Abstract
Objective
Whether obesity without metabolic syndrome (i.e., “metabolically healthy” obesity) confers similar or less metabolic risk remains controversial.
Methods
We conducted a retrospective 5-year cohort study of 9,721 Japanese subjects (48.5±10.5 years, 4,160 men) in 2004 and reevaluated 5 years later. Subjects were excluded if they were hypertensive, diabetic, or were receiving medications for dyslipidemia and/or gout or hyperuricemia in 2004. Study subjects were categorized according to baseline BMI of ≥25 kg/m2(overweight/obesity) and <25 kg/m2(lean/normal) and also whether they had metabolic syndrome. The cumulative incidences of hypertension and diabetes over 5 years between groups were assessed. A second analysis evaluated whether baseline hyperuricemia provided additional risk.
Results
Subjects with overweight/obesity but without metabolic syndrome carried increased cumulative incidences of hypertension(14.6% vs 7.2%, p<0.001) and diabetes(2.6% vs 1.1%, p=0.004) over 5 years compared to lean/normal subjects without metabolic syndrome. Overweight/obesity conferred an increased risk for diabetes even in individuals with normal fasting blood glucose. Hyperuricemia became an independent risk factor for developing hypertension over 5 years in lean/normal subjects without metabolic syndrome. A 1 mg/dL increase in serum uric acid carried increased risk for hypertension(19%) and diabetes(27%).
Conclusions
“Metabolically healthy” obesity and hyperuricemia confers increased risk for hypertension and diabetes.
Keywords: Obesity, Metabolic Syndrome, Cardiovascular Risk, Epidemiology
Introduction
Metabolic syndrome is an important risk factor for cardiovascular disease (1), but current definitions of metabolic syndrome do not consider body mass index (BMI) (2, 3, 4, 5) because waist circumference is a better predictor of total body fat than BMI (6). While obesity is recognized as an independent risk factor for hypertension and diabetes mellitus (DM) (7, 8, 9, 10), the classical approach in obesity research is to control for the various metabolic risk factors, many of which may be causally linked to obesity. An alternative approach would be to perform longitudinal analyses in which subjects with obesity are stratified at baseline into those with or without metabolic syndrome. This is especially important as the concept of a metabolically healthy population with obesity is widely recognized (11, 12, 13, 14, 15).
We tested the hypothesis that “metabolically healthy” obesity, defined as obesity in the absence of metabolic syndrome, still carried increased risk for hypertension and DM by a longitudinal study design. Moreover, we performed a second analysis to determine if hyperuricemia provided an additional risk for developing hypertension or DM because of the strong relationship of serum uric acid with obesity, hypertension, and DM (16).
Methods
Study design and study subjects
This study was a large-scale, single-center, retrospective cohort study in Japan. We used the database at the Center for Preventive Medicine, St. Luke’s International Hospital, Tokyo, Japan. This study population was considered as an ‘apparently healthy population’ since they came to the center by themselves to have annual regular health checkup without specific complaints, and also provided a general history of comorbidities. We have previously published studies using this database (17, 18, 19, 20, 21, 22, 23). The study subjects were similar to our previous studies, but this study design, inclusion and exclusion criteria, and outcomes were different from our previous studies.
We enrolled study subjects between ages 30 and 85 years old whose data were available at 2004 and 2009. Of 13,201 subjects who enrolled in 2004 and had follow-up data in 2009, 5 subjects were excluded due to missing waist circumference data, age <30 (n=121), age >85 (n=10), the presence of hypertension (2,599 subjects), DM (575 subjects), or treatment for dyslipidemia (658 subjects) or hyperuricemia and/or gout (373 subjects). A total of 9,721 study subjects were analyzed (Figure 1). The subjects were cross-classified into four groups as ‘BMI of ≥25 kg/m2 (overweight/obesity) and BMI of <25 kg/m2 (lean/normal) and those with and without metabolic syndrome, defined using a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention (IDF); National Heart, Lung, and Blood Institute (NHLBI); American Heart Association (AHA); World Heart Federation (WHF); International Atherosclerosis Society (IAS); and International Association for the Study of Obesity (IASO) (2). Thus, four groups were followed, consisting of: 1) lean/normal without metabolic syndrome group, 2) overweight/obesity without metabolic syndrome group, 3) lean/normal with metabolic syndrome group, and 4) overweight/obesity with metabolic syndrome group at the baseline (in 2004) (Figure 1). We compared cumulative incidences of hypertension and DM over 5 years among the four groups, and calculated odds ratios (ORs) for each disease by crude analysis and after adjustments for age, sex, smoking and drinking habits, chronic kidney disease, BMI and metabolic syndrome category (lean/normal and overweight/obesity with and without metabolic syndrome), and hyperuricemia (or serum uric acid levels). Moreover, we performed a second analysis to determine if hyperuricemia and elevated serum uric acid provided an additional risk for developing hypertension or DM.
Figure 1. Flow diagram of study enrollment.
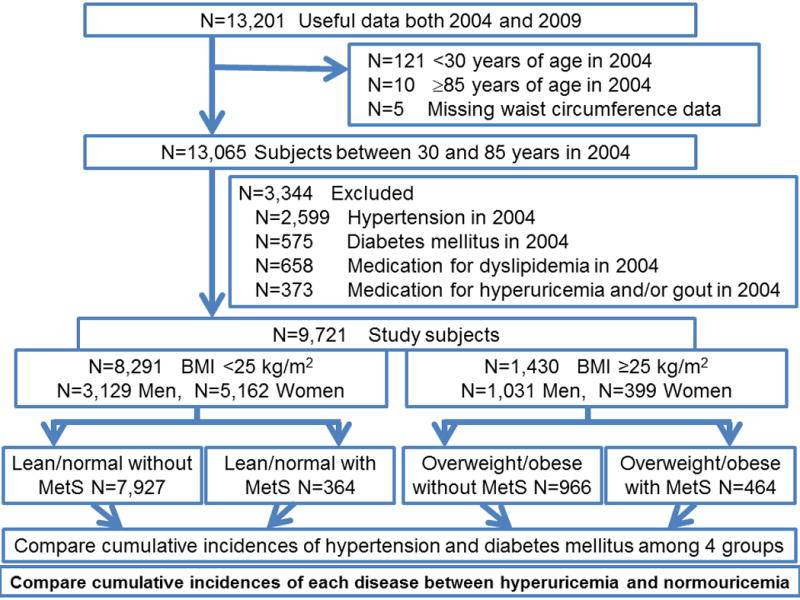
N: number of subjects; BMI: body mass index
1935 subjects had only hypertension. 275 subjects had only diabetes mellitus. 125 subjects had only medication for dyslipidemia. 273 subjects had only medication for hyperuricemia and/or gout.261 subjects had both hypertension and diabetes mellitus. 93 subjects had medication for dyslipidemia and hyperuricemia and/or gout. 322 subjects had hypertension and medication for dyslipidemia. 204 subjects had hypertension and medication for hyperuricemia and/or gout. 58 subjects had hypertension and medication for dyslipidemia and hyperuricemia and/or gout. 84 subjects had diabetes mellitus and medication for dyslipidemia. 36 subjects had diabetes mellitus and medication for hyperuricemia and/or gout. 16 subjects had diabetes mellitus and medication for dyslipidemia and hyperuricemia and/or gout. 54 subjects had hypertension, diabetes mellitus and medication for dyslipidemia. 25 subjects had hypertension, diabetes mellitus and medication for hyperuricemia and/or gout. 14 subjects had hypertension, diabetes mellitus and medication for dyslipidemia and hyperuricemia and/or gout.
