Abstract
Study Design
Meta-analysis of randomized controlled trials.
Objective
To evaluate the effectiveness of perioperative supplemental ketamine to reduce postoperative opioid analgesic consumption following spine surgery.
Summary of Background Data
Although low-dose supplemental ketamine has been known to reduce pain after surgery, there is conflicting evidence regarding whether ketamine can be effective to reduce opioid consumption following spine surgery.
Methods
Comprehensive search of PubMed, the Cochrane Central Register of Controlled Trials for prospective randomized controlled trials (RCTs), Web of Science, and Scopus. Patients that received supplemental ketamine were compared to the control group in terms of postoperative morphine equivalent consumption, pain scores, and adverse events. Mean differences (MD) and 95% confidence intervals (CI) were used to describe continuous outcomes. Odds Ratios (OR) and 95% CIs were applied to dichotomous outcomes.
Results
A total of 14 RCTs comprising 649 patients were selected for inclusion into the meta-analysis. Patients that were administered adjunctive ketamine exhibited less cumulative morphine equivalent consumption at 4, 8, 12, and 24 hours following spine surgery (all ps<0.05). The ketamine group also reported lower postoperative pain scores at 6, 12, and 24 hours (all ps<0.05). None of the adverse events studied attained statistical significance (all ps>0.05).
Conclusions
Supplemental perioperative ketamine reduces postoperative opioid consumption up to 24 hours following spine surgery.
Level of Evidence
1
Keywords: ketamine, analgesic, analgesia, spine surgery, postoperative pain, complication, meta-analysis, randomized controlled trial
INTRODUCTION
Ketamine is a commonly used anesthetic agent that binds N-methyl-d-aspartate receptors (NMDAR) in addition to several opioid receptors (μ, δ, and κ)1–2. In addition, supplemental low-dose ketamine can be used for analgesia3. In general, adjuvant perioperative ketamine has been reported to reduce morphine consumption in the first 24 hours following surgery with little to no adverse events4. Vague feelings, blurred vision, and hallucinations are the adverse events most commonly encountered1. Ketamine may be administered as a single dose, continuous intravenous (IV) infusion, intravenous patient-controlled analgesia (IV-PCA), or epidural infusion2. It may be administered preoperatively, intraoperatively, and/or postoperatively1. Although supplemental ketamine has been studied broadly in a variety of procedures and operations, there is no consensus regarding the effectiveness of adjunctive ketamine analgesic use specifically in spine surgery. Thus, the primary objective of this study was to determine whether perioperative low-dose ketamine reduced opioid consumption after spine surgery. Secondary goals included determining if ketamine use affected postoperative pain scores and if administration of ketamine was linked to higher rates of complications.
MATERIALS AND METHODS
Systematic Search
A systematic search strategy was designed and tailored to each database with the help of a medical librarian. The following databases were searched for prospective randomized controlled trials (RCTs): PubMed, the Cochrane Central Register of Controlled Trials, Web of Science, and Scopus. The terms “ketamine,” “spine surgery,” and other related terms and word variations were used. The query designed for PubMed can be seen in Appendix A.
Inclusion and Exclusion Criteria
Inclusion and exclusion criteria were defined and agreed upon by all authors a priori. Articles that met the following criteria were eligible for inclusion in the meta-analysis: (1) the article described a human study; (2) ketamine was administered; (3) elective, inpatient spine surgery was performed; (4) the article described a randomized controlled trial; (5) postoperative analgesia was reported; (6) postoperative pain scores were reported (6) postoperative complications were reported; (7) general anesthesia was administered. Articles that met any of the following exclusion criterion were excluded from the meta-analysis: (1) the article described a non-human study; (2) ketamine was only administered for general anesthesia; (3) trauma, outpatient, or non-spine surgery were conducted; (4) the article did not describe a clinical trial; (5) postoperative analgesia was not reported; (6) postoperative pain scores were not reported; (7) general anesthesia was not administered; (8) a treatment or control arm of the trial comprised 10 patients or fewer; (9) non-English language article. The results were updated as of February 4th, 2015.
Article Screening
Duplicate articles were removed from the results of the systematic search of each database. The remaining titles and abstracts were downloaded and screened independently by two authors (A.P. and S.F.) based on the pre-existing inclusion and exclusion criteria. A third author (M.E.) resolved disagreement. The full texts of the articles were then screened independently by A.P. and S.F. and disputes once again resolved by M.E. Kappa scores were used to quantify inter-rater agreement. All articles remaining after the second round of screening were included in the meta-analysis.
Data Abstraction
Data were abstracted from the included studies by one author (A.P.). The corresponding author of each study was contacted individually for additional raw or grouped data; data requests were granted by the corresponding authors of 2 studies5–6. The following study characteristics were recorded: first author, publication year, and number of subjects. Also, the mode, dosage, and timing of ketamine administration were documented. Other recorded data included placebo control (saline) and the primary postoperative analgesic. Outcomes were only analyzed if reported by at least 3 studies. Continuous outcomes included opioid consumption and postoperative pain scores at rest. Dichotomous outcomes included adverse events such as bad dreams, cardiac events, dysphoria, hallucinations, headache, postoperative nausea and vomiting (PONV), pruritus, psychotomimetic effects, respiratory depression, sedation, and urinary retention. In articles that reported more than one treatment arm, treatment arms were combined to create a single pair-wise comparison as described by Higgins et al7.
