Abstract
Endothelium-derived epoxyeicosatrienoic acids (EETs) are fatty acid epoxides that play an important role in the control of vascular tone in selected coronary, renal, carotid, cerebral and skeletal muscle arteries. Vasodilation due to endothelium-dependent smooth muscle hyperpolarization (EDH) has been suggested to involve EETs as a transferable endothelium-derived hyperpolarizing factor. However, this activity may also be due to EETs interacting with the components of other primary EDH-mediated vasodilator mechanisms. Indeed, the transfer of hyperpolarization initiated in the endothelium to the adjacent smooth muscle via gap junction connexins occurs separately or synergistically with the release of K+ ions at discrete myoendothelial microdomain signalling sites. The net effects of such activity are smooth muscle hyperpolarization, closure of voltage-dependent Ca2+ channels, phospholipase C deactivation and vasodilation. The spatially localized and key components of the microdomain signalling complex are the inositol 1,4,5-trisphosphate receptor-mediated endoplasmic reticulum Ca2+ store, Ca2+-activated K+ (KCa), transient receptor potential (TRP) and inward-rectifying K+ channels, gap junctions and the smooth muscle Na+/K+-ATPase. Of these, TRP channels and connexins are key endothelial effector targets modulated by EETs. In an integrated manner, endogenous EETs enhance extracellular Ca2+ influx (thereby amplifying and prolonging KCa-mediated endothelial hyperpolarization) and also facilitate the conduction of this hyperpolarization to spatially remote vessel regions. The contribution of EETs and the receptor and channel subtypes involved in EDH-related microdomain signalling, as a candidate for a universal EDH-mediated vasodilator mechanism, vary with vascular bed, species, development and disease and thus represent potentially selective targets for modulating specific artery function.
Keywords: Calcium-activated potassium channel, Cytochrome P450 epoxygenases, Endothelium-derived hyperpolarization, Gap junction, Transient receptor potential channel
Introduction and consensus: a brief history
Endothelial cells underlie the key components of arterial contraction and relaxation and are thus essential for the control of vessel tone, blood flow and pressure [64, 175, 180]. The importance of the endothelium for vessel relaxation in response to the muscarinic agonist and neurotransmitter acetylcholine (ACh) was first demonstrated in studies of rabbit aorta [65, 104], with the biosynthesis and release of nitric oxide (NO) later found to be the dominant endothelium-derived relaxing factor (EDRF) mechanism, at least in the conduit vessels [97, 109, 151]. An EDRF mechanism was also alluded to from the observations of Bolton and co-workers [15], where the esterase-insensitive muscarinic agonist carbachol initiated hyperpolarization and relaxation of guinea pig mesenteric arterial smooth muscle through a process that also required an intact endothelium. Subsequently, endothelium-derived smooth muscle hyperpolarization (EDH) was found to be evoked by various agonists in different arteries, which ultimately led to the hypothesis of a role for an endothelium-derived hyperpolarizing factor (EDHF) [8, 9, 28, 56, 95, 191]. The net effects of EDH/EDHF activity are smooth muscle hyperpolarization, closure of voltage-dependent Ca2+ channels, phospholipase C deactivation [102] and vasodilation [54, 55, 175].
A number of molecular species, including epoxyeicosatrienoic acids (EETs; see Fig. 1), the endocannabinoid anandamide, K+ ions, hydrogen peroxide (H2O2), C-type natriuretic peptide (CNP), nitroxyl, hydrogen sulphide, adenosine and NO, have since been reported to act as EDHFs in particular arteries and states and under specific experimental conditions [3, 21, 25, 47, 94, 121, 131, 132, 143, 147, 164], but none has emerged as a ubiquitous paracrine regulator of smooth muscle membrane potential and vascular tone. Indeed, the relevance of H2O2 as a transferable EDHF has been questioned in murine mesenteric and femoral arteries ([153] and [125], respectively), human mesenteric artery [24], rat saphenous and femoral arteries ([169] and [118], respectively) and in rabbit iliac artery [46, 70] (see also [51, 168, 196] for review). However, notably, in coronary artery disease [139], H2O2 has been suggested to compensate for the lack of NO-mediated vasodilator activity and thus, in some vascular beds, may act in an apparent EDH manner in disease. Of note, in rabbit iliac artery, applied H2O2 (threshold, 10–30 μM) or selective elevation in endothelium-generated H2O2 potentiates EDH-type relaxation in a manner consistent with the ability of this reactive oxygen species (ROS) to promote Ca2+ mobilization from endothelial endoplasmic reticulum (ER) stores, thereby enhancing Ca2+-activated K+ channel (KCa)-mediated hyperpolarization that conducts to the smooth muscle via gap junctions [45, 46, 70]. In addition, H2O2 can modulate intercellular communication directly by altering connexin (Cx) oxidation/phosphorylation status, with specific implications for gap junction-dependent EDH activity (see [34, 168] for review). In these examples, rather than mediating smooth muscle hyperpolarization directly, H2O2 interacts with the integral components of EDH signalling, which can thus account for its apparent role in such activity. Of interest, anandamide [156] and CNP [69, 134, 176] have been subsequently shown not to act as EDHFs.
Fig. 1.
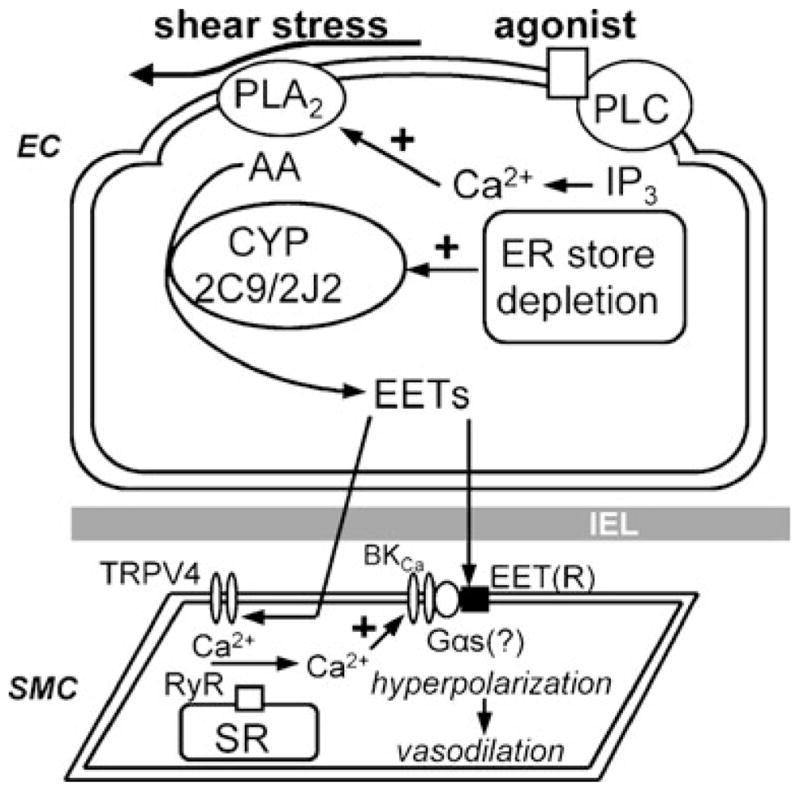
Mechanisms of epoxyeicosatrienoic acid (EET) synthesis and transferable endothelium-derived hyperpolarizing factor (EDHF) action. Endothelial stimulus can result in arachidonic acid (AA) and other lipid substrate-mediated cytochrome P450 (CYP)-dependent synthesis and release of EETs, which are transferred across the internal elastic lamina (IEL) where they induce smooth muscle hyperpolarization directly and potentially via large conductance Ca2+-activated K+ channels (BKCa) and/or an EETs-receptor(R)-mediated mechanism. In specific vessels, EETs activate smooth muscle (SMC) vanilloid type 4 transient receptor potential channels (TRPV4) to induce Ca2+ influx that in turn activates sarcoplasmic reticulum (SR) ryanodine receptors (RyRs) to induce spatially localized Ca2+ sparks, thereby initiating hyperpolarizing BKCa activity, or, alternatively, via binding to the putative EET(R) which induces BKCa activity via a potential Gαs coupled mechanism. ER endoplasmic reticulum, IP3 inositol 1,4,5-trisphosphate, PLA2 phospholipase A2, PLC phospholipase C
Consensus on the nature of EDH/EDHF involves its generation and release from the endothelium and transfer across the internal elastic lamina (IEL; and/or via holes therein) to the effector smooth muscle, independent of NO and prostanoids, as electrical and/or metabolic transfer. A ubiquitous initiating step in this activity is an elevation in endothelial [Ca2+]i, followed by hyperpolarization of the endothelium [34]. Endothelial hyperpolarization can be evoked by both mechanical and chemical stimuli, such as fluid shear stress, agonists and sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) inhibitors, and is mediated by the opening of small and intermediate (and, in some cases, large)-conductance, KCa channels, designated as SKCa (KCa2.3, SK3; SKCa3; KCNN3), IKCa (KCa3.1, SK4, KCa4, IK1, KCNN4) and BKCa (KCa1.1, Slo1/MaxiK, KCNMA1), respectively, which are selectively blocked by apamin, TRAM and iberiotoxin, respectively [34, 44, 46, 77, 149, 211].
Further integral components of EDH-mediated signalling involve KCa-linked inositol 1,4,5-trisphosphate receptor (IP3R)-mediated ER Ca2+ store, inwardly rectifying K+ (Kir) and transient receptor potential (TRP) channels and smooth muscle Na+/K+-ATPase activity [54, 174]; and the distribution and activity of these components show both similarities and differences between vessels and vessel states. In many vascular beds, intercellular communication via gap junctions underpins the spread of hyperpolarization between endothelial and sub-intimal smooth muscle cells (i.e. through myoendothelial gap junctions; [34, 55, 174] for review). Notably, a critical role for EETs in the control of vasodilator tone is intimately linked with the regulation of endothelial Ca2+ homeostasis, endothelial hyperpolarization and gap junctional communication [61, 160, 209]. However, such activity is in general distinct from a direct role for EETs as a putative transferable EDHF.
The aim of this review is to propose a universally general microdomain signalling mechanism as the basis for EDH-mediated vasodilation and to clarify how the role of EETs links with this mechanism. The hypotheses examined are that EDH/EDHF activity is due to the electrical and/or metabolic transfer of current and localized K+ ion activity at myoendothelial microdomain sites and that in specific vascular beds EETs modulate components of the microdomain signalling complex.
Connexins, gap junctions and their regulation
Connexins (Cxs) are the protein monomer constituents of gap junctions, and their activity is modulated by multiple mechanisms [82, 106, 150, 187, 212] that include several signalling molecules and pathways common to those involved in the highly localized myoendothelial microdomain signalling that underlies EDH-mediated relaxation in specific arteries (see comparative reviews of [54, 55, 82, 106, 150, 175, 187, 212]). Indeed, given the close spatial localization of plasmalemmal Cxs and IP3R on ER (as an IP3-sensitive Ca2+ store), KCa, TRP and Kir channels and the Na+/K+-ATPase, Cx-related signalling processes have a significant potential to modulate a number of EDH signalling components [54, 55, 174]. These include the transfer of signalling molecules, including IP3, cyclic adenosine monophosphate (cAMP), adenosine diphosphate (ADP) and adenosine tris-phosphate (ATP), the modulation of [Ca2+]i and (specific) kinase-mediated phosphorylation ([82, 106] for review).
The primary role of gap junctions is the direct and selective intercellular transfer of current and selected metabolites and signalling molecules [82, 106, 150, 187, 212]. In the membranes of adjacent cells, six individual Cxs form a connexon (hexamer) hemichannel with a central pore. These connexons can then dock to form a low-resistance channel that directly couples neighbouring cells (Fig. 2) to facilitate chemical (up to ~1 kDa) and electrical continuity [82, 106, 150, 187, 212]. Notably, the various Cx subtypes expressed in the vessel wall (see below) are not universally compatible with one another in forming connexons, nor the subsequent intercellular channels [82, 106, 150, 187, 212]. Given that the homotypic gap junction channels have distinct conductance and gating properties depending on the single Cx subtype from which they are constructed [34, 82, 87, 106, 150, 187, 212], those Cxs that do connect (i.e. to form heterotypic or heteromeric channels) may confer significant functional plasticity. Connexons that consist of a single or a mixture of two types of Cx are termed homomeric and heteromeric, respectively. In turn, homomeric and heteromeric connexons can dock to form homotypic and heterotypic gap junctions (of the same and different homomeric connexons only, respectively), or a mixture of two heteromeric connexons to form a heteromeric gap junction. These potentially numerous permutations in gap junction Cx composition can therefore confer highly diverse biophysical properties and thus have significant implications for function [82, 212].
Fig. 2.