Definition of metabolic syndrome, hypertension, diabetes mellitus, hyperuricemia, and chronic kidney disease
Metabolic syndrome was defined according to a joint interim statement of IDF; NHLBI; AHA; WHF; IAS; and IASO (2). Metabolic syndrome definition was a condition when a person had three or more of the following five measurements: 1) Abdominal obesity: IDF and WHO recommended waist circumference of 90 cm or above in men, and 80 cm or above in women for Japanese, 2) Triglyceride level of 150 mg/dL of blood or greater, 3) High-density lipoprotein (HDL) cholesterol of less than 40 mg/dL in men or less than 50 mg/dL in women, 4) Systolic blood pressure of 130 mmHg or greater, or diastolic blood pressure of 85 mmHg or greater, 5) Fasting glucose of 100 mg/dL or greater.
Hypertension was defined as the subjects who were on current antihypertensive medication and/or systolic blood pressure of ≥140 mmHg and/or diastolic blood pressure of ≥90 mmHg according to the Japanese Society of Hypertension guidelines (JSH 2014) (24). Blood pressure readings were obtained using an automatic brachial sphygmomanometer (OMRON Corporation, Kyoto, Japan). Two blood pressure examinations were taken after the participant had been seated and resting quietly for more than five minutes with feet on the ground and back supported. The mean systolic and diastolic blood pressure of each of the subjects were calculated from the recorded measurements. DM was defined as the subjects who had current DM on medication and/or HbA1c (National Glycohemoglobin Standardization Program) of ≥6.5% according to International Expert Committee. Hyperuricemia was defined as >7.0 mg/dL of serum uric acid in men and ≥6.0 mg/dL in women as the standard definition for most studies (25). The definition in men was attributed to Japanese guideline for the management of hyperuricemia and gout: second edition (26). However, compared to men, serum uric acid levels are lower in women because female hormones decrease serum uric acid levels (27). Thus, we defined hyperuricemia in women as ≥6.0 mg/dL as is commonly used in many other study populations (28, 29, 30, 31). Chronic kidney disease was defined as estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2. We calculated eGFR using the Japanese GFR equation: eGFR (mL/min/1.73m2) = 194 × serum creatinine−1.094 × age−0.287 (×0.739 if woman) (32). Drinking habits was defined as positive if the individual drank habitually (daily), while an absence of drinking or social drinking was considered negative. Smoking habits defined both current smokers and past smokers as positive.
Statistical analysis
The statistically significant level was set α =0.05, and all statistical analyses were two-sided. Data are expressed as mean ± standard derivation or as percent frequency unless otherwise specified. Comparisons between two groups were performed with Student t-tests for normally distributed variables, and χ2 analyses for categorical data. Multiple comparisons based on Tukey’s method were performed for pairwise comparisons. The risk factors for incident hypertension and DM over 5 years were evaluated both by crude models and by multivariable logistic regression models with adjustments for age, sex, smoking and drinking habits, chronic kidney disease, BMI and metabolic syndrome category (lean/normal and overweight/obesity with and without metabolic syndrome), and hyperuricemia (or serum uric acid), and the ORs for each disease were analyzed in each group. All analyses were also stratified by sex because the distribution of serum uric acid levels differed between men and women. We also compared the cumulative incidences of hypertension and DM between hyperuricemia and normouricemia in each group. All the statistical analyses were performed by the SPSS Statistics software (IBM SPSS Statistics version 22 for Windows; IBM, New York, USA).
Ethical considerations
We adhered to the principles of the Declaration of Helsinki. Consents were obtained from all subjects by a comprehensive agreement method provided by St. Luke’s International Hospital. All data were collected and compiled in a protected computer database. Individual data were anonymous without identifiable personal information. St. Luke’s International Hospital Ethics Committee approved the protocol for this study. No patients were involved in setting the research question or outcome measures, nor were they involved in the design and implementation of the study. There are no plans to involve patients in dissemination.
Results
Demographics
Subjects were divided into four groups; 7,927 lean/normal without metabolic syndrome, 966 overweight/obesity without metabolic syndrome, 364 lean/normal with metabolic syndrome, and 464 overweight/obesity with metabolic syndrome (Table 1). Lean/normal with metabolic syndrome group was significantly older than the other groups (p<0.001), but the other three groups had no significant differences of age. Overweight/obesity groups had significantly higher height, weight, BMI, waist circumference, higher prevalence of smoking and drinking habits, and higher levels of CRP and serum uric acid compared to lean/normal groups (p<0.001). Subjects with metabolic syndrome had significantly higher systolic and diastolic blood pressure, and higher levels of fasting blood glucose hemoglobin A1c, low-density lipoprotein cholesterol, and triglyceride but lower high-density lipoprotein cholesterol compared to subjects without metabolic syndrome (p<0.001) (Table 1 Total). When we compared the baseline characteristics of subjects stratified by sex, the results were similar (Table 1 Men and Women).
Table 1. Demographics of study subjects.
The baseline characteristics of subjects are shown following stratification by cross-classified four groups as ‘BMI of ≥25 kg/m2 (overweight/obesity) and BMI of < 25 kg/m2 (lean/normal) and those with and without metabolic syndrome.
| Total | Total | Lean/normal without MetS | Overweight/obesity without MetS | Lean/normal with MetS | Overweight/obesity with MetS | p |
|---|---|---|---|---|---|---|
| Number of subjects | 9,721 | 7,927 | 966 | 364 | 464 | |
| Male sex | 42.8% | 37.3% | 70.0% | 46.7% | 76.5% | |
| Age | 48.5±10.5 | 48.1±10.5 | 48.3±10.0 | 55.9±9.5 | 49.2±10.6 | <0.001 |
