Abstract
Background
Poor sleep quality among people with chronic low back pain appears to be related to worse pain, affect, poor physical function and pain catastrophizing. The causal direction between poor sleep and pain remains an open question, however, as does whether sleep quality exerts effects on low back pain differently across the course of the day.
Purpose
This daily diary study examined lagged temporal associations between prior night sleep quality and subsequent day pain, affect, physical function and pain catastrophizing, the reverse lagged temporal associations between prior day pain-related factors and subsequent night sleep quality, and whether the time of day during which an assessment was made moderated these temporal associations.
Methods
Chronic low back pain patients (n = 105) completed structured electronic diary assessments five times per day for 14 days. Items included patient ratings of their pain, affect, physical function and pain catastrophizing.
Results
Collapsed across all observations, poorer sleep quality was significantly related to higher pain ratings, higher negative affect, lower positive affect, poorer physical function and higher pain catastrophizing. Lagged analyses averaged across the day revealed that poorer prior night sleep quality significantly predicted greater next day patient ratings of pain, and poorer physical function and higher pain catastrophizing. Prior poorer night sleep quality significantly predicted greater reports of pain, and poorer physical function, and higher pain catastrophizing, especially during the early part of the day. Sleep Quality × Time of Day interactions showed that poor sleepers reported high pain, and negative mood and low function uniformly across the day, whereas good sleepers reported relatively good mornings, but showed pain, affect and function levels comparable to poor sleepers by the end of the day. Analyses of the reverse causal pathway were mostly nonsignificant.
Conclusions
Sleep quality appears related not only to pain intensity but also to a wide range of patient mood and function factors. A good night’s sleep also appears to offer only temporary respite, suggesting that comprehensive interventions for chronic low back pain not only should include attention to sleep problems but also focus on problems with pain appraisals and coping.
Keywords: Chronic low back pain, daily diary, sleep quality, pain, physical function, lagged relationships, negative affect, positive affect, pain catastrophizing
Chronic low back pain (CLBP) is often associated with poor quality of life, negative affect, and functional impairment. Many people with chronic pain conditions also endorse poor sleep quality [1]. For example, Tang and colleagues [2] found a 53 percent prevalence of clinical insomnia among chronic pain patients, which is a rate 18 times higher than that of healthy controls. One set of questions about the temporal associations between poor sleep and pain revolves around causal direction [3]. It is still an open question whether poor sleep predominantly affects subsequent pain for people with chronic pain or vice versa. As well, very little is known about the extent to which sleep quality is related to other pain-related outcomes, such as psychological and physical function. That is, it is not definitively known whether poor sleep predicts lower physical function, whether lower physical function predicts poor sleep, or whether reciprocal relationships exist.
Daily diary studies of temporal precedence between pain and sleep have consistently linked poor sleep quality to greater pain intensity the subsequent day across diverse samples including adults with low back pain [4], adults with heterogenous chronic pain [5], adolescents with chronic pain [6], and a sample with a range of pain syndromes including CLBP, facial pain, and fibromyalgia [7]. However, the reverse association of prior day pain intensity predicting subsequent sleep quality has emerged less consistently [5,6]. Results from other studies have further suggested that the relationship between sleep quality and pain may be reciprocal in separate studies of adults with low back pain [4], women with back pain, facial pain, or fibromyalgia [8], and women with fibromyalgia [9]. Clearly, the issue of causal direction cannot be settled without better understanding of temporal precedence.
One contributor to these discrepant findings could be temporal proximity [4]. Pain levels vary considerably over the course of a day [10]. Many daily diary studies that have included repeated assessments of daily pain have analyzed means averaged across the whole day [4, 6], obscuring temporal effects of pain on sleep and vice versa. Alsaadi and colleagues [4] showed that pain upon waking appeared more strongly associated with subsequent sleep quality than the daily average of pain intensity. Conversely, both Alsaadi and colleagues [4], and Tang and colleagues [5], found that the relationship between sleep parameters and subsequent pain intensity appeared to decrease over the course of the day. Because results of Alsaadi [4] and Tang [5] suggest the ill-effects of poor sleep may dissipate over the course of the day, more frequent assessment of pain and emotional and behavioral functioning is necessary, not only for establishing causal direction but also for isolating distal versus proximal effects.
The literature is also limited by a near exclusive focus on relationships between sleep quality and pain intensity. Cognitive behavioral models of chronic pain emphasize that the experience of pain is complex and that one needs to go beyond simply looking at pain intensity to examine how pain is influenced (and influences) somatic (i.e., nociceptive input), cognitive (i.e., pain catastrophizing), emotional (i.e., negative affect), and behavioral (i.e., pain interference, downtime) domains. To understand the full impact of poor sleep quality on the well-being of people with chronic pain, it may therefore be necessary to assess additional domains beyond pain intensity. Indeed, Kothari and colleagues [11] report that poor sleep has detrimental effects not only on subsequent pain intensity but also on pain interference and negative mood. The reverse may also be the case, as suggested by results of Tang et al. [5], who showed that cognitive arousal prior to bedtime was related to poor sleep.
The present study aimed to extend findings regarding temporal associations between sleep quality and pain-related factors by frequently assessing multiple pain-related domains\in a daily diary study of 105 CLBP patients. Patients completed diary assessments five times per day for 14 days. This breadth and depth of assessment allowed us to examine sleep to pain/function, and pain/function to sleep pathways. We hypothesized that poor sleep quality would be related to higher levels of a number of pain-related domains, including higher levels of patient-reported pain intensity, pain interference, physical activity, downtime, negative mood and pain catastrophizing. We also hypothesized that sleep to pain/function, and pain/function to sleep relationships would depend on the time of day of the assessments. For instance, 9:00 pm pain intensity may predict subsequent sleep, whereas 9:00 am pain intensity may not. Therefore, we tested the interaction of sleep quality and timing of assessments on pain-related outcomes throughout the day. Simple slopes and regions of significance were calculated to probe the nature of the interactions.
Method
Participants
One hundred and twenty-one CLBP patients were recruited through referrals from staff at the pain clinics of Rush University Medical Center in Chicago, IL, Duke University Medical Center in Durham, NC, Memorial Hospital in South Bend, IN, and through advertisements in local newspapers and flyers provided at various health care agencies. Each participant received $150 for completion of the study. The protocol was approved by the Institutional Review Boards at Rush University Medical Center, Duke University Medical Center, and University of Notre Dame.
Patient inclusion criteria were: a) pain of the lower back stemming from degenerative disk disease, spinal stenosis, or disk herniation (radiculopathy subcategory), or muscular or ligamentous strain (chronic myofascial pain subcategory); b) pain duration of at least 6 months with an average intensity of at least 3/10 (with 0 being “no pain” and 10 “the worst pain possible”); and c) age between 18 and 70 years.