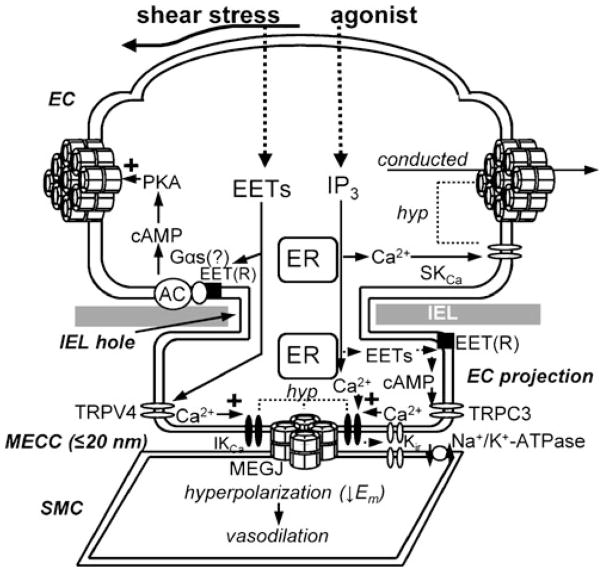
Interaction of epoxyeicosatrienoic acids (EETs) with myoendothelial microdomain signalling complex components. Distinct from a transferable hyperpolarizing factor, endothelial EETs can interact with key EDH signalling components associated with myoendothelial close contact sites (MECCs) that form myoendothelial microdomain signalling complexes integral to EDH activity. EET-mediated activation of TRP channel subtypes located on the endothelium, such as TRP canonical type 3 and TRP vanilloid type 4 (TRPC3 and TRPV4, respectively), can increase endothelial intermediate and small conductance Ca2+-activated K+ channel (I/SKCa) activity to induce endothelial hyperpolarization (hyp) that is conducted to the SMC via gap junctions, thereby inducing vasodilation. Efflux of K+ following endothelial S/IKCa activation (and in some cases Kir) stimulates the smooth muscle Na+/K+-ATPase to promote SMC hyperpolarization. Additionally, endothelial EETs can promote the longitudinal spread of endothelial hyperpolarization over distance by enhancing gap junction Cx activity via a cAMP/PKA-dependent mechanism. AC adenylyl cyclase, cAMP cyclic adenosine monophosphate, EC endothelial cell, ER endoplasmic reticulum, IEL internal elastic lamina, MEGJ myoendothelial gap junction, PKA protein kinase A
In the arteries, Cxs 37, 40, 43 and, to a lesser extent, 45 are expressed, albeit with a specific and often differential distribution and expression within and between junctions, arteries, species, development, ageing and disease [34, 87, 106]. Modulation of the interdependent mechanisms that underlie Cx activity is a significant factor in the plasticity of functional mechanisms in arteries [106]. Gap junction Cx activity, as charge and size selectivity, pore conductance and chemical and voltage gating, is regulated at a number of levels by short- and long-term effects [82, 87, 187, 212]. Short-term effects include regulation via post-translational modifications such as phosphorylation, nitrosylation, glycosylation and S-glutathionylation at single and multiple sites depending on the Cx and conditions ([189, 190], for examples; see also [82, 106, 187, 212]). Longer-term effects include those associated with gene expression and protein synthesis and gap junction formation, involving trafficking, hexamerization and membrane insertion, with the latter processes occurring with a half-life of 1–3 h [82, 87, 106, 212].
Related to a number of the above mechanisms and dependent on the specific kinase and phosphorylation site, Cxs are differentially modulated [82, 87, 106, 150]. For example, phosphorylation via protein kinase C reduces Cx40 and Cx43 gap junctional coupling, whilst phosphorylation of these Cxs via cAMP-dependent protein kinase (PKA), protein kinase B (Akt) and mitogen-activated protein kinase enhances such coupling ([5, 167, 190, 201]; also see [106] for review). In addition, differential modulation of Cx activity can also occur via changes in pH ([82, 187] for review); including a pH-dependent alkalization effect of blocking Cxs and preventing gap junction-dependent EDH [13]. Gap junction Cx function can also be modulated by ROS, such as H2O2 ([34, 51, 168] for review) and [Ca2+]i ([126, 150, 158] for review), and by NO [12, 122], scaffolding protein interactions ([106] for review) and oxygen tension/ischemia [105], as well as via the downstream effects of inflammatory mediators such as cytokines and lipopolysaccharide ([110, 119]; see also [198] for review).
Gap junctions and the transfer of endothelial hyperpolarization
In addition to the physical and metabolic coupling of vascular endothelial and smooth muscle cells, myoendothelial gap junctions facilitate the direct transfer of current between these cell layers and underlie a key aspect of EDH-mediated relaxation ([34, 55, 174] for review). In a syncytial manner, current transfer can further spread via radial and longitudinal homocellular smooth muscle and endothelial gap junctions [27, 78, 177, 213], where it is critical for the coordination of conducted signals over distance ([34, 89, 210] for review; Fig. 2).
For electrical signalling at myoendothelial gap junctions, individual projections between endothelial and smooth muscle cells may have single or multiple gap junction plaques with single or multiple Cx subtypes, which would thus confer significant potential functional plasticity for these sites. Anatomically, myoendothelial gap junctions have been demonstrated with electron microscopy in several vascular beds (summarized in Table 1 in [174]). The specific Cx/s at these junctions has/have also been demonstrated with immunoelectron and confocal microscopy in a more limited number of beds (per below). In addition, in arteries from humans, rabbits and rodents, the anatomical composition of the Cx subtype/s at these gap junctions has also been inferred via the use of synthetic ‘Cx-mimetic’ (or ‘gap’) peptides. These peptides target Cx-specific variations in 13 and 11 amino acid sequences located within the first and second extracellular loops (Gap26 and Gap27 domains, respectively) of the Cx protein ([26, 207]; for review, see [34, 52]) and are reported to block the docking of Cxs in adjacent cells and thereby prevent intercellular gap junctional communication in an apparently Cx-specific manner ([34, 55] for review).
The role of gap junctions in EDH-mediated relaxation has been inferred by correlative anatomical and functional studies involving the use of confocal and ultrastructural observations, membrane potential recording from dye-identified endothelial and/or smooth muscle cells and the use of tracer dyes and Cx-mimetic peptides as well as other putative gap junction targeted agents (see Section “Agonist-evoked, EET-mediated activation of TRPC”), combined with additional pharmacological intervention ([34, 55] for review). For example, the presence and absence of myoendothelial gap junctions corresponds with the patency of EDH to be transferred to the smooth muscle, or not, in the rat mesenteric and saphenous arteries, respectively [177]. Furthermore, Cx-mimetic peptides typically attenuate EDH-mediated vasodilator activity ([34, 55] for review). In the arteries from subcutaneous fat of pregnant patients, for example, Cx-mimetic peptides and ultrastructural data suggest that EDH in this vessel is dependent on Cx43 myoendothelial gap junctions that arise from endothelial or smooth muscle-derived projections [115, 124], with similar peptide data supporting the presence of Cxs37 and/or 43 at myoendothelial gap junctions of rat intrapulmonary arteries [12]. In addition, following selective loading of the endothelium with hydrophilic tracer dyes, such as calcein and carboxyfluorescein-AM, dye transfer to the smooth muscle is attenuated by putative gap junction block with Cx-mimetic peptides [12, 78] and the licorice derivatives, carbenoxolone and 18α-glycyrrhetinic acid [108], supporting the dependency of the EDH on myoendothelial gap junctional coupling.
The expression of specific Cx subtype/s at the myoendothelial gap junctions and the properties of the related endothelial and/or smooth muscle cell projection show both similarities and differences between arteries and species. In the mesenteric artery from humans, the endothelial or smooth muscle cell-derived projections (at a density of ~1.5:1, respectively) have Cx40 gap junctions that are coupling the two cell layers [24]. Comparatively, in the mesenteric artery of the rat and exclusively on endothelial cell-derived projections, the gap junctions consist of Cxs37 and 40 (with Cx43 being absent) [98, 130, 172, 173, 177], with myoendothelial gap junctions in the rat caudal cerebellar artery (as the asymmetric branch of the basilar artery) having the same Cx composition as the rat mesenteric artery [80]. This diversity in Cx distribution is also reflected between species and vascular beds, with myoendothelial gap junctions in the murine cremaster arteriole being likely to consist of Cxs37, 40 and 43 [98].
Notably, in addition to myoendothelial gap junction block, EDH and dye coupling can be attenuated by inhibitors of adenylyl cyclase (AC) and PKA, whereas cAMP phosphodiesterase inhibitors (which increase the bioavailability of endogenous cAMP by preventing nucleotide hydrolysis) enhance conducted smooth muscle hyperpolarization in regions longitudinally remote from the site of focal endothelial stimulation ([78]; for review, see [76]). These data suggest that elevations in cAMP play a permissive role in the relay of endothelial hyperpolarization into and through the media [34, 78, 133, 194]. Indeed, two of the primary vascular Cxs (40 and 43) have been reported to be direct targets of cAMP/PKA-mediated phosphorylation via a mechanism that serves to not only result in a transient-enhanced conductance of pre-formed in situ gap junctions but also to promote the rapid assembly of connexons and their recruitment to the plasmalemma where they are incorporated into the pre-existing plaques [34, 114, 160, 185, 195].
Myoendothelial microdomain signalling and EDH
The precise role of myoendothelial gap junctions as the basis for contact-mediated EDH is potentially confounded by the anatomical demonstration of morphologically distinct close contacts of less than or equal to ~20 nm between the endothelium and smooth muscle. Such myoendothelial close contacts (MECCs) are apparently devoid of the pentalaminar appearance that is a feature of gap junction plaques, with methodological limitations being a potential contributing factor for this observation (see discussion in [174]). Based on ultrastructural data, MECCs are a characteristic feature of arteries from all primary animal models and humans and facilitate physical and metabolic, but not necessarily, electrical coupling. The precise morphology of the projections on which MECCs occur is artery-specific and can also differ in any given artery (see Figure 2 in [174] for example) and depending on the artery, projections can be ~50 to 200 nm in diameter and ~0.2 to 1.5 μm in length and pass through the IEL. Endothelial projections with a bulbous ‘mushroom’-shaped end and, hence, have a large surface area to volume ratio, are typically associated with MECCs (Fig. 2), but such contacts can also occur on projections from the smooth muscle as well as on projections from both endothelial and smooth muscle cells (summarized in Table 1 in [174]). Indeed, the morphological diversity of MECC-related projections, such as surface area and organelle content, may be associated with specific, although as yet, uncharacterized function.
Myoendothelial gap junctions and their associated MECCs are dynamic in nature, with the mechanisms underlying their formation and degradation being alluded to from a number of studies, supporting their involvement in vascular function in health, disease, development and differentiation. For example, in vascular disease, the patency of such sites can be altered in a vascular bed-specific manner. In the saphenous and mesenteric arteries of control and diet-induced obese hypertensive rats, myoendothelial gap junction presence and activity are upregulated and unchanged in these two beds, respectively [23, 81], whilst in control juvenile rat saphenous artery, myoendothelial gap junctions are numerous, but are absent in adult [169]. These observations are also reflected in artificial co-culture systems, such as in murine aortic endothelial and smooth muscle cells where MECCs are numerous [99, 101], and are present in the intact aorta of juvenile but absent in mature rodents [171]. In the murine aortic co-culture model, MECC formation is dependent on plasminogen activator inhibitor-1 action, where it is implicated in the formation of cell processes and the regulation of matrix patency as a potential target for therapy [85].
The endothelial cell modulation of smooth muscle cell phenotype (and vice versa) has also been alluded to from vascular co-culture models of artery function, where it has been suggested to be intimately involved in differentiation, development and disease (e.g. in bovine, human and porcine aorta and porcine carotid artery [162, 206]; see also [66] for review). However, few studies have examined a role for gap junctions in this response. In bovine aortic co-culture, close contact sites between endothelial and smooth muscle cells were described by Saunders and D’Amore [178] and were suggested to be involved in the regulation of vascular growth and function. Apparent heterocellular gap junctions between these co-cultured bovine aortic cells were first demonstrated by Fillinger et al. [58], with a speculated role consistent with Saunders and D’Amore [178]. This concept was supported by Jacot and Wong [103] with endothelium-mediated platelet-derived growth factor-smooth muscle action being suggested to be due to such junctions. In a similar manner, in co-cultured rat pulmonary artery-derived endothelial and smooth muscle cells, projections from either or both cells have Cx43 myoendothelial gap junctions (and potentially, the other pulmonary artery Cxs37 and 40) [12, 111]. These sites are suggested to facilitate endothelium-mediated 5-hydroxytryptamine (serotonin) transfer to the smooth muscle, which initiates, in part, transforming growth factor-β1-dependent smooth muscle cell differentiation to induce medial thickening in vascular disease [67]. Similar contacts between co-cultured human umbilical cord vein endothelial (HUVECs) and smooth muscle cells apparently facilitate bidirectional cross-talk to sense hemodynamic forces such as shear stress and to modulate adhesion molecule expression [31, 32].
In light of the above discussion and given the structure and location of the projections associated with MECCs and myoendothelial gap junctions, a potential mechanosensing role is possible for these sites in intact arteries. Indeed, a mechanosensory role for TRP and IKCa channels has been demonstrated in vascular cells ([38, 84, 88, 112]; and for review, [217]), and these channels are localized to a proportion of myoendothelial microdomain signalling sites in intact arteries ([4, 40, 100, 179, 186]; and for review, [174]), thus supporting such a proposition.
From a historical perspective, Moore and Ruska [141], Fawcett [53] and Rhodin [165] speculated that the endothelium plays an integral role in the control of vessel tone via the activity of MECCs. However, a definitive link between the control of tone and MECCs has not been established; although if it is assumed that gap junctions are present at such sites, they may be the basis for a universal contact-mediated EDH mechanism. Regardless, the presence of gap junctions at vascular MECCs was not established until 15 years after Rhodin [165], being first demonstrated by Spagnoli and coworkers (in rabbit carotid artery; [188]) and Taugner and coworkers (in renal arteries of the mouse and tree shrew; [193]), with the latter group suggesting different functional roles for MECCs derived from anatomically distinct projections, including humoral and electrical coupling and tension-sensing. Notably, like the myoendothelial gap junction incidence ([168] for review; see also [23, 169]), the density of MECCs has been suggested to increase with decreased vessel size [113, 166, 193], with the increased myoendothelial gap junction density having been suggested to reflect the general relative and increased contribution of EDH to endothelium-dependent relaxation in smaller over larger diameter vessels [10, 90, 181]. Thus, these observations are consistent with the potential MECC and myoendothelial gap junction involvement in a universal basic EDH mechanism and reflect a key aspect of a hypothesis of the present review (Fig. 2).
