Abstract
The nitrogen dioxide/oxides of nitrogen (NO2/NOX) ratio is an important surrogate for NO to NO2 chemistry in dispersion models when estimating NOX impacts in a near-road environment. Existing dispersion models use different techniques and assumptions to represent NO to NO2 conversion and do not fully characterize all of the important atmospheric chemical and mechanical processes. Thus, “real-world” ambient measurements must be analyzed to assess the behavior of NO2/NOX ratios near roadways. An examination of NO2/NOX ratio data from a field study conducted in Las Vegas, Nevada (NV), from mid-December, 2008 through mid-December, 2009 provides insights into the appropriateness of assumptions about the NO2/NOX ratio included in dispersion models. Data analysis indicates multiple factors affect the downwind NO2/NOX ratio. These include spatial gradient, background ozone (O3), source emissions of NO and NO2, and background NO2/NOX ratio. Analysis of the NO2/NOX ratio spatial gradient indicates that under high O3 conditions, the change in the ratio is fairly constant once a certain O3 threshold (≥ 30 ppb) is reached. However, under low O3 conditions (< 30 ppb), there are differences between weekdays and weekends, most likely due to a decline in O3 concentrations during the weekday morning hours, reducing the O3 available to titrate the emitted NO, allowing lower NO2/NOX ratios. These results suggest that under high O3 conditions, NOX chemistry is driving the NO2/NOX ratios whereas under low O3 conditions, atmospheric mixing is the driving factor.
Keywords: NO, NO2, NOX, near-source, air pollution, motor vehicle emissions
Graphical Abstract
1. Introduction
1.1. Emitted NO2/NOX Ratios
Urban area oxides of nitrogen (NOX) emissions are primarily from fuel combustion sources such as power generation facilities and transportation sources (U.S. EPA, 2010a). In the U.S., for calendar year 2010, NOX emissions from transportation sources comprised 38% of the national inventory. In some urban areas, NOX emissions from transportation sources (e.g., highway vehicles) may contribute substantially more to the area’s total NOX emissions than other sources due to the area’s mix of anthropogenic emission sources. While U.S. NOX emissions from highway vehicles have been declining for many years, a 63% decrease from 2003 to 2016, NOX emissions from highway vehicles continue to be a public health concern (U.S. EPA, 2010b, 2016).
Numerous studies have reported either nitric oxide (NO), nitrogen dioxide (NO2), or NOX concentrations for the urban or near-road environment (Alvarez et al., 2008; Beckerman et al., 2008; Carslaw and Beevers, 2005; Clements et al., 2009; Costantini et al., 2016; Gilbert et al., 2003; McAdam et al., 2011; Yasuyuki et al., 2014). Some studies have also reported NO2/NOX ratios for the urban or near-road environment. For example, Richmond-Bryant et al. (2017) reported the average NO2/NOX ratio for the Las Vegas Near Road Study was 0.39 and Costantini, et al. (2016) reported an average NO2/NOX ratio of 0.36 from the Advanced Collaborative Emissions Study (ACES). Some studies have reported very low NO2/NOX ratios. For example, Wild et al. (2017) reported an average on-road NO2/NOX ratio of 0.053 and Jimenez et al. (2000) reported an average NO2/NOX ratio of 0.078. Several factors may be responsible for the wide range of reported NO2/NOX concentrations, such as differences in measurement instrumentation, distance from roadway, vehicle fleet mix, and meteorology. Carslaw et al. (2005) and Mavroidis and Chaloulakou (2011) have reported an upwards trend in the NO2/NOX ratio that may be due to changes in the vehicle fleet mix and emission control technologies. The implication of these differing NO2/NOX ratios, which span nearly a factor of 10, is that the frequently assumed NO2/NOX ratio of 5% by volume is inappropriate for most freeways (Wang et al., 2011). The NO2/NOX ratio may also be impacted by the local vehicle fleet mix, especially for freeways with a large fraction of heavy-duty trucks and the freeway’s diurnal profile (Wang et al., 2011). Thus, a more robust NO2/NOX emission ratio is needed for dispersion modeling scenarios involving the near road environment.
1.2. NO, NO2, NOX Chemistry
NO, NO2, and NOX atmospheric chemistry processes are some of the most important chemical processes that are simulated in dispersion models. The atmospheric chemistry of NO to NO2 conversion is complex and involves multiple chemical and photolytic reactions, as described in Atkinson (2000) and Seinfeld and Pandis (2012). The most common pathway for the conversion of NO to NO2 occurs via oxidation by ozone (O3). This conversion happens relatively quickly and is often assumed to be instantaneous, though the reaction rate can actually be on the order of tens of seconds to a few minutes, depending on the amount of available reactants such as O3 and volatile organic compounds (VOCs), solar energy, and ambient meteorological conditions. The formation of NOY (total reactive nitrogen) species during these atmospheric chemistry reactions includes oxidation of NO2 to nitrate radical (NO3·) and nitric acid (HNO3), as well as the photodecomposition of NO2 back to NO (Atkinson, 2000; Bange et al., 1991; NAS, 1992; Seinfeld and Pandis, 2012).
1.3. Relevance of Chemistry and Field Data to Dispersion Models
Dispersion models are typically used to simulate real-world atmospheric and chemical processes such as physical characteristics of source emissions, atmospheric transport, photochemical reactions, deposition, and meteorological interactions [e.g., NO2 concentrations] (Turner, 1979; U.S. EPA, 2011b). In roadway applications these models may be used to determine source impacts over relatively small spatial scales from the source to the receptor (e.g., fom a freeway or other major arterial highway to a neighborhood a few hundred meters distant) (Benson, 1984; Berkowicz et al., 2011; CERC, 2015a, b, c; Hanrahan, 1999; Härkönen et al., 1996; U.S. EPA, 2014a, b). Estimating the initial partitioning of NO and NO2, the total NOX, and available O3 is important when modeling near-road scenario impacts, since atmospheric and chemical reaction processes vary over the course of a day (Wang et al., 2011). Analysis of field data can shed light on limitations in the models for development of strategies to model NO to NO2 conversion more accurately.
1.4. Near Road Study
Understanding near-road NO2 impacts are important due to the number of people living in close proximity to major transportation sources (e.g., four-lane highway, railroad, or airport) (U.S. EPA, 2010b). It is estimated that approximately 45 million people live within 100 m of a major transportation source (U.S. EPA, 2010b). Numerous health effects (e.g., asthma, cardiovascular, and non-asthma respiratory symptoms) from populations living near major transportation facilities have been reported by multiple investigative studies (HEI, 2010; Janssen et al., 2003; Mejía et al., 2011; Van Roosbroeck et al., 2007). Due to these health concerns, the U.S. Environmental Protection Agency (EPA) in 2010 revised the primary NO2 National Ambient Air Quality Standard (NAAQS) to require U.S. state and local environmental agencies to install and operate near-road NO2 ambient air quality monitoring stations (U.S. EPA, 2010b). The NAAQS revision purposely required ambient monitoring sites near high volume freeways since this source type is expected to most frequently have the highest NO2 concentrations in an urban area.
