing group we compared the cumulative weight-based PHT/FOS dose with tPHT serum levels obtained during the first day of treatment. In the LD only group there was a significant difference between the [3–15) mg/kg cumulative dose subgroup and each of the other two subgroups ([15–20) mg/kg, p<0.0005; [20–55) mg/kg, p<0.0005). When LD was supplemented by the maintenance dose (LD+MD(s) group), significant tPHT serum level differences were observed between [20–55) mg/kg cumulative dose subgroup and each of the two lower cumulative dose subgroups ([3–15) mg/kg, p=0.0008; [15–20) mg/kg, p=0.0006). When Multiple LDs were given, the only significant tPHT serum level difference was between the [3–15) mg/kg and [20–55) mg/kg cumulative dose subgroups (p=0.03). (Fig. 1)
When comparing weight-based subgroups across different medication regimens the only significant difference in tPHT serum levels was noted in a cumulative dose of [15–20) mg/kg between those in the single LD and those in the LD+MD(s)c subgroups (p=0.001).
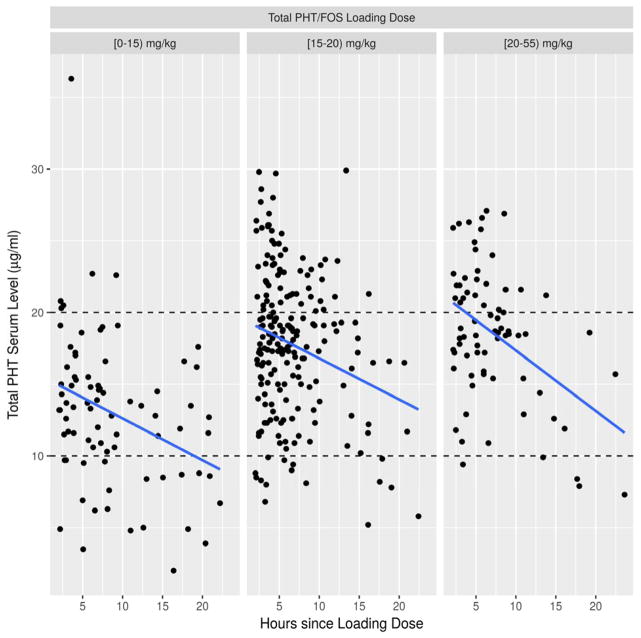
In addition, Table 2 demonstrates a breakdown of serum tPHT levels by either <10, 10–20 or ≥ 20 μg/mL for a given cumulative PHT/FOS dose respective to the time of the blood draw after the LD. Statistically significant associations between higher cumulative PHT dose and higher resulting tPHT serum level was detected in the LD Only group for hours 2–6 (p<0.0007), 6–10 (p=0.0131) and 10–14 (p=0.0092) as well as hours 14–18 (p=0.0253) for the LD+MD(s) group. Overall, total PHT serum levels ≥ 20 μg/mL were present in only 26% when additional MDs were given and 23% when more than one loading dose was administered over the course of one day. In 12% of the LD+MD(s) group and 6% of the multiple LDs group, tPHT levels were <10 μg/mL (Table 2). Figure 2 demonstrates distribution of the tPHT serum levels in relation to the time after a single loading dose.
Table 2. Total Phenytoin (tPHT) serum levels for a given cumulative PHT/Fosphenytoin dose conditional on the time of blood draw from the initial loading dose for each of the medication groups. Please use colors for publication.