Data Normalization
With the help of biostatisticians, abstracted data were normalized prior to analysis. Opioid consumption was analyzed after converting all non-morphine primary postoperative analgesics such as fentanyl (100:1 potency8) and hydromorphone (5:1 potency9) to morphine equivalents. This was necessary for 5 studies10–14. Due to differences in the reporting of opioid consumption, estimation of the average cumulative morphine equivalent consumption was necessary for several studies. In 4 studies, we standardized weight-adjusted means and standard deviations by multiplying by average weight and using the product of the variances to produce a crude estimate of the standard deviation6,10,15–16. In one study, the cumulative opioid consumption was estimated from infusion rate15. In trials that did not report cumulative opioid consumption but described opioids consumed over select time periods (e.g. 0–24hrs, 24–48hrs…), the average cumulative analgesic was calculated and the standard deviations were imputed by entering the largest available standard deviations, thereby producing a conservative estimate. A series of sensitivity analyses were conducted to determine the effect of these estimations.
At rest pain scores were reported on a 0–10 scale by 8 studies6,11,13,15–19, 0–5 scale by 2 studies20–21, and 0–100 scale by 4 studies5,10,13–14. Means and standard deviations of pain scores were normalized to a 0–10 scale prior to comparison.
Normalization was required for the following dichotomous outcomes: unpleasant dreams, cardiac events, dysphoria, hallucinations, PONV, and sedation. PONV was reported as a combined event in 5 studies and separately (experiences of nausea and/or number of emesis) in 3 studies. In the latter case, the incidence of vomiting was recorded preferentially. Cardiac events included arrhythmia, circulatory depression, and/or major changes in heart rate or blood pressure. Several studies (n=3) reported dysphoria while 7 reported unpleasant dreams and 8 reported hallucinations separately. Although sedation was analyzed as a dichotomous outcome, sedation was reported as a continuous outcome in 3 articles. In such cases, the corresponding author was contacted for additional data; supplementary data were obtained for 2 studies. Sedation was recorded for any score of 5 or above (on a 0–5 scale), mention of deep sedation, and/or description of response only with any, repeated, or painful stimuli in accordance with guidelines established by the American Society of Anesthesiologists (ASA)22.
Statistical Analysis
Microsoft® Excel for Mac Version 15.11.12 and Review Manager 5.3.5 for Mac were used to conduct the analysis23. The Cochrane Handbook was used as a reference7. For continuous outcomes such as opioid consumption, postoperative pain scores, and length of stay, mean differences (MD) and 95% confidence intervals (CI) were calculated. Odds ratio (OR) and 95% CI were calculated for dichotomous outcomes (adverse events). Chi-squared analysis was used to test for heterogeneity between studies with a significance value set at 0.10 in order to more accurately detect significant heterogeneity24. Heterogeneity was further quantified by applying the I2 test with values exceeding 50% indicating considerable or substantial heterogeneity7. The random effects model was used to incorporate between-studies heterogeneity for comparisons with phetero<0.10 and I2>50%; otherwise, the fixed effects model was used7. Before incorporating supplementary data obtained from corresponding authors, sensitivity analyses were conducted.
RESULTS
Systematic Search and Article Screening
The systematic search yielded 1846 articles from PubMed (n=618), Web of Science (n=413), Scopus (n=208), and the Cochrane Central Register of Controlled Trials (n=607). A total of 696 duplicate publications were removed. The remaining 1150 articles were reviewed by title and abstract such that 1127 were excluded (κ=0.6). The remaining 23 articles were screening for eligibility based on full-text review. Another 9 articles were discarded and a final tally of 14 articles were included in the qualitative and quantitative synthesis (κ=0.5). Figure 1 diagrams the article screening process.
Figure 1.
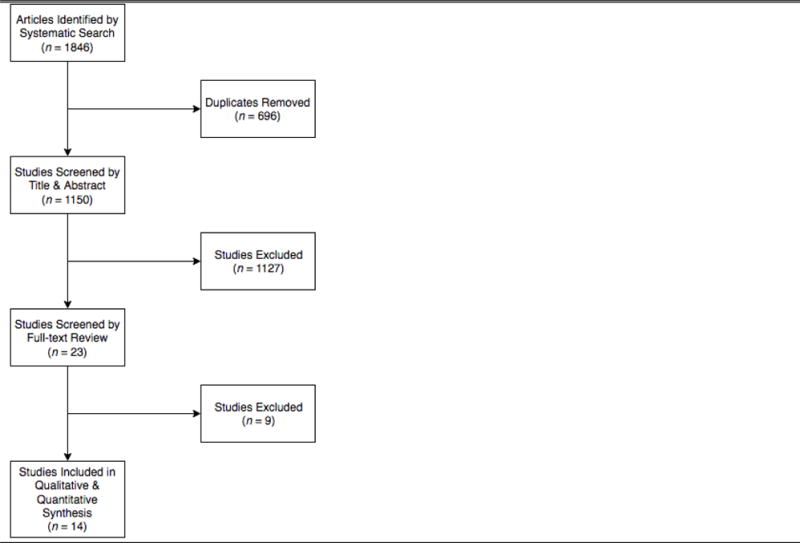
Flow Diagram
Included Studies
A total of 14 randomized controlled trials comprising 649 patients were included in the meta-analysis. Study characteristics are provided in Table 1.