Ambient air measurements alone may not always be sufficient for air quality management applications due to technical and resource considerations (U.S. EPA, 2011a). Integrated or continuous ambient air measurements can be resource intensive thus there is a need to use dispersion models to simulate real-world atmospheric and chemical processes. The NOX data collected from a long-duration study conducted in Las Vegas, Nevada (NV) from mid-December 2008 through mid-December 2009 (Kimbrough et al., 2013b) provides a unique opportunity to examine NO to NO2 conversion rates to guide recommendations for photochemical reaction implementation in dispersion models. In this paper, we focus on identifying relationships between the NO2/NOX ratio using ambient measurements and meteorological and chemical processes to better understand which factors are the most important for NO to NO2 conversion in the near-road environment.
2. Methods
2.1. Dispersion Models – Treatment of NO to NO2 conversion
Since NO is rapidly converted to NO2 in the atmosphere, dispersion models cannot directly model NO. Instead, dispersion models use the sum of NO and NO2 (NOX) as an inert pollutant for modeling and use parameterizations of NOX chemistry to determine the speciation between NO and NO2. Thus, the NO2/NOX ratio, which is an expression of the speciation between NO and NO2, can be used as a surrogate for the NO to NO2 conversion and the chemistry occurring in the atmosphere. The most simplistic implementation of NO to NO2 conversion is full conversion. Intermediate options are based on the generic behavior of NO2 and NOX. For example, one method in AERMOD (American Meteorological Society/Environmental Protection Agency Regulatory Model) (U.S. EPA, 2014b) uses an ambient ratio technique that computes the NO2/NOX ratios based on a sixth-order regression of ambient NO2 and NOX data (Podrez, 2015). Utilizing this method, NO2/NOX ratios have a maximum of 0.9 at lower NOX levels and a minimum of 0.2 at higher NOX levels. This method accounts for proximity to the source–NO2/NOX ratios are lower when close to the source in distance and time (i.e., fresh emissions) while NO2/NOX ratios are higher in aged plumes after chemical conversion. However, not all dispersion models replicate the NO to NO2 conversion process in the same manner, and some models such as AERMOD have additional options for modeling the NO to NO2 conversion with greater complexity. AERMOD employs advanced techniques such as the Ozone Limiting Method (OLM) (Cole and Summerhays, 1979) and the Plume Volume Molar Ratio Method (PVMRM) (Hanrahan, 1999) to account for the initial speciation of NO and NO2, the concentration of NO, and available O3.
Table 1 lists several Gaussian dispersion models that have been applied to NOX modeling with a focus on the chemical and physical factors that are taken into account in dispersion models typically applied to near-road sources. Each of the Gaussian dispersion models listed in Table 1, AERMOD, CALINE4, ADMS-5, ADMS-Urban, ADMS-Roads, CAR-FMI, OML-Highway, and OSPM, implement NOX partitioning differently. The physical dilution of the plume is controlled by a number of factors that affect the local turbulence and are accounted for in dispersion models, including wind speed, the mixing height, and turbulent wind fluctuations (e.g., friction velocity, u*). The chemical transformations that are included in most of the dispersion models are focused on the reaction between O3 and NO (Benson, 1984; Berkowicz et al., 2011; Cole and Summerhays, 1979; Hanrahan, 1999; Härkönen et al., 1996; Venkatram et al., 1994), which is controlled by availability of NO and O3, the temperature, available sunlight, and reaction time for chemical transformations to occur. Given these important physical and chemical parameters that are considered in the modeling regimes, the analysis herein focuses on considering relationships between measured (e.g., wind speed and O3 concentration) and estimated parameters (e.g., mixing height and u*) with the NO2/NOX ratios at each site and the changes in the NO2/NOX ratios along the downwind measurement sites.
Table 1.
Summary of dispersion models’ treatment of NO-to-NO2 conversion.
| Model | Developer | Usage | NOX | Chemistry |
|---|---|---|---|---|
| AERMOD | US EPA | US EPA’s Preferred Regulatory Model for stationary, area and mobile sources | Employs a three-tier approach: Tier 1 methodology assumes full conversion of NO to NO2; Tier 2 ARM2 NO2/NOX ratios have a maximum of 0.9 at lower NOX levels and a minimum of 0.2 at higher NOX levels; Tier 3 OLM and PVMRM NO conversion to NO2 based on titration by O3, but limits the maximum NO2/NOX ratio to 0.9. | (Cole and Summerhays, 1979; Hanrahan, 1999; Podrez, 2015; U.S. EPA, 2011a, 2012, 2014a) |
|
| ||||
| CALINE4 | California Department of Transportation (Caltrans) | Evaluate air quality impacts from transportation facilities (i.e., line sources) | Employs the discrete parcel method (DPM); determines NO reaction with O3 based on plume transit times and reaction rates for NO2 photo dissociation and NO titration by O3; initial NO2/NOX = 0.075. | (Benson, 1984) |
|
| ||||
| ADMS-5 | Cambridge Environmental Research Consultants (CERC) | ADMS is a suite of models that include: ADMS-5, ADMS-Urban, and ADMS-Roads. Model suite can evaluate air quality impacts from stationary, area, and mobile emission sources. | Employs a subset of the Generic Reaction Set (GRS) to model NOX chemistry, excluding hydrocarbons in the reaction scheme. Model default assumptions: 1) background pollutants are instantaneously mixed; 2) background concentration values are adjusted to ensure equilibrium conditions during daytime hours; and 3) background concentration values are not adjusted for nighttime conditions. | (CERC, 2015a; Venkatram et al., 1994) |
|
|
|
|||
| ADMS Urban | Employs the full GRS (seven reactions) associated with O3 production, including bulk reactive organic compounds and NOX removal pathways. NO conversion is a function of the background O3, the temperature, ambient sunlight, transport time, and the plume volume. | (CERC, 2015c; Venkatram et al., 1994) | ||
|
|
||||
| ADMS Roads | (CERC, 2015b; Venkatram et al., 1994) | |||
|
| ||||
| Contaminants in the Air from a Road - Finnish Meteorological Institute (CAR-FMI) | Finnish Meteorological Institute (FMI) | Evaluate air quality impacts from open road networks | Employs a modified form of DPM within its chemical reaction subcomponents. Model assumptions include: 1) instantaneous and uniform mixing of the plume and ambient air; 2) no chemical reaction interactions or physical mixing process reactions within plume; and 3) chemical reactivity of volatile organic compounds (VOCs) or other compounds are ignored. | (Härkönen et al., 1996) |
|
| ||||
| OML-Highway | Department of Environmental Science at Aarhus University | Evaluate air quality impacts from street canyons | Both models employ the same NO2 chemistry scheme: solution of the steady-state approximation between NO, NO2, and O3. | (Berkowicz et al., 2011; Olesen et al., 2015) |
|
|
||||
| OSPM | Evaluate air quality impacts from area and mobile sources | |||
2.2. Field campaign, analytical instruments, and measurement data
This work utilizes data from a field study conducted in collaboration with the U.S. Department of Transportation (DOT) Federal Highway Administration (FHWA) along I-15 in Las Vegas, NV from mid-December 2008 thru mid-December 2009. The study collected continuous and integrated ambient air quality samples for an extensive number of pollutant species (Kimbrough et al., 2013a; Kimbrough et al., 2013b). Measurements discussed herein include NO2 and NOX (SI, Table S1) and are based on data collected at all four of the air measurement stations (SI, Fig. S1) utilized by the study [100 meter (m) west of I-15; and three stations east of I-15: 20 m roadside, 100 m, 300 m] (Kimbrough et al., 2013a; Kimbrough et al., 2013b). Ecotech model 9841T continuous gas analyzers measured ambient NO, NO2 and NOX (Ecotech Pty. Ltd., Knoxfield, Victoria, Australia). WinAQMS/WinCollect software (Ecotech Pty. Ltd., Knoxfield, Victoria, Australia) logged the 5-minute-averaged data (Kimbrough et al., 2013a; Kimbrough et al., 2013b). The continuous gas analyzers were configured to run nightly zero and span points. These analyzer checks typically ran between the hours of midnight and 2 am every day during the course of the study. As a consequence, the study has substantially fewer 5-minute data points for these hours—midnight to 2 am. These hours were chosen for the nightly zero and span points when traffic volumes were low as the study design protocol called for the instrumentation to be operational during peak traffic conditions (FHWA, 2006). Over the course of the field campaign, approximately 100,000 5-minute data points per pollutant per measurement site were logged. Additional details are discussed in the Supplemental Information.