| Height (cm) | 163.3±8.5 | 162.7±8.2 | 166.0.±8.5 | 163.0±9.8 | 168.4±8.5 | <0.001 |
| Weight (kg) | 58.9±11.3 | 55.8±9.0 | 73.6±8.3 | 61.4±9.1 | 78.1±9.7 | <0.001 |
| Body mass index (kg/m2) | 21.9±2.9 | 21.0±2.1 | 26.7±1.6 | 23.0±1.4 | 27.5±2.1 | <0.001 |
| Waist circumference (cm) | 79.6±8.6 | 77.1±7.0 | 90.6±5.8 | 85.7±4.7 | 94.3±5.6 | <0.001 |
| Systolic BP (mmHg) | 112.8±13.1 | 110.8±12.6 | 117.8±10.7 | 125.4±11.8 | 126.2±10.2 | <0.001 |
| Diastolic BP (mmHg) | 70.3±8.8 | 69.1±8.5 | 73.6±7.2 | 77.5±8.0 | 79.0±6.8 | <0.001 |
| Pulse rate (bpm) | 72.8±10.1 | 73.0±10.2 | 70.0±8.7 | 75.9±11.5 | 73.0±9.1 | <0.001 |
| Smoking | 35.9% | 33.2% | 47.3% | 41.2% | 55.8% | <0.001 |
| Drinking habits | 41.5% | 40.2% | 48.0% | 40.9% | 49.4% | <0.001 |
| Fasting blood glucose (g/dL) | 96.8±8.5 | 95.5±7.9 | 99.4±8.2 | 105.6±8.6 | 106.0±8.3 | <0.001 |
| Hemoglobin A1c (%) | 4.94±0.33 | 4.92±0.32 | 4.99±0.32 | 5.17±0.35 | 5.15±0.35 | <0.001 |
| Total cholesterol (mg/dL) | 204.4±33.3 | 202.5±33.0 | 209.1±32.0 | 219.6±36.5 | 214.0±33.4 | <0.001 |
| LDL cholesterol (mg/dL) | 116±29 | 117.9±29.7 | 115.3±29.1 | 129.8±28.6 | 129.3±30.4 | <0.001 |
| HDL cholesterol (mg/dL) | 64.0±15.9 | 66.7±15.4 | 55.6±11.9 | 51.5±14.2 | 47.1±11.3 | <0.001 |
| Triglyceride (mg/dL) | 92.1±66.1 | 79.7±47.3 | 111.8±57.0 | 179.8±120.3 | 193.1±125.0 | <0.001 |
| BUN (mg/dL) | 13.7±3.3 | 13.7±3.3 | 13.9±3.1 | 14.2±3.2 | 14.0±3.0 | 0.003 |
| Serum creatinine (mg/dL) | 0.671±0.177 | 0.659±0.180 | 0.735±0.150 | 0.671±0.144 | 0.745±0.144 | <0.001 |
| eGFR (mL/min/1.73m2) | 87.0±15.2 | 87.5±15.3 | 85.2±14.8 | 84.1±14.7 | 85.0±14.7 | <0.001 |
| CRP (mg/dL) | 0.140±0.260 | 0.133±0.238 | 0.162±0.311 | 0.157±0.343 | 0.206±0.389 | <0.001 |
| Serum uric acid (mg/dL) | 5.17±1.37 | 4.97±1.29 | 6.04±1.38 | 5.67±1.24 | 6.40±1.38 | <0.001 |
|
| ||||||
| Men | Total | Lean/normal without MetS | Overweight/obesity without MetS | Lean/normal with MetS | Overweight/obesity with MetS | p |
|
| ||||||
| Number of subjects | 4,160 | 2,959 | 676 | 170 | 355 | |
| Age | 49.5±11.0 | 49.7±11.2 | 48.1±10.3 | 55.1±10.1 | 48.0±10.1 | <0.001 |
| Height (cm) | 170.5±6.0 | 170.4±6.0 | 169.9±6.1 | 170.6±7.0 | 171.9±5.8 | <0.001 |
| Weight (kg) | 68.0±9.2 | 64.4±6.8 | 76.6±7.1 | 68.7±6.3 | 81.1±7.8 | <0.001 |
| Body mass index (kg/m2) | 23.4±2.6 | 22.1±1.8 | 26.5±1.4 | 23.6±1.1 | 27.4±1.9 | <0.001 |
| Waist circumference (cm) | 83.8±7.4 | 80.8±5.7 | 90.4±5.3 | 87.3±4.5 | 94.3±5.0 | <0.001 |
| Systolic BP (mmHg) | 116.9±11.9 | 114.9±11.7 | 118.6±10.3 | 126.5±11.0 | 125.8±10.2 | <0.001 |
| Diastolic BP (mmHg) | 73.4±8.0 | 72.2±7.9 | 74.4±7.0 | 78.7±7.5 | 79.1±6.8 | <0.001 |
| Pulse rate (bpm) | 70.5±9.5 | 70.6±9.6 | 68.8±8.7 | 73.1±10.3 | 71.8±8.9 | <0.001 |
| Smoking | 67.9% | 59.7% | 59.5% | 72.4% | 67.9% | <0.001 |
| Drinking habits | 60.2% | 60.3% | 60.5% | 61.2% | 58.0% | 0.85 |
| Fasting blood glucose (g/dL) | 100.5±8.7 | 99.3±8.2 | 100.7±8.1 | 108.2±9.2 | 106.5±8.5 | <0.001 |
| Hemoglobin A1c (%) | 4.97±0.33 | 4.94±0.32 | 4.98±0.32 | 5.18±0.35 | 5.12±0.35 | <0.001 |
| Total cholestrol (mg/dL) | 203.6±31.9 | 201.5±31.3 | 206.2±31.1 | 212.3±37.4 | 211.4±33.3 | <0.001 |
| LDL cholesterol (mg/dL) | 122.1±28.9 | 120.0±28.3 | 128.5±28.6 | 122.3±32.0 | 127.1±30.2 | <0.001 |
| HDL cholesterol (mg/dL) | 55.7±13.3 | 58.2±13,3 | 52.8±11.0 | 45.8±11.7 | 44.9±9.7 | <0.001 |
| Triglyceride (mg/dL) | 119.0±83.1 | 101.8±60.5 | 121.7±61.8 | 223.0±146.1 | 207.4±132.9 | <0.001 |
| BUN (mg/dL) | 14.5±3.2 | 14.6±3.3 | 14.2±3.0 | 14.4±3.1 | 14.2±3.0 | 0.003 |
| Serum creatinine (mg/dL) | 0.794±0.120 | 0.792±0.121 | 0.804±0.115 | 0.786±0.109 | 0.797±0.116 | 0.089 |
| eGFR (mL/min/1.73m2) | 84.3±14.5 | 84.5±14.6 | 83.7±14.4 | 82.3±14.1 | 84.6±14.6 | 0.17 |
| CRP (mg/dL) | 0.159±0.310 | 0.152±0.301 | 0.169±0.363 | 0.144±0.176 | 0.201±0.327 | 0.030 |
| Serum uric acid (mg/dL) | 6.20±1.20 | 6.04±1.16 | 6.56±1.23 | 6.42±1.07 | 6.74±1.30 | <0.001 |
|
| ||||||
| Women | Total | Lean/normal without MetS | Overweight/obesity without MetS | Lean/normal with MetS | Overweight/obesity with MetS | p |
|
| ||||||
| Number of subjects | 5,561 | 4,968 | 290 | 194 | 109 | |
| Age | 47.7±10.1 | 47.2±9.9 | 48.9±9.3 | 56.6±9.0 | 53.2±11.4 | <0.001 |
| Height (cm) | 157.9±5.5 | 158.0±5.4 | 156.7.±5.5 | 156.3±6.4 | 157.0±5.1 | <0.001 |
| Weight (kg) | 52.1±7.2 | 50.7±5.6 | 66.6±6.6 | 55.0±5.7 | 68.3±8.8 | <0.001 |
| Body mass index (kg/m2) | 20.9±2.7 | 20.3±2.0 | 27.1±1.9 | 22.5±1.5 | 27.6±2.6 | <0.001 |
| Waist circumference (cm) | 76.5±8.1 | 74.9±6.7 | 90.8±6.7 | 84.3±4.4 | 94.2±7.2 | <0.001 |
| Systolic BP (mmHg) | 109.7±13.1 | 108.3±12.5 | 116.0±11.4 | 124.5±12.4 | 127.5±10.1 | <0.001 |
| Diastolic BP (mmHg) | 68.0±8.6 | 67.3±8.3 | 71.7±7.5 | 76.4±8.2 | 78.6±6.9 | <0.001 |
| Pulse rate (bpm) | 74.6±10.2 | 74.5±10.2 | 72.9±7.9 | 78.4±12.0 | 77.0±8.7 | <0.001 |
| Smoking | 17.3% | 17.3% | 19.0% | 13.9% | 16.5% | 0.54 |
| Drinking habits | 27.4% | 28.2% | 19.0% | 23.2% | 21.1% | 0.001 |
| Fasting blood glucose (g/dL) | 94.0±7.3 | 93.3±6.8 | 96.4±7.5 | 103.3±7.4 | 104.5±7.4 | <0.001 |
| Hemoglobin A1c (%) | 4.92±0.33 | 4.90±0.32 | 5.03±0.32 | 5.17±0.35 | 5.24±0.32 | <0.001 |
| Total cholesterol (mg/dL) | 205.0±34.3 | 203.2±33.9 | 215.7±33.3 | 226.0±34.4 | 222.4±32.6 | <0.001 |
| LDL cholesterol (mg/dL) | 114.8±29.9 | 112.5±29.2 | 132.7±28.4 | 135.9±29.0 | 136.5±30.2 | <0.001 |
| HDL cholesterol (mg/dL) | 70.3±14.7 | 71.7±14.3 | 62.0±11.5 | 56.4±14.3 | 54.1±13.1 | <0.001 |
| Triglyceride (mg/dL) | 71.9±39.0 | 66.6±30.6 | 88.8±34.0 | 141.9±93.7 | 146.6±78.7 | <0.001 |
| BUN (mg/dL) | 13.2±3.2 | 13.1±3.2 | 13.2±3.3 | 13.9±3.3 | 13.5±3.0 | 0.003 |
| Serum creatinine (mg/dL) | 0.578±0.155 | 0.579±0.161 | 0.573±0.080 | 0.570±0.080 | 0.577±0.086 | 0.80 |
| eGFR (mL/min/1.73m2) | 89.1±15.4 | 89.3±15.4 | 88.9±15.0 | 85.7±15.0 | 86.5±14.9 | 0.003 |
| CRP (mg/dL) | 0.126±0.214 | 0.122±0.190 | 0.146±0.116 | 0.168±0.440 | 0.225±0.55 | <0.001 |
| Serum uric acid (mg/dL) | 4.40±0.90 | 4.34±0.87 | 4.84±0.89 | 5.00±0.97 | 5.27±0.96 | <0.001 |
BMI: body mass index; BP: blood pressure, bpm: beats per minute, LDL: low density lipoprotein, HDL: high density lipoprotein, BUN: blood urea nitrogen, eGFR: estimated glomerular filtration rate, CRP: C-reactive protein, p: probability
Data are presented as mean ± standard deviation.
