Abstract
Background
Cardiovascular disease (CVD) imparts a heavy economic burden on our health care system. Evidence regarding the long-term costs following comprehensive CVD screening is limited.
Objective
We calculated 10-year health care costs for 6,814 asymptomatic participants enrolled in the National Institutes of Health–National Heart, Lung, and Blood Institute–sponsored Multi-Ethnic Study of Atherosclerosis (MESA).
Methods
Cumulative 10-year costs for CVD medications, office visits, diagnostic procedures, coronary revascularization, and hospitalizations were calculated from detailed follow-up data. Costs were derived using Medicare nationwide and zip code–specific costs, inflation-corrected, discounted at 3%/year, and presented in 2014 U.S. dollars.
Results
Risk factor prevalence increased dramatically and, by 10 years, diabetes, hypertension, and dyslipidemia was reported in 19%, 57%, and 53%, respectively. Self-reported symptoms (i.e., chest pain or shortness of breath) were common (~40% of enrollees). At 10-years, approximately one-third of enrollees reported having an echocardiogram or exercise test while 7% underwent invasive coronary angiography. These utilization patterns resulted in 10-year health care costs of $23,142. The largest proportion of costs was associated with CVD medication use (78%). Approximately $2 of every $10 was spent for outpatient visits and diagnostic testing among the elderly, obese, those with a high-sensitivity C-reactive protein >3 mg/l or coronary artery calcium score (CACS) ≥400. Costs varied widely from <$7,700 for low-risk (Framingham Risk Score [FRS] <6%, 0 CACS, and normal glucose measurements at baseline) to >$35,800 for high-risk (persons with diabetes, FRS ≥20%, or CACS ≥400) subgroups. Among high-risk enrollees, CVD costs accounted for $74 of the $155 million consumed by MESA participants.
Conclusions
Longitudinal patterns of health care resource use following screening reveal new evidence on the economic burden of treatment and testing patterns not previously reported. Maintenance of a healthy population has the potential to markedly reduce the economic burden of CVD among asymptomatic individuals.
Keywords: asymptomatic, cardiovascular disease screening, economics, long-term follow-up
Introduction
In the United States, nearly 1 in 3 (~80 million) adults have some form of cardiovascular disease (CVD), which imparts a heavy economic burden, including estimated direct costs of approximately $444 billion (1–4). The CVD costs of care are continuing to rise, with the current costs for treatment accounting for nearly $1 of every $6 spent on health care (2). The evidence to date on the economic implications of CVD screening is less well developed. Screening for CVD has the potential to improve clinical outcomes through effective detection of CVD risk and to intensify preventive efforts among asymptomatic individuals. Other forms of screening, such as for lung, breast, and colon cancer, form the basis for preventive health, with robust economic evidence and documentation of the expense of downstream disease states (5). Heretofore, the evidence base on the impact of CVD testing on long-term health care expenditures among asymptomatic, apparently healthy individuals has been unknown. The aim of this analysis was to estimate 10-year CVD costs based on detailed resource consumption patterns collected within the National Institutes of Health (NIH)–National Heart, Lung, and Blood Institute (NHLBI)–sponsored Multi-Ethnic Study of Atherosclerosis (MESA) (6,7).
Methods
MESA Enrollment Criteria
Enrollment criteria and CVD testing procedures for the MESA have been previously reported in detail (6,7). In brief, a total of 6,814 asymptomatic, apparently healthy individuals (age range: 44 to 84 years) were enrolled. This substudy was approved by the MESA policy and publications committee and undertaken with a data use agreement between Emory University and the University of Washington.
Collection of Baseline Traditional Risk Factors
During the baseline visit, participants were queried as to the history of cardiac risk factors. Self-reported history of diabetes, hyperlipidemia, and hypertension, a family history of CVD, and smoking history was recorded. At this time, lipids, glucose, and blood pressure were measured; details of the methodology have been published (6,8). A Framingham Risk Score (FRS) was calculated on each enrollee, including categories of 10-year predicted risk of <6%, 6% to 9.9%, 10% to 19.9%, and ≥20% (9). Use of a total of 46 drugs classes, such as sulfonylurea, beta-blocker, and calcium-channel blocker therapies, were collected in MESA.
Nontraditional CVD Testing
At the baseline visit, MESA enrollees had measurement of high-sensitivity C-reactive protein (hsCRP) using standardized methodology (10). High- and low-risk hsCRP was defined as >3 and ≤3 mg/dl, respectively (11). At the baseline visit, all participants underwent coronary artery calcium scoring (CACS). The protocol and methods for CACS were performed using standardized methodologies (6,7,10). For this analysis, CACS was categorized as 0, 1 to 10, 11 to 99, 100 to 399, 400 to 999, and ≥1,000, respectively.
Data Collection of Socioeconomic Factors
During the baseline visit, detailed socioeconomic status data was collected including: marital status; employment status; highest level of education achieved; household income; and health insurance coverage. These variables were candidates for covariate adjustment in the cost models, as they are established determinants of health care utilization.
Follow-Up Methodologies
MESA collected detailed follow-up hospitalization, medication usage, and varied patterns of resource consumption throughout follow-up. In total, MESA participants returned for a total of 4 additional clinic examinations every 2 years. During each follow-up visit, a detailed history of intercurrent office visits, CVD diagnostic (e.g., exercise stress testing) and invasive (diagnostic coronary angiography and coronary revascularization) procedures, CVD hospitalizations, as well as current and revised prescribed CVD medications was ascertained. Medicare nationwide mean payments for diagnostic procedures, coronary revascularization, and hospitalizations are provided in Online Appendix 1. CVD medications included medications for hypertension, diabetes, and dyslipidemia such as diuretic agents, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, insulin, and statins. Anti-ischemic and heart failure (HF) medication usage was also collected (e.g., nitrates, ACE inhibitors). In the years when an in-person clinic examination was not scheduled, participants completed follow-up information through a detailed telephone interview. Similar to the clinic visit, data on medication, procedures, and hospitalizations was collected annually. Specific questions on changes in drug therapy and intensification or reduction in dosing were collected annually. During each follow-up contact, an enrollee was queried as to whether a new diagnosis of hypertension, diabetes, or dyslipidemia was assigned by their overseeing health care provider. In year 1 of follow-up, participants were queried as to whether they had discussed their MESA test results with their primary care physician. MESA investigators did not provide treatment or procedural guidance based on test findings.