Quality control checks to the 5-minute measurement data included criteria to remove invalid data (e.g., flow rates out of range, instrument errors, calibration data points). The 5-minute measurement data for NO2 and NOX were then arithmetically averaged to achieve an hour time resolution consistent with AERMET meteorological output and the typical time step of a dispersion model.
During the yearlong Las Vegas near-road field study, there were 6849 hours of valid hourly measurements. To isolate the roadway as the source of interest we begin our analyses with the 2668 (39.0%) hours when winds were from the west across the roadway (see SI, Table S3); this dataset was subset further when we began to examine the NOx measurements in 3.2.
Traffic counts (SI, Table S2) were supplied by the Nevada Department of Transportation Regional Transportation Commission (RTC). The RTC used Wavetronix Radar units with data reported in 15-minute increments. These measurements were summed to have an hour time resolution consistent with AERMET meteorological output and the typical time step of a dispersion model.
2.3. Meteorological parameters
Assessment of the near-road impact of air pollutants was determined by the assignment of four wind directional categories: (1) downwind (winds from the west)–winds coming from 210° – 330° and wind speeds > 1 m/s; (2) upwind (winds from the east)–winds coming from 30° – 150° and wind speeds > 1 m/s; (3) parallel (wind from the north/south)– winds coming from 150° – 210°, 330° – 360°, or 0° – 30° and wind speeds > 1 m/s; and (4) low speed winds from all directions (wind speeds < 1 m/s) (Kimbrough et al., 2013a; Kimbrough et al., 2013b). These winds are defined as the sector encompassing 60 degrees on either side of the normal to I-15. In these hours, there were three measurement stations downwind of the roadway and a fourth measurement station upwind of the roadway to examine a background concentration.
Meteorological parameters collected at the location of the field study were compared with meteorological parameters from the National Weather Service (NWS) at Las Vegas airport (LAS) (i.e., McCarran International Airport). This comparison indicted that the LAS meteorological dataset was the most consistent and representative dataset available. Therefore, this analysis uses LAS meteorological parameters. These parameters include wind speed, wind direction, air temperature, relative humidity, precipitation, and cloud cover. The LAS meteorological station is part of the Automated Surface Observing Systems (ASOS) and has a one-minute temporal resolution for wind speed and direction. The distance from the near road site is 1.5 km. Upper air data from the Universal RAwinsonde OBservation (RAOB) station in Mercury/Desert Rock, NV (KDRA, elevation 1006 m) was used as the primary upper air station. One-Minute ASOS wind data were processed for input to American Meteorological Society (AMS) /Environmental Protection Agency Meteorology (AERMET) (U.S. EPA, 2004) using AERMINUTE for the study period of mid-December 2008 through mid-December 2009. Upper air and surface data were processed through AERMET to obtain hourly averages of surface and upper air meteorological parameters (NOAA, 2015).
2.4. O3 monitor data
The Clark County FRM monitoring network, which includes O3 monitors, is operated by the Clark County Department of Air Quality (DAQ). These Federal Reference Method (FRM) monitors are operated in accordance with Title 40, Part 53 of the Code of Federal Regulations (CFR) by Clark County DAQ. The network operates under a quality assurance project plan (QAPP) and is subject to a Performance Evaluation Program (PEP) audit with results submitted to EPA’s Air Quality System (AQS) database (Clark County DAQEM, 2012).
Data from two O3 monitor locations were chosen for this analysis. Two criteria for the selection included proximity to the study site and these monitors strattle the same roadway, I-15, just north of the field site, thus they would serve as an upwind and a downwind location. The location of O3 monitors nearest to the near-road measurement sites that would serve both criteria are shown in SI, Fig. S2. These Clark County DAQ sites are the Paul Meyer site-approximately 7.4 km west, northwest of measurement sites and the Orr Site-approximately 6.6 km north, northeast of measurement sites. The Paul Meyer site is located in a local park, situated in a large residential area, while the Orr monitor is located at an elementary school, approximately 3.5 km east of Las Vegas Blvd, and situated in a mixed commercial and residential area. The data from both monitors were combined to create a representative “upwind” O3 data set. This was done by using the wind direction to select data from the upwind monitor during perpendicular wind conditions and average the data from both monitors during parallel wind conditions.
3. Results and Discussion
3.1. Diurnal patterns – traffic, NO2/NOX ratios, and O3
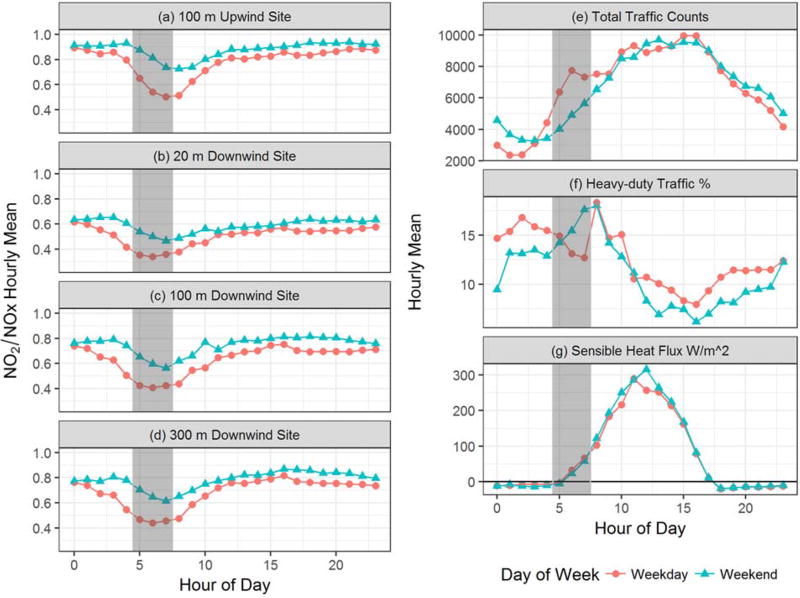
While most U.S. cities exhibit a bimodal traffic pattern, Las Vegas traffic does not exhibit this same bimodal traffic pattern (i.e., home-to-work and work-to-home). As reported in Kimbrough et al. (2013b) and Kimbrough et al. (2014), Las Vegas traffic at the field site exhibits a trimodal traffic distribution. This atypical traffic distribution is believed to be the result of several factors: (1) the city is a recreation destination for many travelers; (2) shift changes occur in the city later or earlier in the day, depending on the employer; (3) the study site was adjacent to an interstate (I-15) that carries both inter- and intrastate traffic; and (4) I-15 is considered a part of the North American Free Trade Agreement (NAFTA) corridor, thus supporting freight goods movement along the freeway. Additionally, there is a notable difference in fleet mix between weekday and weekend traffic, with a large fluctuation in the truck percentage throughout the day and a significant difference in truck percentage between weekday and weekend days (as shown in Fig. 1) and in particular, the weekday and weekend hours of 5 am to 7 am (gray bars in Fig. 1).