The ultrastructural morphology of MECCs (summarized in Table 1 and Figures 1–4 in [174]) reflects the basic structure of well-characterized myoendothelial microdomain signalling sites, such as those at a proportion of IEL holes in murine and rat mesenteric arteries [36, 50, 81, 117, 173, 179, 186], with some evidence for their role in such activity in the human mesenteric artery [23]. In murine and rat mesenteric arteries, there is a close spatial localization of an IP3R-ER Ca2+ store and KCa, TRP and Kir and gap junction Cx channels, and the smooth muscle Na+/K+-ATPase (summarized, Figure 3 in [179]). Such sites facilitate the synergistic and/or separate transfer of current and localized K+ signalling that underlies EDH activity (Fig. 2), as presently characterized in several vascular beds.
The variation in the structure of channels, receptors and accessory proteins is a significant factor for their activity and consequently, artery function [54, 55, 91]. The significant heterogeneity in EDH mechanisms and associated vessel function within and between arteries, species, development, ageing and disease [54, 55] could in theory be due to variable and differential expression, distribution and activity of the above-mentioned channel and receptor subtypes at the localized myoendothelial microdomain signalling sites. Furthermore, given the critical nature of stromal-interacting molecule 1 and 2 accessory proteins as an ER membrane Ca2+ sensor and Orai 1–3 store-operated Ca2+ influx channel protein subunits in modulating the selected TRP channel function, including TRP canonical type 3 (TRPC3 [1, 73, 142]; see also [152, 183] for review), such proteins are likely to play a significant role in myoendothelial microdomain signalling. Similarly, a role for the different forms of EDH signalling proteins, such as monomeric and heteromultimeric forms and splice variants, and their mechanisms of activation and suppression [11, 93, 138, 148, 197, 205, 208, 215], may also be critical at such localized sites.
Notably, in human, murine and rat mesenteric arteries, in murine and rat cremaster arteries and in porcine coronary and rat middle cerebral arteries, transferable K+ and EETs, as transferable EDHFs, and gap junctions facilitate EDH and vessel relaxation [23, 24, 33, 36, 40–43, 47, 50, 74, 81, 135, 136, 161, 163, 177, 179, 186, 211].
Intrinsic regulation of endothelial function by EETs
A role for EETs in EDH-mediated activity was first demonstrated by Campbell et al. [21]. In response to various physiological stimuli, including shear and cyclic stretch, as well as experimental agonists, such as bradykinin and ACh, endothelial cells variably synthesize four EET regioisomers (5,6-EET, 8,9-EET, 11,12-EETand 14,15-EET) predominantly via the CYP 2C and 2J isoenzymes from various lipid substrates that include arachidonic, linoleic, eicosapentaenoic and docosahexaenoic acids (Fig. 1) [20, 60]. Bioavailability is determined primarily by cyclooxygenases (COX; selectively for 5,6-EET) and soluble epoxide hydrolases (sEH; for all EET regioisomers), which metabolise EETs into prostanoids and corresponding dihydroxyeicosatrienoic acid regioisomers, respectively [20, 123].
Studies specifically designed to examine the potential transferable smooth muscle actions of endothelial EETs (and thereby clarify their role as EDHFs) have often employed tandem bioassays involving serial perfusion or effluent cascade in which agonist-mediated stimuli results in an endothelium-intact ‘donor’ artery evoking hyperpolarization and dilation of a downstream/isolated endothelium-denuded ‘detector’ artery, as evidenced in bovine and porcine coronary arteries and human coronary arterioles [21, 72, 116, 159]. Similar methodological approaches have also led to the proposal that EETs, as EDHFs, are produced and released in response to elevations in shear stress in murine and rat gracilis muscle arterioles (for example in [94]).
However, given that smooth muscle BKCa activation is thought to underpin the EDHF-type activity of EETs (Fig. 1) and that bradykinin-evoked, EET-mediated smooth muscle hyperpolarization is unaffected by iberiotoxin alone in the isolated intact porcine coronary artery [211], the physiological relevance of EETs as a transferable EDHF is questionable in specific vascular beds (see [20] for review). Indeed, in the porcine coronary artery, applied 11,12-EET mimics the effect of bradykinin by evoking an SKCa- and IKCa-dependent endothelial hyperpolarization, which is consistent with the suggestion that endogenous EETs evoke [211] an endothelial hyperpolarization that conducts to the media via myoendothelial gap junctions [7]. Nevertheless, in selected arteries, EDH-type responses are attenuated to a similar extent by either iberiotoxin or inhibitors of CYPs, thereby collectively supporting a physiological role for EETs as a transferable EDHF and/or in modulating endothelial function directly in specific vascular beds ([20] for review). Notably, BKCa has only rarely been described in the intact artery endothelium [170]. In addition, the idea that eicosanoids modulate endothelial function and facilitate vasodilation is supported by observations in a number of arteries, including rat caudal and mesenteric [22, 138], rabbit mesenteric and iliac [62, 194], murine mesenteric [43] and porcine coronary and pulmonary arteries [49, 63], where responses to applied EETs are attenuated or abolished by endothelial removal.
Shear stress-evoked, EET-mediated activation of transient receptor potential vanilloid 4 (TRPV4) channels
Evidence that EETs modulate endothelial function directly [75] preceded the first demonstration of their apparent role in endothelium-dependent vasodilation as an EDHF [21]. Treatment of cultured endothelial cells from bovine coronary artery, human umbilical cord and porcine aorta with applied 5,6-EET or 11,12-EET evokes a cyclooxygenase-independent influx of extracellular Ca2+ and subsequent hyperpolarization [75, 92], with 5,6-EET stimulating an extracellular Ca2+ influx through TRPV4 in isolated murine aortic endothelial cells [209]. Eicosanoid-mediated activation of TRPV4 (Fig. 2) appears to occur in a regioisomer-specific manner, with 5,6-EET and (to a lesser extent) 8,9-EET promoting activity and 14,15-EET being ineffective [202, 209]; whereas activation by 11,12-EET may be tissue-specific, since this regioisomer promotes activity in the murine mesenteric circulation, but fails to activate TRPV4 in isolated murine aortic endothelial cells [43, 186, 202, 209].
The activation of endothelial TRPV4 may be a particularly important mechanism for fluid shear stress (flow)-mediated vasodilation [17, 112, 123, 137], which is arguably the most significant mode of endothelial stimulation in vivo (in the intact vasculature). In murine carotid arteries, for example, flow-induced EDH-type dilation is attenuated either by the CYP inhibitor N-methylsulfonyl-6-(2-propargyloxyphenyl) hexanamide (MS-PPOH) or the non-selective TRP channel blocker ruthenium red, with no further inhibition evoked following the administration of these agents in combination. However, in TRPV4-deficient mice, EDH-type relaxations are selectively diminished and unaffected by MS-PPOH, leading to the proposal that the shear-induced activation of endothelial TRPV4 requires intermediary signalling by EETs [123]. A support for this scenario is suggested from HUVEC studies, where TRPV4 is retained within perinuclear regions when cells are maintained under static conditions and translocated to regions of the plasmalemma during flow adaptation (12-dyn/cm2 for 24 h), which induces CYP expression and offsets the rapid decrease in epoxygenase levels that follows cell isolation [59, 123]. Notably, immunohistochemical approaches have demonstrated that TRPV4 is absent from the media of the murine carotid artery [123], with the action of endogenous EETs in this vessel therefore being endothelial and thus distinct from a putative EDHF. In murine mesenteric resistance arteries (in which TRPV4 is expressed in both the endothelium and media), however, 11,12-EET induces both endothelium-dependent and -independent hyperpolarization and relaxation (with each component contributing ~50 % to the total dilator response) through apamin+TRAM- and iberiotoxin-sensitive mechanisms, respectively [43]. Collectively, these observations are indicative of heterogeneous and differential TRPV4 distribution and function between different vascular regions [43, 123], and the relative physiological importance of endothelial EETs action could, at least in part, depend on the distribution of TRP channels in the vessel wall as well as the vascular region under study.
Based on data from several studies, EETs may also play a role in shear-evoked, TRPV4-dependent dilation in the human coronary microcirculation where TRPV4-dependent synthesis and release of mitochondrial-derived H2O2 (which then activates smooth muscle BKCa channels) underpin flow-induced arteriolar dilation [17, 121, 139, 218], responses that are nevertheless also attenuated by CYP inhibitors [140]. However, although recently suggested in the human coronary vasculature [37], whether EETs are required for flow-induced TRPV4 activation (or endothelial TRP channel activity in general) requires further clarification. Indeed, arachidonic acid may directly activate endothelial TRPV4 to induce EDH-mediated dilation in intact human coronary arterioles, although, in contrast to EETs [75, 92], this effect requires prior endothelial hyperpolarization [220]. Notably, exogenous EETs are less potent than exogenous arachidonic acid in activating TRPV4 in these vessels, and the ability of the putative CYP inhibitors 17-octadecyanoic acid (17-ODYA) and MS-PPOH to attenuate arachidonic acid-induced Ca2+ entry and dilation does not reflect reductions in EET biosynthesis, which is collectively suggestive of a novel mechanism of TRPV4 activation [220]. In the light of these findings, endogenous arachidonic acid could, in theory, facilitate EET-dependent, TRPV4-mediated Ca2+ entry by sustaining channel activity at more negative potentials. The physiological significance of this mechanism does, however, require further clarification because the ability of miconazole to attenuate flow-induced dilation in the human coronary microcirculation [140] could potentially reflect antagonist activity, rather than CYP inhibition [29], as suggested for the above inhibitors [220].
In large arteries (in which the contribution of EDH is generally, but not universally, minimal), similar mechanisms may also underlie flow-induced NO synthesis. In rat middle cerebral and cremaster arteries, for example, shear-evoked, NO-dependent dilation is attenuated either by ruthenium red or following inhibition of phospholipase A2 (PLA2) thereby preventing the formation of endogenous arachidonic acid [112]. In pressurised rat middle cerebral arteries, a PLA2-dependent mechanism also underlies the ruthenium red-sensitive elevation in endothelial Ca2+ following lumenal delivery of the purinergic receptor agonist uridine triphosphate [128], suggesting that the dilator action of specific agonists may also require an increase in TRPV4 activity. Indeed, in murine mesenteric resistance arteries, increased TRPV4 activity may also be required for ACh-induced relaxation, as suggested by the decreased response to this agonist in TRPV4-knockout mice [43, 219].
The variability in endothelium-dependent relaxation to applied EETs between different vascular beds is reflected by the differential contribution and blocking of NO synthesis and SKCa and IKCa as well as by agents that are reported to prevent gap junction function [34, 43, 63, 86, 96, 194]. These data collectively suggest a potential general property in vascular beds in which EET-mediated EDH activity occurs. Notably however, EET-mediated TRPV4 activation is unlikely to be a universal mechanism underlying flow-induced dilation, as NO-independent dilation in rat small coronary artery is unaffected by 17-ODYA [200]. Furthermore, co-transfection studies of human embryonic kidney (HEK 293) cells have demonstrated that TRPV4 can form heteromeric channel complexes with TRPC1 proteins, which prolongs extracellular Ca2+ influx relative to homotypic TRPV4 channels [127]. However, it remains to be determined if these channels are present in native/intact arterial endothelial cells and whether EETs influence the gating of such channels.
Agonist-evoked, EET-mediated activation of TRPC
A further autocrine action of endothelium-derived EETs involves their activation of TRPC channels in endothelial cells (Fig. 2). For example, the bradykinin-evoked sustained increase in [Ca2+]i in HUVECs, as well as in these cells overexpressing CYP 2C9, is enhanced following the administration of the sEH inhibitor 1-adamantyl-3-cyclohexylurea, which prolongs KCa-dependent hyperpolarization in a manner that is reversed by the selective CYP 2C9 inhibitor sulfaphenazole [61]. In the same manner, in CYP 2C9-overexpressing HUVECs, bradykinin and 11,12-EET (but not 14,15-EET) induce rapid (within 30 s) translocation of either TRPC3 or TRPC6 channels from perinuclear regions to caveolin-1-rich plasmalemmal domains through a mechanism that is prevented by RpcAMPs (a PKA inhibitor), the EET antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EE5ZE) and ruthenium red [61]. By contrast, neither shear stress nor 5,6-EET activate TRPC6, leading to the proposal that the mode of endothelial stimulation as well as the predominant endogenous regioisomers produced in any given artery are likely to be a prerequisite for which TRP channels are activated [123].
The ability of agonists to activate TRPC channels via the formation of EETs is consistent with findings in porcine coronary artery. In this scenario, EETs facilitate bradykinin-mediated activation of endothelial SKCa and IKCa, which subsequently induces hyperpolarization that conducts to the media via gap junctions [211]. Indeed, this particular action of EETs masks their ability to induce iberiotoxin-sensitive smooth muscle hyperpolarization (i.e. their EDHF action), which is only apparent in preparations incubated continuously with apamin and TRAM [211], suggesting that in some cases local autocrine actions of EETs are more efficacious than their classic ‘EDHF’-type role, as suggested above. Considering these observations, the conclusion drawn from a number of studies using tandem bioassays (i.e. that EETs are transferable EDHFs; see also Section “Intrinsic regulation of endothelial function by EETs”) is debatable, given that the donor endothelium and detector smooth muscle are electrically uncoupled in such experimental setups. Notably, the formation of EETs in porcine coronary artery does not require an initiating endothelial hyperpolarization per se, since iberiotoxin-sensitive smooth muscle hyperpolarization persists in the continuous presence of SKCa and IKCa blockers [211], which is consistent with the earlier demonstration that the increased CYP activity that follows the administration of agonists or SERCA inhibitors is intimately linked to depletion of IP3-sensitive intracellular Ca2+ stores (Fig. 1); with hyperpolarization occurring secondarily [92]. By contrast, 11,12-EET does not affect endothelial Ca2+ in rat cremaster arteries even when administered at supraphysiological concentrations (10 μM) [136], yet still causes vasodilation, although in some cases fails to do so, even in preparations with an intact endothelium [96, 194].