Exclusion criteria for patients were: a) current alcohol or substance abuse problems, or meeting DSM-IV criteria for alcohol or substance abuse or dependence (within the past 12 months); b) past or current psychotic or bipolar disorders; c) inability to understand English well enough to complete questionnaires; d) acute suicidality; and e) meeting criteria for obsessive-compulsive disorder or posttraumatic stress disorder within the past 2 years. A further exclusion criterion for patients was if their pain complaint was due to certain medical conditions (e.g., cancer, rheumatoid arthritis), migraine or tension headache, fibromyalgia, or complex regional pain syndrome.
Inclusion and exclusion criteria were assessed using a detailed medical and psychosocial history, including administration of the Mood Disorder, Psychotic Screening, and Substance Use Disorders modules of the Structured Clinical Interview for DSM-IV Axis I Disorders - Non-Patient Edition (SCID-IV/NP) [12].
Of the 121 patients recruited, eight patients declined to participate in the diary portion of the study, three patients withdrew before completing 14 days of data collection, four patients lost data due to PDA malfunctions, and one patient’s data were lost due to failure to upload it from the PDA at an appropriate time. Thus, the final sample was 105 patients. Female patients comprised 48.6% of the sample (n = 51). Demographic characteristics of patients not included in this investigation did not differ significantly from those who were included. See Table 1.
Table 1.
Modeling Daily Average Pain Intensity
| Level 1 Components: | Pain Intensity Intercept |
| Person Mean Centered Sleep Quality from the previous night | |
| Level 1 Residual | |
| Level 2 Components: | Patient Pain Intensity Intercept Deviation |
| Demographic Covariates (Employment; Disability Compensation) | |
| Level 2 Residual | |
Note. Level 1 components refer to within person processes linking sleep quality to subsequent pain-related factors measured the next day. These were entered as fixed effects. Level 2 components refer to between subject processes. A random effect allowed average pain intensity to vary across participants. Demographic covariates including employment and disability compensation were entered as fixed effects.
Electronic Diary
The PDA program signaled participants to complete five assessments each day, starting at 8:50 am and occurring every three hours until 8:50 pm. Frequent assessments were used because they help minimize retrospective bias in ratings [13]. Daily diary data obtained in this manner also appears to suffer little from reactivity effects that are sometimes caused by monitoring [14–15]. Variability in ratings within the day also can be captured well by this method [16]. Previous studies support the reliability, validity, and compliance with electronic diary strategies when used to assess pain, affect, and behavior [13–16]. Electronic diaries with time-stamped entries also allowed us to accurately assess when ratings were made, a process that cannot be accomplished with paper diary methods [15]. Finally, PDA technology allowed us to use branching algorithms that reduced participant burden by withholding irrelevant items.
Patients completed electronic diary measures for 14 consecutive days. We used the Experience Sampling Program (ESP) [17] on handheld Palm® Zire 22 PDAs, running the Palm OS platform. The PDA program was protected from participants altering the items or alarm times.
Measures
Sleep Quality
At their first diary entry of the day, participants rated their sleep quality the previous night. Specifically, participants were prompted, “Rate the overall quality of your sleep.” Participants responded using a 5 point likert-type scale (0 = not at all restful; 1 = a little restful; 2 = somewhat restful, 3= very restful, and 4 = extremely restful).
Patient-reported pain-related variables
At each assessment, patients also rated “how intense was your pain,” “to what degree did your pain interfere with you being physically active,” and “how much did you rest (sit, lie down) because of your pain” during the past 3 hours. Responses were made on 9-point scales with anchors at 0 (not at all), 2 (somewhat), 4 (much), 6 (very much), and 8 (extremely).
Patient state negative and positive affect
At each assessment, patients rated the extent to which they felt anxious, on edge, uneasy, sad, helpless, and discouraged during the past 3 hours, and these items were summed to create a composite Negative Affect rating. Patients also rated the extent to which they felt happy, lively, and cheerful over the past three hours, and these items were summed to create a composite Positive Affect rating. Items were derived from the Profile of Mood States-15 [18]. Responses were made on 9-point scales with anchors at 0 (not at all), 2 (somewhat), 4 (much), 6 (very much), and 8 (extremely). The scales demonstrated internal consistency (Negative Affect α = .96; Positive Affect α = .95).
Patient-reported pain catastrophizing
At each assessment, patients also rated, “When you felt pain during the past 3 hours, to what degree did you feel afraid that the pain may get worse?”, “When you felt pain during the past 3 hours, to what degree did you keep thinking about how much it hurts?, and “When you felt pain during the past 3 hours, to what degree did you feel that the pain was awful and overwhelming?” Items were adapted from the Pain catastrophizing Subscale of the Coping Strategies Questionnaire [19]. Responses were made on 9-point scales with anchors at 0 (not at all), 2 (somewhat), 4 (much), 6 (very much), and 8 (extremely). The scale demonstrated internal consistency (α = .94)
Procedure
Patients who inquired about participation underwent screening procedures over the telephone. Eligible patients attended an initial session, during which they signed IRB-approved consent forms to participate and completed questionnaires. Patients were instructed to carry the PDAs with them throughout the day for 14 consecutive days. Research assistants described and defined terms and items contained in the diary s and provided participants with printed instructions for later reference. Participants were asked to phone the research assistants with any problems or questions.
Starting at 8:50 am, and then again every three hours until 8:50 pm, participants were prompted by the PDA alarm to complete assessments. Participants had 15 minutes following this alert in which to respond to the PDA and the diary items. After the initial alarm, the PDA would emit a signal every 30-seconds until participants responded. Participants were also given the option to tap the screen to dismiss the alarms and delay the signal as long as they completed the assessment within 15 minutes. If participants did not respond in any way within 15 minutes of the original prompt, the time period was coded as missing data. The data for each assessment session was time stamped. After 14 days of data collection, participants returned the PDA, data were downloaded, and participants were debriefed.
Data Preparation
All item responses submitted past the 15-minute response interval were discarded. After deleting these responses, out of the 7,350 possible total diary responses, 80.01 – 87.06% were complete. For the possible 1470 sleep ratings 99.11% were rated by patients. This amount of complete data is in the range typically found in other electronic diary studies involving patients with pain [20].