Fig. 1.
Weekday and weekend diurnal variations of (a–d) mean hourly NO2 to NOX ratio at the four near-road measurement locations, (e) total traffic counts, (f) percent heavy-duty traffic counts, (g) sensible heat flux (W/m2). Hours used are only when winds are from the west; the hours between 5 and 7 of the day are highlighted with a grey vertical bar on all plots to highlight the hours of the morning atmospheric transition from stable to convective conditions and where there is a notable difference in the total traffic volumes and heavy-duty traffic percentage between weekdays and weekends on I-15.
The differences in the traffic counts and vehicle distributions between the weekdays and weekends allow for an important assessment of the impact of emissions versus meteorology and chemistry in controlling NOX concentrations and speciation. These differences result in the diurnal profiles shown in Fig. 1. A distinct difference in the NO2/NOX ratio for weekdays versus weekends can be observed, with the largest differences observed in the morning hours between 5 am and 7 am. This difference is also reported by Kimbrough et al. (2013a) as an increase in NO2 concentrations during the early morning when there was a significant increase in traffic counts. Although traffic counts were still increasing, increased atmospheric mixing begins to occur between 7 and 8 am, which acts to decrease NO2 concentrations as traffic emissions are mixed into a deeper boundary layer (Kimbrough et al., 2013a). The differences in the profiles in Fig. 1 suggest that there is a brief period of time in the morning when increased traffic emissions can have a substantial impact on NO2 concentrations. After this window, NOx and NO2 emissions are rapidly diluted within a deeper boundary layer, resulting in an increase in the NO2/NOX ratios to values much more similar to regional background.
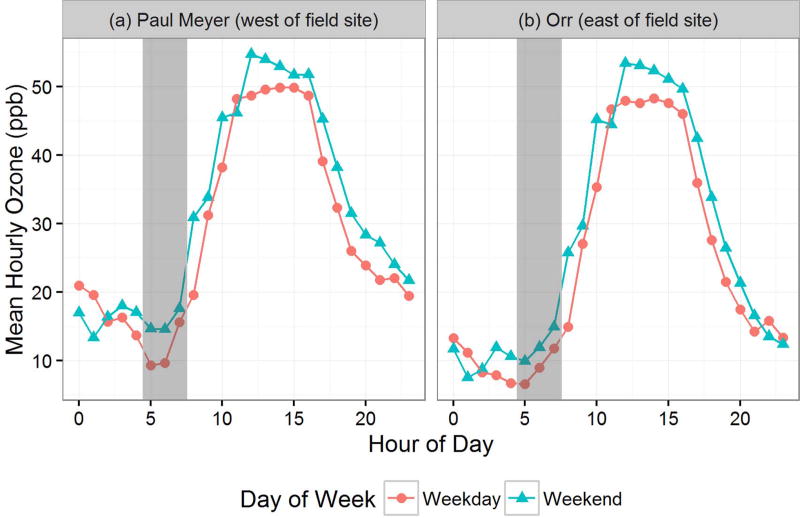
The drop in the NO2/NOX ratio between 5 am and 7 am corresponds with a drop in O3 concentration (gray bars in Fig. 2), presumably more pronounced during the weekdays due to the greater traffic volumes during these weekday hours (Fig. 1). The dip in O3 during the early morning hours from 5 am to 7 am may be due to an area-wide increase in traffic at the start of the work day. This decrease in early morning O3 is apparent at the upwind O3 site (Paul Meyer – 7.4 k west, northwest of measurement sites) that coincides with both low NO2/NOX ratios and higher total NO2 concentrations at all of near road measurement sites. While the ratios and NO2 concentrations at the upwind site may be impacted by the upwind dispersion from the I-15 roadway, given the differences in weekday and weekend O3 at the upwind Paul Meyer O3 site and the subsequent low O3 on both weekend and weekday mornings at the downwind Orr O3 site, it seems likely that this early morning (5 am to 7 am) condition is an area-wide characteristic.
Fig. 2.
O3 measurements for: (a) Paul Meyer (west, northwest of measurement sites) and (b) Orr site (east of measurement sites)—when winds are from the west. Mean hourly values are differentiated by weekday vs. weekend. The hours between 5 and 7 of the day are highlighted with a grey vertical bar on all plots to highlight the hours of the morning atmospheric transition from stable to convective conditions and where there is notable difference in the mean hourly O3 measurements.
Overall, O3 concentrations are highest during the daytime (approximately 55 ppb) and lowest during the nighttime (approximately 5 ppb). O3 concentrations are approximately the same between the measurement locations to the east and west of I-15. However, there is noticeably higher O3 (Paul Meyer O3) to the west of the measurement sites during the late night (approximately 20 ppb at midnight) and early morning hours (approximately 15 ppb at 6 a.m.), presumably due to O3 titration (reaction of O3 in the presence of NO to form NO2) from the additional vehicular NO emissions measured near the downwind sites due to the predominately westerly winds during nighttime hours.
3.2. Spatial gradient results: conversion of NO to NO2
The spatial rate of conversion and the diurnal pattern of conversion of NO to NO2 is fairly consistent across the three downwind sites, as indicated by the NO2/NOX ratio (Fig. 1). Less than 1% of the hours at the 300 m site have a measured ratio equal to one (SI, Fig. S3), thus while the chemical transition from NO to NO2 is occurring throughout the measurement locations, full conversion of all NO to NO2 generally does not occur within 300 m of the roadway. The ratio at the 20 m site is lower than at the other sites for all hours of the day, showing that NO comprises a higher percentage of NOX near the roadway compared with the proportion of NO in NOX downwind. The NO2 concentration decreases from the 20 to 100 to 300 m measurement sites (SI, Fig. S4), as has been reported elsewhere by Kimbrough et al. (2013a) and Richmond-Bryant et al. (2017). Other studies have reported similar results at other sites (Clements et al., 2009; Karner et al., 2010; Roorda-Knape et al., 1998). As the roadway plume travels westward between the 20 m to the 300 m downwind sites, the concentration of NO2 decreases on average by 31%, and the concentration of NOX decreases on average by 54%. At the same time, the ratio of NO2/NOX increases on average by 26%. A decrease in the NO2 concentration coupled with an increase in the NO2/NOX ratio suggests that photochemical conversion of NO to NO2 is occurring over this distance, but the contribution of NO to NO2 conversion is smaller than the reduction in NO2 related to dilution with background, which would account for the decrease in NOX. We further subset the data by examining the change in NOX measured at each site. In SI, Fig S5, we note that there are some hours where NOX increases between the downwind sites, indicating a source of NOX between the sites. These hours were eliminated, and only hours where measured NOX remained constant or decreased (due to other processes such as deposition) are further analyzed–data are shown in (SI, Fig S5) Quadrant 3 (Q3), see subset summary in SI, Table S3. From this point further we only analyzing the subset with winds from the west and in Q3.