Cumulative incidences of hypertension and diabetes mellitus among lean/normal and overweight/obesity with and without metabolic syndrome
The cumulative incidences of hypertension and DM over 5 years are shown in Table 2. Lean/normal subjects without metabolic syndrome group had lowest cumulative incidence of hypertension and DM among the four groups (p<0.001). Overweight/obesity without metabolic syndrome group had significantly higher cumulative incidence of hypertension (14.6% vs 7.2%, p<0.001) and DM (2.6% vs 1.1%, p=0.004) compared to lean/normal without metabolic syndrome group, but significantly lower cumulative incidence of hypertension (14.6% vs 28.0%, p<0.001) and DM (2.6% vs 7.1%, p<0.001) compared to lean/normal with metabolic syndrome group. Lean/normal with metabolic syndrome group did not have significant differences of cumulative incidence of hypertension (28.0% vs 28.4%, p=1.00) and DM (7.1% vs 9.5%, p=0.057) compared to overweight/obesity with metabolic syndrome group. We also conducted the same analyses stratified by sex, the results were almost the same, but there was no significant differences of cumulative incidences of DM between lean/normal without metabolic syndrome group and overweight/obesity without metabolic syndrome group both in men (1.8% vs 3.0%, p=0.38) and women (0.6% vs 1.7%, p=0.25).
Table 2.
Cumulative incidences of hypertension and diabetes mellitus over 5 years between study groups
| Total | N | Incidence of hypertension | Incidence of diabetes |
|---|---|---|---|
|
|
|||
| 1) Lean/normal without MetS (presence of 0-2 risk factors) | 7,927 | 7.2% | 1.1% |
| 2) Overweight/obesity without MetS (presence of 0-2 risk factors) | 966 | 14.6% | 2.6% |
| 3) Lean/normal with MetS | 364 | 28.0% | 7.1% |
| 4) Overweight/obesity with MetS | 464 | 28.4% | 9.5% |
|
| |||
| p value | 9,721 | <0.001 | <0.001 |
| Men | |||
| 1) Lean/normal without MetS (presence of 0-2 risk factors) | 2,959 | 10.1% | 1.8% |
| 2) Overweight/obesity without MetS (presence of 0-2 risk factors) | 676 | 14.8% | 3.0% |
| 3) Lean/normal with MetS | 170 | 28.8% | 7.6% |
| 4) Overweight/obesity with MetS | 355 | 26.8% | 9.6% |
|
| |||
| p value | 4,160 | <0.001 | <0.001 |
| Women | |||
| 1) Lean/normal without MetS (presence of 0-2 risk factors) | 4,968 | 5.5% | 0.6% |
| 2) Overweight/obesity without MetS (presence of 0-2 risk factors) | 290 | 14.1% | 1.7% |
| 3) Lean/normal with MetS | 194 | 27.3% | 6.7% |
| 4) Overweight/obesity with MetS | 109 | 33.9% | 9.2% |
|
| |||
| p value | 5,561 | <0.001 | <0.001 |
BMI: body mass index, High BMI means BMI ≥25 kg/m2
Total: There are significant differences of cumulative incidences of hypertension between 1) lean/normal without metabolic syndrome group and 2) overweight/obesity without metabolic syndrome group (p<0.001), between 1) and 3) lean/normal with metabolic syndrome group (p<0.001), between 1) and 4) overweight/obesity with metabolic syndrome group (p<0.001), between 2) and 3) (p<0.001), and between 2) and 4) (p<0.001), but there is no significant differences between 3) and 4) (p=1.0) by analysis using Tukey’s methods.
There are significant differences of cumulative incidences of diabetes mellitus between 1) and 2) (p=0.004), between 1) and 3) (p<0.001), between 1) and 4) (p<0.001), between 2) and 3) (p<0.001), and between 2) and 4) (p<0.001), but there is no significant differences between 3) and 4) (p=0.058) by analysis using Tukey’s methods.
Men: There are significant differences of cumulative incidences of hypertension between 1) and 2) (p=0.006), between 1) and 3) (p<0.001), between 1) and 4) (p<0.001), between 2) and 3) (p<0.001), and between 2) and 4) (p<0.001), but there is no significant differences between 3) and 4) (p=0.91) by analysis using Tukey’s methods.
There are significant differences of cumulative incidences of diabetes mellitus between 1) and 3) (p<0.001), between 1) and 4) (p<0.001), between 2) and 3) (p=0.006), and between 2) and 4) (p<0.001), but there is no significant differences between 1) and 2) (p=0.38) and between 3) and 4) (p=0.60) by analysis using Tukey’s methods.
Women: There are significant differences of cumulative incidences of hypertension between 1) and 2) (p<0.001), between 1) and 3) (p<0.001), between 1) and 4) (p<0.001), between 2) and 3) (p<0.001), and between 2) and 4) (p<0.001), but there is no significant differences between 3) and 4) (p=0.13) by analysis using Tukey’s methods.
There are significant differences of cumulative incidences of diabetes mellitus between 1) and 3) (p<0.001), between 1) and 4) (p<0.001), between 2) and 3) (p<0.001), and between 2) and 4) (p<0.001), but there is no significant differences between 1) and 2) (p=0.25) and between 3) and 4) (p=0.17) by analysis using Tukey’s methods.
Effect of hyperuricemia and normouricemia on outcomes
We compared the cumulative incidences of hypertension and DM over 5 years between hyperuricemia and normouricemia in each group by χ2 analyses (Figure 2). Only in the lean/normal without metabolic syndrome group, was hyperuricemia a significant risk factor for incident hypertension (12.6% vs 6.7%, p<0.001) and DM (2.0% vs 1.0%, p=0.014) compared to normouricemia. Stratified by sex, hyperuricemia had a significantly higher cumulative incidence of hypertension both in men (12.5% vs 9.6%, p=0.050) and women (12.9% vs 5.2%, p<0.001), but hyperuricemia carried a significant risk for DM only in women (2.0% vs 0.5%, p=0.031) (Figure 3). We also calculated the cumulative incidence of hypertension and DM over 5 years for each serum uric acid level by sex (Figure 4). Levels of more than 6.0 mg/dL of serum uric acid in men and 5.0 mg/dL in women are associated with higher risk for developing hypertension and DM compared to mean prevalence of these conditions in the overall population. The results suggest a threshold of 5.0 mg/dL of serum uric acid in women and 6.0 mg/dL in men that carries an increased risk for developing hypertension and DM.
Figure 2. Cumulative incidences of hypertension and diabetes mellitus over 5 years between hyperuricemia and normouricemia in each group.