Analysis
In addition to traditional between-subjects descriptive statistics, individual person means were computed within subjects across repeated measure variables. Bivariate correlations assessed the overall associations between these variables. In order to assess the impacts of changes in sleep and changes in pain-related domains on one another, within subject fluctuations were calculated by subtracting individual means from their raw data (i.e., Person Mean Centering). For example, if a patient reported an average sleep quality of 2, but on a specific instance reported a sleep quality of 3, that single instance would be 1 when person mean centered. If that same patient reported a sleep quality of 1, that instance would be −1 when person mean centered. Thus scores reflect individual improvements and declines in sleep quality from one’s own average. When person mean centered variables are entered as covariates into models, the models reflect how change in the independent variable (e.g. sleep) predicts change in the dependent variable (e.g. pain intensity). Hierarchical linear models (HLM) using maximum likelihood estimation were fit to assess the longitudinal associations between night-to-night changes in patient reported sleep quality and pain-related domains.
Although estimation of effect size within HLM has been debated, Cohen’s f2 was computed in order to provide an estimate of effect size for the association sleep quality and pain-related domains (see Selya et al. [21] for description of f2 computations). Given the multiple HLMs computed, a Bonferroni correction was applied (i.e. p <.05 ÷ 77 HLMs = p <.00065) to provide a more conservative criterion for statistical significance.
The first set of analyses explored the association between night-to-night changes in sleep quality and subsequent pain-related outcomes averaged across the day. Five daily pain intensity ratings were averaged to calculate the dependent variable that was then regressed on the patient’s person mean centered sleep quality rating. Here a two-level model is used to account for repeated measures (Level 1) nested within patients (Level 2). A random intercept was computed to account for the possibility that individuals could differ from one another with regard to their average overall pain. An example of this reduced form equation is as follows (see also Table 1 for all components in full form):
Next, we examined whether sleep quality predicted pain-related variables at each momentary assessment point throughout the day. An example of this equation is as follows:
Interaction effects based on time of day were then explored to determine whether the impact of sleep quality varies over the course of the day. Here a three-level model was used with repeated measures (Level 1) nested within days (Level 2) nested within patients (Level 3). The intercept was set as random to vary across days and patients. An example of this equation is as follows:
In the event that significant time of day interactions were observed, regions of significance were assessed. Such analyses would indicate the time of day during which sleep quality became significantly associated with, or became no longer significantly associated with, pain-related factors. These analyses were conducted using the Preacher, Curran and Bauer web utility [22].
Separate models also explored the reverse lagged relationships wherein pain-related variables predicted subsequent night sleep quality. These analyses included the assessment of changes in pain related outcomes averaged across the day to predict subsequent sleep quality. For example, the five daily pain intensity ratings were averaged, and then this average was subtracted from the patients overall pain mean. This produced an estimate of the degree to which the patient’s daily pain was less than or greater than that patient’s normal pain.
An example of this level-one equation is as follows:
Finally, recognizing that the impact of day time pain may vary by time of day, we computed separate models predicting sleep quality across the 5 daily assessments. An example of this level-one equation is as follows:
Results
Associations Among Average Sleep Quality and Average Pain-Related Domains
Descriptive statistics are presented in Table 2. The average sleep quality rating across patients and across observations was 1.92 (SD = .99), suggesting that on average patients experienced “somewhat restful” sleep. Average pain outcomes reported by patients fell in the low to moderate range. When collapsed across all observations, daily ratings of sleep quality were correlated significantly with patient daily ratings of pain intensity, interference, downtime, positive affect, negative affect and pain catastrophizing (Table 3). The direction of relationships indicate that patients who reported better average sleep tended to experience less pain and pain-related impairment over the course of the 14-day study than those reporting worse average sleep.
Table 2.
Demographic Characteristics
| Patient | |
|---|---|
| Gender (female) | 48.6% (n = 51) |
| Age in years (M, SD) | 46.30 (12.1) |
| Hispanic | 4.8% (n = 5) |
| African American | 15.2% (n = 16) |
| Caucasian | 80.0% (n = 84) |
| Employed | 40.0% (n = 42) |
| Disability Insurance | 34.3% (n = 36) |
| Length of Marriage (M, SD) | 14.30 (14.0) |
| Pain Duration (M, SD) | 9.04 years (7.8) |
Table 3.
Means, Standard Deviations and Correlations among study variables
| M | SD | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|
| 1 Sleep Quality | 1.92 | 0.66 | - | |||||
| 2 Pain Intensity | 3.09 | 1.63 | −.35** | |||||
| 3 Pain Interference | 2.69 | 1.86 | −.35** | .85** | ||||
| 4 Downtime | 2.43 | 1.48 | −.21* | .62** | .72** | |||
| 5 Negative Affect | 7.01 | 7.12 | −.34** | .62** | .57** | .49** | ||
| 6 Positive Affect | 7.75 | .48 | .49** | −.16 | −.18 | −.17 | −.34* | |
| 7 Pain Catastrophizing | 5.63 | 4.84 | −.27** | .78** | .76** | .60** | .73** | −.28** |
Note.
p<.05,
p <.01.
Sleep was rated on a five-point scale item 0 (not at all restful) to 4 (extremely restful). Pain Intensity, Interference and Downtime were rated on 9-point scales with anchors at 0 (not at all) to 8 (extremely). The Negative Affect Scale included six items and the Positive Affect scales included three items each using the same 9-point rating scale. The Pain Catastrophizing Scale was three items and used the same 9-point rating scale.
Associations Between Prior Night Sleep Quality and Subsequent Day Pain-Related Domains
Relationships between patient ratings of prior night sleep quality and averaged levels of pain-related factors the following day appear in Table 4. Gender, age, employment, and disability compensations were assessed as potential confounders. Age and gender were not significantly associated with any of the pain-related factors and were not included as covariates. Disability compensation was associated with all pain-related factors, and employment status was significantly associated with pain interference. In general, participants receiving workers compensation or social security disability benefits reported better pain-related outcomes. Patients working full or part time reported less pain interference than those who were unemployed. After accounting for significant potential confounders, fluctuations in sleep quality were significantly associated with subsequent pain intensity, negative affect, positive affect and pain catastrophizing across the following day.
Table 4.