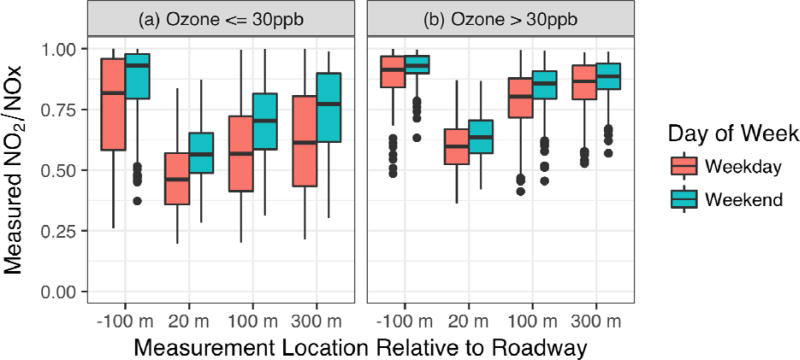
We present the distribution of the NO2/NOX ratios as a function of the measurement location in Fig. 3. The measured ratio is also separated by weekday versus weekend to show the influence of the difference in traffic and fleet mix that was presented in Fig. 1(e) and (f). The distributions shown in Fig. 3 suggest that there is a non-negligible difference in the emissions and mixing conditions between weekday and weekend, likely due to a higher concentration of fresh NO near the roadway on weekdays. The change in the ratio from weekday to weekend is fairly small at all near-road sites during the higher O3 conditions (panel B in Fig. 3). However, during low O3 conditions, there is a notable difference between weekdays and weekends. On weekdays, there is a decline in the O3 in the morning hours (as shown in Fig. 2), which means that during these early morning hours, less O3 is available to titrate the emitted NO, thus allowing for the lower ratios of NO2/NOX as shown in Fig. 3.
Fig. 3.
Measured NO2/NOX ratios at each measurement location during the Las Vegas field study for all hours; (a) left-hand panel contains only hours where the measured O3 at the Paul Meyer monitoring site is ≤ 30 ppb, and (b) right-hand panel contains only hours where measured O3 at the Paul Meyer monitoring site is > 30 ppb. Values are differentiated by weekday vs. weekend. The box represents the middle 50% of the data, extending from the 25th to the 75th percentiles; the horizontal line through the center of the box is the median; the whiskers represent 1.5*IQR (the inter-Quartile Range, the range from the 25th to 75th percentiles); the points are outliers beyond 1.5*IQR. Data presented are in subset with winds from the west and in Q3.
It should be noted that under low O3 conditions, one might expect the difference in the weekday vs weekend NO2/NOX ratio to be less pronounced than is shown by Fig. 3. This difference may be due to the later onset of morning traffic on the weekend. During the early morning hours when traffic impacts are most visible, there is a greater influence from vehicles during the weekday than on the weekend, when more background O3 is available for the conversion of NO to NO2.
3.3. Relationships between NO2/NOX ratios and key model parameters
We continue the focus on time periods when winds are from the west and data points in Q3 (see SI, Fig S5). These time periods coincide with only hours where measured NOX remained constant or decreased between the downwind sites. Any deviations from these conditions generally imply that either the wind is not consistently from the roadway to the downwind monitors during the course of the hour or that there is an intermediate emission source between the downwind monitors that disrupts the anticipated behavior of the ambient concentrations (e.g. truck freight movement in parking lot near monitors).
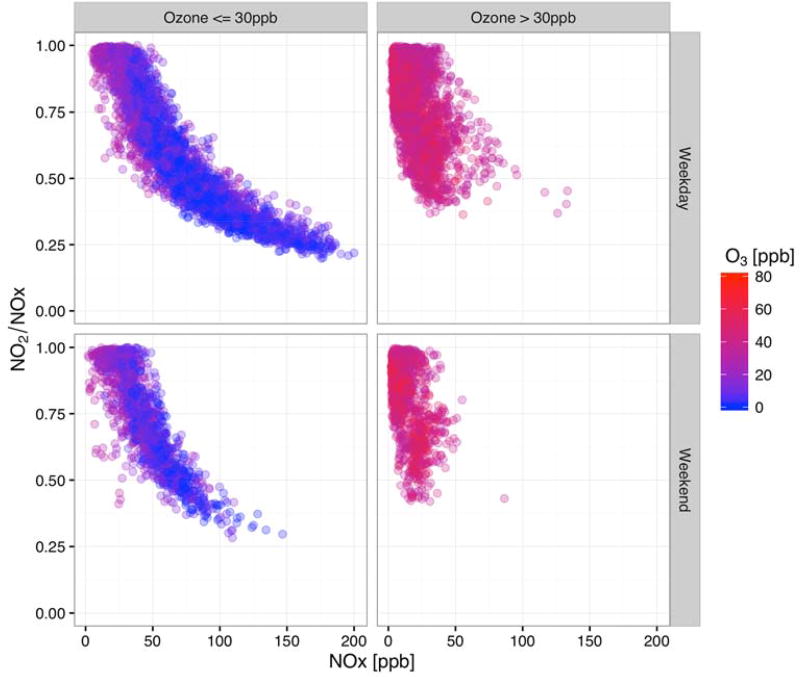
The NO2/NOX ratio as a function of the total NOX, which is shown in Fig. 4, is a common way to examine the NOX speciation. An analysis by Podrez (2015) shows that there is a characteristic behavior of the NO2/NOX ratio in ambient data from EPA’s AQS with an exponential decrease in the maximum NO2/NOX ratios as the total NOX increases. We observed a similar decrease in the ratio with increasing NOX (Fig 4) during low (≤ 30 ppb) O3 conditions. However, there is a very different trend for measured NO2/NOX during high (>30 ppb) O3 conditions. This different pattern may be related to limitations in the domain of NOX values present during high O3 periods. The small amount of data where NOX > 50 ppb in high O3 periods suggest that a decaying trend could be present, although that potential trend is unlikely to exhibit the same rate of decay as for the low O3 periods.
Fig. 4.
NO2/NOX ratio as a function of NOX for all measurements sites when winds are from the west and in Q3, colors are based on the O3 concentration; top row shows weekdays, bottom row shows weekends; Right column shows high O3 (>30 ppb) and the left column shows low O3 (≤ 30 ppb).
The basic interpretation of the curve for low O3 conditions in Fig. 4 is that the highest NOX levels indicate the most direct and least diluted impacts from the roadway and the decreasing NOX represents increasing dilution (and reaction) towards background levels of NOX and the atmospheric steady state apportioning between NO2 and NO. This interpretation suggests that the on-road NO2/NOX ratio, which would result from the direct emissions from vehicles in addition to incoming background air concentrations, is approximately 0.2. This is the lowest ratio shown at the 20 m site (see Fig. 1(b) and Fig. 4) (Richmond-Bryant et al., 2017). Interestingly, the maximum NOX and minimum NO2/NOX ratios are similar at the other three sites as well. However, the maximum measured ratios are approximately 0.8 at the 20 m site (see Fig. 1(b) and SI, Fig. S3), with zero data points reaching an NO2/NOX ratio of 1.0 at the 100 m site (see Fig. 1(c) and SI, Fig. S3), and a small amount of data showing NO2/NOX ratios of 1.0 at the 300 and 100 m upwind sites (see Fig. 1(a), Fig. 1(d), and SI, Fig. S3).