The numbers reflect tPTH serum levels divided as <10/10–20/≥ 20 μg/mL. Subgroup totals are in parentheses. (LD= Loading Dose; MD= Maintenance Dose). Colored are the groups with statistically significant difference.
| PHT/FOS Dosing Groups (Number of values) | Cumulative PHT/FOS dose [mg/kg) and total number of values (N) | 2–6 hours post LD | 6–10 hours post LD | 10–14 hours post LD | 14–18 hours post LD | 18–20 hours post LD | 20–24 hours post LD |
|---|---|---|---|---|---|---|---|
| LD only (355) | |||||||
| [3,15) (79) | 6/24/4 | 4/16/2 | 3/2/- | 3/5/- | 2/3/- | 3/2/- | |
| [15,20) (202) | 7/69/42 | 3/36/11 | -/8/8 | 3/9/1 | 1/1/- | 1/2/- | |
| [20,55) (74) | 1/19/19 | -/12/8 | 1/5/2 | 2/2/- | -/1/- | 1/1/- | |
| LD+MD(s) (137) | |||||||
| [3,15) (12) | -/-/- | -/1/1 | -/2/- | 3/3/- | -/-/- | 1/1/- | |
| [15,20) (42) | -/1/- | -/-/1 | -/12/4 | 1/11/- | 2/2/- | 3/5/- | |
| [20,55) (83) | -/-/1 | -/2/8 | 2/9/11 | 1/15/2 | -/4/2 | 4/16/6 | |
| Multiple LDs (53) | |||||||
| [3,15) (7) | -/-/- | -/1/- | 1/1/- | -/1/- | -/1/- | 1/-/1 | |
| [15,20) (8) | -/-/- | -/1/- | -/2/- | -/2/- | -/-/- | -/3/- | |
| [20,55) (38) | -/2/3 | 1/5/3 | -/10/3 | -/4/2 | -/1/- | -/4/- |
Figure 2. Total PHT (tPHT) serum levels obtained two to 24 hours in a group receiving a single Phenytoin (PHT)/Fosphenytoin (FOS) loading dose.
The weight-based loading dose is divided into [3–15), [15–20), or [20–51) mg/kg subgroups. X axis: hours since loading dose. Y axis: total PHT serum level (μg/ml). Blue lines represent linear model fit.
Relationship between fixed non-weight-based PHT/FOS loading doses and subsequent tPHT serum levels
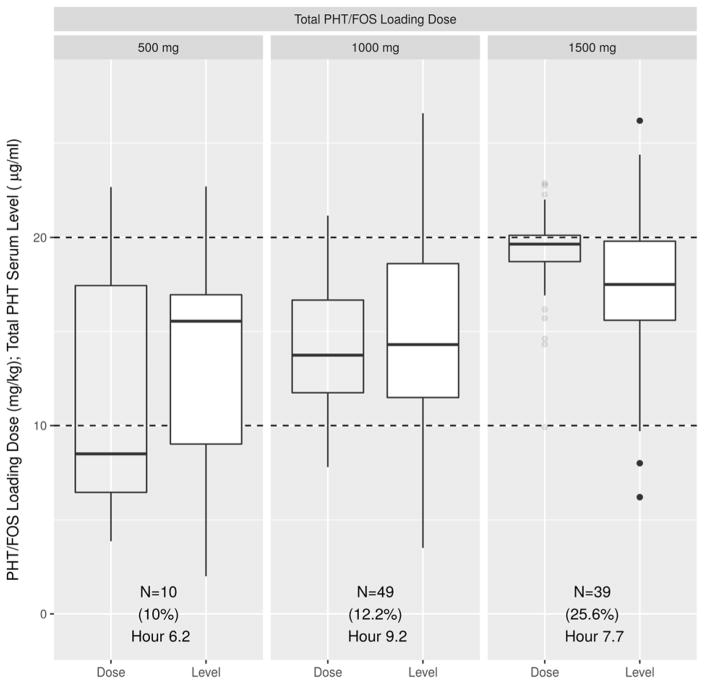
Within the sample, 199 patients received a single fixed PHT/FOS loading dose in the amount of either 500, 1,000 or 1,500 mg/PE. Ninety-eight of them (45.2%) had a subsequent tPHT serum level obtained between 2 and 24 hours post infusion. Figure 3 demonstrates the calculated weight-based LDs for each group and the resulting tPHT serum levels obtained between 2 and 24 hours post load. Pair-wise comparison of either weight-based doses or tPHT serum levels between the sexes in each group showed no significant differences.