Data on CVD hospitalization was collected similar to the procedural and visit data. Angina or HF hospitalization, coronary revascularization, acute myocardial infarction (MI), or stroke underwent a detailed event adjudication by the MESA morbidity and mortality committee, as detailed in an online manual (12). The timing for MI, HF, angina, coronary revascularization, and resuscitated cardiac arrest was collected. During follow-up, all enrollees were queried as to new-onset chest pain during the preceding year of follow-up and adjudicated angina was defined as an episode of ischemic pain, tightness, pressure, or discomfort in the chest, arm, or jaw, when co-occurring with a prescribed anti-ischemic therapy, documented angiographic obstructive coronary artery disease, an ischemic electrocardiogram, or imaging abnormality. Each enrollee was also queried with regard to the presence of shortness of breath symptoms. The criteria for adjudicated, symptomatic HF was documented HF symptoms and receiving treatment (with diuretic agents, vasodilator agents, beta-blockers, or ACE inhibitors) or imaging evidence of a dilated left ventricular or reduced left ventricular ejection fraction.
CVD Health Care Costs
The Centers for Medicare and Medicaid Services pharmacy charges (zip code–specific for each site) and nationwide reimbursement rates for office visits, CVD procedures, and hospitalizations were collected. Common prescribing doses were examined and aggregated by drug class (13). Based on the common prescribing doses, median drug pricing was derived using the site- or zip code–specific, online drug pricing. Online Appendix 2 provides a range of costs for CVD medications across the participating sites. Generic pricing for antihypertensive (e.g., diuretic agents, ACE inhibitors, beta-blockers), cholesterol-lowering (e.g., statins), and diabetes (e.g., metformin) drugs were collected (Online Appendix 3). All fees were derived by the year of reported use during the 10 years of follow-up. Costs for outpatient services were derived from the Outpatient Prospective Payment amounts based on Healthcare Common Procedure Codes using the Outpatient PC Pricer system for nationwide mean fees (14). A similar approach was applied to define in-hospital procedures (PC Pricer Prospective Payment System estimator) (15). Payment codes included: diagnosis related groups 66 to 69, 222 to 223, 232, 236 to 238, 280 to 285, 293, 311, 313; ambulatory payment classifications 96, 99, 336, 337, 602; and current procedural terminology 80053, 80061, 83519, 85380, 85384, 86140, 93015, 93350, 93454 to 93459. An MI hospitalization that occurred within 1 week of a percutaneous coronary intervention or coronary artery bypass surgery was assigned the relevant Medicare payment. Hospital costs varied and were higher when associated with complications. We applied mean costs, for example, for acute MI. When a range of hospital costs for a given admission were applied, our results did not vary. All costs were inflation-adjusted using the medical care component of the consumer price index (16). The unit cost is given in 2014 U.S. dollars. Costs were discounted at 3%/year to reflect the lower economic value of deferred expenses. When a cost was assigned to a given year of follow-up, discounting was based on the remaining years of follow-up through 10 years. As enrollment occurred from 2000 to 2002, patient-specific costs varied by the year of follow-up (e.g., a year 1 cost may be from 2001 to 2003 for a given patient). Costs for medications, outpatient visits, procedures, coronary revascularization, and hospitalizations were summed for each MESA enrollee. Nonsurviving enrollees had costs accumulated until their deaths.
Statistical Analysis
All analyses were performed on MESA-approved and locked SAS files. Descriptive statistics (mean ± 95% confidence interval [CI] or percentage for continuous and categorical variables) were recorded. Chi-square and Student t-tests were used to compare MESA subgroups. We plotted the cumulative (unadjusted) costs through 10 years of follow-up aggregated by costs for CVD medications, outpatient visits, diagnostic procedures, coronary revascularization, and hospitalization. Unadjusted costs for CVD medications, outpatient visits, diagnostic procedures, coronary revascularization, and hospitalizations were plotted for women and men by age, FRS, CACS, and hsCRP subgroups, respectively.
A multivariate linear regression model was calculated to identify predictors of elevated 10-year health care costs. We log-transformed the 10-year cost to normalize the distribution of the values. The primary analysis was undertaken using the log-transformed cost variable for evaluation of statistical significance. An additional linear regression model using (nontransformed) cost as the dependent measure was used to derive adjusted cost values from the unstandardized beta coefficients. Socioeconomic covariates in the model were education, income, and health insurance coverage. From the linear regression model, the model r2 was calculated. Predicted costs were then compared across MESA subgroups (i.e., by CACS, FRS, age, BMI, hsCRP, hypertension, and diabetes). We then defined: a low-risk subgroup as those with a low-risk FRS, CACS = 0, and normal glucose values (n = 1,182); a high-risk subgroup as those with a high-risk FRS, CACS ≥400, or diabetes (n = 2,520); and a very high-risk subgroup with a high-risk FRS, CACS ≥400, and diabetes (n = 126). Among these subgroups, we plotted cumulative costs and 95% CIs for women and men. We then calculated the proportion of total costs for CVD medications, diagnostic procedures, outpatient visits, coronary revascularization, and hospitalizations. In a secondary analysis, we applied the lowest-cost, generic pricing for hypertension, dyslipidemia, and diabetic medication costs (Online Appendix 3). Cost savings were calculated as the difference between brand name and generic pricing.
Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, North Carolina) and IBM SPSS version 24.0 (IBM, Armonk, New York).
Results
Socioeconomic Characteristics of the MESA (Table 1)
Table 1.
Socioeconomic Characteristics That Influence Health Care Utilization
| N = 6,814 | |
|---|---|
|
| |
| Age deciles (yrs) | |
| 45–54 | 28.6 |
| 55–64 | 27.7 |
| 65–74 | 29.6 |
| 75–84 | 14.2 |
|
| |
| Sex | |
| Women | 52.8 |
| Men | 47.2 |
|
| |
| Race | |
| African American | 27.8 |
| Chinese | 11.8 |
| Hispanic | 22.0 |
| Caucasian | 38.5 |
| Marital status | |
| Married | 60.6 |
| Widowed | 13.1 |
| Divorced | 13.6 |
| Separated | 3.7 |
| Not married | 8.2 |
|
| |
| Education | |
| <9th grade | 11.0 |
| Grades 9–11 | 7.0 |
| High school | 18.2 |
| Some college | 28.5 |
| Bachelor’s degree | 17.3 |
| Graduate school | 18.0 |
|
| |
| Employment | |
| Homemaker | 11.5 |
| Full-time | 38.4 |
| Part-time | 8.6 |
| Unemployed | 2.3 |
| Retired | 38.1 |
|
| |
| Family income† | |
| <$20,000 | 23.9 |
| $20,000–$29,900 | 13.6 |
| $30,000–$39,900 | 13.1 |
| $40,000–$49,900 | 9.8 |
| $50,000–$74,900 | 17.0 |
| $75,000–$99,900 | 9.1 |
| ≥$100,000 | 13.5 |
|
| |
| Health insurance | |
| Private | 69.3 |
| Medicare | 35.9 |
| Medicaid | 7.0 |
| None | 9.0 |
|
| |
| Regular source of medical care | |
| Doctor’s office or clinic | 92.8 |
| Hospital or emergency department | 3.1 |
Values are %.