Complicating the interpretation of the curve for low O3 conditions in Fig. 4 may be the availability of either O3 or NOX (and NO) to drive the conversion of NO to NO2. At lower O3 concentrations, the fraction of NO that is converted to NO2 may be dependent upon available O3, (i.e., O3 limited conditions). At lower NOx (and NO) concentrations, there may be sufficient O3 to convert NO to NO2, but at higher NOx (and NO) concentrations, there may be insufficient O3 to convert available NO to NO2. Thus, even with minimal to no mixing, one may be able to observe a relationship where, as NO levels increase, a smaller fraction of NO is converted to NO2 (NOX limited conditions). As shown in Fig. S4, higher NO2 concentrations occur during early morning hours as traffic volume is increasing. Thus, time of day influences the NO2/NOX ratio.
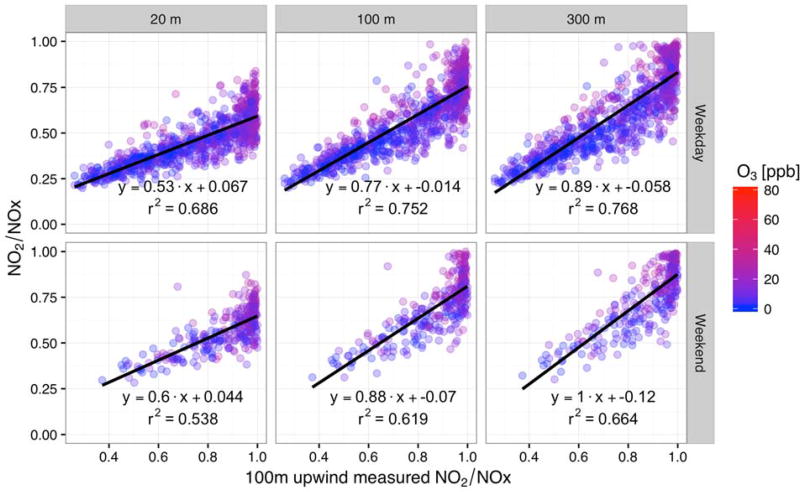
Without modeling the emissions, the relative impact of the roadway versus the background air is not immediately clear and as a result, it can be difficult from the analysis above to identify the importance of the background conditions in the plume mixing. To understand the role of background versus direct roadway impacts and the degree of mixing, we also examine relationships between the NO2/NOX ratio and upwind concentrations and changes in the upwind concentrations and the downwind sites for low O3 conditions, as shown in Fig. 5. There is a clear positive correlation between the measured ratios at the downwind sites as compared to the upwind site, providing further evidence that in low O3 conditions, the dominant atmospheric process that determines the measured NO2/NOX ratios is dilution. When O3 is not available and mixing is the only controlling factor, the rate of change can be estimated based on the background ratio.
Fig. 5.
NO2/NOX ratio as a function of NO2/NOx as measured at the 100 m upwind site when winds are from the west and in Q3, colors are based on the O3 concentration, where only low O3 (≤ 30 ppb) conditions are shown; top row are weekdays, bottom row are weekends; Left-hand column shows the 20 m measurement site, center column shows the 100 m measurement site, and the right-hand columns shows the 300 m measurement site.
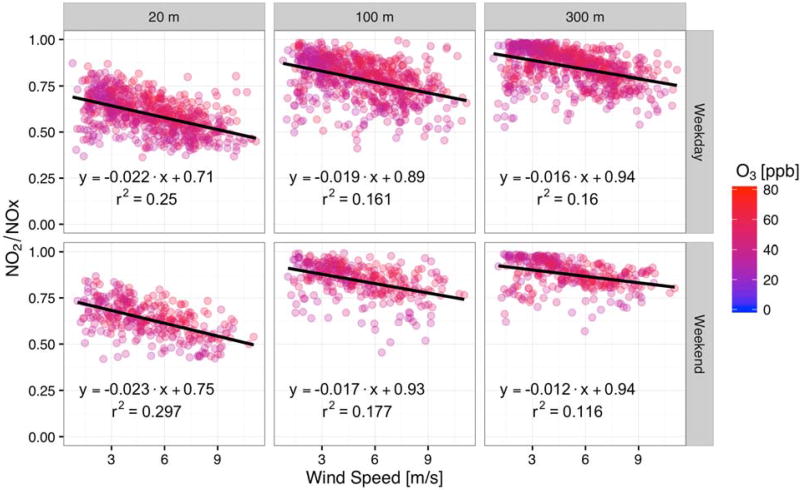
High O3 conditions show very different behavior, as was shown in Fig. 4, with the NO2/NOX ratio not being correlated to the total NOX. While the presence of O3 is the controlling factor for NO titration, there are several factors that impact the rate of mixing with background. For example, the wind speed is a first-order variable (i.e., measured or calculated directly from measurements) that is an important model input to estimate the amount of mixing in a plume. We further investigate this by presenting NO2/NOX ratios for only high O3 conditions in Fig. 6 as a function of wind speed. The negative slope suggests that for these high O3 conditions, the travel time needed to go from the roadway to the measurement locations allows chemistry to occur. This chemistry needs to be accounted for to estimate the NO2/NOX ratio.
Fig. 6.
NO2/NOX ratio as a function of wind speed when winds are from the west and in Q3, colors are based on the O3 concentration, where only high O3 (> 30 ppb) conditions are shown; top row shows weekdays, bottom row shows weekends. Left-hand column shows the 20 m measurement site, center column shows the 100 m measurement site, and the right-hand column shows the 300 m measurement site.
In Fig. 6, for all three downwind sites, there is a consistent, but weak negative correlation between the wind speed and the ratios for the high O3 cases. The rate of change is fairly constant across various wind speeds, though the correlation coefficient is not particularly high. There are two potential causes for this relationship: 1) high wind speeds indicate faster transport times, resulting in less time for the O3/NO reaction to occur, which would result in a negative correlation between the two (note the increasing ratios at subsequent downwind sites and how the extra transport time can be important for this factor); and 2) increased wind speeds would suggest more turbulence and increased mixing, entraining more O3 and background air (with higher ratios) into the plume, both of which would result in a positive correlation between the ratio and wind speeds. Since the overall relationship here is negative, the suggestion is that the reaction rate component is more important than the mixing component when O3 is available. Similar results have been reported by Richmond-Bryant et al. (2017). However, the results reported by Richmond-Bryant et al. (2017) indicated that other factors may be important and suggests a more in-depth analysis with additional data is needed to explore additional scenarios that could lead to more insights into the factors that drive the NO2/NOX ratio.
For the low O3 conditions, there is no apparent causal relationship between the wind speeds and the NO2/NOX ratio (SI, Fig. S6). This lack of a causal relationship is somewhat surprising, as discussed above. Faster wind speeds would mix towards background faster, but the limited range of wind speeds (almost all data have speeds less than 3 m/s) may be the limiting factor in deriving a meaningful relationship from this subset of data.