Mixed Model Regressions of Sleep Quality with Subsequent Pain Outcomes
| Pain Interference | Pain Intensity | Negative Affect | Downtime | Pain Catastrophizing | Positive Affect | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | p | f2 | B | p | f2 | B | p | f2 | B | p | f2 | B | p | f2 | B | p | f2 | |
| Intercept | 2.89 | .00000 | 2.65 | .00000 | 5.54 | .00000 | 2.03 | .00000 | 4.60 | .00000 | 8.58 | .0000 | ||||||
| Sleep Quality | −.09 | .00134 | .00 | −.16 | .00000 | .01 | −.66 | .00000 | .01 | −.10 | .00128 | .00 | −.36 | .00000 | .01 | .36 | .0000 | .01 |
| Compensation | ||||||||||||||||||
| Workers Comp | 3.00 | .00007 | 2.96 | .00001 | 10.93 | .00042 | .93 | .13602 | 6.91 | .00102 | −4.28 | .0057 | ||||||
| SSDI | 1.47 | .00133 | 1.49 | .00007 | 4.65 | .00565 | 1.43 | .00006 | 3.26 | .00454 | −2.08 | .0140 | ||||||
| Other Insurance | −.64 | .26140 | −.08 | .87104 | −.67 | .75907 | .40 | .38218 | .11 | .94062 | −2.69 | .0166 | ||||||
| No Compensation | ||||||||||||||||||
| Employment | ||||||||||||||||||
| Full time | −1.04 | .01092 | ||||||||||||||||
| Part time | −1.37 | .01948 | ||||||||||||||||
| Student | −.62 | .16880 | ||||||||||||||||
| Unemployed | - | - | ||||||||||||||||
Note. SSDI = Social Security Disability Insurance.
f2 is an estimate of effect size and refers to variance accounted for by sleep quality.
After adjustment for confounders, results indicated that when patients experienced a night of sleep that was better than their own average, they reported significantly lower levels of pain intensity, downtime, pain catastrophizing, and negative affect, and higher levels of positive affect. Sleep quality was significantly associated with subsequent pain interference and downtime the following day by conventional standards, but not after correction for multiple tests. Effect size estimates indicated that on the temporal associations of individual changes in sleep quality with subsequent pain intensity, pain catastrophizing, negative affect, and positive affect the following day were of small magnitude (f2 = .01).
Hour by Hour Analysis of Sleep Quality and Pain-Related Domains
Averaging pain outcomes across the day may mask hour-to-hour variability, and so associations of sleep with subsequent pain-related domains across each assessment period are reported in Table 4. These findings indicate that sleep quality is associated with subsequent pain-related domains at various times throughout the day, with all significant associations (after a Bonferroni correction of p<.00065) for pain intensity, pain interference, negative affect, and pain catastrophizing emerging in the morning hours. Effect sizes for pain intensity (f2 = .05), pain catastrophizing (f2 = .05), and negative affect (f2 = .06) assessed at 8:50 AM were stronger than when averaged across the entire day, but were still small in terms of magnitude. Sleep quality was positively associated with positive affect measured at 11:50 AM (f2 = .01). Other temporal associations assessed later in the day were significant by conventional standards (p<.01 and p <.05), however, the effect sizes were of smaller magnitudes (f2 = .01), and not significant after the Bonferroni correction.
Interaction of Sleep Quality and Time of Day Predicting Pain-Related Domains
The variability in significant associations between sleep quality and pain-related domains across the day supported the hypothesis that the impact of sleep quality on pain-related domains may differ depending on the time of day. To fully test this notion, Sleep Quality × Time of Day interactions were tested using all assessment points for each day. Table 6 presents Sleep Quality × Time of Day mixed model regressions that yielded significant interaction terms. Results indicated that Time of Day significantly moderated the temporal associations between within-subject changes in sleep quality, and subsequent patient reported pain intensity (B = .03, p <.00065), pain interference (B = .01, p =.0280), pain catastrophizing (B = .07, p <.00065), and negative affect (B = .09, p < .00065). Time of Day did not significantly moderate the temporal associations of within-subject changes in sleep quality with downtime (B = −.01 SE = .01, p = .149) or with positive affect (B = −.01 SE = .01, p = .317).
Table 6.
Mixed Model Regressions of the Interaction of Sleep Quality and Time of Day Moderations Table
| Pain Intensity | Pain Interference |
Pain Catastrophizing | Negative Affect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | B | SE | p | B | SE | p | |
| Sleep Quality | −.32 | .04 | <.00001 | −.15 | .05 | .0013 | −.74 | .10 | <.00001 | −1.17 | .16 | <.00001 |
| Time of Day | .02 | .00 | <.00001 | .04 | .00 | <.00001 | .05 | .01 | <.00001 | .07 | .01 | <.00001 |
| Interaction | .03 | .01 | <.00001 | .01 | .01 | .0280 | .07 | .01 | <.00001 | .09 | .02 | <.00001 |
Note. Time of Day was measured by centering the time of assessment at 8:50 AM. The Interaction term refers to the interaction of Sleep Quality and Time of Day.
Probing Interactions with Simple Slopes and Regions of Significance
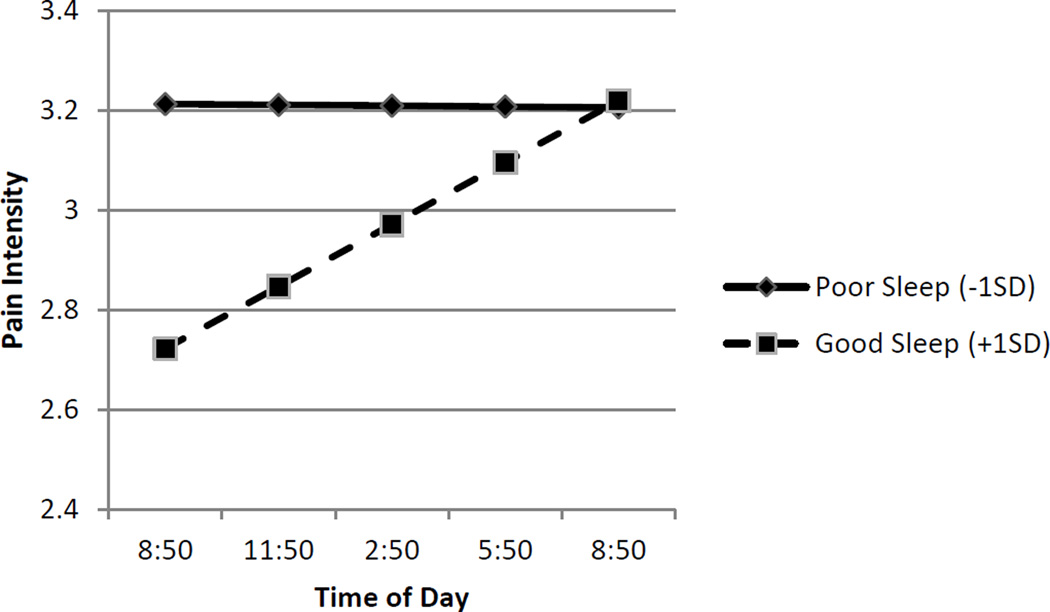
To dissect the significant interactions, simple effects were computed, following recommendations of Aiken and West (1991), to estimate the effect of time of day on pain-related factors when sleep quality was good and poor. Because each individual has their own individual within-subject standard deviation, “good sleep quality” was operationalized as 1 SD above the overall person-centered mean (i.e. +.76 units of sleep quality), and “poor sleep quality” was operationalized as 1 SD below the overall person-centered mean (i.e., −.76 units of sleep quality). This operationalization was used to provide a visual summary of the data in Figures 1 through 4. Figure 1 depicts the interaction of within-subject changes in sleep quality and pain intensity. Following evenings of poor sleep quality (−1 SD), patients endorsed higher levels of pain intensity (simple intercept B = 3.21, p < .01) that were relatively stable across the day (simple slope B= .00, p = .916). However, following nights of good sleep quality (+1 SD), participants reported lower levels of pain intensity (simple intercept B = 2.72, p < .01), that significantly increased over the course of the day (simple slope B = .04, p < .01). Thus, by the end of the day participants reported comparable levels of pain intensity regardless of whether their sleep quality was poorer or better than their own average. Analyses of regions of significance indicated that the relationship between sleep quality and pain intensity was no longer significant 9.21 hours after the 9 am assessment, or at approximately 6 pm. The shape of the effect is depicted in Figure 1, and it suggests that the interaction between sleep quality and time of day was due to the beneficial effects of good sleep quality decreasing over the course of the day.