Variables include a nonresponse category and do not sum to 100%. In some cases, multiple responses are included, such as for health insurance. Due to rounding, not all categories sum to 100%.
Income ranges were based on 2000-2002 statements based on the year of enrollment.
Among the 6,814 MESA enrollees, the median age was 62 years, 53% were women, with 10 years of follow-up data reported. Enrollment included diverse representation of African-American (28%), Chinese (12%), and Hispanic (22%) individuals. Only 35% of MESA participants completed a college education with a bachelor’s degree or higher, and only 38% were employed full-time. Approximately one-half of MESA enrollees had a family income <$40,000/year. Of the MESA participants, 69% had private health insurance and 93% reported a doctor’s office or clinic as their regular source of medical care.
Risk Factor Diagnosis and Treatment on the Baseline Visit (Table 2)
Table 2.
Baseline FRS, Traditional and Nontraditional Risk Factor Measurements, Preventive Medication Usage, and Follow-Up Physician Discussions Regarding MESA Findings
| Self-reported | |
| Dyslipidemia | 37.3 |
| Hypertension | 39.3 |
| Diabetes | 11.3 |
|
| |
| BMI (kg/m2) | 28 (25–31) |
|
| |
| Examination diabetes categories | |
| Treated diabetes | 10.0 |
| Untreated diabetes | 2.6 |
| Impaired fasting glucose | 13.8 |
|
| |
| FRS – 10-yr predicted risk | |
| <6% | 24.1 |
| 6–9.9% | 17.4 |
| 10–19.9% | 28.6 |
| ≥20% | 29.9 |
|
| |
| Preventive medication use | |
| Aspirin (regular use ≥3 times/week) | 23.9 |
| Hypertension | 33.3 |
| Lipid-lowering | 16.2 |
| Diabetes | 9.8 |
| None | 43.4 |
|
| |
| hsCRP | |
| ≤3 mg/l | 63.9 |
| >3 mg/l | 36.1 |
|
| |
| CACS | |
| 0 | 50.1 |
| 1–10 | 7.5 |
| 11–99 | 18.9 |
| 100–399 | 13.6 |
| 400–999 | 6.2 |
| ≥1,000 | 3.8 |
|
| |
| Discussion of MESA findings with primary care physician | 54.8 |
Values are % or median (interquartile range).
BMI = body mass index; CACS = coronary artery calcium score; FRS = Framingham Risk Score; hsCRP = high-sensitivity C-reactive protein; MESA = Multi-Ethnic Study of Atherosclerosis
At the baseline examination, 39%, 37%, and 11% of enrollees, respectively, reported a history of hypertension, dyslipidemia, and diabetes. An abnormal fasting glucose or diabetes was reported in 26% of MESA enrollees. Similarly, 45% of enrollees met criteria for hypertension on the baseline examination. Approximately 60% of MESA participants were on a CVD-preventive medication for hypertension, dyslipidemia, or diabetes at the baseline visit. Of the MESA enrollees, 55% reported that they discussed findings from their baseline examination with their primary care physician. Nearly 60% of enrollees had an intermediate-high FRS. A high-risk hsCRP >3 mg/l occurred in 36% of participants, and 10% had a CACS ≥400.
10-Year Cumulative Diagnoses of Diabetes, Hypertension, and Dyslipidemia (Table 3)
Table 3.
Cumulative 10-Year Proportion of Incident Diabetes, Hypertension, and Dyslipidemia Among MESA Participants
| Yrs of Follow-Up | Diabetes | Hypertension | Dyslipidemia |
|---|---|---|---|
| Index | 10.0 | 44.9 | 37.3 |
| 1 | 13.2 | 46.5 | 39.7 |
| 2 | 13.8 | 47.6 | 42.5 |
| 3 | 15.3 | 49.2 | 43.9 |
| 4 | 15.2 | 50.7 | 45.7 |
| 5 | 16.0 | 52.0 | 47.5 |
| 6 | 16.6 | 53.0 | 48.6 |
| 7 | 17.4 | 54.2 | 50.0 |
| 8 | 18.0 | 55.2 | 51.0 |
| 9 | 18.9 | 56.1 | 52.1 |
| 10 | 19.3 | 57.0 | 52.8 |
Values are %.
MESA = Multi-Ethnic Study of Atherosclerosis
At the index examination, 10%, 45%, and 37%, respectively, of MESA participants had diabetes, hypertension, and dyslipidemia. During follow-up, there was a graded increase in the cumulative incidence of diabetes, hypertension, and dyslipidemia. Cumulative 10-year rates of diabetes, hypertension, and dyslipidemia were 19%, 57%, and 53%, respectively.
10-Year Procedural Utilization Data (Table 4)
Table 4.
Cumulative 10-Year Proportion of Follow-Up for Diagnostic Testing Among MESA Participants
| Yrs of Follow-Up | Echocardiogram | Exercise Test | Invasive Angiography | No Follow-Up Testing |
|---|---|---|---|---|
| 1 | 5.9 | 5.4 | 0.9 | 88.2 |
| 2 | 9.3 | 7.6 | 1.5 | 81.2 |
| 3 | 13.6 | 12.1 | 2.2 | 70.6 |
| 4 | 16.5 | 15.2 | 3.0 | 65.1 |
| 5 | 20.6 | 19.3 | 3.8 | 56.2 |
| 6 | 23.6 | 22.3 | 4.5 | 51.1 |
| 7 | 27.4 | 25.6 | 5.2 | 44.3 |
| 8 | 30.8 | 28.1 | 5.7 | 40.3 |
| 9 | 33.5 | 30.5 | 6.4 | 35.8 |
| 10 | 35.6 | 32.1 | 6.9 | 29.5 |
Values are %.