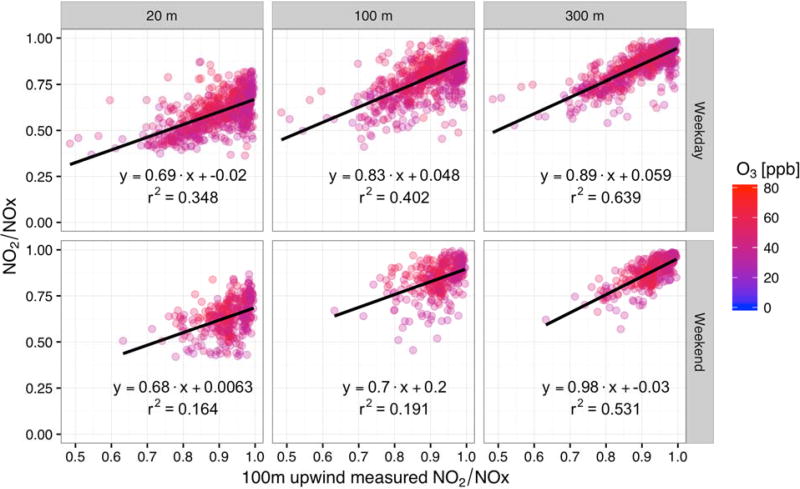
Although chemical reactions will have an influence on the NO2/NOX ratio during high O3 conditions (Fig. 6), NO2/NOX ratio is also shown to be a function of the measured upwind ratio (Fig. 7). The correlations between the NO2/NOX ratio and upwind ratio shown in Fig. 7, similar to Fig. 6, indicate the dilution with background is important for all conditions.
Fig. 7.
NO2/NOX ratio as a function of NO2/NOX as measured at the 100 m upwind site when winds are from the west and in Q3. Points are filled by O3 concentration, where only high O3 (> 30 ppb) conditions at the Paul Meyer monitoring site are shown. Top row shows weekdays; bottom row shows weekends. Left-hand column shows the 20 m measurement site, center column shows the 100 m measurement site, and the right-hand columns shows the 300 m measurement site.
Fig. 7 shows the ratio at each downwind site as a function of the upwind ratio during high O3 conditions. There is a correlation between the upwind ratio and the downwind ratio during all O3 conditions, though the relationship is much stronger during low O3 conditions. Similar mixing rates for all conditions are evident in the slopes of the linear regressions of Fig. 5 and Fig. 7, suggesting that there is a balance between background mixing and the titration of NO to NO2. The upwind ratios all tend to be higher during high O3 conditions, which is expected during daytime conditions and based on the steady-state between O3, NO, and NO2. This results in a strong tendency towards NO2, which also helps form additional O3 in the presence of VOCs. However, the lack of variability in the upwind ratios prohibits a higher correlation coefficient. Nonetheless, there is still a positive relationship for this case. For the low O3 case, there is a strong correlation between the upwind ratio and the downwind ratio at all sites, with a slope of approximately 0.5 at the 20 m site and a slope approaching 1 at the 300 m site. Additionally, the R2 values in Fig. 7 suggest that under low O3 conditions, the ratios at the 20 m site are approximately 15–35% controlled by the upwind concentrations and 65–85% controlled by the measured NO2 concentrations. Conversely, conditions at the 300 m site are closest to background conditions, i.e., there is very little roadway influence on the ratio. These results suggest that when modeling the NOX partitioning from roadway impacts based on an NO2/NOX ratio partitioning (e.g., the ARM2 approach in AERMOD), it is important to account for background conditions in this partitioning, rather than determining an NO2/NOX ratio based on modeled impacts alone.
Meteorological processes, as shown in Richmond-Bryant et al. (2017), play an important role in observed concentrations of NO2 and NOX. We explored correlations between NO2/NOX ratio, temperature, u*, traffic volumes, mixing height, Monin-Obukhov length, and estimated plume volume (SI, Figs. S7 – S13). Although we see some trends in variables such as u*, as this parameter is derived from wind speed, the relationships are much more pronounced with the first-order variables of wind speed and background ratio than any of the other parameters we tested. A linear relationship occurs between measured NO2/NOX at the downwind sites, wind speed (transport time), and background measured NO2/NOX.
3.4. Implications for Chemistry Methods in Gaussian Models
The Gaussian models, including AERMOD, CALINE4, ADMS-5, ADMS-Urban, ADMS-Roads, OLM-Highway, and CAR-FMI, have different NO to NO2 conversion rate schemes for modeling NOX. Features of these various schemes include chemical reaction sets of varying complexity, entrainment of background NOx and O3, and plume interactions.
When O3 is present in sufficient quantities (Figs. 6 and 7 suggest over 30 ppb), it is clear that some explicit accounting for O3 chemistry is required. Of these models, only the ARM2 method incorporated into AERMOD does not take into account any explicit O3 chemistry. The correlations expressed in Fig. 6 strongly suggest that when O3 chemistry occurs, a reaction rate should be considered. OLM and PVMRM in AERMOD, which consider only the forward reaction forming NO2 from NO, are the only explicit chemistry schemes that do not account for the transport time when determining the total reaction rate between O3 and NO. The remaining models (ADMS, CALINE4, CAR-FMI, and OLM/OS PM) consider chemical reactions beyond just the reaction between O3 and NO, including the photo dissociation of NO2 to NO. Some of the models incorporate a consideration of transport time/reaction rates when accounting for these reactions.
Our analysis presented in Fig. 7 shows that the composite mix of fresh emissions and dilution with background are major factors in determining the ambient NO2/NOX ratio. Fig. 7 also shows that estimating NOX chemistry only within the emissions plume may not fully account for the composite NOX partitioning. There are three aspects of the composite plume that are important. First, the background NOX concentrations should be considered when equilibrium between the various species is computed. The NOx algorithms, in ADMS CALINE4, CAR-FMI, and OLM/OSPM, have as inputs background NOX concentrations and include NOX/O3 equilibrium calculations to varying degrees of complexity. Second, the amount of O3 entrained into the plume, and thus the amount of O3 actually available to contribute to NOX/O3 equilibrium calculations, is important. While these five models have fairly detailed computations of NOX/O3 equilibrium, they do not limit available O3 from entrainment. Within an individual plume, all five assume that all the background O3 is immediately available to participate in NOX chemistry (i.e., they do not consider the plume volume and the amount of entrained O3). Although PVMRM in AERMOD lacks the chemistry complexities present in other models, it is the only model that explicitly takes into account the amount of entrained O3 in individual plumes. Third, competition for O3 from multiple plumes should also be considered. ADMS, CALINE4, CAR-FMI, and OLM/OSPM do not consider O3 competition between plumes, assuming that all background O3 is available for NOX chemistry in each plume even though they may have significant overlap. In this respect, the OLM and PVMRM algorithms in AERMOD are more advanced than these other models, as both account for O3 competition between plumes. While our analysis cannot examine the importance of these aspects to O3 competition directly, the discovery of the importance of O3 chemistry strongly suggests that the amount of O3 available to participate in NOX chemistry would also be important.
Thus, while ADMS, CALINE4, CAR-FMI, and OLM/OSPM consider more chemical reactions, OLM and PVMRM have more accurate estimates of the O3 available for NOX chemistry. Thus, none of the available models fully take into account all of the important aspects of NOX chemistry. These results emphasize the importance of selecting the appropriate model for the intended application, because no single model fully accounts for all major considerations in determining the speciation of NOX.