Table 1.
Study Characteristics
| First author | Publication Year | Number of Subjects | Ketamine | Control Saline | Primary postoperative analgesic | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ketamine | Control | Mode | Dosage | Timing | Analgesic | Mode | |||
| Javery | 1996 | 22 | 20 | IV-PCA | 1 mg ml−1 | Postoperative | Morphine | IV-PCA | |
| Aveline | 2006 | 23 | 23 | Bolus | 0.15 mg kg−1 | Intraoperative | Morphine | IV-PCA | |
| Engelhardt | 2008 | 16 | 18 | Bolus Continuous infusion |
0.5 mg/kg 4 μg kg−1 min−1 |
Intraoperative Intraoperative |
Saline | Morphine | IV-PCA |
| Yamauchi | 2008 | 88 | 44 | Bolus Continuous infusion |
1 mg kg−1 42–83 μg kg−1 min−1 |
Intraoperative Intraoperative |
Saline | Fentanyl | IV-PCA |
| Hadi | 2009 | 20 | 20 | Continuous infusion | 4 μg kg−1 min−1 | Intraoperative | Morphine | IV-PCA | |
| Subramaniam | 2011 | 15 | 15 | Bolus Continuous infusion |
0.15 mg kg−1 2 μg kg−1 min−1 |
Intraoperative Intraoperative Postoperative |
Saline | Hydromorphone | IV-PCA |
| Abrishamkar | 2012 | 22 | 23 | Continuous infusion | 0.5 mg kg−1 hr−1 | Postoperative | Morphine | IV | |
| Pacreu | 2012 | 10 | 10 | Bolus Continuous infusion IV-PCA |
0.5 mg kg−1 2.5 μg kg−1 min−1 0.5 mg |
Intraoperative Intraoperative Postoperative |
Saline | Methadone | IV-PCA |
| Yeom | 2012 | 20 | 20 | Bolus IV-PCA |
0.2 mg/kg 30 μg ml−1 kg−1 |
Intraoperative Postoperative |
Saline | Fentanyl | IV-PCA |
| Hadi | 2013 | 30 | 15 | Continuous infusion | 1 μg kg−1 min−1 | Intraoperative Postoperative |
Saline | Morphine | IV |
| Kim | 2013 | 35 | 17 | Bolus Continuous infusion |
0.5 mg kg 1–2 μg kg−1 min−1 |
Intraoperative Postoperative |
Saline | Fentanyl | IV-PCA |
| Nitta | 2013 | 12 | 12 | Bolus IV-PCA |
10 mg kg−1 2 mg kg−1 hr−1 |
Intraoperative Postoperative |
Morphine | IV-PCA | |
| Song | 2013 | 25 | 24 | Continuous infusion IV-PCA |
0.3 mg kg−1 3 mg kg−1 |
Intraoperative Postoperative |
Saline | Fentanyl | IV-PCA |
| Pestieau | 2014 | 29 | 21 | Bolus Continuous infusion Continuous infusion |
0.5 mg kg−1 0.2 mg kg−1 hr−1 0.1 mg kg−1 hr−1 |
Intraoperative Intraoperative Postoperative |
Saline | Morphine | IV-PCA |
Morphine Equivalent Consumption
Postoperative morphine equivalent consumption and postoperative pain scores are documented in Table 2. Patients in the ketamine group exhibited significantly less morphine equivalent consumption 4 hours following surgery (MD: −5.69, 95% CI: −10.73 to −0.65, p=0.03). At 8 hours, the ketamine group again was associated with lower opioid consumption (MD: −8.16, 95% CI: −10.54 to −5.78, p<0.001). Patients in the ketamine group also consumed fewer cumulative morphine equivalents at 12 hours after surgery as well (MD: −7.06, 95% CI: −12.99 to −1.13, p=0.02). The difference in cumulative opioid consumption was most pronounced at 24 hours following surgery, with patients that were administered ketamine consuming significantly fewer morphine equivalents (MD: −14.38, 95% CI: −18.13 to −10.62, p<0.001). However, the difference in opioid consumption was no longer significant at 36 hours (MD: −8.64, 95% CI: −18.62 to 1.33, p=0.09). At 48 hours, patients in the ketamine group had consumed more morphine equivalents compared to control, though this difference was not statistically significant (MD: 2.39, 95% CI: −10.42 to 15.21, p=0.71). Forest plots for morphine equivalent consumption at 12, 24, and 48 hours are reported in Figures 2, 3, and 4, respectively.
Table 2.