Figure 1.
Link between Sleep Quality and Subsequent Pain Intensity is Moderated by Time of Day. Sleep quality refers to within-subject changes in sleep quality. Poor sleep (solid line) is identified as one Standard Deviation below the average person centered mean. Good sleep (dashed line) is identified +1 SD refers to one Standard Deviation above the average person centered mean. Good sleep (dashed line) is associated lower pain intensity in the morning hours, but this pain intensity increases over the course of the day.
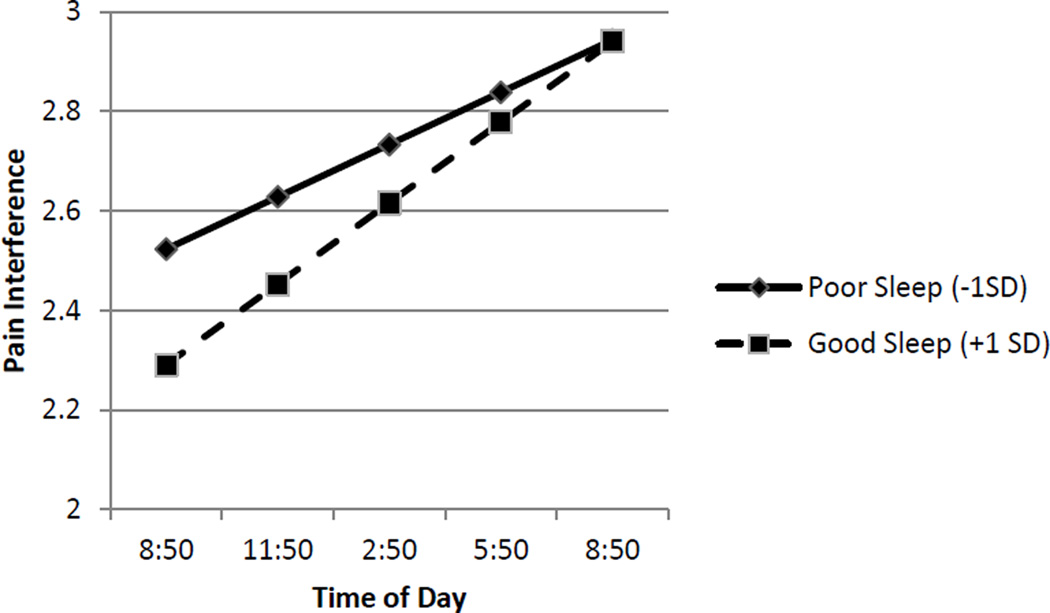
Figure 4.
Link between Sleep Quality and Subsequent Pain Interference is Moderated by Time of Day. Sleep quality refers to within-subject changes in sleep quality. Poor sleep (solid line) is identified as one Standard Deviation below the average person centered mean. Good sleep (dashed line) is identified +1 SD refers to one Standard Deviation above the average person centered mean. Good sleep (dashed line) is associated lower pain interference in the morning hours. Pain interference increases over the course of the day for both groups.
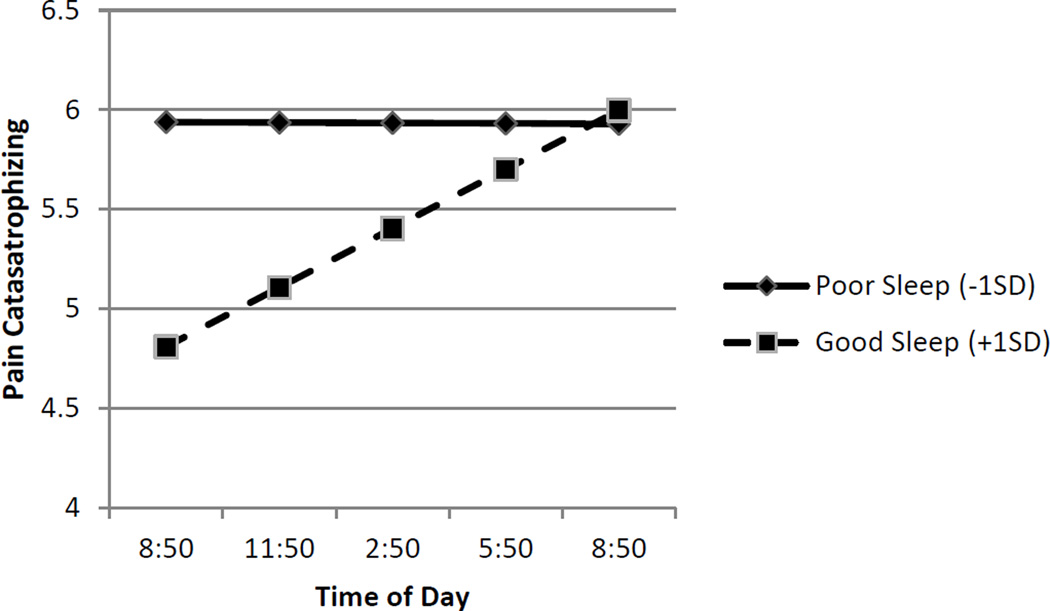
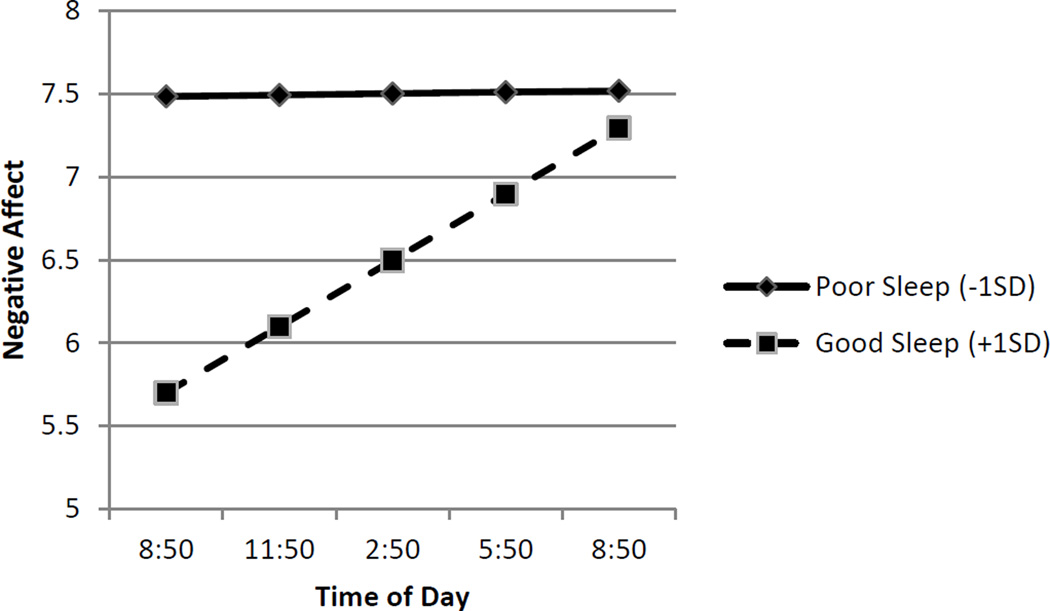
Similar patterns were observed for pain catastrophizing and negative affect (see Figures 2 and 3). When sleep quality was poor (− 1 SD), patients reported higher and stable levels of pain catastrophizing (simple intercept B = 5.34, p < .01; simple slope B = .01, p = .334) and negative affect (simple intercept B = 7.48, p < .01; simple slope B =.00, p = .884). Following evenings of good sleep quality (+1 SD), patients reported lower but significantly increasing levels of pain catastrophizing (simple intercept B = 5.41, p < .01; simple slope B = .09, p < .01) and negative affect (simple intercept B = 5.70, p < .01; simple slope B = .13, p < .01) over the course of the day. Analyses of regions of significance indicated that the relationship between sleep quality and pain catastrophizing was no longer significant 8.81 hours after the morning assessment, that is, by approximately 6 pm. For negative affect, the relationship was no longer significant 10.37 hours after the morning assessment, that is, by approximately 7 pm. As in the case of pain intensity, the shape of the effects (see Figures 2 and 3) suggests that the interaction between sleep quality and time of day was due to the beneficial effects of sleep decreasing over the course of the day.
Figure 2.
Link between Sleep Quality and Subsequent Pain Catastrophizing is Moderated by Time of Day. Sleep quality refers to within-subject changes in sleep quality. Poor sleep (solid line) is identified as one Standard Deviation below the average person centered mean. Good sleep (dashed line) is identified +1 SD refers to one Standard Deviation above the average person centered mean. Good sleep (dashed line) is associated lower pain catastrophizing in the morning hours, but this pain catastrophizing increases over the course of the day.
Figure 3.
Link between Sleep Quality and Subsequent Negative Affect is Moderated by Time of Day. Sleep quality refers to within-subject changes in sleep quality. Poor sleep (solid line) is identified as one Standard Deviation below the average person centered mean. Good sleep (dashed line) is identified +1 SD refers to one Standard Deviation above the average person centered mean. Good sleep (dashed line) is associated lower negative in the morning hours, but this negative affect increases over the course of the day.