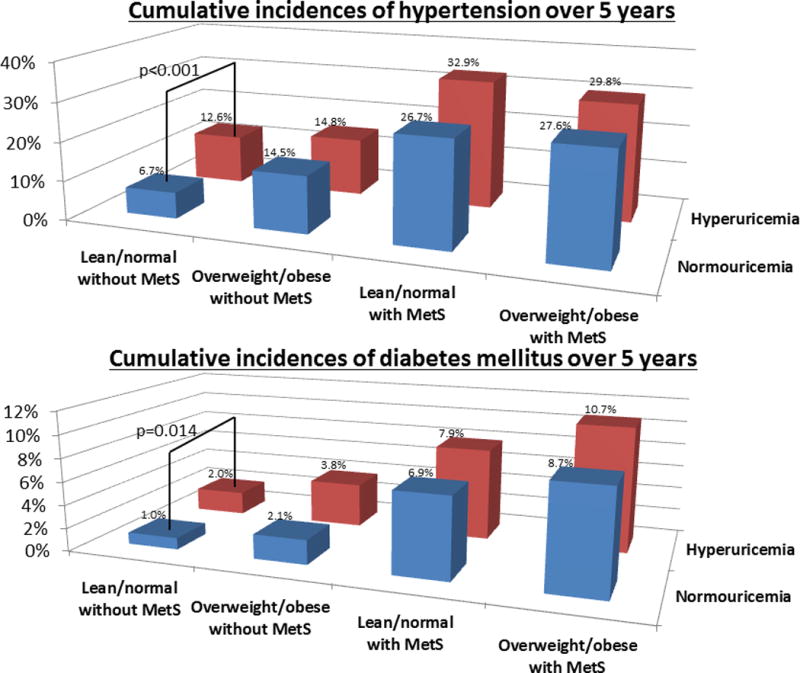
MetS: metabolic syndrome
The number of subjects in normouricemia: 7,173 subjects in lean/normal without metabolic syndrome, 702 subjects in overweight/obesity without metabolic syndrome, 288 subjects in lean/normal with metabolic syndrome, and 286 subjects in overweight/obesity with metabolic syndrome.
The number of subjects in hyperuricemia: 754 subjects in lean/normal without metabolic syndrome, 264 subjects in overweight/obesity without metabolic syndrome, 76 subjects in lean/normal with metabolic syndrome, and 178 subjects in overweight/obesity with metabolic syndrome.
The bars show significant difference of cumulative incidences of hypertension and diabetes mellitus between hyperuricemia and normouricemia by χ2 analyses.
Figure 3. Cumulative incidences of hypertension and diabetes mellitus over 5 years between hyperuricemia and normouricemia in each group by sex.
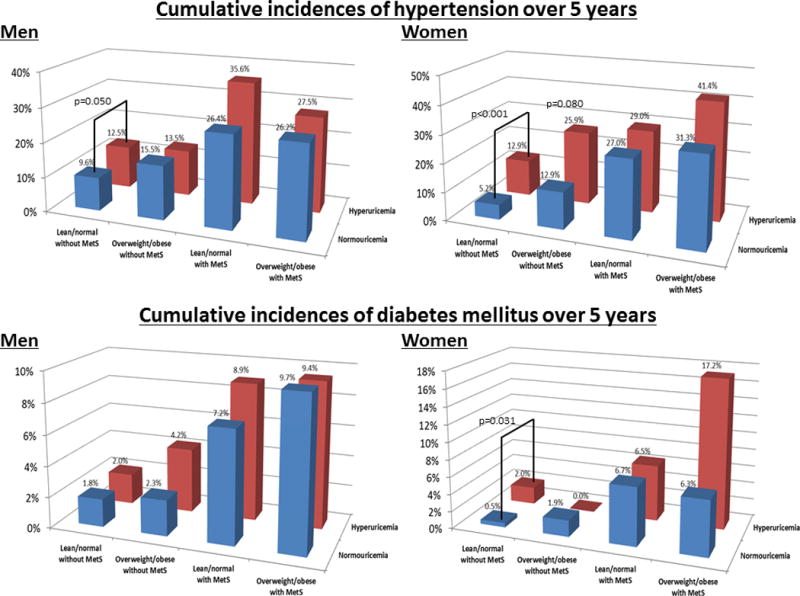
The number of subjects in normouricemia: 2,406 men and 4,767 women in lean/normal without metabolic syndrome, 439 men and 263 women in overweight/obesity without metabolic syndrome, 125 men and 163 women in lean/normal with metabolic syndrome, and 206 men and 80 women in overweight/obesity with metabolic syndrome.
The number of subjects in hyperuricemia: 553 men and 201 women in lean/normal without metabolic syndrome, 237 men and 27 women in overweight/obesity without metabolic syndrome, 45 men and 31 women in lean/normal with metabolic syndrome, and 149 men and 29 women in overweight/obesity with metabolic syndrome.
The bars show significant difference of cumulative incidences of hypertension and diabetes mellitus between hyperuricemia and normouricemia by χ2 analyses.
Figure 4. Cumulative incidences of hypertension and diabetes mellitus over 5 years in each serum uric acid level by sex.

The number of women in serum uric acid of <3.0, 3.0-3.9, 4.0-4.9, 5.0-5.9, 6.0-6.9, and 7.0- were 256, 1,451, 2,503, 1,063, 249, and 39, respectively. The number of men in serum uric acid of <4.0, 4.0-4.9, 5.0-5.9, 6.0-6.9, 7.0-7.9, and 8.0- were 143, 421, 1,178, 1,323, 798, and 297, respectively. Blue lines show mean cumulative incidence of hypertension (women 7.3%, men 13.1%) and red lines shows mean cumulative incidence of diabetes mellitus (women 1.0%, men 2.9%).
Multivariable analyses
We calculated ORs for hypertension and DM by crude analysis and after adjustments for age, sex, smoking and drinking habits, chronic kidney disease, BMI and metabolic syndrome category (lean/normal and overweight/obesity with and without metabolic syndrome), and hyperuricemia (or serum uric acid levels) (Table 3). Male sex is an independent risk for hypertension [OR: 1.31; 95% confidence interval (CI), 1.10-1.55] and DM (OR: 1.89; 95% CI, 1.28-2.78) after multivariable adjustments. Compared with lean/normal without metabolic syndrome group, overweight/obesity without metabolic syndrome group had a 1.93-fold greater odds for hypertension (95% CI, 1.57-2.38) and a 1.94-fold greater odds for DM (95% CI, 1.22-3.10), lean/normal with metabolic syndrome group had a 3.53-fold greater odds for hypertension (95% CI, 2.75-4.55) and a 4.68-fold greater odds for DM (95% CI, 2.93-7.46), and overweight/obesity with metabolic syndrome group had a 4.27-fold greater odds for hypertension (95% CI, 3.38-5.39) and a 7.10-fold greater odds for DM (95% CI, 4.47-10.6) after multivariable adjustments. Every 1 mg/dL increase in serum uric acid was associated with a 19% increased risk for developing hypertension (95% CI, 1.11-1.27) and a 27% increased risk for developing DM (95% CI, 1.10-1.45) in adjusted analyses. We also performed the same analyses stratified by sex. In men, we observed a 12% increased risk for developing hypertension (95% CI, 1.03-1.21) and a 21% increased risk for developing DM (95% CI, 1.03-1.43) for every 1 mg/dL increase in serum uric acid in adjusted analyses. In women, we observed a 29% increased risk for developing hypertension (95% CI, 1.14-1.45) and a 34% increased risk for developing DM (95% CI, 1.01-1.79) for every 1 mg/dL increase in serum uric acid in adjusted analyses.
Table 3.