A potential limitation for the ability of endothelium-derived EETs to promote smooth muscle hyperpolarization via their autocrine actions may be the extent of gap junctional coupling. In healthy rat mesenteric resistance arteries, relaxation to 11,12-EET is abolished by iberiotoxin and is unaffected following the removal of the endothelium, whereas in the same vessels from CCl4-induced cirrhotic rats (in which endothelial Cx40 and 43 mRNA and protein expression are apparently selectively elevated), relaxation is markedly enhanced. This latter response has an additional endothelium-dependent, 18α-glycyrrhetinic acid-sensitive component that masks the inhibitory action of iberiotoxin [14]. Physiologically, in rat mesenteric artery, EDH-type relaxation to ACh in healthy rats is insensitive to miconazole (which acts as a non-selective EET antagonist and CYP blocker) [29] but is attenuated by 18α-glycyrrhetinic acid [35, 48, 90, 130], whereas in cirrhotic rats, relaxation to ACh is enhanced, with miconazole and 18α-glycyrrhetinic acid showing similar efficacy in attenuating relaxation [14]. Collectively, these findings suggest that the gap junctional coupling may be required for the autocrine actions of EETs to modulate vessel tone. In support of this interpretation, arachidonic acid- and bradykinin-evoked EDH-type relaxation in rat renal microvessels (in which Cxs37, 40 and 43 are expressed in the endothelium) [16] are abolished following treatment with either miconazole, MS-PPOH, apamin or 18α-glycyrrhetinic acid [199]. Notably, however, a caveat to this and similar studies that use licorice derivatives 18α/β-glycyrrhetinic acid and carbenoxolone to examine vascular gap junction effects is that these agents have significant non-gap junctional effects [6, 33, 79, 83, 129, 192].
EETs, cAMP and gap junctional communication
As alluded to the above discussion, in addition to promoting endothelial hyperpolarization, EETs may also play a permissive role in electrotonic conduction via gap junctions. In cultured porcine coronary artery endothelial cells, bradykinin exerts a biphasic effect on electrical and Lucifer yellow dye coupling, consisting of an initial rapid increase followed by a sustained decrease [160]. The initial phase in coupling is prevented by selective CYP2C9 block with sulphaphenazole, but not with apamin and charybdotoxin (respective SKCa and I/BKCa block), which is indicative of a hyperpolarization-independent increase in epoxygenase activity, and is mimicked by applied 11,12-EET [160]. In a similar manner to the modulation of gap junction Cxs described above, bradykinin-enhanced coupling is prevented by inhibitors of AC and PKA, while the AC activator forskolin enhances coupling and is associated with a time-dependent recruitment of Cx43 to membrane [160], thus confirming the role of a cAMP-dependent mechanism (Fig. 2). By contrast, the subsequent sustained decrease in coupling is mediated by extracellular-regulated kinases (ERK1/2) which do not require EETs or KCa channel activation [160].
Notably, the vascular endothelium facilitates the longitudinal propagation of membrane potential changes in vessels over distance ([7, 89, 184] for review) and also promotes the radial spread of endothelial hyperpolarization into the media. Indeed, given that gap junction plaques between neighbouring endothelial cells are larger (per Fig. 2) than myoendothelial and homocellular smooth muscle plaques and that the endothelium behaves as a low-resistance ‘current sink’ [7, 76], the ability of EETs to promote interendothelial coupling is thus consistent with their having an integral role in the coordination of vasomotor responses and control of tone. Although a role for EETs in modulating radial coupling via myoendothelial gap junctions has not yet been confirmed, their ability to activate AC and increase cAMP is consistent with the findings that this nucleotide plays a permissive role in gap junction-dependent vasodilation in response to agonists and SERCA inhibitors (see Section “Connexins, gap junctions and their regulation”). Notably, all four EET regioisomers at physiological concentrations elevate cAMP levels in endothelial cells, at least in the preparations from the human saphenous vein and bovine aorta [146].
The EET receptor and intracellular EET binding sites
The involvement of a putative EET receptor in the control of vasodilator tone is generally accepted, but the receptor itself has not yet been formally identified (for review, see [155]). However, the existence of such a receptor and its involvement in the control of tone has been alluded to by several studies. Analogues of 14,15-EET demonstrate particular structural and stereoisomeric features required for EETs-mediated vasodilator activity, as well as for antagonist activity, thereby indicating a highly selective binding site(s) (for detail, see [20]). In addition, the ability of the cell-impermeable EET antagonist, 14,15-epoxyeicosa-5(Z)-enoic acid methylsulfonylimide (14,15-EE5ZE-mSl), to attenuate 5,6-EET- and 14,15-EET-mediated vessel relaxation is consistent with EETs’ interaction with a cell surface protein [71, 211]. Furthermore, in a number of tissues, EETs-mediated BKCa activation requires guanine triphosphate (GTP)-dependent ADP-ribosylation of Gαs proteins (Fig. 1); and the involvement of a Gαs-coupled mechanism is consistent with EETs elevating cAMP [20, 61, 120]. Indeed, EETs can bind to defined low affinity sites, including prostaglandin E (EP2), cannabinoid, TP and dopamine receptors, as well as the fatty acid binding protein peroxisomal proliferator-activated receptor-α, but this occurs at EET concentrations that are significantly higher than those generally required to evoke relaxation, supporting the ability of EETs to regulate vascular tone following their occupation of alternative higher affinity binding sites [29]. Corroborating these data, the combined methodology of photoaffinity labelling with electrophoretic isolation and radioautographic detection using an analogue of 14,15-EET with a photoactive azido group (20-iodo-14, 15-epoxyeicosa-8(Z)-enoyl-3-azidophenylsulfonamide; 20-I-14,15-EE8ZE-APSA) demonstrates the ability of a single 47 kDa protein expressed in vascular cell membranes to bind low nanomolar concentrations of EETs [29]. Consistent with the idea of regioisomer-specific binding sites, 11,12-EET and 14,15-EET potently (IC50, 8–11 nM) inhibit photolabelling of the 47 kDa band by 20-I-14,15-EE8ZE-ASPA, but inhibition by 8,9-EET occurs at higher concentrations (IC50, ~440 nM), and 14,15-DHET is ineffective [29]. Additionally, functional studies demonstrate the agonist and vasoactive properties of 20-I-14,15-EE8ZE-ASPA that are indistinguishable from applied 14,15-EET, which is indicative of a common binding site [29]. In support of both autocrine and paracrine actions of EETs, this high affinity 47-kDa protein is expressed in both endothelial and smooth muscle membranes of bovine coronary arteries, albeit at higher levels in the latter [29]. While these findings support the existence of an EET receptor (without formally identifying it), it is currently unknown whether multiple regioisomer-specific subtypes exist and, in turn, what their potential regional- and species-specific distribution patterns might be. Indeed, the ability of some vessels to relax to 11,12-EET, but not to 14,15-EET [30, 157], and the ability of 14,15-EE5ZE-mSl to attenuate responses to 14,15-EET, but not to 11,12-EET [71], may indicate the presence of multiple subtypes of EET receptor with variable regioisomer selectivity. Of note, it has also been suggested that 14,15-EET may exert its effect via stereo-specific cell surface binding site/s [214].
In addition to cell surface receptors, EETs have been suggested to activate TRPV4 through an arachidonate recognition sequence located near the intracellular N terminus of this channel protein and thus act as direct agonists, as has already been demonstrated for TRP melastatin type 2 channels [42, 145]. Indeed, 14,15-EEZE attenuates relaxation to all EETs, but 14,15-EE5ZE-mSl only attenuates those evoked by 5,6-EET and 14,15-EET [71, 211], raising the possibility of EET recognition sites being located within the cell and at the plasmalemma. It is unlikely, however, that EETs share a common binding site with the selective synthetic TRPV4 agonist 4α-phorbol 12,13-didecanoate (4α-PDD), as substitution of tyr555 (located at the N-terminal of the third trans-membrane domain) with alanine or deletion of leu584 and trp586 blocks activation by 4α-PDD, but not by hypotonic cell swelling (which activates PLA2), arachidonic acid or 5,6-EET [203, 204]. In rat cerebral artery smooth muscle, the idea that EETs act as direct TRPV4 agonists is supported by the EET-mediated increase in BKCa activity that occurs secondarily to an increase in TRPV4-dependent Ca2+ influx and SR ryanodine receptor (RyR) activation, thereby increasing intra-cellular Ca2+ release to modulate channel activity (Fig. 1) [42]. Thus, G-protein coupled mechanisms that gate BKCa following the occupation of a putative EETs receptor/s are unlikely to be a universal mechanism for hyperpolarization and the associated vessel relaxation.
In addition, IP3 sensitizes TRPV4 to activation by 5,6-EET by evoking a physical interaction between IP3R subtype 3 and a specific sequence within the C-terminal of TRPV4 that coincides with its Ca2+-calmodulin binding domain [68]. Thus, in the continuous presence of IP3, TRPV4 can be activated synergistically by low concentrations (≤1 nM) of 5,6-EET, and therefore eicosanoid activation of TRPV4 can occur even when PLA2 activity is low [57, 68]. In further support of direct binding of 5,6-EET to TRPV4, PKA has been shown to have a sensitizing action on TRPV4, rather than directly activating the channel [57], as has been demonstrated for TRPC3 and TRPC6 ([61]; also see Section “Shear stress-evoked, EET-mediated activation of transient receptor potential vanilloid 4 (TRPV4) channels”).
The ongoing development of regioisomer-specific EET antagonists will likely clarify the role of individual EET regioisomers. Notably, 14,15-EE5ZE is metabolised within the cell by sEH to its corresponding diol form (i.e. 14,15-DHE5ZE) which alters antagonist activity to one of high selectivity against 14,15-EET [18]. Another antagonist, 20-hydroxy-11,12-epoxyeicosa-8(Z)-enoic acid, markedly attenuates bovine coronary arterial relaxation to 11,12-EET and partially inhibits responses to 14,15-EET, whereas its sEH-derived hydration product, 11,12,20-trihydroxyeicosa-8[Z]-enoic acid, selectively attenuates responses to 11,12-EET, with no effect against other regioisomers [19]. It is currently unknown whether these latter two antagonists are cell-permeable and, thus, whether they can act as antagonists of purported intracellular recognition sites. Selective antagonists for 5,6-EET (the most potent and efficacious agonist of TRPV4) and 8,9-EET are still to be developed.
Conclusions
In several vascular beds, the endothelial cells produce EETs in response to in vivo stimuli, including cyclic stretch and shear stress [59, 123], which is indicative of their physiological role in vasodilation. This review supports the hypothesis that endothelium-derived EETs act as integral modulators of myoendothelial microdomain signalling components, which underlie EDH activity in a potentially universal manner. Indeed, these autocrine actions of EETs may be of significant physiological relevance over their apparent classic action as a transferable EDHF [20, 54], where such activity masked the direct EETs-mediated smooth muscle hyperpolarization in vessels where myoendothelial gap junction Cxs are a critical facilitating mechanism [34, 55, 174].
The role of microdomain signalling at putative MECC sites in murine mesenteric arteries is highlighted by the activity of Ca2+ ‘pulsars’ and ‘sparklets’ following IP3R and TRPV4 activation, respectively, that is associated with IKCa activation and endothelium-dependent vasodilation [117, 186]. Thus, in a similar manner to the localized Cx-ER-IP3R-IKCa-TRPC3 myoendothelial microdomain signalling in rat mesenteric artery [179] and, potentially, at a similar complex associated with TRPV4 in rat cremaster arterioles [4, 136], such spatially restricted activity plays an integral role in the activation of TRPV4 by endogenous EETs as well as other TRP channel subtypes that are activated by EETs, such as TRPC3 and TRPC6. However, it is currently unknown whether the major endothelial EET-synthesizing epoxygenases (i.e. CYPs 2C and 2J) are preferentially expressed at MECCs, where they could, in theory, optimize the biological role of EETs at such sites.
Notably, putative high affinity EET receptors have yet to be formally identified; although evidence that candidate receptor proteins display regioisomer-selective binding and affinity [29] is potentially indicative of the existence of such multiple receptor isoforms. Tissue-specific variation in expression and distribution of such receptors would thus be expected to contribute to the functional plasticity proposed for myoendothelial microdomain signalling (except perhaps for TRPV4, for which EETs are proposed as endogenous ligands) and reflected by characteristic heterogeneity in EDH-mediated activity. In addition, the ability of EETs to potentiate intercellular coupling between neighbouring endothelial cells [160] is suggestive of their potential role in promoting low-resistance longitudinal propagation of hyperpolarization along the endothelium over distance as well as their potential to amplify the radial spread of endothelial hyperpolarization into the vessel wall via myoendothelial Cxs. Furthermore, whether sEH inhibitors increase the biological role of EETs as regulators of myoendothelial microdomain signalling remains to be elucidated. Indeed, pharmacological enhancement of the bioavailability of EETs through sEH inhibition is currently being investigated with a view not only to improve endothelial function but to also enhance vasculoprotection potentially against a range of cardiovascular disease states (for review, see [144]).