Postoperative morphine equivalent consumption and postoperative pain score
| Outcome | # of studies | Sample size: ketamine (control) | Analysis Model | MD (95% CI) | p Hetero | p Effect |
|---|---|---|---|---|---|---|
| Morphine equivalents (4hrs) | 3 | 54 (56) | Random Effects | −5.69 (−10.73 to −0.65) | 0.02 | 0.03 |
| Morphine equivalents (8hrs) | 3 | 54 (56) | Fixed Effects | −8.16 (−10.54 to −5.78) | 0.34 | <0.001 |
| Morphine equivalents (12hrs) | 7 | 208 (152) | Random Effects | −7.06 (−12.99 to −1.13) | <0.001 | 0.02 |
| Morphine equivalents (24hrs) | 13 | 357 (272) | Random Effects | −14.38 (−18.13 to −10.62) | <0.001 | <0.001 |
| Morphine equivalents (36hrs) | 3 | 51 (52) | Fixed Effects | −8.64 (−18.62 to 1.33) | 0.18 | 0.09 |
| Morphine equivalents (48hrs) | 7 | 205 (155) | Random Effects | 2.39 (−10.42 to 15.21) | 0.04 | 0.71 |
| Pain scores (0h) | 4 | 78 (80) | Fixed Effects | −0.18 (−0.69 to 0.33) | 0.60 | 0.48 |
| Pain scores (1hrs) | 5 | 158 (96) | Random Effects | −1.02 (−2.46 to 0.42) | <0.001 | 0.16 |
| Pain scores (6hrs) | 5 | 200 (123) | Random Effects | −1.18 (−1.67 to −0.69) | 0.03 | <0.001 |
| Pain scores (12hrs) | 6 | 196 (139) | Random Effects | −1.01 (−1.51 to −0.52) | 0.03 | <0.001 |
| Pain scores (24hrs) | 10 | 302 (202) | Random Effects | −1.27 (−1.70 to −0.84) | 0.007 | <0.001 |
| Pain scores (36hrs) | 4 | 143 (102) | Random Effects | 0.15 (−1.15 to 1.44) | 0.002 | 0.83 |
| Pain scores (48hrs) | 7 | 227 (160) | Random Effects | −0.35 (−0.96 to 0.26) | 0.04 | 0.26 |
| Pain scores (72hrs) | 3 | 133 (83) | Fixed Effects | 0.04 (−0.36 to 0.43) | 0.05 | 0.85 |
MD: mean difference; CI: confidence interval; p: p-value
Figure 2.
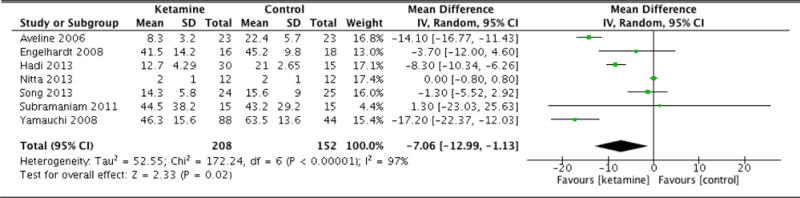
Morphine equivalent consumption at 12 hours
Figure 3.
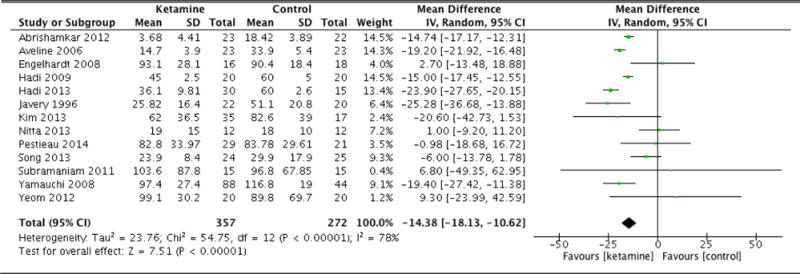
Morphine equivalent consumption at 24 hours
Figure 4.
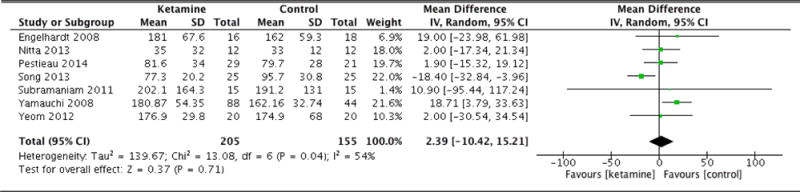
Morphine equivalent consumption at 48 hours
Pain Scores
Immediately following surgery, there was no statistical difference in average pain scores between groups (MD: −0.18, 95% CI: −0.69 to 0.33, p=0.48). At 1 hour, once again there was no significant difference (MD: −1.02, 95% CI: −2.46 to 0.42, p=0.16). However, at 6 hours following surgery, patients in the ketamine group indicated significantly lower pain scores compared to control (MD: −1.18, 95% CI: −1.67 to −0.69, p<0.001). The ketamine group was also associated with lower average pain scores at 12 hours (MD: −1.01, 95% CI: −1.51 to −0.52, p<0.001). At 24 hours, patients in the ketamine group continued to report lower pain scores compared to control (MD: −1.27, 95% CI: −1.70 to −0.84, p<0.001). However, by 36 hours, there was no statistically significant difference between the pain scores of each group (MD: 0.15, 95% CI: −1.15 to 1.44, p=0.83). At 48 hours, the difference in pain scores was again not statistically significant (MD: −0.35, 95% CI: −0.96 to 0.26, p=0.26). By 72 hours, there was nearly no difference in average pain scores between groups (MD: 0.04, 95% CI: −0.36 to 0.43, p=0.85). Forest plots for postoperative pain scores at 12, 24, and 48 hours are reported in Figures 5, 6, and 7, respectively.