Risk for developing hypertension and diabetes mellitus.
| Total (n=9,721) | Hypertension
|
Diabetes mellitus
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude
|
Adjusted*
|
Crude
|
Adjusted*
|
|||||||||
| OR | 95% CI | p | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Lean/normal without MetS | Reference | Reference | Reference | Reference | ||||||||
| Overweight/obesity without MetS | 2.19 | 1.80–2.67 | <0.001 | 1.93 | 1.57–2.38 | <0.001 | 2.48 | 1.58–3.90 | <0.001 | 1.94 | 1.22–3.10 | 0.005 |
| Lean/normal with MetS | 5.00 | 3.91–6.38 | <0.001 | 3.53 | 2.75–4.55 | <0.001 | 7.18 | 4.57–11.3 | <0.001 | 4.68 | 2.93–7.46 | <0.001 |
| Overweight/obesity with MetS | 5.10 | 4.10–6.35 | <0.001 | 4.27 | 3.38–5.39 | <0.001 | 9.78 | 6.80–14.3 | <0.001 | 7.10 | 4.74–10.6 | <0.001 |
| Hyperuricemia | 2.10 | 1.78–2.47 | <0.001 | 1.36 | 1.13–1.63 | 0.001 | 2.64 | 1.89–3.68 | <0.001 | 1.38 | 0.96–1.99 | 0.080 |
| Serum uric acid (per 1 mg/dL increased) | 1.36 | 1.30–1.43 | <0.001 | 1.19 | 1.11–1.27 | <0.001 | 1.55 | 1.40–1.72 | <0.001 | 1.27 | 1.10–1.45 | <0.001 |
| Sex (men) | 1.92 | 1.68–2.20 | <0.001 | 1.31 | 1.10–1.55 | 0.002 | 2.84 | 2.07–3.90 | <0.001 | 1.89 | 1.28–2.78 | 0.001 |
|
| ||||||||||||
| Men (n=4,160) | ||||||||||||
| Lean/normal without MetS | Reference | Reference | Reference | Reference | ||||||||
| Overweight/obesity without MetS | 1.54 | 1.21–1.96 | <0.001 | 1.60 | 1.25–2.06 | <0.001 | 1.64 | 0.98–2.76 | 0.062 | 1.71 | 1.005–2.90 | 0.048 |
| Lean/normal with MetS | 3.59 | 2.52–5.11 | <0.001 | 3.00 | 2.10–4.31 | <0.001 | 4.45 | 2.38–8.33 | <0.001 | 3.36 | 2.54–14.5 | <0.001 |
| Overweight/obesity with MetS | 3.24 | 2.49–4.22 | <0.001 | 3.43 | 2.61–4.52 | <0.001 | 5.70 | 3.65–8.89 | <0.001 | 5.86 | 0.090–5.96 | <0.001 |
| Hyperuricemia | 1.38 | 1.13–1.69 | 0.002 | 1.24 | 1.002–1.53 | 0.048 | 1.56 | 1.06–2.30 | 0.025 | 1.24 | 0.82–1.88 | 0.30 |
| Serum uric acid (per 1 mg/dL increased) | 1.70 | 1.53–1.90 | <0.001 | 1.12 | 1.03–1.21 | 0.006 | 1.30 | 1.12–1.51 | <0.001 | 1.21 | 1.03–1.43 | 0.017 |
|
| ||||||||||||
| Women (n=5,561) | ||||||||||||
| Lean/normal without MetS | Reference | Reference | Reference | Reference | ||||||||
| Overweight/obesity without MetS | 2.83 | 1.99–4.03 | <0.001 | 2.71 | 1.89–3.90 | <0.001 | 2.89 | 1.11–7.50 | 0.029 | 2.38 | 0.91–6.23 | 0.077 |
| Lean/normal with MetS | 6.46 | 4.61–9.07 | <0.001 | 3.91 | 2.74–5.59 | <0.001 | 11.8 | 6.06–23.0 | <0.001 | 6.56 | 3.24–13.3 | <0.001 |
| Overweight/obesity with MetS | 8.84 | 5.84–13.4 | <0.001 | 6.28 | 4.01–9.78 | <0.001 | 16.6 | 7.91–34.9 | <0.001 | 9.46 | 4.21–21.3 | <0.001 |
| Hyperuricemia | 3.25 | 2.37–4.45 | <0.001 | 1.63 | 1.15–2.31 | 0.006 | 4.42 | 2.27–8.61 | <0.001 | 1.75 | 0.84–3.65 | 0.138 |
| Serum uric acid (per 1 mg/dL increased) | 1.16 | 1.08–1.25 | <0.001 | 1.29 | 1.14–1.45 | <0.001 | 1.97 | 1.52–2.55 | <0.001 | 1.34 | 1.01–1.79 | 0.042 |
n: number of subjects; MetS: metabolic syndrome; OR: odds ratio; CI: confidence interval
Data adjusted for age, sex, smoking and drinking habits, chronic kidney disease, body mass index and metabolic syndrome category (lean/normal and overweight/obesity with and without metabolic syndrome), and hyperuricemia (or serum uric acid).
Risk for hypertension in subjects with normal blood pressure and for DM in subjects with normal fasting blood glucose
Since a baseline elevated blood pressure may increase the risk for hypertension, and since a baseline impaired fasting glucose level might predict development of DM, we performed a separate analysis in which we evaluated whether obesity without these findings could predict hypertension and DM, respectively.
Of 9,721 subjects (4,160 men) in this study, 8,411 subjects (3,365 men) had normal blood pressure (<130/85 mmHg) and 6,502 subjects (2,073 men) had normal blood glucose (<100 mg/dL). We calculated ORs for hypertension in the 8,411 subjects with normal blood pressure and for DM in the 6,502 subjects with normal blood glucose after adjustments for age, sex, smoking and drinking habits, chronic kidney disease, BMI and metabolic syndrome category (lean/normal and overweight/obesity with and without metabolic syndrome), and hyperuricemia (Table 4). In the subjects with normal blood pressure, the overweight/obesity without metabolic syndrome group were not a risk for hypertension compared with lean/normal subjects without metabolic syndrome after multivariable adjustments (p=0.59). In contrast, in the subjects with normal blood glucose, the presence of overweight/obesity without metabolic syndrome still carried an 11.4-fold greater odds for developing DM (95% CI, 2.49-51.9) compared with lean/normal subjects without metabolic syndrome after multivariable adjustments.
Table 4.
Risk for developing hypertension in subjects with normal blood pressure (a) and risk for developing diabetes mellitus in subjects with normal fasting blood glucose (b)
| a) Normal blood pressure | Hypertension
|
|||||||
|---|---|---|---|---|---|---|---|---|
| (n=8,411, 3,365 men) | Crude
|
Adjusted*
|
||||||
| OR | 95% CI | p | OR | 95% CI | P | |||
| Lean/normal without MetS | Reference | Reference | ||||||
| Overweight/obesity without MetS | 2.04 | 1.57–2.66 | <0.001 | 0.90 | 0.62–1.31 | 0.59 | ||
| Lean/normal with MetS | 2.72 | 1.62–4.56 | <0.001 | 1.67 | 0.98–2.85 | 0.058 | ||
| Overweight/obesity with MetS | 3.33 | 2.20–5.03 | <0.001 | 1.28 | 0.77–2.14 | 0.34 | ||
| b) Normal fasting blood glucose | Diabetes mellitus
|
|||||
|---|---|---|---|---|---|---|
| (n=6,502, 2,073 men) | Crude
|
Adjusted*
|
||||
| OR | 95% CI | p | OR | 95% CI | P | |
| Lean/normal without MetS | Reference | Reference | ||||
| Overweight/obesity without MetS | 5.60 | 2.36–13.3 | <0.001 | 11.4 | 2.49–51.9 | 0.002 |
| Lean/normal with MetS | 8.42 | 1.09–65.1 | 0.041 | 5.67 | 0.67–47.8 | 0.11 |
| Overweight/obesity with MetS | 5.53 | 0.72–42.5 | 0.10 | 15.1 | 1.25–182.5 | 0.033 |
n: number of subjects; MetS: metabolic syndrome; OR: odds ratio; CI: confidence interval
Data adjusted for age, sex, smoking and drinking habits, chronic kidney disease, body mass index and metabolic syndrome category (lean/normal and overweight/obesity with and without metabolic syndrome), and hyperuricemia (or serum uric acid).