Figure 3. Preset loading dose of 500, 1000 or 1500 mg/PE converted to weight-based value and correlated with subsequent total phenytoin serum levels obtained between 2 and 24 hours post infusion.
The clear boxplots represent Phenytoin weight-based loading doses (mg/kg for each of the present loading dose value). The white boxplots represent the corresponding serum tPHT levels (μg/mL) obtained between 2 and 24 hours post infusion. Below each boxplot is the total number of values in each subgroup (N), the percent of tPHT serum levels ≥ 20 μg/mL (%) and the last hour when tPHT level of ≥ 20 μg/mL was recorded.
Correlation between total and free PHT levels
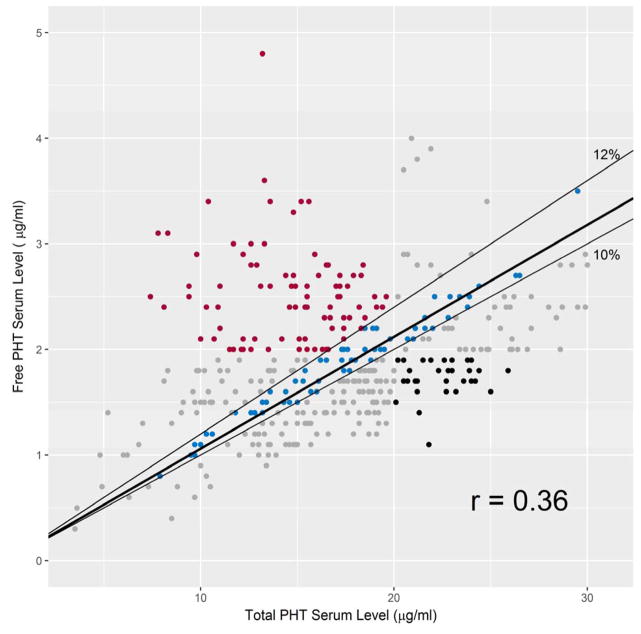
We examined the correlation between simultaneously drawn total and free serum PHT (frPHT) levels (N=393). The median proportion of free to total PHT levels was 10% (IQR=6%; max/min 5%/40%) with a mean of 12% (SD=5%). The correlation coefficient of these two measures equaled 0.36. Figure 4 shows the corresponding frPHT and tPHT levels. Two hundred and seven (53%) corresponding values had a free to total PHT level ratio within 10–12%. In 65 (17%) cases frPHT level was > 2 μg/ml, but tPHT level < 20 μg/ml and in 35 (9%) cases frPHT < 2 μg/mL had a corresponding tPHT level ≥ 20 μg/mL.
Figure 4. Correlation between free and total concomitant Phenytoin (PHT) serum levels.
Color should be used in print.
X axis: total PHT serum level (μg/mL). Y axis: free PHT serum level (μg/mL). The thin black lines border free to total PHT serum level ratios of 10% and 12%. The thick black line is the linear model fit to the data. The data points colored blue if the ratio of free to total PHT level is within 10–12%; red with frPHT level > 2 μg/mL and tPHT level < 20 μg/mL; black if frPHT < 2 μg/mL and tPHT level ≥ 20μg/mL.
Discussion
We evaluated the association between weight based PHT/FOS loading dose with and without additional maintenance or loading doses with total PHT serum levels drawn within 2–24 hours post first infusion in PHT-naïve patients managed in the acute hospital setting.