MESA = Multi-Ethnic Study of Atherosclerosis
By 10 years of follow-up, approximately one-third of MESA enrollees underwent an echocardiogram or exercise test. At the end of follow-up, 70% of MESA participant had one or more noninvasive diagnostic procedures. The cumulative 10-year rate of invasive angiography was 6.9%.
10-Year Self-Reported Chest Pain or Dyspnea Symptoms (Table 5)
Table 5.
Annual Proportion of Self-Reported Chest Pain and Shortness of Breath
| Yrs of Follow-Up | Chest Pain | Shortness of Breath |
|---|---|---|
| Index | 0.0 | 0.0 |
| 1 | 9.8 | 12.9 |
| 2 | 10.7 | 14.3 |
| 3 | 10.6 | 14.6 |
| 4 | 10.3 | 15.1 |
| 5 | 11.2 | 14.9 |
| 6 | 9.6 | 13.3 |
| 7 | 9.8 | 13.3 |
| 8 | 8.8 | 14.8 |
| 9 | 10.5 | 16.3 |
| 10 | 8.9% | 15.1% |
| Cumulative rate | 38.7 | 43.3% |
| Adjudicated rate | 9.6 with angina | 6.9 with heart failure |
Values are %. Across all Multi-Ethnic Study of Atherosclerosis participants, the cumulative 10-year rate of adjudicated angina was 3.7% and for heart failure was 2.9%, but was 9.6% and 6.9% for those with self-reported chest pain and shortness of breath.
The cumulative rate of self-reported chest pain at 10 years was 39%, whereas the 10-year rate of adjudicated angina was 9.6%. Of note, 79% and 39% of MESA enrollees with and without angina, respectively, underwent exercise testing (p < 0.0001). Similarly, 55% and 4% of MESA enrollees with and without angina underwent invasive coronary angiography during 10 years of follow-up (p < 0.0001). The odds of invasive angiography were 8-fold higher (95% CI: 5.8 to 11.4) among patients experiencing an acute MI or resuscitated cardiac arrest during follow-up (p < 0.0001). Nearly one-third of individuals undergoing invasive coronary angiography also had documented coronary revascularization (including 54 coronary bypass surgeries and 79 percutaneous coronary interventions).
The cumulative rate of self-reported shortness of breath at 10 years was 43%, whereas the 10-year rate of adjudicated HF was 6.9%. For those with and without HF symptoms, 77% and 35% of enrollees, respectively, reported having an echocardiogram performed during follow-up (p < 0.0001).
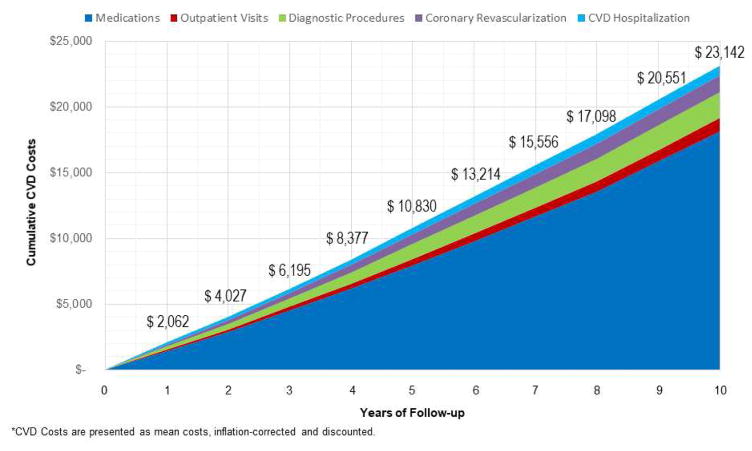
10-Year CVD Costs (Figure 1)
Figure 1. Cumulative Mean Per-Patient CVD Costs for Medications, Diagnostic Procedures, Outpatient Visits, Coronary Revascularization, and Hospitalization among 6,814 MESA Participants.
At each year of follow-up, the mean per-patient cost values for medications, visits, diagnostic procedures, coronary revascularization, and hospitalization are reported. The mean value at each year of follow-up is reported above the cumulative total costs. All cost values are rounded to the nearest whole number. CVD = cardiovascular disease; MESA = Multi-Ethnic Study of Atherosclerosis.
All costs presented in Figures 1 and 2 are unadjusted. The mean cumulative 10-year health care costs were $23,142 and summed to >$155 million for all enrollees. Cumulative health care costs ranged from $0 to $274,582; with 10th, 25th, 50th, 75th, and 90th percentile costs, respectively, of $592, $2,026, $13,349, $33,026, and $56,824. Over time, CVD medication costs represented a growing proportion of cumulative costs. At 10 years of follow-up, 78% of cumulative costs were associated with CVD medication usage. When generic pricing was applied to treatment for diabetes, hypertension, and dyslipidemia, total costs for CVD medications were reduced by ~$45 million, representing a 36.3% cost savings. By applying generic pricing, the proportional costs for CVD medications would be reduced to 71% of the total CVD health care costs. The proportion of cumulative costs were 4.3%, 8.6%, 5.4%, and 3.3%, respectively, for outpatient visits, diagnostic procedures, coronary revascularization, and CVD hospitalizations.