Future studies will also provide a focus on the potential ability of EETs to activate other endothelial TRP channel subtypes and microdomain components. Notably, recent studies of dorsal root ganglion neurones have demonstrated that nanomolar concentrations of 5,6-EET activate TRP ankyrin 1 channels (TRPA1) [182]. Indeed, the suggested density of TRPA1 at IEL holes as potential myoendothelial contact sites in rat cerebral arteries may be associated with their activation and role in endothelium-dependent dilation ([40]; see also [39] for review). In addition, a functional association (and therefore a potential spatial interaction) between TRPC3 and IP3R has been reported in cultured bovine pulmonary artery endothelial cells [107] and in isolated smooth muscle cells of rabbit coronary and rat cerebral artery [2, 154]. In uterine arterial endothelial cells, such an interaction is increased in pregnancy without alteration in the expression of either TRPC3 or IP3, but is associated with increased expression of cPLA2 and Cx43 activities [216]. However, whether the increased density of an EET-TRPC3-IP3R-driven myoendothelial microdomain signalling complex underpins augmented dilator responses in pregnancy remains to be determined.
The differential distribution, expression and activity of microdomain components are proposed to contribute to the apparent diversity of EDH mechanisms within and between vascular beds, species, ageing, development and disease. The EETs-mediated microdomain hypothesis is consistent with a role for these epoxides and K+ as being integral for EDH-dependent vasodilation in the sense that where EETs are an apparent transferable EDHF, their interaction with the receptor and channel subtypes and their associated signalling mechanisms and Ca2+ stores may facilitate EDH-dependent microdomain signalling, contributing to a putative universal EDH mechanism. The differential expression, distribution and function of myoendothelial microdomain components in different arteries represent potentially selective targets for modulating artery function in a specific manner.
Acknowledgments
This work was supported by grants from the Monfort Excellence Award (Monfort Family Foundation) and the National Institutes of Health to S. Earley (R01HL091905) and the National Health and Medical Research Council of Australia to S.L. Sandow (APP1048885).
Contributor Information
David C. Ellinsworth, Bristol Heart Institute, University of Bristol, Queens Building Level 7, Bristol Royal Infirmary, Upper Maudlin Street, Bristol BS2 8HW, UK
Scott Earley, Biomedical Sciences, Colorado State University, Fort Collins, CO 80523, USA.
Timothy V. Murphy, Department of Physiology, School of Medical Sciences, University of New South Wales, Sydney, New South Wales 2052, Australia
Shaun L. Sandow, Department of Physiology, School of Medical Sciences, University of New South Wales, Sydney, New South Wales 2052, Australia
References
- 1.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103:1289–1299. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adebiyi A, Zhao G, Narayanan D, Thomas-Gatewood CM, Bannister JP, Jaggar JH. Isoform-selective physical coupling of TRPC3 channels to IP3 receptors in smooth muscle cells regulates arterial contractility. Circ Res. 2010;106:1603–1612. doi: 10.1161/CIRCRESAHA.110.216804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews KL, Irvine JC, Tare M, Apostolopoulos J, Favaloro JL, Triggle CR, Kemp-Harper BK. A role for nitroxyl (HNO) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. Br J Pharmacol. 2009;157:540–550. doi: 10.1111/j.1476-5381.2009.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci USA. 2012;109:18174–18179. doi: 10.1073/pnas.1211946109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begandt D, Bintig W, Oberheide K, Schlie S, Ngezahayo A. Dipyridamole increases gap junction coupling in bovine GM-7373 aortic endothelial cells by a cAMP-protein kinase A dependent pathway. J Bioenerg Biomembr. 2010;42:79–84. doi: 10.1007/s10863-009-9262-2. [DOI] [PubMed] [Google Scholar]
- 6.Behringer EJ, Socha MJ, Polo-Parada L, Segal SS. Electrical conduction along endothelial cell tubes from mouse feed arteries: confounding actions of glycyrrhetinic acid derivatives. Br J Pharmacol. 2012;166:774–787. doi: 10.1111/j.1476-5381.2011.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beny JL. Information networks in the arterial wall. News Physiol Sci. 1999;14:68–73. doi: 10.1152/physiologyonline.1999.14.2.68. [DOI] [PubMed] [Google Scholar]
- 8.Beny JL, Brunet PC. Electrophysiological and mechanical effects of substance P and acetylcholine on rabbit aorta. J Physiol. 1988;398:277–289. doi: 10.1113/jphysiol.1988.sp017042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beny JL, Brunet PC, Huggel H. Effect of mechanical stimulation, substance P and vasoactive intestinal polypeptide on the electrical and mechanical activities of circular smooth muscles from pig coronary arteries contracted with acetylcholine: role of endothelium. Pharmacology. 1986;33:61–68. doi: 10.1159/000138202. [DOI] [PubMed] [Google Scholar]
- 10.Berman RS, Martin PE, Evans WH, Griffith TM. Relative contributions of NO and gap junctional communication to endothelium-dependent relaxations of rabbit resistance arteries vary with vessel size. Microvasc Res. 2002;63:115–128. doi: 10.1006/mvre.2001.2352. [DOI] [PubMed] [Google Scholar]
- 11.Beyer E, Berthoud V. The family of connexin genes. In: Harris A, Locke D, editors. Connexins: a guide. Chapter 1. Humana; New York: 2009. pp. 3–26. [Google Scholar]
- 12.Billaud M, Marthan R, Savineau JP, Guibert C. Vascular smooth muscle modulates endothelial control of vasoreactivity via reactive oxygen species production through myoendothelial communications. PLoS One. 2009;4:e6432. doi: 10.1371/journal.pone.0006432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boedtkjer E, Kim S, Aalkjaer C. Endothelial alkalinisation inhibits gap junction communication and endothelium-derived hyperpolarisations in mouse mesenteric arteries. J Physiol. 2013;591:1447–1461. doi: 10.1113/jphysiol.2012.247478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolognesi M, Zampieri F, Di Pascoli M, Verardo A, Turato C, Calabrese F, Lunardi F, Pontisso P, Angeli P, Merkel C, Gatta A, Sacerdoti D. Increased myoendothelial gap junctions mediate the enhanced response to epoxyeicosatrienoic acid and acetylcholine in mesenteric arterial vessels of cirrhotic rats. Liver Int. 2011;31:881–890. doi: 10.1111/j.1478-3231.2011.02509.x. [DOI] [PubMed] [Google Scholar]
- 15.Bolton TB, Lang RJ, Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braunstein TH, Sorensen CM, Holstein-Rathlou NH. Connexin abundance in resistance vessels from the renal microcirculation in normo- and hypertensive rats. APMIS. 2009;117:268–276. doi: 10.1111/j.1600-0463.2009.02432.x. [DOI] [PubMed] [Google Scholar]
- 17.Bubolz AH, Mendoza SA, Zheng X, Zinkevich NS, Li R, Gutterman DD, Zhang DX. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca2+ entry and mitochondrial ROS signaling. Am J Physiol. 2012;302:H634–H642. doi: 10.1152/ajpheart.00717.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bukhari IA, Gauthier KM, Jagadeesh SG, Sangras B, Falck JR, Campbell WB. 14,15-Dihydroxy-eicosa-5(Z)-enoic acid selectively inhibits 14,15-epoxyeicosatrienoic acid-induced relaxations in bovine coronary arteries. J Pharmacol Exp Ther. 2010;336:47–55. doi: 10.1124/jpet.110.169797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bukhari IA, Shah AJ, Gauthier KM, Walsh KA, Koduru SR, Imig JD, Falck JR, Campbell WB. 11,12,20-Trihydroxy-eicosa-8(Z)-enoic acid: a selective inhibitor of 11,12-EET-induced relaxations of bovine coronary and rat mesenteric arteries. Am J Physiol. 2012;302:H1574–H1583. doi: 10.1152/ajpheart.01122.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 2010;459:881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 22.Carroll MA, Garcia MP, Falck JR, McGiff JC. 5,6-Epoxyeicosatrienoic acid, a novel arachidonate metabolite. Mechanism of vasoactivity in the rat. Circ Res. 1990;67:1082–1088. doi: 10.1161/01.res.67.5.1082. [DOI] [PubMed] [Google Scholar]
- 23.Chadha PS, Haddock RE, Howitt L, Morris MJ, Murphy TV, Grayson TH, Sandow SL. Obesity up-regulates intermediate conductance calcium-activated potassium channels and myoendothelial gap junctions to maintain endothelial vasodilator function. J Pharmacol Exp Ther. 2010;335:284–293. doi: 10.1124/jpet.110.167593. [DOI] [PubMed] [Google Scholar]
- 24.Chadha PS, Liu L, Rikard-Bell M, Senadheera S, Howitt L, Bertrand RL, Grayson TH, Murphy TV, Sandow SL. Endothelium-dependent vasodilation in human mesenteric artery is primarily mediated by myoendothelial gap junctions intermediate conductance calcium-activated K+ channel and nitric oxide. J Pharmacol Exp Ther. 2011;336:701–708. doi: 10.1124/jpet.110.165795. [DOI] [PubMed] [Google Scholar]
- 25.Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci USA. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaytor AT, Evans WH, Griffith TM. Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries. J Physiol. 1997;503:99–110. doi: 10.1111/j.1469-7793.1997.099bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaytor AT, Evans WH, Griffith TM. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J Physiol. 1998;508:561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen G, Suzuki H, Weston AH. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol. 1988;95:1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Falck JR, Manthati VL, Jat JL, Campbell WB. 20-Iodo-14,15-epoxyeicosa-8(Z)-enoyl-3-azidophenylsulfonamide: photoaffinity labeling of a 14,15-epoxyeicosatrienoic acid receptor. Biochemistry. 2011;50:3840–3848. doi: 10.1021/bi102070w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng MK, Doumad AB, Jiang H, Falck JR, McGiff JC, Carroll MA. Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br J Pharmacol. 2004;141:441–448. doi: 10.1038/sj.bjp.0705640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiu JJ, Chen LJ, Lee CI, Lee PL, Lee DY, Tsai MC, Lin CW, Usami S, Chien S. Mechanisms of induction of endothelial cell E-selectin expression by smooth muscle cells and its inhibition by shear stress. Blood. 2007;110:519–528. doi: 10.1182/blood-2006-08-040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu JJ, Chen LJ, Lee PL, Lee CI, Lo LW, Usami S, Chien S. Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood. 2003;101:2667–2674. doi: 10.1182/blood-2002-08-2560. [DOI] [PubMed] [Google Scholar]
- 33.Coleman HA, Tare M, Parkington HC. K+ currents underlying the action of endothelium-derived hyperpolarizing factor in guinea-pig, rat and human blood vessels. J Physiol. 2001;531:359–373. doi: 10.1111/j.1469-7793.2001.0359i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Wit C, Griffith TM. Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses. Pflugers Arch. 2010;459:897–914. doi: 10.1007/s00424-010-0830-4. [DOI] [PubMed] [Google Scholar]
- 35.Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res. 2008;102:1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dora KA, Sandow SL, Gallagher NT, Takano H, Rummery NM, Hill CE, Garland CJ. Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J Vasc Res. 2003;40:480–490. doi: 10.1159/000074549. [DOI] [PubMed] [Google Scholar]
- 37.Durand MJ, Gutterman DD. Diversity in mechanisms of endothelium-dependent vasodilation in health and disease. Microcirculation. 2013;20:239–247. doi: 10.1111/micc.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dyachenko V, Rueckschloss U, Isenberg G. Modulation of cardiac mechanosensitive ion channels involves superoxide, nitric oxide and peroxynitrite. Cell Calcium. 2009;45:55–64. doi: 10.1016/j.ceca.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Earley S. TRPA1 channels in the vasculature. Br J Pharmacol. 2012;167:13–22. doi: 10.1111/j.1476-5381.2012.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-activated K+ channels. Circ Res. 2009;104:987–994. doi: 10.1161/CIRCRESAHA.108.189530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Earley S, Gonzales AL, Garcia ZI. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol Pharmacol. 2010;77:612–620. doi: 10.1124/mol.109.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 43.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol. 2009;297:H1096–H1102. doi: 10.1152/ajpheart.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards DH, Chaytor AT, Bakker LM, Griffith TM. Modulation of gap-junction-dependent arterial relaxation by ascorbic acid. J Vasc Res. 2007;44:410–422. doi: 10.1159/000104254. [DOI] [PubMed] [Google Scholar]