Discussion
The primary finding of this study was that “metabolically healthy” obesity, defined as overweight/obesity without metabolic syndrome, carried increased cumulative incidences of hypertension (14.6% vs 7.2%, p<0.001) and DM (2.6% vs 1.1%, p=0.004) over 5 years compared to lean/normal without metabolic syndrome. This result confirms other studies (7, 8, 9, 10) and suggests that “metabolically healthy” obesity is a misnomer as it is still associated with increased risk for hypertension and DM. Second, our study documented that hyperuricemia is an independent risk factor for developing hypertension and DM in the overall population. However, when stratified by the four groups as BMI of ≥25 kg/m2 (overweight/obesity) and BMI of < 25 kg/m2 (lean/normal) and those with and without metabolic syndrome, hyperuricemia was only an independent risk for the development of hypertension and DM only in the lean/normal without metabolic syndrome group. The result may relate to hyperuricemia being causally linked with obesity and metabolic syndrome, and therefore not independent of its components (33, 34).
While overweight/obesity was an important risk factor for hypertension and DM in the group without metabolic syndrome, it did not confer independent risk for incident hypertension and DM in subjects with metabolic syndrome. This may be ascribed to BMI and increased abdominal circumference (a feature of metabolic syndrome) both reflecting a state of increased fat storage. In the group without metabolic syndrome, overweight/obesity carried a nearly 2-fold risk for incident hypertension and DM after multivariable adjustments.
An important observation was that in subjects without metabolic syndrome, the presence of overweight/obesity carried increased risk for the development of hypertension and DM (Table 2 and 3). However, the subjects who were overweight/obesity and/or had metabolic syndrome had higher baseline blood pressure and fasting blood glucose than lean/normal subjects without metabolic syndrome (Table 1), which may increase the risk for hypertension and DM. Some of these subjects with overweight/obesity had elevated blood pressure despite not qualifying as having metabolic syndrome, which might be expected to predict the development of hypertension. Likewise, some subjects with overweight/obesity who did not qualify as having metabolic syndrome also had elevated fasting blood glucose that might influence the risk for DM. Thus, to address this, we performed a separate analysis to determine if subjects with overweight/obesity who did not have metabolic syndrome and had normal blood pressure (defined as <130/85 mmHg on no antihypertensive agent) still carried risk for hypertension, and similarly, whether a normal blood glucose (defined as fasting blood glucose <100 mg/dL) in subjects with overweight/obesity but without metabolic syndrome carried risk for DM (Table 4). The primary finding was that overweight/obesity with a normal blood glucose still conveyed an 11-fold risk for developing DM after multivariable adjustments but that the risk for developing hypertension in subjects with overweight/obesity and with normal blood pressure at baseline was not significant. This emphasizes the key role obesity itself has in driving DM. In contrast, baseline blood pressure in subjects overweight/obesity but without metabolic syndrome is more important for developing hypertension compared to overweight/obesity itself.
In subjects who were lean/normal and without metabolic syndrome, hyperuricemia became an important risk factor for hypertension and DM. Thus, our studies show the importance of BMI and serum uric acid in identifying people at risk for developing hypertension and DM with hyperuricemia carrying a more important role in lean/normal subjects, whereas once overweight/obesity and metabolic syndrome develop, the latter factors become dominant.
This study has some limitations. First, there are two definitions for abdominal obesity using in Japan. Japanese Obesity Society (JOS) recommended waist circumference of 85 cm or above in men, and 90 cm or above in women (35). However, this study used abdominal obesity definition per IDF and WHO recommendation; waist circumference of 90 cm or above in men, and 80 cm or above in women (4, 36). A sensitivity analysis using JOS definition rather than IDF and WHO definition provided similar results. Second, this study was a retrospective, single center study, which may have introduced selection bias. Furthermore, since subjects were those who sought a health examination, the study subjects may have more interested in health issues than the general population, especially since the health checkup was not covered by insurance. However, our medical check-up systems is open to everyone and the number of our study subjects was large, which suggests that we can generalize our results to Japanese. Our study was based on a homogenous population, and further studies of other ethnicities are needed. Third, our study did not assess the risk for developing dyslipidemia due to it being part of the diagnostic criteria for metabolic syndrome. This is different from predicting hypertension or DM, as the criteria for metabolic syndrome involves prehypertension and impaired fasting blood glucose rather than hypertension and DM per se. However, high blood pressure, hyperglycemia and lipid abnormality have mutual influences, and we should account for the importance of lipid abnormality. Finally, this longitudinal study lacks time-to-event data which precluded survival analysis.
The results of this study add to the growing evidence base demonstrating that being obesity is associated with increased risk of metabolic disease as compared to matched individuals without obesity. We do not believe the use of the term “metabolically healthy” obesity is useful either for understanding the development of chronic disease or for prioritizing treatment of obesity. Designating some individuals as “metabolically healthy” suggests that intervention in these individuals should not be prioritized compared to metabolically unhealthy obesity. Given that “metabolically healthy” obesity have a higher risk of developing metabolic disease than their lean counterparts, and that obesity increases risks of other many non-metabolic conditions such as orthopedic problems (37) and increased risk of depression and other mood disorders (38), it is inappropriate to suggest that this group is healthy. In fact, this group might present a very cost-effect opportunity for intervention since it is possible that less intense interventions may be effective in this population. We think it is time to stop thinking of any individuals with obesity as healthy.
Conclusion
“Metabolically healthy” obesity, defined as obesity in the absence of metabolic syndrome, confers increased risk for hypertension and diabetes mellitus. An elevated serum uric acid levels also confers a risk for hypertension and diabetes mellitus in lean/normal subjects, but not in subjects with overweight/obesity or those with metabolic syndrome.
What is already known about this subject?
Obesity is recognized as an independent risk factor for hypertension and diabetes.
However, the concept of a metabolically healthy population with obesity is widely recognized.
Metabolic syndrome is an important risk factor for cardiovascular disease, but many of metabolic risk factors are causally linked with obesity.
What does this study add?
This study is to clarify whether subjects with obesity in the absence of metabolic syndrome confers similar or less compared to subjects without obesity.
Subjects with obesity but without metabolic syndrome carried more than 2-fold increased cumulative incidences of hypertension and diabetes mellitus over 5 years compared to lean/normal subjects without metabolic syndrome.
Hyperuricemia was an independent risk factor for developing hypertension in the lean/normal subjects without metabolic syndrome but not in subjects with obesity those with metabolic syndrome.
Acknowledgments
The authors thank the patients and all staff in the Center for Preventive Medicine, St. Luke’s International Hospital, for assistance with data collection.
Funding Statements: There is no source of funding in this study.
Dr. Kuwabara reports the grant for studying abroad from Federation of National Public Service Personnel Mutual Aid Association in Japan; Dr. Johnson has equity with XORT Therapeutics that is developing novel xanthine oxidase inhibitors and with Colorado Research Partners LLC that is developing inhibitors of fructose metabolism. In addition, Dr. Johnson is an inventor on several patents licensed to XORT Therapeutics. (US Patent No 7,799,794, US Patent No. 8,557,831).
Abbreviations list
- BMI
body mass index
- DM
diabetes mellitus
- ORs
odds ratios
- IDF
the International Diabetes Federation Task Force on Epidemiology and Prevention
- NHLBI
National Heart, Lung, and Blood Institute
- AHA
American Heart Association
- WHF
World Heart Federation
- IAS
International Atherosclerosis Society
- IASO
International Association for the Study of Obesity
- eGFR
estimated glomerular filtration rate
Footnotes
Disclosures: The remaining authors have nothing to disclose.