Figure 5.
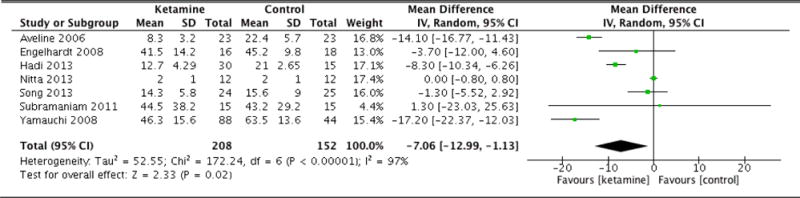
Postoperative pain scores at 12 hours
Figure 6.
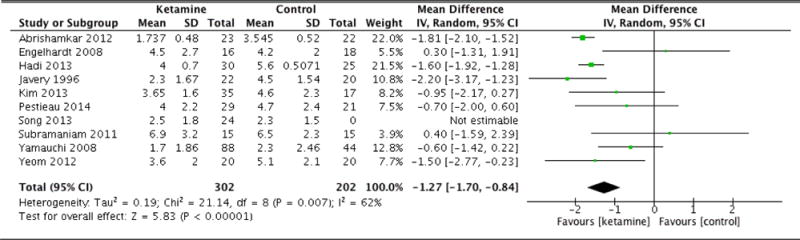
Postoperative pain scores at 24 hours
Figure 7.
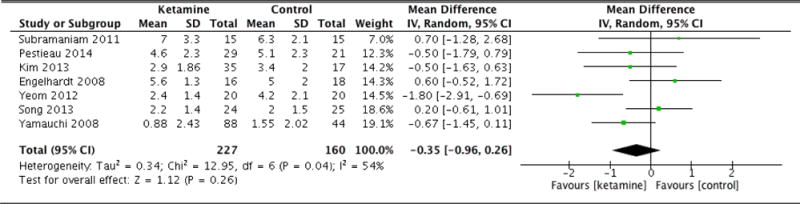
Postoperative pain scores at 48 hours
Adverse Events
Adverse events are documented in Table 3. Unpleasant dreams were reported in 2 patients administered ketamine compared to none in the control group (OR: 3.19, 95% CI: 0.32 to 31.81, p=0.32). Cardiac events were reported in 1 case in the control group compared to none in the ketamine group (OR: 0.31, 95% CI: 0.01 to 8.28, p=0.49). Dysphoria occurred in 2 patients that received ketamine and in 3 patients in the control group (OR: 0.64, 95% CI: 0. 09 to 4.27, p=0.64). Patients that were given ketamine experienced twice as many hallucinations (4 events) compared to control (2 events) (OR: 1.58, 95% CI: 0.30 to 8.43, p=0.59). Headache was experienced in 5 patients that received ketamine compared to 3 patients in the control cohort (OR: 1.34, 95% CI: 0.30 to 6.01, p=0.71). Incidence of PONV occurred in 42 and 41 patients in the ketamine group and control group, respectively (OR: 0.80, 95% CI: 0.44 to 1.45, p=0.46). Pruritus was experienced by 14 patients given ketamine and 11 patients in the control group (OR: 2.14, 95% CI: 0.64 to 7.21, p=0.22). Psychotomimetic events occurred in 3 patients administered ketamine but in 0 patients in the control group (OR: 3.95, 95% CI: 0.19 to 81.49, p=0.37). Respiratory depression was found in 2 ketamine patients compared to 4 control patients (OR: 0.42, 95% CI: 0.06 to 2.77, p=0.37). Patients that received ketamine experienced more sedation (5 events) compared to control (3 events) (OR: 1.34, 95% CI: 0.29 to 6.24, p=0.71). Urinary retention was not encountered in the ketamine group but occurred once in the control group (OR: 0.31, 95% CI: 0.01 to 8.28, p=0.49).
Table 3.