- 45.Edwards DH, Li Y, Ellinsworth DC, Griffith TM. The effect of inorganic arsenic on endothelium-dependent relaxation: role of NADPH oxidase and hydrogen peroxide. Toxicology. 2013;306:50–58. doi: 10.1016/j.tox.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edwards DH, Li Y, Griffith TM. Hydrogen peroxide potentiates the EDHF phenomenon by promoting endothelial Ca2+ mobilization. Arterioscler Thromb Vasc Biol. 2008;28:1774–1781. doi: 10.1161/ATVBAHA.108.172692. [DOI] [PubMed] [Google Scholar]
- 47.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 48.Edwards G, Feletou M, Gardener MJ, Thollon C, Vanhoutte PM, Weston AH. Role of gap junctions in the responses to EDHF in rat and guinea-pig small arteries. Br J Pharmacol. 1999;128:1788–1794. doi: 10.1038/sj.bjp.0703009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards G, Thollon C, Gardener MJ, Feletou M, Vilaine J, Vanhoutte PM, Weston AH. Role of gap junctions and EETs in endothelium-dependent hyperpolarization of porcine coronary artery. Br J Pharmacol. 2000;129:1145–1154. doi: 10.1038/sj.bjp.0703188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellis A, Goto K, Chaston DJ, Brackenbury TD, Meaney KR, Falck JR, Wojcikiewicz RJ, Hill CE. Enalapril treatment alters the contribution of epoxyeicosatrienoic acids but not gap junctions to endothelium-derived hyperpolarizing factor activity in mesenteric arteries of spontaneously hypertensive rats. J Pharmacol Exp Ther. 2009;330:413–422. doi: 10.1124/jpet.109.152116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellis A, Triggle CR. Endothelium-derived reactive oxygen species: their relationship to endothelium-dependent hyperpolarization and vascular tone. Can J Physiol Pharmacol. 2003;81:1013–1028. doi: 10.1139/y03-106. [DOI] [PubMed] [Google Scholar]
- 52.Evans WH, Bultynck G, Leybaert L. Manipulating connexin communication channels: use of peptidomimetics and the translational outputs. J Membr Biol. 2012;245:437–449. doi: 10.1007/s00232-012-9488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fawcett DW. The fine structure of capillaries, arterioles and small arteries. In: Reynolds SRM, Zweifach BW, editors. The microcirculation. University of Illinois Press; Urbana: 1959. pp. 1–27. [Google Scholar]
- 54.Feletou M. Part 1: multiple functions of the endothelial cells—focus on endothelium-derived vasoactive mediators. In: Granger DN, Granger J, editors. The endothelium. Morgan and Claypool Life Sciences; San Rafael: 2011. [PubMed] [Google Scholar]
- 55.Feletou M. Part 2: EDHF-mediated responses “the classical pathway”. In: Granger DN, Granger J, editors. The endothelium. Morgan and Claypool Life Sciences; San Rafael: 2011. [PubMed] [Google Scholar]
- 56.Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988;93:515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernandes J, Lorenzo IM, Andrade YN, Garcia-Elias A, Serra SA, Fernandez-Fernandez JM, Valverde MA. IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5′-6′-epoxyeicosatrienoic acid. J Gen Physiol. 2008;131:i2. doi: 10.1085/JGP1315OIA2. [DOI] [PubMed] [Google Scholar]
- 58.Fillinger MF, Sampson LN, Cronenwett JL, Powell RJ, Wagner RJ. Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. J Surg Res. 1997;67:169–178. doi: 10.1006/jsre.1996.4978. [DOI] [PubMed] [Google Scholar]
- 59.Fisslthaler B, Popp R, Michaelis UR, Kiss L, Fleming I, Busse R. Cyclic stretch enhances the expression and activity of coronary endothelium-derived hyperpolarizing factor synthase. Hypertension. 2001;38:1427–1432. doi: 10.1161/hy1201.096532. [DOI] [PubMed] [Google Scholar]
- 60.Fleming I. The cytochrome P450 pathway in angiogenesis and endothelial cell biology. Cancer Metastasis Rev. 2011;30:541–555. doi: 10.1007/s10555-011-9302-3. [DOI] [PubMed] [Google Scholar]
- 61.Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau C, Hammock BD, Busse R. Epoxyeicosatrienoic acids regulate TRP channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:2612–2618. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- 62.Fujimoto S, Ikegami Y, Isaka M, Kato T, Nishimura K, Itoh T. K+ channel blockers and cytochrome P450 inhibitors on acetylcholine-induced, endothelium-dependent relaxation in rabbit mesenteric artery. Eur J Pharmacol. 1999;384:7–15. doi: 10.1016/s0014-2999(99)00663-9. [DOI] [PubMed] [Google Scholar]
- 63.Fuloria M, Smith TK, Aschner JL. Role of 5,6-epoxyeicosatrienoic acid in the regulation of newborn piglet pulmonary vascular tone. Am J Physiol. 2002;283:L383–L389. doi: 10.1152/ajplung.00444.2001. [DOI] [PubMed] [Google Scholar]
- 64.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- 65.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 66.Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 67.Gairhe S, Bauer NN, Gebb SA, McMurtry IF. Serotonin passes through myoendothelial gap junctions to promote pulmonary arterial smooth muscle cell differentiation. Am J Physiol. 2012;303:L767–L777. doi: 10.1152/ajplung.00183.2012. [DOI] [PubMed] [Google Scholar]
- 68.Garcia-Elias A, Lorenzo IM, Vicente R, Valverde MA. IP3 receptor binds to and sensitizes TRPV4 channel to osmotic stimuli via a calmodulin-binding site. J Biol Chem. 2008;283:31284–31288. doi: 10.1074/jbc.C800184200. [DOI] [PubMed] [Google Scholar]
- 69.Garland CJ, Dora KA. Evidence against C-type natriuretic peptide as an arterial ‘EDHF’. Br J Pharmacol. 2008;153:4–5. doi: 10.1038/sj.bjp.0707520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garry A, Edwards DH, Fallis IF, Jenkins RL, Griffith TM. Ascorbic acid and tetrahydrobiopterin potentiate the EDHF phenomenon by generating hydrogen peroxide. Cardiovasc Res. 2009;84:218–226. doi: 10.1093/cvr/cvp235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gauthier KM, Jagadeesh SG, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic-mSI: a 14,15- and 5,6-EET antagonist in bovine coronary arteries. Hypertension. 2003;42:555–561. doi: 10.1161/01.HYP.0000091265.94045.C7. [DOI] [PubMed] [Google Scholar]
- 72.Gebremedhin D, Harder DR, Pratt PF, Campbell WB. Bioassay of an endothelium-derived hyperpolarizing factor from bovine coronary arteries: role of a cytochrome P450 metabolite. J Vasc Res. 1998;35:274–284. doi: 10.1159/000025594. [DOI] [PubMed] [Google Scholar]
- 73.Giachini FR, Chiao CW, Carneiro FS, Lima VV, Carneiro ZN, Dorrance AM, Tostes RC, Webb RC. Increased activation of stromal interaction molecule-1/Orai-1 in aorta from hypertensive rats: a novel insight into vascular dysfunction. Hypertension. 2009;53:409–416. doi: 10.1161/HYPERTENSIONAHA.108.124404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goto K, Fujii K, Kansui Y, Abe I, Iida M. Critical role of gap junctions in endothelium-dependent hyperpolarization in rat mesenteric arteries. Clin Exp Pharmacol Physiol. 2002;29:595–602. doi: 10.1046/j.1440-1681.2002.03689.x. [DOI] [PubMed] [Google Scholar]
- 75.Graier WF, Simecek S, Sturek M. Cytochrome P450 mono-oxygenase-regulated signalling of Ca2+ entry in human and bovine endothelial cells. J Physiol. 1995;482:259–274. doi: 10.1113/jphysiol.1995.sp020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol. 2004;141:881–903. doi: 10.1038/sj.bjp.0705698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Griffith TM, Chaytor AT, Bakker LM, Edwards DH. 5-Methyltetrahydrofolate and tetrahydrobiopterin can modulate electrotonically mediated endothelium-dependent vascular relaxation. Proc Natl Acad Sci USA. 2005;102:7008–7013. doi: 10.1073/pnas.0408919102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Griffith TM, Chaytor AT, Taylor HJ, Giddings BD, Edwards DH. cAMP facilitates EDHF-type relaxations in conduit arteries by enhancing electrotonic conduction via gap junctions. Proc Natl Acad Sci USA. 2002;99:6392–6397. doi: 10.1073/pnas.092089799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guan BC, Si JQ, Jiang ZG. Blockade of gap junction coupling by glycyrrhetinic acids in guinea pig cochlear artery: a whole-cell voltage- and current-clamp study. Br J Pharmacol. 2007;151:1049–1060. doi: 10.1038/sj.bjp.0707244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haddock RE, Grayson TH, Brackenbury TD, Meaney KR, Neylon CB, Sandow SL, Hill CE. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am J Physiol. 2006;291:H2047–H2056. doi: 10.1152/ajpheart.00484.2006. [DOI] [PubMed] [Google Scholar]
- 81.Haddock RE, Grayson TH, Morris MJ, Howitt L, Chadha PS, Sandow SL. Diet-induced obesity impairs endothelium-derived hyperpolarization via altered potassium channel signaling mechanisms. PLoS One. 2011;6:e16423. doi: 10.1371/journal.pone.0016423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 83.Harris D, Martin PE, Evans WH, Kendall DA, Griffith TM, Randall MD. Role of gap junctions in endothelium-derived hyperpolarizing factor responses and mechanisms of K+-relaxation. Eur J Pharmacol. 2000;402:119–128. doi: 10.1016/s0014-2999(00)00512-4. [DOI] [PubMed] [Google Scholar]
- 84.Hayabuchi Y, Nakaya Y, Mawatari K, Inoue M, Sakata M, Kagami S. Cell membrane stretch activates intermediate-conductance Ca2+-activated K+ channels in arterial smooth muscle cells. Hear Vessel. 2011;26:91–100. doi: 10.1007/s00380-010-0025-0. [DOI] [PubMed] [Google Scholar]
- 85.Heberlein KR, Straub AC, Best AK, Greyson MA, Looft-Wilson RC, Sharma PR, Meher A, Leitinger N, Isakson BE. Plasminogen activator inhibitor-1 regulates myoendothelial junction formation. Circ Res. 2010;106:1092–1102. doi: 10.1161/CIRCRESAHA.109.215723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, da Costa GA, Huang Y, Luft FC, Gollasch M. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol. 2009;29:54–60. doi: 10.1161/ATVBAHA.108.171298. [DOI] [PubMed] [Google Scholar]
- 87.Herve JC, Derangeon M, Sarrouilhe D, Giepmans BN, Bourmeyster N. Gap junctional channels are parts of multiprotein complexes. Biochim Biophys Acta. 2012;1818:1844–1865. doi: 10.1016/j.bbamem.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 88.Hilgers RH, Janssen GM, Fazzi GE, De Mey JG. Twenty-four-hour exposure to altered blood flow modifies endothelial Ca2+-activated K+ channels in rat mesenteric arteries. J Pharmacol Exp Ther. 2012;333:210–217. doi: 10.1124/jpet.109.161448. [DOI] [PubMed] [Google Scholar]
- 89.Hill CE. Long distance conduction of vasodilation: a passive or regenerative process? Microcirculation. 2012;19:379–390. doi: 10.1111/j.1549-8719.2012.00169.x. [DOI] [PubMed] [Google Scholar]
- 90.Hill CE, Hickey H, Sandow SL. Role of gap junctions in acetylcholine-induced vasodilation of proximal and distal arteries of the rat mesentery. J Auton Nerv Syst. 2000;81:122–127. doi: 10.1016/s0165-1838(00)00113-2. [DOI] [PubMed] [Google Scholar]
- 91.Hill CE, Phillips JK, Sandow SL. Heterogeneous control of blood flow amongst different vascular beds. Med Res Rev. 2001;21:1–60. doi: 10.1002/1098-1128(200101)21:1<1::aid-med1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 92.Hoebel BG, Kostner GM, Graier WF. Activation of microsomal cytochrome P450 mono-oxygenase by Ca2+ store depletion and its contribution to Ca2+ entry in porcine aortic endothelial cells. Br J Pharmacol. 1997;121:1579–1588. doi: 10.1038/sj.bjp.0701304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Horinouchi T, Higashi T, Higa T, Terada K, Mai Y, Aoyagi H, Hatate C, Nepal P, Horiguchi M, Harada T, Miwa S. Different binding property of STIM1 and its novel splice variant STIM1L to Orai1, TRPC3, and TRPC6 channels. Biochem Biophys Res Commun. 2012;428:252–258. doi: 10.1016/j.bbrc.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 94.Huang A, Sun D, Jacobson A, Carroll MA, Falck JR, Kaley G. Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. Circ Res. 2005;96:376–383. doi: 10.1161/01.RES.0000155332.17783.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang AH, Busse R, Bassenge E. Endothelium-dependent hyperpolarization of smooth muscle cells in rabbit femoral arteries is not mediated by EDRF (nitric oxide) Naunyn-Schmiedebergs Arch Pharmacol. 1988;338:438–442. doi: 10.1007/BF00172124. [DOI] [PubMed] [Google Scholar]
- 96.Hutcheson IR, Chaytor AT, Evans WH, Griffith TM. Nitric oxide-independent relaxations to acetylcholine and A23187 involve different routes of heterocellular communication. Role of gap junctions and phospholipase A2. Circ Res. 1999;84:53–63. doi: 10.1161/01.res.84.1.53. [DOI] [PubMed] [Google Scholar]
- 97.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Isakson BE, Best AK, Duling BR. Incidence of protein on actin bridges between endothelium and smooth muscle in arterioles demonstrates heterogeneous connexin expression and phosphorylation. Am J Physiol. 2008;294:H2898–H2904. doi: 10.1152/ajpheart.91488.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Isakson BE, Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res. 2005;97:44–51. doi: 10.1161/01.RES.0000173461.36221.2e. [DOI] [PubMed] [Google Scholar]
- 100.Isakson BE, Duling BR. Organization of IP3-R1 and TRPC3 at the myoendothelial junction may influence polarized calcium signaling. Exp Biol Proc. 2006;786:783. Abstract. [Google Scholar]