The initial loading dose was not highly predictive of whether a tPHT serum level of ≥ 20 μg/mL would be achieved. As expected, those receiving a cumulative medication dose in the 20–55 mg/kg range had a greater chance of attaining an adequate tPHT level (Fig. 1, Table 2). The data also demonstrate that even despite an adequate weight-based single loading dose, 63 % in 15–20 mg/kg and 51 % in 20–55 mg/kg loading dose subgroups did not attain a tPHT serum level of ≥ 20μg/ml even within the first 6 hours of treatment (Fig. 2, Table 2). “We also observed that some patients attained tPHT serum levels ≥ 20 μg/mL despite low weight-based loading dose, supporting the need for close drug level monitoring.” These observations confirm the complexity of PHT pharmacokinetics, particularly when applied to the acutely sick hospital based patient population. Whereas total PHT serum levels between 10 and 20 μg/mL may be acceptable for chronic management of infrequent seizures or seizure prevention, published practical data based on clinical experience advocates for targeting higher serum tPHT levels particularly when treating uncontrolled seizures7. If medication efficacy is indeed serum level-dependent, sub- or low therapeutic serum tPHT levels more likely would be associated with failure in preventing seizure recurrence12 or terminating status epilepticus13, 14. When seizures recur, the assumption that an adequate serum medication level was achieved solely based on the loading dose value without a documented serum tPHT level could lead to an erroneous attribution of medication inefficacy. Since PHT levels are influenced by concomitant medications, critical illness status and pharmacogenomics, precise mathematical adjustments for all factors are not available. As such measurements of the serum tPHT levels become a necessity within the first day of use.
When comparing different cumulative doses (3–15, 15–20 and 20–55 mg/kg) among the different dosing regimens (LD only, LD+MD(s) and multiple LDs), the only difference was observed in 15–20 mg/kg cumulative dose subgroup between LD only and LD+MD(s). This is explained by the fact most serum levels in the LD group were obtained during the first 14 hours post LD in the LD only group and 10 hours and after in the LD+MD(s) group (Table 2). It also suggests that average maintenance dose of 112 mg (Table 1) is insufficient to maintain the tPHT serum level ≥ 20 μg/mL in the second half of the 24 hour period. Absence of the differences between other groups indicates that it is the cumulative dose of the medication, whether given either as a single load or as a loading dose with additional doses, determines tPHT serum levels over the first 24 hours. Additional investigation evaluating appropriate timing of initiation and the amount of the maintenance dosing of PHT/FOS after the LD in PHT-naïve patients is needed.
In our population, those receiving the fixed 1,500 mg LD had a correlated weight-based dose range of 10 to 23 mg/kg. On average, all groups were under-dosed as evidenced from the weight-based calculations in Figure 3. In the United States, the average weight for men is 89 kg and 76 kg for women15. If the FOS dose is capped at 1,500 mg as per recent recommendations5, a substantial portion of patients will be under-dosed. As such we advise against the use of preset 500, 1,000 or 1,500 mg PHT/FOS loading doses or limiting the dose of FOS to 1,500 mg (PE).
Lastly, the analysis of the relationship between free and total serum PHT levels is complex. Our data demonstrate an overall weak correlation with wide variability in the ratio of unbound pharmacologically active to total PHT serum levels. In patients with higher than expected free PHT serum levels but with therapeutic total PHT levels (Fig. 4, red points), making the decision to increase the dose of the medication solely based on a tPHT serum level could potentially augment medication toxicity due to zero order PHT elimination kinetics. On the other hand in patients with disproportionally high total but therapeutic free PHT serum levels, the decision to decrease or hold a dose of medication could lead to an unnecessary decrease in frPHT levels and potentially seizures. Although the median for the unbound plasma fraction of phenytoin in our population is close to previously reported value16, the correlation coefficient between the free and total serum PHT levels is low. This weak correlation may be explained in part by hypoalbuminemia as well as use of other medications with high protein bounding capacity17 or effect on PHT pharmacokinetics through either induction or inhibition of the hepatic cytochrome P450 enzymes involved in PHT metabolism. While the Sheiner-Tozer equation18 is used to calculate tPHT levels in hypoalbunimic patients, precise adjustments for other factors are not available. Since the correlation between PHT concentration in the cerebrospinal fluid and frPHT is more reliable than that of the tPHT level19, serum frPHT is the preferred method of drug monitoring as it more accurately reflects specific medication fraction available to the brain.