Adverse events
| Outcome | # of studies | Events [Ketamine total] | Events [Control total] | Analysis Model | OR (95% CI) | p Hetero | p Effect |
|---|---|---|---|---|---|---|---|
| Unpleasant dreams | 7 | 2 [235] | 0 [168] | Fixed Effects | 3.19 (0.32 to 31.81) | 0.99 | 0.32 |
| Cardiac events | 4 | 0 [144] | 1 [92] | Random Effects | 0.31 (0.01 to 8.28) | n/a | 0.49 |
| Dysphoria | 3 | 2 [66] | 3 [65] | Fixed Effects | 0.64 (0.09 to 4.27) | 0.22 | 0.64 |
| Hallucination | 9 | 4 [215] | 2 [176] | Fixed Effects | 1.58 (0.30 to 8.43) | 0.43 | 0.59 |
| Headache | 3 | 6 [60] | 3 [42] | Fixed Effects | 1.34 (0.30 to 6.01) | 0.58 | 0.71 |
| PONV | 7 | 42 [161] | 41 [130] | Fixed Effects | 0.80 (0.44 to 1.45) | 0.12 | 0.46 |
| Pruritus | 4 | 14 [129] | 11 [87] | Fixed Effects | 2.14 (0.64 to 7.21) | 0.84 | 0.22 |
| Psychotomimetic effects | 3 | 3 [64] | 0 [50] | Random Effects | 3.95 (0.19 to 81.49) | n/a | 0.37 |
| Respiratory depression | 8 | 2 [219] | 4 [165] | Random Effects | 0.42 (0.06 to 2.77) | n/a | 0.37 |
| Sedation | 4 | 5 [89] | 3 [73] | Fixed Effects | 1.34 (0.29 to 6.24) | 0.55 | 0.71 |
| Urinary retention | 3 | 0 [126] | 1 [82] | Random Effects | 0.31 (0.01 to 8.28) | n/a | 0.49 |
OR: odds ratio; CI: confidence interval; p: p-value; PONV: postoperative nausea and vomiting
DISCUSSION
Ketamine has been discussed in the literature extensively as a supplemental analgesic for perioperative pain control. Although several reviews have reported a statistically significant decrease in opioid analgesic consumption with use of ketamine, whether this holds true for spine surgery is not certain4,25. According to the findings of this meta-analysis, addition of supplemental ketamine yielded a significant reduction in postoperative morphine equivalent consumption at 4 hours, 8 hours, 12 hours, and 24 hours postoperatively. However, this statistically significant opioid sparing effect was no longer apparent at 36 hours following surgery. By 48 hours after surgery, patients that had been administered ketamine actually had a greater cumulative opioid consumption compared to their counterparts, though this was not considered statistically significant.
With regards to postoperative pain, patients in the ketamine group reported lower pain scores, on average, throughout the postoperative period (at 0, 1, 6, 12, 24, 36, 48, and 72 hours after surgery). Although the difference in pain scores immediately after surgery (0–1 hour) was not significant, patients in the ketamine reported significantly lower average pain scores at 6, 12, and 24 hours following surgery. This reduction in pain scores mirrors the reduction in cumulative morphine equivalent consumption during the first postoperative day. In addition, any difference in pain scores after 24 hours (36, 48, and 72 hours after surgery) was not significant. As a result, the significant reduction in morphine equivalent consumption by patients in the ketamine group during the first 24 hours following surgery coincided with significantly reduced pain scores during that same period, with the exception of the first postoperative hour. These findings suggest that the analgesia provide by supplemental low-dose ketamine may be confined to the first 24 hours following spine surgery.
Notably, this meta-analysis showed the opiate-sparing effects and postoperative pain reduction due to ketamine demonstrated efficacy exclusively within the first 24 hours postoperatiely. This ‘window effect’ may stem from differences in timing, dose, or mode of ketamine among trials. For example, ketamine was administered solely intraoperatively in several trials; given that the half-life of ketamine is approximately 186 minutes, analgesic effects may dissipate soon after intraoperative infusion is terminated26. Another possible explanation may be dosing: Pestieau et al reduced ketamine dosage when transitioning from the intraoperative to postoperative period16. Furthermore, in several trials, continuous IV infusions during the intraoperative period were replaced by IV-PCA postoperatively, introducing variables such as patient decision-making and differences in lockout intervals. Finally, opiate-tolerant patients may respond differently to ketamine, most notably at 48 hours and beyond. For example, Subramaniam et al. included subjects with pre-operative opiate use, finding low-dose ketamine to be ineffective11. Although many trials excluded patients with pre-operative narcotic use, other did not acknowledge pre-operative opiate consumption, leaving it unclear as to whether opiate tolerant patients were included and if so, how many. As a result, if chronic opiate use mitigated ketamine analgesia, the magnitude of this effect is indeterminate with the available data to include.
Delirium has been reported to occur in 5–15% of patients undergoing non-cardiac surgery and ketamine has been noted to cause delirium27–28. Although delirium was not explicitly reported by the trials included in this meta-analysis, several adverse events commonly associated with delirium were studied: unpleasant dreams, hallucinations, psychotomimetic events, and dysphoria. None of the aforementioned complications achieved statistical significance. These findings are contrary to previous reports. For example, ketamine as an anesthetic agent has been associated with dysphoria in as many as 10–20% of adult patients after a variety of surgical procedures29. However, sub-anesthetic doses of ketamine, such as those that may be used for supplemental analgesia, has been reported to reduce the occurrence of dysphoria. Also, ketamine has previously been associated with a reduction in postoperative nausea and vomiting, though no such difference was detected in this study4,30. In terms of cardiac events, headaches, respiratory depression, and urinary retention, there were no significant differences between groups.
Recent trends in spine surgery involve eschewing opiates in favor of non-opiate agents due to suboptimal opiate-related side effects2. One such non-opiate agent is ketamine, which has been noted to prevent opiate-induced hyperalgesia, the paradoxical heightened sensitive to pain following opiate exposure31–32. Given the short-term reduction in both morphine equivalent consumption and postoperative pain scores without an increase in complication rates, this meta-analysis offers an informed choice with new knowledge supporting either use or non-use of ketamine in the perioperative period based on individual interpretation of the objective results.