- 101.Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res. 2007;100:246–254. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- 102.Itoh T, Seki N, Suzuki S, Ito S, Kajikuri J, Kuriyama H. Membrane hyperpolarization inhibits agonist-induced synthesis of inositol 1,4,5-trisphosphate in rabbit mesenteric artery. J Physiol. 1992;451:307–328. doi: 10.1113/jphysiol.1992.sp019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jacot JG, Wong JY. Endothelial injury induces vascular smooth muscle cell proliferation in highly localized regions of a direct contact co-culture system. Cell Biochem Biophys. 2008;52:37–46. doi: 10.1007/s12013-008-9023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jelliffe RW. Dilator and constrictor effects of acetylcholine on isolated rabbit aortic chains. J Pharmacol Exp Ther. 1962;135:349–353. [PubMed] [Google Scholar]
- 105.Johansen D, Cruciani V, Sundset R, Ytrehus K, Mikalsen SO. Ischemia induces closure of gap junctional channels and opening of hemichannels in heart-derived cells and tissue. Cell Physiol Biochem. 2011;28:103–114. doi: 10.1159/000331719. [DOI] [PubMed] [Google Scholar]
- 106.Johnstone S, Isakson B, Locke D. Biological and biophysical properties of vascular connexin channels. Int Rev Cell Mol Biol. 2009;278:69–118. doi: 10.1016/S1937-6448(09)78002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kamouchi M, Philipp S, Flockerzi V, Wissenbach U, Mamin A, Raeymaekers L, Eggermont J, Droogmans G, Nilius B. Properties of heterologously expressed hTRP3 channels in bovine pulmonary artery endothelial cells. J Physiol. 1999;518:345–358. doi: 10.1111/j.1469-7793.1999.0345p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium. 2008;44:135–146. doi: 10.1016/j.ceca.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Khan MT, Furchgott RF. Additional evidence that endothelium-derived relaxing factor is nitric oxide. In: Rand CJ, Raper C, editors. Pharmacology. Elsevier; Amsterdam: 1987. pp. 341–344. [Google Scholar]
- 110.Klee P, Allagnat F, Pontes H, Cederroth M, Charollais A, Caille D, Britan A, Haefliger JA, Meda P. Connexins protect mouse pancreatic beta cells against apoptosis. J Clin Invest. 2011;121:4870–4879. doi: 10.1172/JCI40509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ko YS, Yeh HI, Rothery S, Dupont E, Coppen SR, Severs NJ. Connexin make-up of endothelial gap junctions in the rat pulmonary artery as revealed by immunoconfocal microscopy and triple-label immunogold electron microscopy. J Histochem Cytochem. 1999;47:683–692. doi: 10.1177/002215549904700510. [DOI] [PubMed] [Google Scholar]
- 112.Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol. 2006;26:1495–1502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- 113.Kristek F, Gerova M. Myoendothelial relations in the conduit coronary artery of the dog and rabbit. J Vasc Res. 1992;29:29–32. doi: 10.1159/000158928. [DOI] [PubMed] [Google Scholar]
- 114.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lang NN, Luksha L, Newby DE, Kublickiene K. Connexin 43 mediates endothelium-derived hyperpolarizing factor-induced vasodilatation in subcutaneous resistance arteries from healthy pregnant women. Am J Physiol. 2007;292:H1026–H1032. doi: 10.1152/ajpheart.00797.2006. [DOI] [PubMed] [Google Scholar]
- 116.Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res. 2008;102:59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leung HS, Leung FP, Yao X, Ko WH, Chen ZY, Vanhoutte PM, Huang Y. Endothelial mediators of the acetylcholine-induced relaxation of the rat femoral artery. Vasc Pharmacol. 2006;44:299–308. doi: 10.1016/j.vph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 119.Li K, Yao J, Shi L, Sawada N, Chi Y, Yan Q, Matsue H, Kitamura M, Takeda M. Reciprocal regulation between proinflammatory cytokine-induced inducible NO synthase (iNOS) and connexin43 in bladder smooth muscle cells. J Biol Chem. 2011;286:41552–41562. doi: 10.1074/jbc.M111.274449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li PL, Campbell WB. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ Res. 1997;80:877–884. doi: 10.1161/01.res.80.6.877. [DOI] [PubMed] [Google Scholar]
- 121.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res. 2011;108:566–573. doi: 10.1161/CIRCRESAHA.110.237636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Looft-Wilson RC, Billaud M, Johnstone SR, Straub AC, Isakson BE. Interaction between nitric oxide signaling and gap junctions: effects on vascular function. Biochim Biophys Acta. 2012;1818:1895–1902. doi: 10.1016/j.bbamem.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res. 2008;80:445–452. doi: 10.1093/cvr/cvn207. [DOI] [PubMed] [Google Scholar]
- 124.Luksha L, Nisell H, Luksha N, Kublickas M, Hultenby K, Kublickiene K. Endothelium-derived hyperpolarizing factor in preeclampsia: heterogeneous contribution, mechanisms, and morphological prerequisites. Am J Physiol. 2008;294:R510–R519. doi: 10.1152/ajpregu.00458.2007. [DOI] [PubMed] [Google Scholar]
- 125.Luksha L, Poston L, Gustafsson JA, Hultenby K, Kublickiene K. The oestrogen receptor beta contributes to sex related differences in endothelial function of murine small arteries via EDHF. J Physiol. 2006;577:945–955. doi: 10.1113/jphysiol.2006.121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lurtz MM, Louis CF. Intracellular calcium regulation of connexin43. Am J Physiol. 2007;293:C1806–C1813. doi: 10.1152/ajpcell.00630.2006. [DOI] [PubMed] [Google Scholar]
- 127.Ma X, Cheng KT, Wong CO, O’Neil RG, Birnbaumer L, Ambudkar IS, Yao X. Heteromeric TRPV4-C1 channels contribute to store-operated Ca2+ entry in vascular endothelial cells. Cell Calcium. 2010;50:502–509. doi: 10.1016/j.ceca.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marrelli SP, O’Neil RG, Brown RC, Bryan RM., Jr PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol. 2007;292:H1390–H1397. doi: 10.1152/ajpheart.01006.2006. [DOI] [PubMed] [Google Scholar]
- 129.Matchkov VV, Rahman A, Peng H, Nilsson H, Aalkjaer C. Junctional and nonjunctional effects of heptanol and glycyrrhetinic acid derivates in rat mesenteric small arteries. Br J Pharmacol. 2004;142:961–972. doi: 10.1038/sj.bjp.0705870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 131.Matoba T, Shimokawa H, Kubota H, Morikawa K, Fujiki T, Kunihiro I, Mukai Y, Hirakawa Y, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun. 2002;290:909–913. doi: 10.1006/bbrc.2001.6278. [DOI] [PubMed] [Google Scholar]
- 132.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Matsumoto T, Kobayashi T, Kamata K. Alterations in EDHF-type relaxation and phosphodiesterase activity in mesenteric arteries from diabetic rats. Am J Physiol. 2003;285:H283–H291. doi: 10.1152/ajpheart.00954.2002. [DOI] [PubMed] [Google Scholar]
- 134.McGuire JJ, Hollenberg MD, Bennett BM, Triggle CR. Hyperpolarization of murine small caliber mesenteric arteries by activation of endothelial proteinase-activated receptor 2. Can J Physiol Pharmacol. 2004;82:1103–1112. doi: 10.1139/y04-121. [DOI] [PubMed] [Google Scholar]
- 135.McNeish AJ, Sandow SL, Neylon CB, Chen MX, Dora KA, Garland CJ. Evidence for involvement of both IKCa and SKCa channels in hyperpolarizing responses of the rat middle cerebral artery. Stroke. 2006;37:1277–1282. doi: 10.1161/01.STR.0000217307.71231.43. [DOI] [PubMed] [Google Scholar]
- 136.McSherry IN, Sandow SL, Campbell WB, Falck JR, Hill MA, Dora KA. A role for heterocellular coupling and EETs in dilation of rat cremaster arteries. Microcirculation. 2006;13:119–130. doi: 10.1080/10739680500466400. [DOI] [PubMed] [Google Scholar]
- 137.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol. 2010;298:H466–H476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miller AW, Dimitropoulou C, Han G, White RE, Busija DW, Carrier GO. Epoxyeicosatrienoic acid-induced relaxation is impaired in insulin resistance. Am J Physiol. 2001;281:H1524–H1531. doi: 10.1152/ajpheart.2001.281.4.H1524. [DOI] [PubMed] [Google Scholar]
- 139.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003;92:e31–e40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 140.Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca2+-activated K+ channels. Circulation. 2001;103:1992–1998. doi: 10.1161/01.cir.103.15.1992. [DOI] [PubMed] [Google Scholar]
- 141.Moore DH, Ruska H. The fine structure of capillaries and small arteries. J Biophys Biochem Cytol. 1957;3:457–462. doi: 10.1083/jcb.3.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mumtaz S, Burdyga G, Borisova L, Wray S, Burdyga T. The mechanism of agonist induced Ca2+ signalling in intact endothelial cells studied confocally in in situ arteries. Cell Calcium. 2011;49:66–77. doi: 10.1016/j.ceca.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ni GH, Chen JF, Chen XP, Yang TL. Soluble epoxide hydrolase: a promising therapeutic target for cardiovascular diseases. Die Pharmazie. 2011;66:153–157. [PubMed] [Google Scholar]
- 145.Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol. 2004;286:C195–205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- 146.Node K, Ruan XL, Dai J, Yang SX, Graham L, Zeldin DC, Liao JK. Activation of Galpha s mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem. 2001;276:15983–15989. doi: 10.1074/jbc.M100439200. [DOI] [PubMed] [Google Scholar]
- 147.Ohta M, Toyama K, Gutterman DD, Campbell WB, Lemaitre V, Teraoka R, Miura H. Ecto-5′-nucleotidase, CD73, is an endothelium-derived hyperpolarizing factor synthase. Arterioscler Thromb Vasc Biol. 2013;33:629–636. doi: 10.1161/ATVBAHA.112.300600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ohya S, Niwa S, Yanagi A, Fukuyo Y, Yamamura H, Imaizumi Y. Involvement of dominant-negative spliced variants of the intermediate conductance Ca2+-activated K+ channel, KCa3.1, in immune function of lymphoid cells. J Biol Chem. 2011;286:16940–16952. doi: 10.1074/jbc.M110.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- 150.Orellana JA, Sanchez HA, Schalper KA, Figueroa V, Saez JC. Regulation of intercellular calcium signaling through calcium interactions with connexin-based channels. Adv Exp Med Biol. 2012;740:777–794. doi: 10.1007/978-94-007-2888-2_34. [DOI] [PubMed] [Google Scholar]
- 151.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 152.Pani B, Bollimuntha S, Singh BB. The TR (i)P to Ca2+ signaling just got STIMy: an update on STIM1 activated TRPC channels. Front Biosci. 2012;17:805–823. doi: 10.2741/3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pannirselvam M, Ding H, Anderson TJ, Triggle CR. Pharmacological characteristics of endothelium-derived hyperpolarizing factor-mediated relaxation of small mesenteric arteries from db/db mice. Eur J Pharmacol. 2006;551:98–107. doi: 10.1016/j.ejphar.2006.08.086. [DOI] [PubMed] [Google Scholar]
- 154.Peppiatt-Wildman CM, Albert AP, Saleh SN, Large WA. Endothelin-1 activates a Ca2+-permeable cation channel with TRPC3 and TRPC7 properties in rabbit coronary artery myocytes. J Physiol. 2007;580:755–764. doi: 10.1113/jphysiol.2006.126656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pfister SL, Gauthier KM, Campbell WB. Vascular pharmacology of epoxyeicosatrienoic acids. Adv Pharmacol. 2010;60:27–59. doi: 10.1016/B978-0-12-385061-4.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Plane F, Holland M, Waldron GJ, Garland CJ, Boyle JP. Evidence that anandamide and EDHF act via different mechanisms in rat isolated mesenteric arteries. Br J Pharmacol. 1997;121:1509–1511. doi: 10.1038/sj.bjp.0701361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pomposiello SI, Quilley J, Carroll MA, Falck JR, McGiff JC. 5,6-Epoxyeicosatrienoic acid mediates the enhanced renal vasodilation to arachidonic acid in the SHR. Hypertension. 2003;42:548–554. doi: 10.1161/01.HYP.0000090095.87899.36. [DOI] [PubMed] [Google Scholar]
- 158.Ponsaerts R, Wang N, Himpens B, Leybaert L, Bultynck G. The contractile system as a negative regulator of the connexin 43 hemichannel. Biol Cell. 2012;104:367–377. doi: 10.1111/boc.201100079. [DOI] [PubMed] [Google Scholar]
- 159.Popp R, Bauersachs J, Hecker M, Fleming I, Busse R. A transferable, beta-naphthoflavone-inducible, hyperpolarizing factor is synthesized by native and cultured porcine coronary endothelial cells. J Physiol. 1996;497:699–709. doi: 10.1113/jphysiol.1996.sp021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Popp R, Brandes RP, Ott G, Busse R, Fleming I. Dynamic modulation of interendothelial gap junctional communication by 11,12-epoxyeicosatrienoic acid. Circ Res. 2002;90:800–806. doi: 10.1161/01.res.0000015328.20581.d6. [DOI] [PubMed] [Google Scholar]
- 161.Potocnik SJ, McSherry I, Ding H, Murphy TV, Kotecha N, Dora KA, Yuill KH, Triggle CR, Hill MA. Endothelium-dependent vasodilation in myogenically active mouse skeletal muscle arterioles: role of EDH and K+ channels. Microcirculation. 2009;16:377–390. doi: 10.1080/10739680902804042. [DOI] [PubMed] [Google Scholar]