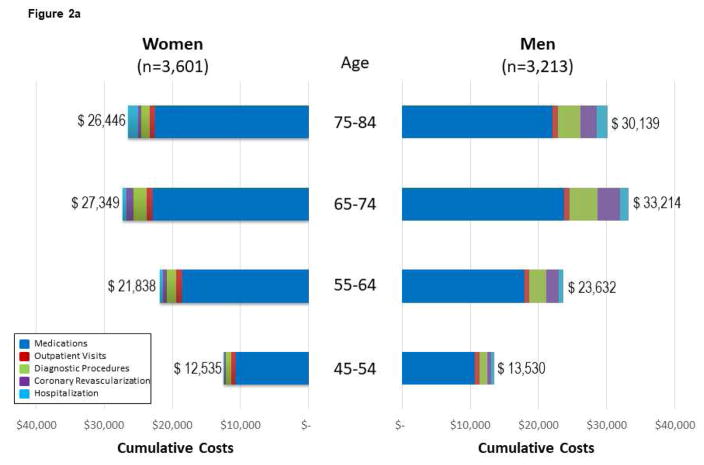
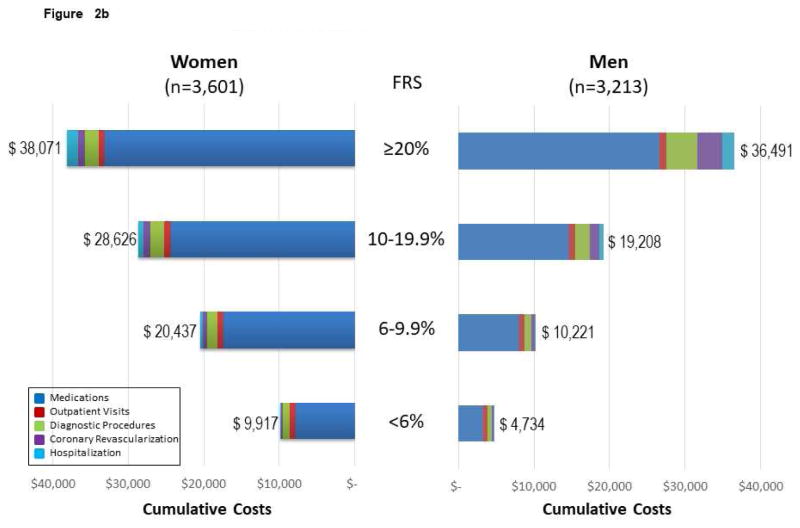
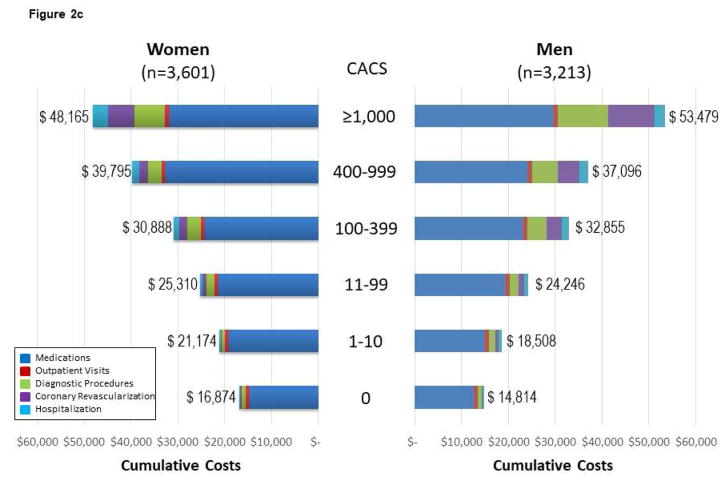
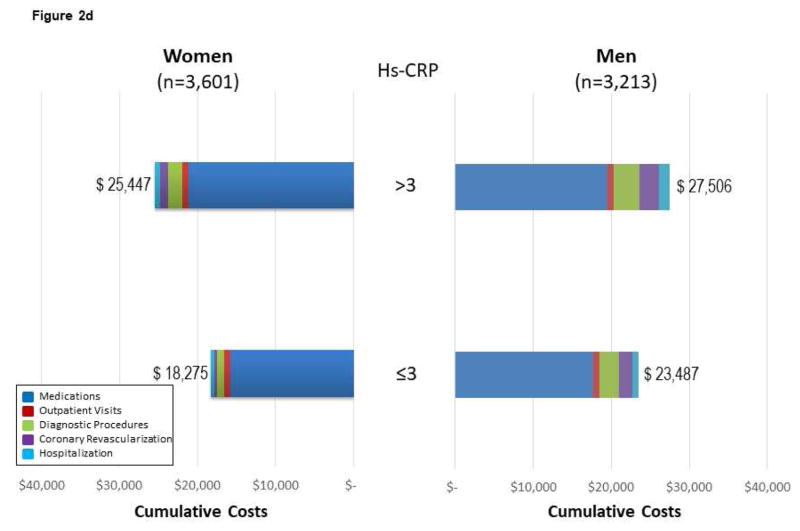
Figure 2. Follow-up CVD Costs* for Medications, Diagnostic Procedures, Outpatient Visits, Coronary Revascularization, and Hospitalization Among MESA Subgroups.
(A) Follow-up CVD costs for medications, diagnostic procedures, outpatient visits, coronary revascularization, and hospitalization among 6,814 MESA participants across age and sex subgroups. Costs are reported by sex, as data support variable cost patterns among women and men. For Figure 2, the cumulative costs by subgroups of medications, visits, diagnostic procedures, coronary revascularization, and hospitalization are reported. The cumulative costs across the age ranges support higher cost/resource consumption patterns. (B) Cumulative follow-up CVD costs* for medications, diagnostic procedures, outpatient visits, coronary revascularization, and hospitalization among 6,814 mesa participants across Framingham Risk Score (FRS) subgroups of women and men. The cumulative costs across the FRS subgroups support higher cost/resource consumption patterns among higher-risk individuals. The presented costs are unadjusted consumption patterns among older individuals. The presented costs are unadjusted. (C) Follow-up CVD costs* for medications, diagnostic procedures, outpatient visits, coronary revascularization, and hospitalization among 6,814 MESA participants across CACS subgroups of women and men. The cumulative costs across the CACS subgroups support higher cost/resource consumption patterns among individuals with more extensive CAC. Please note that this x-axis extends through $60,000 versus $40,000 for the age, FRS, and hsCRP analyses. The presented costs are unadjusted. (D) Follow-up CVD costs* for medications, diagnostic procedures, outpatient visits, coronary revascularization, and hospitalization among 6,814 MESA participants across hsCRP subgroups of women and men. The cumulative costs among low- and high-risk hsCRP subgroups report slightly higher cost/resource consumption patterns within these higher risk individuals. Noteworthy is the higher costs associated with medications for those individuals with high-risk hsCRP. The presented costs are unadjusted. CACS = coronary artery calcium score; FRS = Framingham Risk Score; hsCRP = high-sensitivity C-reactive protein. Other abbreviations as in Figure 1.
Costs varied widely and increased with advancing age for women and men (Figure 2A). MESA participants <65 years of age had much lower health care costs. Similarly, costs increased from low- to high-risk FRS subgroups (Figure 2B). Across the FRS subgroups, women had higher cumulative costs. Women were older across FRS subgroups by 6, 9, 7, and 2 years for low, average, intermediate, and high FRS enrollees, respectively (p < 0.0001).
A similar pattern of health care costs was noted across low- to high-risk CACS (Figure 2C), and hsCRP (Figure 2D) subgroups. Among those with a high-risk hsCRP, new diagnoses for dyslipidemia, hypertension, and diabetes contributed to higher CVD medication costs. For those with a high-risk hsCRP, the rate of diagnosis for hypertension, diabetes, and dyslipidemia increased 1.7-, 2.9-, and 4.9-fold, respectively, over the 10 years of follow-up.