Limitations
Despite an exhaustive and systematic search of multiple databases, it is possible that there exist trials of ketamine use that were not included in this study. Furthermore, of the trials that were included, there was often significant heterogeneity in the comparisons. For example, the modes of administration included single bolus (n=9), continuous infusion (n=10), and IV-PCA (n=5). Dosages also differed considerably, weight-adjusted doses were utilized in several trials (n=4), and timing of administration varied from intraoperatively (n=4), postoperatively (n=2) trials, and both intra- and postoperatively (n=8). This study was not designed to evaluate dose-dependency so although supplemental ketamine yielded a significant reduction in morphine equivalent consumption for the first 24 hours, the optimal dose remains unclear. Furthermore, combining low- and high-dose treatment arms may have produced a moderating effect. Similarly, this meta-analysis was not structured to compare the mode or timing of ketamine administration, but determining the optimal dose and/or the ideal timing of intravenous ketamine may constitute avenues for future prospective studies. Although none of the adverse events obtained statistical significance, it is possible that this study was not adequately powered to detect differences in complication rates as evidence by several instances in which either the ketamine group or control contained no patients that experienced a complication.
Conclusion
This meta-analysis of supplemental ketamine for analgesia in spine surgery found a consistent opioid analgesic sparing effect over the course of 24 hours following surgery. With the exception of the first hour, this reduction in analgesic consumption coincided with significant reduced pain scores. Notably, significant reductions in both morphine equivalent consumption and pain scores did not persist beyond the first 24 hours, suggesting that the analgesic effect of supplemental ketamine may be limited to this timeframe. Furthermore, there was significant heterogeneity associated with the trials that were included in this study. Although this study indicates that low-dose supplemental ketamine may be useful for short-term analgesia following spine surgery, this meta-analysis was not designed to evaluate dose, timing, or mode of ketamine administration, all of which constitute avenues for future research.
Supplementary Material
KEY POINTS.
Supplemental ketamine reduced cumulative morphine equivalent consumption at 4, 8, 12, and 24 hours after spine surgery.
Supplemental ketamine reduced postoperative pain scores at 6, 12, and 24 hours after spine surgery.
Supplemental ketamine did not predispose spine surgery patients to an increased odds of experiencing unpleasant dreams, cardiac events, dysphoria, hallucinations, postoperative nausea or vomiting, pruritus, psychotomimetic events, respiratory depression, sedation, or urinary retention.
Acknowledgments
Linda Suk-Ling Murphy, MLIS for devising comprehensive search strategy
This work was partially supported by National Institutes of Health grant UL1 TR001414 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Lishi Zhang, MS – statistical support
Yanjun “Alice” Chen, MS – statistical support
Financial Support: None.
Footnotes
Previous Presentation: None.
Conflicts of Interest: None.
- The device(s)/drug(s) is/are FDA-approved or approved by corresponding national agency for this indication.
References
- 1.Weinbroum AA. Non-opioid IV adjuvants in the perioperative period: Pharmacological and clinical aspects of ketamine and gabapentinoids. Pharmacol Res. 2012;65(4):411–29. doi: 10.1016/j.phrs.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Rivkin A, Rivkin MA. Perioperative nonopioid agents for pain control in spinal surgery. Am J Health Syst Pharm. 2014;71(21):1845–47. doi: 10.2146/ajhp130688. [DOI] [PubMed] [Google Scholar]
- 3.Jouguelet-Lacoste J, La Colla L, Schilling D, Chelly JE. The use of intravenous infusion or single dose of low-dose ketamine for postoperative analgesia: a review of the current literature. Pain Med. 2015;16(12):383–403. doi: 10.1111/pme.12619. [DOI] [PubMed] [Google Scholar]
- 4.Bell FR, Dahl JB, Moore RA, Kalso E. Peri-operative ketamine for acute post-operative pain: a quantitative and qualitative systematic review (Cochrane review) Acta Anaesthesiol Scand. 2005;49:1405–1428. doi: 10.1111/j.1399-6576.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- 5.Aveline C, Le Hetet H, Vautier P, Gautier JF, Bonnet F. Peroperative ketamine and morphine for postoperative pain control after lumbar disk surgery. Eur J Pain. 2006;10(7):653–8. doi: 10.1016/j.ejpain.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Engelhardt T, Zaarour C, Naser B, Pehora C, de Ruiter J, Howard A, Crawford MW. Intraoperative low-dose ketamine does not prevent a remifentanil-induced increase in morphine requirement after pediatric scoliosis surgery. Anesth Analg. 2008;107(4):1170–5. doi: 10.1213/ane.0b013e318183919e. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. Version 5.1.0 [updated Mach 2011] Available from www.handbook.cochrane.org. [Google Scholar]