The shape of the interaction was different in the case of pain interference (See Figure 4). When sleep quality was poor (−1 SD), pain interference was relatively low but increased significantly over the course of the day (simple intercept B = 2.52, p < .01, simple slope B = .40, p= < .01). When sleep quality was good (+1 SD), pain interference was also relatively lower, but increased at a higher rate (simple intercept B = 2.29, p < .01; simple slope B = .05, p < .01) over the course of the day than when sleep quality was poor. Regions of significance analyses indicated that the relationship between sleep quality and pain interference was no longer significant 7.04 hours after the 9 am assessment, that is, by approximately 4 pm. See Figure 4.
In general, the small or nonsignificant relationships between Time of Day and patient reported pain-related domains for those reporting poor sleep, coupled with significant positive relationships between Time of Day and pain-related domains for those reporting good sleep, suggest that the beneficial impact of good sleep quality was most pronounced in the morning (i.e., lower levels of pain and pain related functional impairments). The benefits for those reporting good sleep, however, appeared to erode over the course of the day, but good sleep nevertheless appeared to exert protective effects for most of the day.
Associations Between Prior Day Pain-Related Domains and Subsequent Night Sleep Quality
Tests of the reverse temporal pathway, from prior pain, affect, physical function, and pain catastrophizing to subsequent sleep, revealed no significant relationships between pain averaged over the course of the prior day and subsequent sleep quality. Only morning (8:50 am) positive affect, and evening (8:50pm) negative affect were significantly associated with subsequent sleep quality at the p<.05 level, indicating very little support for this proposed pathway.
Discussion
Although it is clear that sleep quality and pain intensity are related, especially among people with chronic pain [1], less attention has been devoted to whether sleep quality is related to overall functioning and other important pain-related variables such as mood, catastrophizing, and pain interference. Unclear, as well, is the causal direction of effects. Using daily diary methods, we examined whether prior day pain and function predicted next night sleep quality, and/or whether prior night sleep quality predicted next day pain and function. Results of these lagged analyses were generally consistent with a temporal association in which worse sleep quality the prior night had detrimental effects on pain, affect, physical function and pain catastrophizing the following day. However, time of day of assessments moderated these temporal associations, suggesting that effects of sleep quality were not uniform across the course of the day.
Results of analyses using assessments collapsed across days and times showed that, overall, sleep quality was correlated with patient-reported pain intensity, pain interference, downtime, positive affect, negative affect, and pain catastrophizing in directions indicating that poor sleep was related to greater pain intensity, decreased physical function, and higher levels of negative affect and pain catastrophizing. These results extend past work by revealing that sleep quality is related to a wide range of patient experiences, including how much time patients rest due to pain and the degree to which patients make catastrophic appraisals of pain-related phenomena.