Author contributions: MK, RK, IH and RJJ designed the study; MK and KN collected the data; MK analyzed the data; and MK, JOH, and RJJ were responsible for writing the manuscript. All authors read and approved the final manuscript. MK had full access to all of the data and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Sperling LS, Mechanick JI, Neeland IJ, Herrick CJ, Despres JP, Ndumele CE, et al. The CardioMetabolic Health Alliance: Working Toward a New Care Model for the Metabolic Syndrome. J Am Coll Cardiol. 2015;66:1050–1067. doi: 10.1016/j.jacc.2015.06.1328. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, American Heart A et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Zimmet P, Shaw J, Group IDFETFC The metabolic syndrome–a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 6.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91:4459–4466. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 7.Lee SK, Kim SH, Cho GY, Baik I, Lim HE, Park CG, et al. Obesity phenotype and incident hypertension: a prospective community-based cohort study. J Hypertens. 2013;31:145–151. doi: 10.1097/HJH.0b013e32835a3637. [DOI] [PubMed] [Google Scholar]
- 8.Tirosh A, Shai I, Afek A, Dubnov-Raz G, Ayalon N, Gordon B, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364:1315–1325. doi: 10.1056/NEJMoa1006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang LC, Bai CH, Sun CA, Chen CJ. Prevalence of metabolically healthy obesity and its impacts on incidences of hypertension, diabetes and the metabolic syndrome in Taiwan. Asia Pac J Clin Nutr. 2012;21:227–233. [PubMed] [Google Scholar]
- 10.Heianza Y, Kato K, Kodama S, Suzuki A, Tanaka S, Hanyu O, et al. Stability and changes in metabolically healthy overweight or obesity and risk of future diabetes: Niigata wellness study. Obesity (Silver Spring) 2014;22:2420–2425. doi: 10.1002/oby.20855. [DOI] [PubMed] [Google Scholar]
- 11.Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499–1504. doi: 10.1053/meta.2001.27213. [DOI] [PubMed] [Google Scholar]
- 12.Roberson LL, Aneni EC, Maziak W, Agatston A, Feldman T, Rouseff M, et al. Beyond BMI: The “Metabolically healthy obese” phenotype & its association with clinical/subclinical cardiovascular disease and all-cause mortality – a systematic review. BMC Public Health. 2014;14:14. doi: 10.1186/1471-2458-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 14.Shea JL, Randell EW, Sun G. The prevalence of metabolically healthy obese subjects defined by BMI and dual-energy X-ray absorptiometry. Obesity (Silver Spring) 2011;19:624–630. doi: 10.1038/oby.2010.174. [DOI] [PubMed] [Google Scholar]
- 15.Munoz-Garach A, Cornejo-Pareja I, Tinahones FJ. Does Metabolically Healthy Obesity Exist? Nutrients. 2016;8 doi: 10.3390/nu8060320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwabara M, Hisatome I, Roncal-Jimenez CA, Niwa K, Andres-Hernando A, Jensen T, et al. Increased Serum Sodium and Serum Osmolarity Are Independent Risk Factors for Developing Chronic Kidney Disease; 5 Year Cohort Study. PLoS One. 2017;12:e0169137. doi: 10.1371/journal.pone.0169137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuwabara M, Motoki Y, Sato H, Fujii M, Ichiura K, Kuwabara K, et al. Low frequency of toothbrushing practices is an independent risk factor for diabetes mellitus in male and dyslipidemia in female: A large-scale, 5-year cohort study in Japan. J Cardiol. 2016 doi: 10.1016/j.jjcc.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Kuwabara M, Niwa K, Nishihara S, Nishi Y, Takahashi O, Kario K, et al. Hyperuricemia is an independent competing risk factor for atrial fibrillation. Int J Cardiol. 2017;231:137–142. doi: 10.1016/j.ijcard.2016.11.268. [DOI] [PubMed] [Google Scholar]
- 20.Kuwabara M, Motoki Y, Ichiura K, Fujii M, Inomata C, Sato H, et al. Association between toothbrushing and risk factors for cardiovascular disease: a large-scale, cross-sectional Japanese study. BMJ Open. 2016;6:e009870. doi: 10.1136/bmjopen-2015-009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuwabara M, Niwa K, Nishi Y, Mizuno A, Asano T, Masuda K, et al. Relationship between serum uric acid levels and hypertension among Japanese individuals not treated for hyperuricemia and hypertension. Hypertens Res. 2014;37:785–789. doi: 10.1038/hr.2014.75. [DOI] [PubMed] [Google Scholar]
- 22.Kuwabara M, Bjornstad P, Hisatome I, Niwa K, Roncal-Jimenez CA, Andres-Hernando A, et al. Elevated Serum Uric Acid Level Predicts Rapid Decline in Kidney Function. Am J Nephrol. 2017;45:330–337. doi: 10.1159/000464260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuwabara M, Niwa K, Hisatome I, Nakagawa T, Roncal-Jimenez CA, Andres-Hernando A, et al. Asymptomatic Hyperuricemia Without Comorbidities Predicts Cardiometabolic Diseases: Five-Year Japanese Cohort Study. Hypertension. 2017 doi: 10.1161/HYPERTENSIONAHA.116.08998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014) Hypertens Res. 2014;37:253–390. doi: 10.1038/hr.2014.20. [DOI] [PubMed] [Google Scholar]
- 25.International Expert C. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamanaka H, Japanese Society of G, Nucleic Acid M Japanese guideline for the management of hyperuricemia and gout: second edition. Nucleosides Nucleotides Nucleic Acids. 2011;30:1018–1029. doi: 10.1080/15257770.2011.596496. [DOI] [PubMed] [Google Scholar]
- 27.Kuwabara M. Hyperuricemia, Cardiovascular Disease, and Hypertension. Pulse (Basel) 2016;3:242–252. doi: 10.1159/000443769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai RV, Ahmed MI, Fonarow GC, Filippatos GS, White M, Aban IB, et al. Effect of serum insulin on the association between hyperuricemia and incident heart failure. Am J Cardiol. 2010;106:1134–1138. doi: 10.1016/j.amjcard.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin KC, Lin HY, Chou P. Community based epidemiological study on hyperuricemia and gout in Kin-Hu, Kinmen. J Rheumatol. 2000;27:1045–1050. [PubMed] [Google Scholar]
- 30.Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642–650. [PubMed] [Google Scholar]
- 31.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–3141. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 32.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 33.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 34.Johnson RJ, Tuttle KR. Much ado about nothing, or much to do about something? The continuing controversy over the role of uric acid in cardiovascular disease. Hypertension. 2000;35:E10. doi: 10.1161/01.hyp.35.3.e10. [DOI] [PubMed] [Google Scholar]
- 35.Oka R, Kobayashi J, Yagi K, Tanii H, Miyamoto S, Asano A, et al. Reassessment of the cutoff values of waist circumference and visceral fat area for identifying Japanese subjects at risk for the metabolic syndrome. Diabetes Res Clin Pract. 2008;79:474–481. doi: 10.1016/j.diabres.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Hara K, Matsushita Y, Horikoshi M, Yoshiike N, Yokoyama T, Tanaka H, et al. A proposal for the cutoff point of waist circumference for the diagnosis of metabolic syndrome in the Japanese population. Diabetes Care. 2006;29:1123–1124. doi: 10.2337/diacare.2951123. [DOI] [PubMed] [Google Scholar]
- 37.Parratte S, Pesenti S, Argenson JN. Obesity in orthopedics and trauma surgery. Orthop Traumatol Surg Res. 2014;100:S91–97. doi: 10.1016/j.otsr.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Preiss K, Brennan L, Clarke D. A systematic review of variables associated with the relationship between obesity and depression. Obes Rev. 2013;14:906–918. doi: 10.1111/obr.12052. [DOI] [PubMed] [Google Scholar]