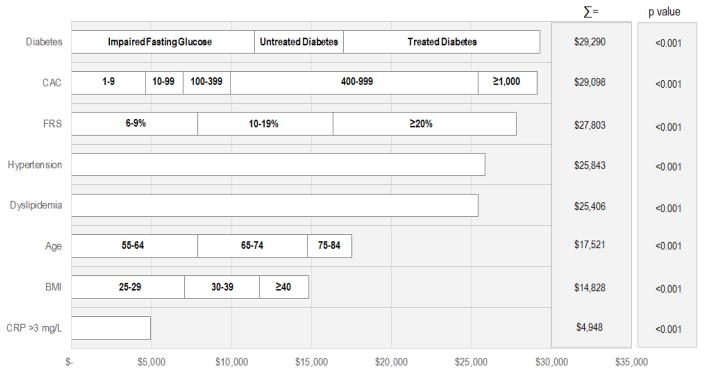
Multivariate Linear Regression Model Estimating 10-Year Health Care Costs (Central Illustration)
Central Illustration. Adjusted 10-Year Health Care Costs by Traditional and Nontraditional CVD Risk Factors.
We first log-transformed 10-year total costs and performed a multivariate linear regression (Model r2 = 0.41; p < 0.001). So that adjusted costs might be presented, results from a multivariate linear model using the log-transformed dependent variable of 10-year total costs are also shown (Model r2 = 0.35; p < 0.001). From this latter model, we present the predicted, unique costs associated with each variable and/or subgroup. For example, MESA enrollees with an impaired fasting glucose had $11,449 higher 10-year costs compared with those with normal glucose values. Moreover, a participant with untreated diabetes at the index evaluation had an additional $5,567 in ten-year health care costs compared with participants with an impaired fasting glucose. Finally, the 10-year health care costs were highest for those with treated diabetes ($29,290). Costs are ranked from highest to lowest. When added to the model, MESA racial/ethnic subgroups had lower costs of CVD; with black, Hispanic, and Chinese participants having $832, $619, and $1,666 lower adjusted 10-year costs (p = 0.46); however, this is not statistically significant. Similarly, enrollees on Medicare, Medicaid, or with private insurance had predicted costs of $2,264 (p = 0.006), $562 (p = 0.63), and $719 (p = 0.30), respectively. BMI = body mass index; CAC = coronary artery calcium; CRP = C-reactive protein; CVD = cardiovascular disease; FRS = Framingham Risk Score; MESA = Multi-Ethnic Study of Atherosclerosis.
We then performed multivariate linear regression modeling to identify significant estimators of 10-year CVD health care costs. The results from a multivariate linear regression using log-transformed and nontransformed cost as the dependent variable reveal similar findings (Online Appendix 4). The Central Illustration plots show adjusted costs. Cumulative costs were high for those with impaired fasting glucose or diabetes. For those with impaired fasting glucose, adjusted costs were $11,449, whereas those with untreated and treated diabetes had 10-year CVD costs of $5,567 and $12,274. Using this analysis, a CACS of 400 to 999 contributed $15,511 in higher costs, and an additional $3,668 in higher costs was estimated for those with a CACS ≥1,000. By comparison, a high-risk hsCRP >3 mg/l added $4,948 to cumulative health care costs.
Proportional Costs for CVD Medications, Visits, Procedures, and Hospitalizations
Table 6 reports the proportion of 10-year health care costs associated with CVD medications, diagnostic procedures, outpatient visits, coronary revascularization, and CVD hospitalization. For all MESA enrollees, CVD medications encumbered the highest proportion of 10-year costs of care; especially for persons with diabetes (87%) and dyslipidemia (90%). The elderly, obese, and those with a high-risk hsCRP > 3 mg/l and a CACS ≥400 had higher proportional costs related to diagnostic procedures and outpatient visits. Approximately 5% of costs for MESA enrollees with a CACS ≥400 were attributed to coronary revascularization. Less than 5% of 10-year health care costs were attributable to CVD hospitalization.
Table 6.
Proportion of 10-Year Costs Attributed to CVD Medications, Diagnostic Procedures, Outpatient Visits, Coronary Revascularization, and CVD Hospitalizations
| CVD Medications | CVD Diagnostic Procedures | Outpatient Visits | Coronary Revascularization | CVD Hospitalizations | |
|---|---|---|---|---|---|
| Diabetes | 86.6 | 4.6 | 3.7 | 1.7 | 3.5 |
| CACS ≥400 | 70.8 | 10.4 | 9.6 | 4.6 | 4.6 |
| High-risk FRS | 76.9 | 7.7 | 9.5 | 2.2 | 3.8 |
| Hypertension | 83.4 | 5.9 | 6.6 | 1.5 | 2.6 |
| Dyslipidemia | 89.9 | 4.3 | 1.5 | 1.6 | 2.7 |
| Age ≥65 yrs | 71.1 | 10.1 | 14.3 | 1.6 | 2.9 |
| BMI ≥30 kg/m2 | 70.3 | 9.6 | 16.8 | 1.4 | 2.0 |
| hsCRP >3 mg/l | 67.4 | 10.8 | 18.1 | 1.3 | 2.4 |
Values are %.
CVD = cardiovascular disease. Other abbreviations as in Table 2.
Low- and High-Cost Subgroups
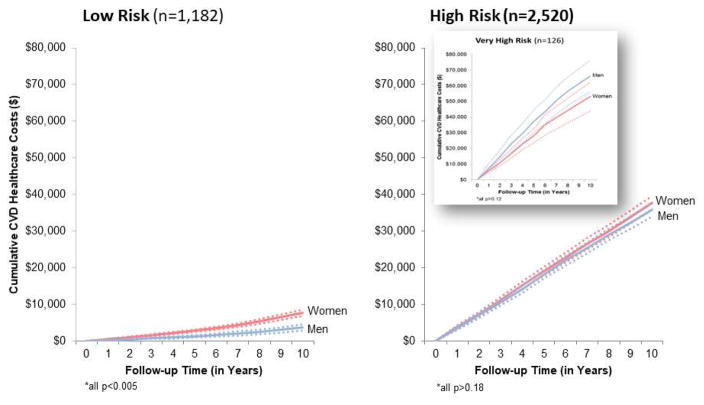
A total of 1,182 MESA enrollees were categorized as low risk (0 CACS, a low FRS, and normal glucose values at baseline) with cumulative 10-year (mean) costs of $7,008, albeit higher in women due to their older age and elevated costs for outpatient visits and CVD medications. Total costs associated with low-risk status were $8.3 million, or 5.2% of the total $155 million for all MESA enrollees. By comparison, among the 2,520 high-risk participants (CACS ≥400, diabetes, or a high-risk FRS), 10-year costs of health care were $37,732 for women and $35,814 for men. Total costs associated with high-risk status were $74 million, or 48% of the $155 million total for all MESA enrollees.