Limitations
Due to the retrospective nature of these data, we could not confirm accurate weights of many participants. As such, dosing might be approximate, but we considered the likely inaccuracies to be small and acceptable in clinical practice 20. In addition, the timing of the serum PHT draw was not predetermined and occurred at different points during the 2–24 hour post-load period. Since this data analysis is based on current clinical practice, we considered this limitation representative of ‘real world’ data. We included a population of broad age distribution, as recommendations on emergent use of PHT/FOS are similar among different age groups21. Furthermore, protein binding and oxidative metabolism of phenytoin is consistent between acutely sick pediatric and adult populations22. Moreover, we did not review concomitant medications and over-the-counter preparations, nor did we analyze concomitant albumin levels. Due to the emergent use of PHT/FOS, such information albeit important may not be readily available in the acute hospital setting. Lastly due to the retrospective approach to these data analysis, we could not accurately correlate sustained seizure termination or seizure recurrence with the PHT serum levels.
Conclusions
Our results confirm that the pharmacokinetics of PHT and FOS are complex and if a specific serum PHT level is targeted, close laboratory surveillance and dose adjustments are required. We suggest that serum PHT levels need to be closely monitored in the first day of use to ensure adequate serum levels are achieved and sustained, particularly if medication is used for the management of acute repetitive seizures or status epilepticus. The maintenance dosing of PHT/FOS may need to be initiated within the first 24 hours to reach sustained and sufficient tPHT serum levels in accordance with goals of care. Additional research is required to provide a pharmacokinetic-based correlation between medication level and the presence of sustained medication efficacy in the post-loading phase. In addition, differences in pharmacokinetic profiles between the sexes and complex drug–drug interactions should be studied in the future.
Highlights.
PHT serum levels of either < or ≥ 20 μg/mL were observed along wide dose ranges
Despite proper dosing, PHT serum level could be low in the first hours of treatment
The correlation between free and total serum PHT levels was weak
Acknowledgments
Funding.
This work was supported by the Clinical & Translational Science Institute (CTSI) of the University of Rochester (to RAG [UL1 TR002001 and TL1 TR002000], to KG T32ES007271 and to OS CTSI award number 3191). We affirm that the work described is consistent with the Journal’s guidelines for ethical publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- PHT
Phenytoin
- FOS
Fosphenytoin
- tPHT
Total serum PHT
- frPHT
Free serum PHT
- IV
Intravenous
- LD
Loading dose
- MD
Maintenance dose
Footnotes
Total serum phenytoin level (tPHT)
Loading Dose (LD)
Maintenance Dose (MD)
Disclosures
Olga Selioutski, DO has received support from SAGE Therapeutics, Sepracor/Sunovion, USL261 for being a primary investigator in industry sponsored clinical trials and from the University of Rochester CTSI award number 3191 from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH).
Katherine Grzesik, MA received award # T32ES007271 from the National Institute of Environmental Health Sciences of the NIH.
Olga Vasilyeva, PharmD, PhD has no conflicts of interest to disclose.
Agust Hilmarsson, MD has no conflicts of interest to disclose.
Lynn Liu, MD has no conflicts of interest to disclose.
James Fessler has no conflicts of interest to disclose.
Robert A. Gross, MD, PhD receives support from the Clinical and Translational Science Award to University of Rochester (educational directorship). He also receives an editorial stipend from the American Academy of Neurology and royalties from a book series.
References
- 1.Lexicomp/Phenytoin Lexi-Drugs. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7489#f_pharmacology-and-pharmacokinetics.