- 162.Powell RJ, Cronenwett JL, Fillinger MF, Wagner RJ, Sampson LN. Endothelial cell modulation of smooth muscle cell morphology and organizational growth pattern. Ann Vasc Surg. 1996;10:4–10. doi: 10.1007/BF02002334. [DOI] [PubMed] [Google Scholar]
- 163.Qian X, Francis M, Solodushko V, Earley S, Taylor MS. Recruitment of dynamic endothelial Ca2+ signals by the TRPA1 channel activator AITC in rat cerebral arteries. Microcirculation. 2012;20:138–148. doi: 10.1111/micc.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Randall MD, Alexander SP, Bennett T, Boyd EA, Fry JR, Gardiner SM, Kemp PA, McCulloch AI, Kendall DA. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem Biophys Res Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- 165.Rhodin JA. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967;18:181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- 166.Roggendorf W, Cervos-Navarro J. Ultrastructure of arterioles in the cat brain. Cell Tissue Res. 1977;178:495–515. doi: 10.1007/BF00219571. [DOI] [PubMed] [Google Scholar]
- 167.Salameh A, Krautblatter S, Karl S, Blanke K, Gomez DR, Dhein S, Pfeiffer D, Janousek J. The signal transduction cascade regulating the expression of the gap junction protein connexin43 by beta-adrenoceptors. Br J Pharmacol. 2009;158:198–208. doi: 10.1111/j.1476-5381.2009.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sandow SL. Factors, fiction and endothelium-derived hyperpolarizing factor. Clin Exp Pharmacol Physiol. 2004;31:563–570. doi: 10.1111/j.1440-1681.2004.04048.x. [DOI] [PubMed] [Google Scholar]
- 169.Sandow SL, Goto K, Rummery NM, Hill CE. Developmental changes in myoendothelial gap junction mediated vasodilator activity in the rat saphenous artery. J Physiol. 2004;556:875–886. doi: 10.1113/jphysiol.2003.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sandow SL, Grayson TH. Limits of isolation and culture: intact vascular endothelium and BKCa. Am J Physiol. 2009;297:H1–H7. doi: 10.1152/ajpheart.00042.2009. [DOI] [PubMed] [Google Scholar]
- 171.Sandow SL, Gzik DJ, Lee RM. Arterial internal elastic lamina holes: relationship to function? J Anat. 2009;214:258–266. doi: 10.1111/j.1469-7580.2008.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 173.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (KCa) and connexins: possible relationship to vasodilator function? J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sandow SL, Senadheera S, Bertrand PP, Murphy TV, Tare M. Myoendothelial contacts, gap junctions, and microdomains: anatomical links to function? Microcirculation. 2012;19:403–415. doi: 10.1111/j.1549-8719.2011.00146.x. [DOI] [PubMed] [Google Scholar]
- 175.Sandow SL, Senadheera S, Grayson TH, Welsh DG, Murphy TV. Calcium and endothelium-mediated vasodilator signaling. Adv Exp Med Biol. 2012;740:811–831. doi: 10.1007/978-94-007-2888-2_36. [DOI] [PubMed] [Google Scholar]
- 176.Sandow SL, Tare M. C-type natriuretic peptide: a new endothelium-derived hyperpolarizing factor? Trends Pharmacol Sci. 2007;28:61–67. doi: 10.1016/j.tips.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 177.Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ Res. 2002;90:1108–1113. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- 178.Saunders KB, D’Amore PA. An in vitro model for cell-cell interactions. In Vitro Cell Dev Biol. 1992;28A:521–528. doi: 10.1007/BF02634136. [DOI] [PubMed] [Google Scholar]
- 179.Senadheera S, Kim Y, Grayson TH, Toemoe S, Kochukov MY, Abramowitz J, Housley GD, Bertrand RL, Chadha PS, Bertrand PP, Murphy TV, Tare M, Birnbaumer L, Marrelli SP, Sandow SL. Transient receptor potential canonical type 3 channels facilitate endothelium-derived hyperpolarization-mediated resistance artery vasodilator activity. Cardiovasc Res. 2012;95:439–447. doi: 10.1093/cvr/cvs208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shimokawa H. Primary endothelial dysfunction: atherosclerosis. J Mol Cell Cardiol. 1999;31:23–37. doi: 10.1006/jmcc.1998.0841. [DOI] [PubMed] [Google Scholar]
- 181.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 182.Sisignano M, Park CK, Angioni C, Zhang DD, von Hehn C, Cobos EJ, Ghasemlou N, Xu ZZ, Kumaran V, Lu R, Grant A, Fischer MJ, Schmidtko A, Reeh P, Ji RR, Woolf CJ, Geisslinger G, Scholich K, Brenneis C. 5,6-EET is released upon neuronal activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals. J Neurosci. 2012;32:6364–6372. doi: 10.1523/JNEUROSCI.5793-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Socha MJ, Behringer EJ, Segal SS. Calcium and electrical signalling along endothelium of the resistance vasculature. Basic Clin Pharmacol Toxicol. 2012;110:80–86. doi: 10.1111/j.1742-7843.2011.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sosinsky GE, Nicholson BJ. Structural organization of gap junction channels. Biochim Biophys Acta. 2005;1711:99–125. doi: 10.1016/j.bbamem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 188.Spagnoli LG, Villaschi S, Neri L, Palmieri G. Gap junctions in myo-endothelial bridges of rabbit carotid arteries. Experientia. 1982;38:124–125. doi: 10.1007/BF01944566. [DOI] [PubMed] [Google Scholar]
- 189.Straub AC, Billaud M, Johnstone SR, Best AK, Yemen S, Dwyer ST, Looft-Wilson R, Lysiak JJ, Gaston B, Palmer L, Isakson BE. Compartmentalized connexin 43 s-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arterioscler Thromb Vasc Biol. 2011;31:399–407. doi: 10.1161/ATVBAHA.110.215939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Straub AC, Johnstone SR, Heberlein KR, Rizzo MJ, Best AK, Boitano S, Isakson BE. Site-specific connexin phosphorylation is associated with reduced heterocellular communication between smooth muscle and endothelium. J Vasc Res. 2010;47:277–286. doi: 10.1159/000265562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Suzuki H. The electrogenic Na-K pump does not contribute to endothelium-dependent hyperpolarization in the rabbit ear artery. Eur J Pharmacol. 1988;156:295–297. doi: 10.1016/0014-2999(88)90337-8. [DOI] [PubMed] [Google Scholar]
- 192.Tare M, Coleman HA, Parkington HC. Glycyrrhetinic derivatives inhibit hyperpolarization in endothelial cells of guinea pig and rat arteries. Am J Physiol. 2002;282:H335–H341. doi: 10.1152/ajpheart.2002.282.1.H335. [DOI] [PubMed] [Google Scholar]
- 193.Taugner R, Kirchheim H, Forssmann WG. Myoendothelial contacts in glomerular arterioles and in renal interlobular arteries of rat, mouse and Tupaia belangeri. Cell Tissue Res. 1984;235:319–325. doi: 10.1007/BF00217856. [DOI] [PubMed] [Google Scholar]
- 194.Taylor HJ, Chaytor AT, Edwards DH, Griffith TM. Gap junction-dependent increases in smooth muscle cAMP underpin the EDHF phenomenon in rabbit arteries. Biochem Biophys Res Commun. 2001;283:583–589. doi: 10.1006/bbrc.2001.4791. [DOI] [PubMed] [Google Scholar]
- 195.TenBroek EM, Lampe PD, Solan JL, Reynhout JK, Johnson RG. Ser364 of connexin43 and the upregulation of gap junction assembly by cAMP. J Cell Biol. 2001;155:1307–1318. doi: 10.1083/jcb.200102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Triggle CR, Samuel SM, Ravishankar S, Marei I, Arunachalam G, Ding H. The endothelium: influencing vascular smooth muscle in many ways. Can J Physiol Pharmacol. 2012;90:713–738. doi: 10.1139/y2012-073. [DOI] [PubMed] [Google Scholar]
- 197.Tuteja D, Rafizadeh S, Timofeyev V, Wang S, Zhang Z, Li N, Mateo RK, Singapuri A, Young JN, Knowlton AA, Chiamvimonvat N. Cardiac small conductance Ca2+−activated K+ channel subunits form heteromultimers via the coiled-coil domains in the C termini of the channels. Circ Res. 2010;107:851–859. doi: 10.1161/CIRCRESAHA.109.215269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tyml K. Role of connexins in microvascular dysfunction during inflammation. Can J Physiol Pharmacol. 2011;89:1–12. doi: 10.1139/y10-099. [DOI] [PubMed] [Google Scholar]
- 199.Udosen IT, Jiang H, Hercule HC, Oyekan AO. Nitric oxide-epoxygenase interactions and arachidonate-induced dilation of rat renal microvessels. Am J Physiol. 2003;285:H2054–H2063. doi: 10.1152/ajpheart.00075.2003. [DOI] [PubMed] [Google Scholar]
- 200.Ueda A, Ohyanagi M, Koida S, Iwasaki T. Enhanced release of endothelium-derived hyperpolarizing factor in small coronary arteries from rats with congestive heart failure. Clin Exp Pharmacol Physiol. 2005;32:615–621. doi: 10.1111/j.0305-1870.2005.04240.x. [DOI] [PubMed] [Google Scholar]
- 201.Vorderwulbecke BJ, Maroski J, Fiedorowicz K, Da Silva-Azevedo L, Marki A, Pries AR, Zakrzewicz A. Regulation of endothelial connexin40 expression by shear stress. Am J Physiol. 2012;302:H143–152. doi: 10.1152/ajpheart.00634.2011. [DOI] [PubMed] [Google Scholar]
- 202.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2+ permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97:908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 203.Vriens J, Owsianik G, Janssens A, Voets T, Nilius B. Determinants of 4 alpha-phorbol sensitivity in transmembrane domains 3 and 4 of the cation channel TRPV4. J Biol Chem. 2007;282:12796–12803. doi: 10.1074/jbc.M610485200. [DOI] [PubMed] [Google Scholar]
- 204.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wagner LE, Joseph SK, Yule DI. Regulation of single inositol 1,4,5-trisphosphate receptor channel activity by protein kinase A phosphorylation. J Physiol. 2008;586:3577–3596. doi: 10.1113/jphysiol.2008.152314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wallace CS, Champion JC, Truskey GA. Adhesion and function of human endothelial cells co-cultured on smooth muscle cells. Ann Biomed Eng. 2007;35:375–386. doi: 10.1007/s10439-006-9230-5. [DOI] [PubMed] [Google Scholar]
- 207.Warner A, Clements DK, Parikh S, Evans WH, DeHaan RL. Specific motifs in the external loops of connexin proteins can determine gap junction formation between chick heart myocytes. J Physiol. 1995;488:721–728. doi: 10.1113/jphysiol.1995.sp021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Waschk DE, Fabian A, Budde T, Schwab A. Dual-color quantum dot detection of a heterotetrameric potassium channel (hKCa3.1) Am J Physiol. 2011;300:C843–C849. doi: 10.1152/ajpcell.00053.2010. [DOI] [PubMed] [Google Scholar]
- 209.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 210.Westcott EB, Segal SS. Perivascular innervation: a multiplicity of roles in vasomotor control and myoendothelial signaling. Microcirculation. 2013;20:217–238. doi: 10.1111/micc.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Weston AH, Feletou M, Vanhoutte PM, Falck JR, Campbell WB, Edwards G. Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: involvement of potassium channel activation and epoxyeicosatrienoic acids. Br J Pharmacol. 2005;145:775–784. doi: 10.1038/sj.bjp.0706256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.White TW, Paul DL. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu Rev Physiol. 1999;61:283–310. doi: 10.1146/annurev.physiol.61.1.283. [DOI] [PubMed] [Google Scholar]
- 213.Wolfle SE, Schmidt VJ, Hoepfl B, Gebert A, Alcolea S, Gros D, de Wit C. Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circ Res. 2007;101:1292–1299. doi: 10.1161/CIRCRESAHA.107.163279. [DOI] [PubMed] [Google Scholar]
- 214.Wong PY, Lin KT, Yan YT, Ahern D, Iles J, Shen SY, Bhatt RK, Falck JR. 14(R),15(S)-epoxyeicosatrienoic acid (14(R),15(S)-EET) receptor in guinea pig mononuclear cell membranes. J Lipid Med. 1993;6:199–208. [PubMed] [Google Scholar]
- 215.Xie A, Aihara Y, Bouryi VA, Nikitina E, Jahromi BS, Zhang ZD, Takahashi M, Macdonald RL. Novel mechanism of endothelin-1-induced vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2007;27:1692–1701. doi: 10.1038/sj.jcbfm.9600471. [DOI] [PubMed] [Google Scholar]
- 216.Yi FX, Boeldt DS, Gifford SM, Sullivan JA, Grummer MA, Magness RR, Bird IM. Pregnancy enhances sustained Ca2+ bursts and endothelial nitric oxide synthase activation in ovine uterine artery endothelial cells through increased connexin 43 function. Biol Reprod. 2010;82:66–75. doi: 10.1095/biolreprod.109.078253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yin J, Kuebler WM. Mechanotransduction by TRP channels: general concepts and specific role in the vasculature. Cell Biochem Biophys. 2010;56:1–18. doi: 10.1007/s12013-009-9067-2. [DOI] [PubMed] [Google Scholar]
- 218.Zhang DX, Gutterman DD. Transient receptor potential channel activation and endothelium-dependent dilation in the systemic circulation. J Cardiovasc Pharmacol. 2011;57:133–139. doi: 10.1097/FJC.0b013e3181fd35d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension. 2009;53:532–538. doi: 10.1161/HYPERTENSIONAHA.108.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zheng X, Zinkevich NS, Gebremedhin D, Gauthier KM, Nishijima Y, Fang J, Wilcox DA, Campbell WB, Gutterman DD, Zhang DX. Arachidonic acid-induced dilation in human coronary arterioles: convergence of signaling mechanisms on endothelial TRPV4-mediated Ca2+ entry. J Am Heart Assoc. 2013;2:e000080. doi: 10.1161/JAHA.113.000080. [DOI] [PMC free article] [PubMed] [Google Scholar]