Discussion
The NIH-NHLBI–sponsored MESA is a landmark investigation reporting on the long-term effectiveness of traditional and nontraditional risk markers for prediction of major CVD events (7,10). Although many risk markers have proven effectiveness at stratifying populations, estimating risk alone is insufficient to describe the clinical burden of diagnosis and treatment for high-risk subsets. Limited information is available on the follow-up resource consumption and cost patterns among asymptomatic individuals following a detailed screening examination collecting measurements of CVD risk factors and nontraditional risk markers, such as with hsCRP and CACS (17–20). Our findings are consistent with prior estimates that there is an increasing economic burden of CVD over time, which is projected to increase to $1.1 trillion in the United States by 2035 (21). This pattern of accelerating health care costs within the MESA reveals that individuals who are screened today will experience suspected coronary artery disease symptoms and new diagnoses prompting more intensive treatment and frequent use of diagnostic procedures.
Cost Findings among MESA Subgroups
Our MESA analysis examining resource consumption patterns reveals dramatic and surprising differences in CVD costs across varied MESA subgroups. We identified a high-risk population cohort with a high FRS, CACS ≥400, or diabetes that consumed nearly one-half of the estimated $155 million for CVD health care costs during the 10 years of follow-up. By comparison, a low-risk population cohort (with normal glucose values, no detectable CACS, and a low risk FRS) consumed only 5% of the CVD health care costs over 10 years. These results are similar to recent findings from the Chicago Heart Association Detection Project noting that low CVD risk individuals had decidedly lower 5-year Medicare costs (22).
Moreover, women often had higher CVD costs, in large part related to their risk factor burden and advanced age compared with men. This relationship between women and men was most apparent when comparing across FRS subgroups where, in low to intermediate FRS subgroups, 10-year costs were ~$10,000 higher among average to intermediate risk women. Numerous reports have challenged the accuracy of the FRS and proposed an underestimation of risk, particularly associated with aging for women. This factor led to comparisons across any FRS subgroup of women having higher CVD costs and more intensive resource consumption patterns compared with men (23).
We also reported a lack of association between race and ethnicity as a primary driver of increased CVD costs. Many reports note the high burden of CVD risk factors and comorbidities that increase CVD event risk among racial and ethnically-diverse patient populations (24). Our analysis revealed that the addition of income, insurance, and education attenuated the impact of race and ethnicity as a significant driver of health care. These data further support that socioeconomic factors influence resource consumption patterns for many priority populations, including MESA enrollees of diverse race and ethnicity (23). Moreover, policies focusing on equitable access to preventive management not only reduce health care disparities, but may also influence the economic burden of CVD care among at-risk minority populations.
Another surprising finding is the high costs (~$5,000) associated with high-risk hsCRP. Certainly, the epidemiological data support an elevated CVD risk that would increase hospitalization and revascularization costs (25–27). Analyses also detailed higher costs for CVD medications among those with hsCRP >3 mg/l, which was likely influenced by trial evidence of therapeutic risk reduction with statin therapy (28).
A final example is the high rate of self-reported chest pain and shortness of breath in this initially apparently healthy, asymptomatic population. Nearly 1 in 10 MESA enrollees reported symptoms that prompted a high rate of diagnostic procedural use when compared with those without such symptoms. This association suggests a heavy burden of suspected symptoms, which may underlie the high rates of diagnostic procedures observed over the past several decades (29).
Policy Implications for Investing in Screening of Apparently Healthy Populations
As health care systems embark on population health strategies, the cost estimates from MESA may provide insight into the cost implications of early screening and targeted preventive programs. From our analysis, we capture a low-risk subgroup with decidedly lower long-term CVD costs when compared with higher-risk MESA subgroups. It is possible that an investment in preventive health programs could be economically advantageous if a sizeable proportion of enrollees maintain their low-risk status for many years. Moreover, depending on the success of preventive programs, investments could be balanced by reduced health care costs for the low-risk patients who require minimal clinical care. As CVD medications encumbered the highest proportional costs, a focus on behavioral or community-wide policies and programs to enhance lifestyle changes may reduce the need (and thus the costs) for CVD medications. There should also be an aim on more widespread use of lower-cost treatments, such as aspirin, to reduce CVD risk while adding minimally to CVD costs (30).
Moreover, implementation of community or health system policies may become more palatable if economic advantages, such as that estimated within MESA, were realized in long-term CVD costs of care. In a recent example, New York City health policy initiatives on improving healthy eating behaviors and tobacco control were associated with reductions in CVD mortality (31). The dramatic differences in cost among MESA subgroups provide insight into the substantive health care costs that could be averted by reducing the burden of risk factors (i.e., CACS) and preventing atherosclerotic disease development.
MESA Limitations
Importantly, contractual agreements with private payers generally have higher payment rates than that of Medicare. Thus, for the nearly one-quarter of MESA enrollees who are not yet eligible for Medicare, costs were underestimated. Although rigorously collected data were ascertained on utilization of health care services, only Medicare data was used in our analyses. Limited diagnostic test results were available. The MESA adjudication process was detailed and provided for ascertainment of CVD hospitalizations and events, as well as symptom data. Data are not available to compare the effectiveness of various screening strategies, as all enrollees underwent a battery of laboratory and imaging procedures.
Conclusions
The longitudinal patterns of health care resource use following traditional and nontraditional CVD testing within the MESA reveal new evidence on the long-term economic burden of follow-up treatment and testing patterns. The economic needs of the adult population with traditional and nontraditional CVD risk markers exceeded $155 million in the MESA. These data illustrate the unique contributions to elevated cost, and the targeted economic burden of various risk factors and markers on total health care costs. Identification of low-risk populations that maintain a healthy weight, glucose, blood pressure, and cholesterol have markedly reduced health care costs. High-risk subgroups with diabetes, a high FRS, or a CACS ≥400 have up to 15-fold higher costs for health care, and targeted early screening and intervention programs could offset the elevated long-term costs for asymptomatic populations.