To replicate and extend past findings that poorer sleep quality leads to subsequent increases in pain, we tested whether prior night sleep quality predicted next day pain intensity, mood, and function with the latter values averaged over the course of the day. Again we found that sleep quality was significantly related to a wide range of patient-reported pain-related domains. Consistent with past reports [5,6], lagged analyses showed that a prior night’s sleep that was poorer than average was related to subsequently higher levels of next day pain intensity. Second, extending past findings, we also found that a poor night’s sleep was related to greater next day negative affect, and pain catastrophizing, as well as lower positive affect. Thus, a person with low back pain following a night of poor sleep may be expected to have a day in which he or she is more irritable, sad, and nervous than usual, spends more time resting due to pain than usual, and may ruminate more about pain and view pain as more overwhelming and uncontrollable than usual. The last may be particularly important in that increased pain catastrophizing following poor sleep may undermine a patient’s ability to cope productively with events or demands that may occur in the course of a day.
As suggested by results of previous studies [4–6], lagged effects involving single or averaged pain values for the next day may obscure possible variability in associations between sleep quality and pain, physical function and pain catastrophizing over the course of the day. We examined these associations for all five daily assessment periods (viz., 9:00 am, 12:00 pm, etc.). Results suggested that analysis of average daily values may have indeed masked important phenomena. Replicating results of Alsaadi and Tang [4–6], we found that the relationship between prior night sleep quality and next day pain intensity was strongest during the morning, becoming nonsignificant by later in the day. Extending their findings, we also found that associations with subsequent negative affect, and pain catastrophizing were strongest in the morning.
The nature of the declining relationships between sleep quality and next day pain-related domains is not immediately clear from examining coefficients describing linear associations, as shown in Table 5. These coefficients could signify that poor sleepers recovered or that good sleepers worsened as the day progressed. To address this issue, we tested Sleep Quality × Time of Day interactions. Results suggested that when patients reported a night of poor sleep they also reported a consistently bad day of elevated pain intensity, pain interference, negative affect and pain catastrophizing. Following a night of better than average sleep, on the other hand, patients reported a good morning of relatively lower pain intensity, negative affect and pain catastrophizing. However, these beneficial effects of good sleep appeared to erode so much that by roughly 6:00 pm, their pain intensity, pain interference, negative affect, and pain catastrophizing were no longer significantly better than for days after nights that they slept poorly. A similar interaction was observed with pain interference, but was not significant after our correction for multiple tests. For pain interference, the erosion effect appeared most pronounced as the beneficial effects of a good night’s sleep had vanished by 4:00 pm. Thus, within-person analyses using average values over the whole day may accurately capture the experience of people having had a poor night’s sleep but miss what may be a critical phenomenon for people who slept well. A better than average night’s sleep may confer salutary effects on the pain and function of people with chronic pain especially during the early part of the day, but these effects may be diminished by the efforts needed to endure physical pain over the course of the day. Good sleep may be necessary for optimal functioning but may not be sufficient to maintain it.
Table 5.
Mixed Model Regressions of Sleep Quality with Subsequent Pain Outcomes
| 8:50 am | 11:50am | 2:50pm | 5:50pm | 8:50pm | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DV Predicted by Sleep | B | f2 | B | f2 | B | f2 | B | f2 | B | f2 |
| Pain Intensity | −.42*** | .05 | −.15** | .01 | −.07 | .00 | −.04 | .00 | −.09 | .00 |
| Pain Interference | −.26*** | .02 | −.07 | .00 | .03 | .00 | −.04 | .00 | −.09 | .00 |
| Negative Affect | −1.52*** | .06 | −.55** | .01 | −.41** | .00 | −.38 | .00 | −.52* | .00 |
| Positive Affect | .34** | .01 | .49*** | .01 | .33** | .01 | .34** | .01 | .31* | .01 |
| Downtime | .04 | .00 | −.13** | .01 | −.10 | .00 | −.10 | .00 | −.17** | .01 |
| Pain Catastrophizing | −.93*** | .05 | −.38** | .01 | −.19 | .00 | −.23 | .00 | −.09 | .00 |
Note.
p<.00065,
p<.01,
p<.05.
DV= Dependent Variable.
All coefficients are unstandardized beta weights. Values reported are unstandardized mixed model regression coefficients. f2 is computed as a measure of effect size, and provides an estimate of variance accounted for by sleep quality. Pain Interference is adjusted for Employment and Disability Compensation. All others are adjusted for Disability Compensation.
We also evaluated the reverse causal pathway (pain → sleep), and the possibility of a reciprocal, negative spiral. Results support conclusions from a recent review [3], suggesting that the pathway leading from increased pain to poor sleep is not as strong as the pathway leading from poor sleep to increased pain. Here, none of the lagged effects between prior day pain-related values averaged over the day and subsequent sleep quality were significant. Indeed, for analyses of each assessment period and subsequent sleep, only two of 36 effects were significant by conventional standards. Despite the intuitive appeal of a high pain intensity day undermining a sound night’s sleep, the weight of extant evidence does not favor this notion. Nevertheless, research efforts should continue. One limitation of many diary studies is that assessments cease at bedtime. Assessment of pain episodes that occur during the night, awaken the patient and thus disturb sleep, may help reveal the much sought after negative spiral in which high pain begets worse sleep which in turn begets worse pain. Preliminary results among patients recovering from orthopedic surgery indicate that pain flares at night are one of the primary disruptors of sleep [23].
The chief limitation of the present study was the exclusive reliance on patient-reported ratings of sleep quality and pain-related factors, and indeed, we used only one item to do so. Studies have included other methods, such as wrist actigraphy or polysomnography to gain more objective measures of sleep quality. Although results have been mixed, future work may benefit from more comprehensive assessment batteries. Effect sizes of the study results suggest that within-subject fluctuations in sleep quality over a two week period account for approximately one percent of the variance in these pain-related phenomena when averaged over the course of the day, and two to six percent of the variance when assessed at 9 AM. Although small in magnitude, given the chronic nature of sleep disturbances and low back pain, it is plausible that these small effects could accumulate into patterns of disability over extended periods of time. The study had ample power to detect such small effects, and suggests that future studies may require large samples with many observations to further elucidate the temporal associations between sleep and pain –related factors.
Accumulating results of temporal associations between sleep quality and pain-related factors among people with chronic pain conditions supports the need to include sleep interventions in chronic pain treatment regimens, especially for people with persistent sleep problems. Recent randomized controlled trials of cognitive-behavioral therapy for insomnia (CBT-I) among people with chronic pain have shown promise, particularly with improving sleep quality [24]. Less consistent have been improvements in pain and function [24]. These smaller improvements for pain and function may be partly due to the effects we report above. Among people with poor sleep, CBT-I may have favorable effects on pain and function that are manifest mostly in the morning. By evening, the benefits have eroded – a phenomenon that may be reflected in small overall improvements in pain and function following CBT-I. Attention to coping with the more general cognitive, emotional, and behavioral effects of chronic pain may also be needed to provide a comprehensive treatment regimen. A few studies [25–27] report efforts to combine CBT-I with more general CBT for chronic pain. Results were mixed and again favored improvements in sleep parameters over more general pain-related improvements. Still, these efforts are promising and support the idea of a hybrid approach that combines attention to sleep problems with attention to appraisal and coping problems. In sum, as may be true for people suffering from many physical ailments, enhancing sleep quality may help to enrich the quality of life for people with chronic low back pain by reducing pain, physical dysfunction and pain catastrophizing.