- 8.Yamauchi M, Asano M, Watanabe M, Iwasaki S, Furuse S, Namiki A. Continuous low-dose ketamine improves the analgesic effects of fentanyl patient-controlled analgesia after cervical spine surgery. Anesth Analg. 2008;107(3):1041–4. doi: 10.1213/ane.0b013e31817f1e4a. [DOI] [PubMed] [Google Scholar]
- 9.Subramaniam K, Akhouri V, Glazer PA, Rachlin J, Kunze L, Cronin M, Desilva D, Asdourian CP, Steinbrook RA. Intra- and postoperative very low dose intravenous ketamine infusion does not increase pain relief after major spine surgery in patients with preoperative narcotic analgesic intake. Pain Med. 2011;12(8):1276–83. doi: 10.1111/j.1526-4637.2011.01144.x. [DOI] [PubMed] [Google Scholar]
- 10.Pacreu S, Candil JF, Moltó L, Carazo J, Galinski SF. The perioperative combination of methadone and ketamine reduces post-operative opioid usage compared with methadone alone. Acta Anaesthesiol Scand. 2012;56:1250–6. doi: 10.1111/j.1399-6576.2012.02743.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Kim SI, Ok SY, Park SY, Kim M-G, Lee S-J, Noh JI, Chun HR, Suh H. Opioid sparing effect of low dose ketamine in patients with intravenous patient-controlled analgesia using fentanyl after lumbar spinal fusion surgery. Korean J Anesthesiol. 2013;64(6):524–8. doi: 10.4097/kjae.2013.64.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song JW, Shim JK, Song Y, Yang SY, Park SJ, Kwak YL. Effect of ketamine as an adjunct to intravenous patient-controlled analgesia, in patients at high risk of postoperative nausea and vomiting undergoing lumbar spinal surgery. Br J Anaesth. 2013;111(4):630–5. doi: 10.1093/bja/aet192. [DOI] [PubMed] [Google Scholar]
- 13.Yeom JH, Chon M-S, Jeon WJ, Shim J-H. Peri-operative ketamine with the ambulatory elastometric infusion pump as an adjuvant to manage acute postoperative pain after spinal fusion in adults: a prospective randomized trial. Korean J Anesthesiol. 2012;63(1):54–8. doi: 10.4097/kjae.2012.63.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pestieau SR, Finkel JC, Junqueira MM, Cheng Y, Lovejoy JF, Wang J, Quezado Z. Prolonged perioperative infusion of low-dose ketamine does not alter opioid use after pediatric scoliosis surgery. Paediatr Anesth. 2014;24(6):582–90. doi: 10.1111/pan.12417. [DOI] [PubMed] [Google Scholar]
- 15.Javery KB, Ussery TW, Steger HG, Colclough GW. Comparison of morphine and morphine with ketamine for postoperative analgesia. Can J Anaesth. 1996;43(3):212–5. doi: 10.1007/BF03011736. [DOI] [PubMed] [Google Scholar]
- 16.Abrishamkar S, Eshraghi N, Feizi A, Talakoub R, Rafiei A, Rahmani P. Analgesic effects of ketamine infusion on postoperative pain after fusion and instrumentation of the lumbar spine: a prospective randomized clinical trial. Med Arh. 2012;66(2):107–10. doi: 10.5455/medarh.2012.66.107-110. [DOI] [PubMed] [Google Scholar]
- 17.Nitta R, Goyagi T, Nishikawa T. Combination of oral clonidine and intravenous low-dose ketamine reduces the consumption of postoperative patient-controlled analgesia morphine after spine surgery. Acta Anaesthesiol Taiwan. 2013;51(1):14–7. doi: 10.1016/j.aat.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Hadi BA, Al Ramadani R, Daas R, Naylor I, Zelko R, Saleh M. The influence of anaesthetic drug selection for scoliosis surgery on the management of intraoperative haemodynamic stability and postoperative pain—pharmaceutical care programme. SAJAA. 2009;15(5):10–4. [Google Scholar]
- 19.Hadi BA, Daas R, Zelkó R. A raondomized, controlled trial of a clinical pharmacist intervention in microdiscectomy surgery – low dose intravenous ketamine as an adjunct to standard therapy. Saudi Pharm J. 2013;21:169–75. doi: 10.1016/j.jsps.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96(4):1004–17. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 21.Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 22.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elia N, Tramer MR. Ketamine and postoperative pain – a quantitative systematic review of randomised trials. Pain. 2005;113:61–70. doi: 10.1016/j.pain.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 24.Field RR, Wall MH. Delirium: past, present, and future. Seminars in Cardiothoracic and Vascular Anesthesia. 2013;17(3):170–9. doi: 10.1177/1089253213476957. [DOI] [PubMed] [Google Scholar]
- 25.Wein S, Spruyt O, Chapman R. Ketamine as a possible cause of delirium. Journal of Pharmacy Practice and Research. 2002;32(3):212–4. [Google Scholar]
- 26.Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26(9):985–1028. doi: 10.1016/j.ajem.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Johnston B, Kaushal A, Cheng D, Zhu F, Martin J. Ketamine added to morphine or hydromorphone patient-controlled analgesic for acute postoperative pain in adults: a systematic review and meta-analysis of randomized trials. Can J Anesth. 2015 doi: 10.1007/s12630-015-0551-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