Figure 3. Cumulative Follow-Up CVD Health Care Costs Among MESA Subjects with Low- and High-Risk Findings.
Cumulative (unadjusted) follow-up CVD health care costs (with 95% confidence intervals, dotted lines) are shown for low-risk (n = 1,182 with low-risk FRS, CACS = 0, and normal glucose values) and high-risk findings (n = 2,520 with CACS ≥400, diabetes, or high-risk FRS). A subset of very high-risk subjects, defined as diabetic patients with a high-risk FRS and CACS ≥400 (n = 126) is plotted as an insert. Abbreviations as in Figures 1 and 2.
PERSPECTIVES.
COMPETENCY IN SYSTEMS-BASED PRACTICE
Among patients screened for CVD, those in high-risk subgroups have up to 15-fold greater costs for health care over the following decade.
TRANSLATIONAL OUTLOOK
Further studies are needed to confirm whether more carefully targeted screening and intervention programs for asymptomatic populations could reduce the elevated resource utilization and costs during long-term follow-up.
Abbreviations and Acronyms
- BMI
body mass index
- CACS
coronary artery calcium score
- CVD
cardiovascular disease
- FRS
Framingham Risk Score
- HF
heart failure
- hsCRP
high-sensitivity C-reactive protein
- MI
myocardial infarction
Footnotes
Disclosures: Support was provided by NIH-NHLBI grant RC1 HL100915-01. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–44. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Blaha MJ, Chiuve SE, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association [Published corrections appear in Circulation 2017;135:e646 and Circulation. 2017;136:e196] Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Curry SJ, Byers T, Hewitt M, editors. Fulfilling the Potential of Cancer Prevention and Early Detection. National Academies Press; Washington, DC: 2003. Available at: https://doi.org/10.17226/10263. [PubMed] [Google Scholar]
- 6.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 7.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 8.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 9.Pasternak RC, Abrams J, Greenland P, Smaha LA, Wilson PW, Houston-Miller N. 34th Bethesda Conference: Task force #1--Identification of coronary heart disease risk: is there a detection gap? J Am Coll Cardiol. 2003;41:1863–74. doi: 10.1016/s0735-1097(03)00358-9. [DOI] [PubMed] [Google Scholar]
- 10.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–95. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ockene IS, Matthews CE, Rifai N, Ridker PM, Reed G, Stanek E. Variability and classification accuracy of serial high-sensitivity C-reactive protein measurements in healthy adults. Clin Chem. 2001;47:444–50. [PubMed] [Google Scholar]
- 12.MESA Coordinating Center. [Accessed January 17, 2018];MESA website. 2018 Available at: https://www.mesa-nhlbi.org.
- 13.Truven Health Analytics. Red Book; [Accessed January, 16, 2018]. truvenhealth.com. Available at: http://www.redbook.com/redbook/ [Google Scholar]
- 14.www.cms.hhs.gov/PCPricer/08_OPPS.asp#TopOfPage.
- 15.www.cms.hhs.gov/PCPricer/03_inpatient.asp#TopOfPage.
- 16. [Accessed January 16, 2018];CPI Inflation Calculator. Available at: data.bls.gov/cgi-bin/cpicalc.pl.
- 17.U.S. Preventive Services Task Force. [Accessed January 16, 2018];Coronary Heart Disease: Screening Using Non-Traditional Risk Factors. 2009 Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/coronary-heart-disease-screening-using-non-traditional-risk-factors.
- 18.Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57:1622–32. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw LJ, Min JK, Budoff M, et al. Induced cardiovascular procedural costs and resource consumption patterns after coronary artery calcium screening: results from the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) study. J Am Coll Cardiol. 2009;54:1258–67. doi: 10.1016/j.jacc.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O'Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46:807–14. doi: 10.1016/j.jacc.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 21.American Heart Association/American Stroke Association. [Accessed January 16, 2018];Cardiovascular Disease: A Costly Burden For America — Projections Through 2035. 2017 Available at: http://www.heart.org/idc/groups/heart-public/@wcm/@adv/documents/downloadable/ucm_491543.pdf.
- 22.Allen NB, Zhao L, Liu L, et al. Favorable cardiovascular health, compression of morbidity, and healthcare costs: forty-year follow-up of the CHA Study (Chicago Heart Association Detection Project in Industry) Circulation. 2017;135:1693–701. doi: 10.1161/CIRCULATIONAHA.116.026252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw LJ, Pepine CJ, Xie J, et al. Quality and equitable health care gaps for women: attributions to sex differences in cardiovascular medicine. J Am Coll Cardiol. 2017;70:373–88. doi: 10.1016/j.jacc.2017.05.051. [DOI] [PubMed] [Google Scholar]
- 24.Yancy CW, Wang TY, Ventura HO, et al. credo Advisory Group. The coalition to reduce racial and ethnic disparities in cardiovascular disease outcomes (credo): why credo matters to cardiologists. J Am Coll Cardiol. 2011;57:245–52. doi: 10.1016/j.jacc.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Rifai N, Pfeffer MA, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98:839–44. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–3. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM. High-sensitivity C-reactive protein, vascular imaging, and vulnerable plaque: more evidence to support trials of antiinflammatory therapy for cardiovascular risk reduction. Circ Cardiovasc Imaging. 2011;4:195–7. doi: 10.1161/CIRCIMAGING.111.965053. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Danielson E, Fonseca FA, et al. JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 29.Mark DB, Anderson JL, Brinker JA, et al. ACC/AHA/ASE/ASNC/HRS/IAC/Mended Hearts/NASCI/RSNA/SAIP/SCAI/SCCT/SCMR/SNMMI 2014 health policy statement on use of noninvasive cardiovascular imaging: a report of the American College of Cardiology Clinical Quality Committee. J Am Coll Cardiol. 2014;63:698–721. doi: 10.1016/j.jacc.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Van't Hof JR, Duval S, Walts A, Kopecky SL, Luepker RV, Hirsch AT. Contemporary primary prevention aspirin use by cardiovascular disease risk: impact of US Preventive Services Task Force Recommendations, 2007–2015: a serial, cross-sectional study. J Am Heart Assoc. 2017;6:e006328. doi: 10.1161/JAHA.117.006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ong P, Lovasi GS, Madsen A, Van Wye G, Demmer RT. Evaluating the effectiveness of New York City health policy initiatives in reducing cardiovascular disease mortality, 1990–2011. Am J Epidemiol. 2017;186:555–63. doi: 10.1093/aje/kwx134. [DOI] [PMC free article] [PubMed] [Google Scholar]