Acknowledgments
The study was funded by the National Institutes of Health (#: 1R01NR010777).
Footnotes
Ethics
This study was approved by the Institutional Review Boards at Rush University Medical Center, Duke University Medical Center, and University of Notre Dame. All participants provided informed consent as part of this study, and the study was conducted according to APA ethical standards.
COI statement
We have no conflicts of interest to report.
Contributor Information
James I. Gerhart, Rush University Medical Center.
John W. Burns, Rush University Medical Center.
Kristina M. Post, University of La Verne.
David A. Smith, University of Notre Dame.
Laura S. Porter, Duke University Medical Center.
Helen J. Burgess, Rush University Medical Center.
Erik Schuster, Rush University Medical Center.
Asokumar Buvanendran, Rush University Medical Center.
Anne Marie Fras, Duke University Medical Center.
Francis J. Keefe, Duke University Medical Center.
References
- 1.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8(2):119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 2.Tang NK, Wright KJ, Salkovskis PM. Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. J Sleep Res. 2007;16(1):85–95. doi: 10.1111/j.1365-2869.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- 3.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alsaadi SM, Mcauley JH, Hush JM, et al. The bidirectional relationship between pain intensity and sleep disturbance/quality in patients with low back pain. Clin J Pain. 2014;30(9):755–765. doi: 10.1097/AJP.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 5.Tang NK, Goodchild CE, Sanborn AN, Howard J, Salkovskis PM. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: a multilevel daily process study. Sleep. 2012;35(5):675A–687A. doi: 10.5665/sleep.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewandowski AS, Palermo TM, De la motte S, Fu R. Temporal daily associations between pain and sleep in adolescents with chronic pain versus healthy adolescents. Pain. 2010;151(1):220–225. doi: 10.1016/j.pain.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'brien EM, Waxenberg LB, Atchison JW, et al. Negative mood mediates the effect of poor sleep on pain among chronic pain patients. Clin J Pain. 2010;26(4):310–319. doi: 10.1097/AJP.0b013e3181c328e9. [DOI] [PubMed] [Google Scholar]
- 8.O'brien EM, Waxenberg LB, Atchison JW, et al. Intraindividual variability in daily sleep and pain ratings among chronic pain patients: bidirectional association and the role of negative mood. Clin J Pain. 2011;27(5):425–433. doi: 10.1097/AJP.0b013e318208c8e4. [DOI] [PubMed] [Google Scholar]
- 9.Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68(2–3):363–368. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- 10.Jamison RN, Brown GK. Validation of hourly pain intensity profiles with chronic pain patients. Pain. 1991;45(2):123–128. doi: 10.1016/0304-3959(91)90176-X. [DOI] [PubMed] [Google Scholar]
- 11.Kothari DJ, Davis MC, Yeung EW, Tennen HA. Positive affect and pain: mediators of the within-day relation linking sleep quality to activity interference in fibromyalgia. Pain. 2015;156(3):540–546. doi: 10.1097/01.j.pain.0000460324.18138.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders- Non-patient edition (SCID-I/NP, Version 2.0) New York: Biometrics Research Department; 1996. [Google Scholar]
- 13.Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine. 1994;16:199–202. [Google Scholar]
- 14.Cruise CE, Broderick J, Porter L, Keall A, Stone AA. Reactive effects of diary self-assessment in chronic pain patients. Pain. 1996;67:253–258. doi: 10.1016/0304-3959(96)03125-9. [DOI] [PubMed] [Google Scholar]
- 15.Jamison RN, Raymond SA, Levine JG, Slawsby EA, Nedeljkovic SS, Katz NP. Electronic diaries for monitoring chronic pain: 1 year validation study. Pain. 2001;91:277–285. doi: 10.1016/S0304-3959(00)00450-4. [DOI] [PubMed] [Google Scholar]
- 16.Peters ML, Sorbi MJ, Kruise DA, Kerssens JJ, Verhaak PFM, Bensing JM. Electronic diary assessment of pain, disability and psychological adaptation in patients differing in duration of pain. Pain. 2000;84:181–192. doi: 10.1016/s0304-3959(99)00206-7. [DOI] [PubMed] [Google Scholar]
- 17.Barrett LF, Barrett DJ. An introduction to computerized experience sampling in psychology. Social Science Computer Review. 2001;19(2):175–185. [Google Scholar]
- 18.Cranford JA, Shrout PE, Iida M, Rafaeli E, Yip T, Bolger N. A procedure for evaluating sensitivity to within-person change: can mood measures in diary studies detect change reliably? Pers Soc Psychol Bull. 2006;32(7):917–929. doi: 10.1177/0146167206287721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17(1):33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 20.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical. Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 21.Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ. A Practical Guide to Calculating Cohen's f(2), a Measure of Local Effect Size, from PROC MIXED. Front Psychol. 2012;3:111. doi: 10.3389/fpsyg.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- 23.Büyükyilmaz FE, Şendir M, Acaroğlu R. Evaluation of night-time pain characteristics and quality of sleep in postoperative Turkish orthopedic patients. Clin Nurs Res. 2011;20(3):326–342. doi: 10.1177/1054773811406110. [DOI] [PubMed] [Google Scholar]
- 24.Finan PH, Buenaver LF, Coryell VT, Smith MT. Cognitive-Behavioral Therapy for Comorbid Insomnia and Chronic Pain. Sleep Med Clin. 2014;9(2):261–274. doi: 10.1016/j.jsmc.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pigeon WR, Moynihan J, Matteson-rusby S, et al. Comparative effectiveness of CBT interventions for co-morbid chronic pain & insomnia: a pilot study. Behav Res Ther. 2012;50(11):685–689. doi: 10.1016/j.brat.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang NK, Goodchild CE, Sanborn AN, Howard J, Salkovskis PM. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: a multilevel daily process study. Sleep. 2012;35(5):675A–687A. doi: 10.5665/sleep.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitiello MV, Mccurry SM, Shortreed SM, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the lifestyles randomized controlled trial. J Am Geriatr Soc. 2013;61(6):947–956. doi: 10.1111/jgs.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]