- 2.Fischer JH, Patel TV, Fischer PA. Fosphenytoin: clinical pharmacokinetics and comparative advantages in the acute treatment of seizures. Clin Pharmacokinet. 2003;42:33–58. doi: 10.2165/00003088-200342010-00002. [DOI] [PubMed] [Google Scholar]
- 3.Meierkord H, Boon P, Engelsen B, et al. EFNS guideline on the management of status epilepticus in adults. Eur J Neuro. 2010;17:348–355. doi: 10.1111/j.1468-1331.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 4.Brophy GM, Bell R, Claassen J, et al. Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the Evaluation and Management of Status Epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 5.Glauser T, Shinnar S, Gloss D, et al. Evidence-Based Guideline: Treatment of Convulsive Status Epilepticus in Children and Adults: Report of the Guideline Committee of the American Epilepsy Society. Epilepsy Currents. 2016;16:48–61. doi: 10.5698/1535-7597-16.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cranford RE, Leppik IE, Patrick B, et al. Intravenous phenytoin: Clinical and pharmacokinetic aspects. Neurology. 1978;28:874–80. doi: 10.1212/wnl.28.9.874. [DOI] [PubMed] [Google Scholar]
- 7.Chen JW, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5:246–56. doi: 10.1016/S1474-4422(06)70374-X. [DOI] [PubMed] [Google Scholar]
- 8.Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans affairs status epilepticus cooperative study group. N Engl J Med. 1998;339:792–8. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 9.Knake S, Hamer HM, Rosenow F. Status epilepticus: A critical review. Epilepsy Behav. 2009;15:1–14. doi: 10.1016/j.yebeh.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Falco-Walter JJ, Bleck T. Treatment of Established Status Epilepticus. J Clin Med. 2016;5:49. doi: 10.3390/jcm5050049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lexicomp/Phenytoin Lexi-Drugs. https://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7489#f_dosages.
- 12.De Santis A, Villani R, Sinisi M, et al. Add-on Phenytoin Fails to Prevent Early Seizures after Surgery for Supratentorial Brain Tumors: A Randomized Controlled Study. Epilepsia. 2002;43:175–182. doi: 10.1046/j.1528-1157.2002.24801.x. [DOI] [PubMed] [Google Scholar]
- 13.Misra UK, Kalita J, Patel R. Sodium valproate vs phenytoin in status epilepticus: A pilot study. Neurology. 2006;67:340–342. doi: 10.1212/01.wnl.0000224880.35053.26. [DOI] [PubMed] [Google Scholar]
- 14.Redecker J, Wittstock M, Rösche J. The efficacy of different kinds of intravenously applied antiepileptic drugs in the treatment of status epilepticus. How can it be determined? Epilepsy Behav. 2017;71:35–38. doi: 10.1016/j.yebeh.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 15.http://www.cdc.gov/nchs/fastats/body-measurements.htm
- 16.Kilpatrick CJ, Wanwimolruk S, Wing M. Plasma concentrations of unbound phenytoin in the management of epilepsy. Br J Clin Pharmacol. 1984;17:539–546. doi: 10.1111/j.1365-2125.1984.tb02387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckley MS, Reeves BA, Barletta JF, Bikin DS. Correlation of Free and Total Phenytoin Serum Concentrations in Critically Ill Patients. Ann Pharmacother. 2016;50:276–81. doi: 10.1177/1060028015627468. [DOI] [PubMed] [Google Scholar]
- 18.Melmon KL. Melmon and Morelli’s Clinical Pharmacology: Basic Principles in Therapeutics. New York: Macmillan; 1978. [Google Scholar]
- 19.Friel PN, Ojemann GA, Rapport RL, et al. Human brain phenytoin: correlation with unbound and total serum concentrations. Epilepsy Res. 1989;3:82–85. doi: 10.1016/0920-1211(89)90072-7. http://dx.doi.org/10.1016/0920-1211(89)90072-7. [DOI] [PubMed] [Google Scholar]
- 20.Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus Intravenous Therapy for Prehospital Status Epilepticus. N Engl J Med. 2012;366:591. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith DM, McGinnis EL, Walleigh DJ, et al. Management of Status Epilepticus in Children. J Clin Med. 2016 Apr;5(4):47. doi: 10.3390/jcm5040047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stowe CD, Lee K, Storgio SA, et al. Altered Phenytoin Pharmacokinetics in Children with Severe, Acute Traumatic Brain Injury. J Clin Pharmacol. 2000;40:1452–1461. [PubMed] [Google Scholar]