4. Conclusions
The results presented here suggest that the ambient NO2/NOX ratio immediately downwind of roadway sources is not entirely a function of the roadway emissions. Instead, even at 20 m from the roadway edge, the ratio is a function of 1) the roadway emissions (i.e., the emitted NO2/NOX ratio), 2) the in-situ and in-transport conversion of NO to NO2, and 3) mixing of emissions with background air, with 2 and 3 being ongoing and competing processes that drive the NO2 concentrations in different directions. The conversion of NO to NO2 and mixing of emissions with background air can impact the changes in baseline NO2 and NOX and the resultant ratio. Thus, a single assumption about the NO2 chemistry cannot account for all of these processes. These results highlight the usefulness of long-duration gradient data in assessing the behavior of NO2/NOX ratios to inform air quality modeling strategies in the near-road environment. These results also support the need for future enhancements to existing models to incorporate ambient background NO2/NOX ratio, emissions NO2/NOX ratio, and O3 concentration into a single NOX chemistry scheme within a dispersion model.
Supplementary Material
Highlights.
Examined NO2/NOX ratio spatial gradients near a major freeway in Las Vegas, NV
NOX chemistry drives the NO2/NOX ratio spatial gradient during high ozone conditions (≥ 30 ppb).
Atmospheric mixing is more important to the NO2/NOX ratio spatial gradient during low ozone conditions (< 30 ppb).
NO2/NOX ratio controlled by three factors: emitted NO2/NOX ratio; conversion of NO-to-NO2; mixing with background air.
Gaussian models have different NO-to-NO2 conversion rate schemes and differing NO2/NOX ratios—none of the models discussed herein employs all three components that are important to estimating the evolution of the NO2/NOX ratio.
Acknowledgments
We thank members of the EPA Near-Road team for their contributions to this project. We also acknowledge the contributions of ARCADIS-US, Inc., staff and the Clark County Department of Air Quality staff to the success of the near-road measurement project.
Footnotes
Disclaimer
The U.S. Environmental Protection Agency, through its Office of Research and Development, partially funded and collaborated in the research described here under Contract No. EP-D-12-044 work assignment 4–10 and 5-05 to the University of North Carolina at Chapel Hill.
This document has been reviewed in accordance with U.S. Environmental Protection Agency policy and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. The views expressed in this journal article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
References
- Alvarez R, Weilenmann M, Favez J-Y. Evidence of increased mass fraction of NO2 within real-world NOX emissions of modern light vehicles — derived from a reliable online measuring method. Atmospheric Environment. 2008;42:4699–4707. http://dx.doi.org/http://dx.doi.org/10.1016/j.atmosenv.2008.01.046. [Google Scholar]
- Atkinson R. Atmospheric Chemistry of VOCs and NOX. Atmospheric Environment. 2000;34:2063–2101. http://dx.doi.org/10.1016/S1352-2310(99)00460-4. [Google Scholar]
- Bange P, Janssen LHJM, Nieuwstadt FTM, Visser H, Erbrink JJ. Improvement of the modelling of daytime nitrogen oxide oxidation in plumes by using instantaneous plume dispersion parameters. Atmospheric Environment. Part A. General Topics. 1991;25:2321–2328. http://dx.doi.org/http://dx.doi.org/10.1016/0960-1686(91)90106-H. [Google Scholar]
- Beckerman B, Jerrett M, Brook JR, Verma DK, Arain MA, Finkelstein MM. Correlation of nitrogen dioxide with other traffic pollutants near a major expressway. Atmospheric Environment. 2008;42:275–290. http://dx.doi.org/10.1016/j.atmosenv.2007.09.042. [Google Scholar]
- Benson PE. Final Report. California Department of Transportation; Sacramento, CA: 1984. [Last Accessed: October 19, 2016]. CALINE4-A Dispersion Model for Predicting Air Pollutant Concentrations Near Roadways. http://www.dot.ca.gov/hq/env/air/documents/C4manual_searchable.pdf. [Google Scholar]
- Berkowicz R, Ketzel M, Lofstrom P, Olesen HR. [Last Accessed: October 2, 2015];NO2 Chemistry Scheme in OSPM and other Danish Models. 2011 http://www2.dmu.dk/AtmosphericEnvironment/Docs/NO2scheme.pdf.
- Carslaw DC. Evidence of an increasing NO2/NOX emissions ratio from road traffic emissions. Atmospheric Environment. 2005;39:4793–4802. http://dx.doi.org/http://dx.doi.org/10.1016/j.atmosenv.2005.06.023. [Google Scholar]
- Carslaw DC, Beevers SD. Estimations of road vehicle primary NO2 exhaust emission fractions using monitoring data in London. Atmospheric Environment. 2005;39:167–177. http://dx.doi.org/http://dx.doi.org/10.1016/j.atmosenv.2004.08.053. [Google Scholar]
- CERC. ADMS-5 User Guide. Cambridge Environmental Research Consultants; Cambridge, United Kingdom: 2015a. [Last Accessed: October 2, 2015]. http://www.cerc.co.uk/environmental-software/user-guides.html. [Google Scholar]
- CERC. ADMS-ROADS User Guide. Cambridge Environmental Research Consultants; Cambridge, United Kingdom: 2015b. [Last Accessed: October 2, 2015]. http://www.cerc.co.uk/environmental-software/user-guides.html. [Google Scholar]
- CERC. ADMS-Urban User Guide. Cambridge Environmental Research Consultants; Cambridge, United Kingdom: 2015c. [Last Accessed: October 2, 2015]. http://www.cerc.co.uk/environmental-software/user-guides.html. [Google Scholar]
- Clark County DAQEM. Clark County Air Quality Data: Select a Monitoring Site in Clark County. Clark County Department of Air Quality & Environmental Management; Las Vegas: 2012. [Last Accessed: September 21, 2012, 2012]. http://ccaqapps5m.co.clark.nv.us/cgi-bin/select_summary.pl. [Google Scholar]
- Clements AL, Jia Y, Denbleyker A, McDonald-Buller E, Fraser MP, Allen DT, Collins DR, Michel E, Pudota J, Sullivan D, Zhu Y. Air pollutant concentrations near three Texas roadways, part II: Chemical characterization and transformation of pollutants. Atmospheric Environment. 2009;43:4523–4534. http://dx.doi.org/http://dx.doi.org/10.1016/j.atmosenv.2009.06.044. [Google Scholar]
- Cole HS, Summerhays JE. A Review of Techniques Available for Estimating Short-Term NO2 concentrations. Journal of the Air Pollution Control Association. 1979;29:812–817. doi: 10.1080/00022470.1979.10470866. http://dx.doi.org/10.1080/00022470.1979.10470866. [DOI] [PubMed] [Google Scholar]
- Costantini MG, Khalek I, McDonald JD, van Erp AM. The Advanced Collaborative Emissions Study (ACES) of 2007- and 2010-Emissions Compliant Heavy-Duty Diesel Engines: Characterization of Emissions and Health Effects. Emission Control Science and Technology. 2016;2:215–227. http://dx.doi.org/10.1007/s40825-016-0046-y. [Google Scholar]
- FHWA. FHWA. Washington, DC: 2006. [Last Accessed: October 19, 2016]. Detailed monitoring protocol for U.S. 95 settlement agreement. http://www.fhwa.dot.gov/environment/air_quality/air_toxics/research_and_analysis/near_road_study/finaldmpjune.pdf. [Google Scholar]
- Gilbert NL, Woodhouse S, Stieb DM, Brook JR. Ambient nitrogen dioxide and distance from a major highway. The Science of The Total Environment. 2003;312:43–46. doi: 10.1016/S0048-9697(03)00228-6. http://www.sciencedirect.com/science/article/B6V78-48XD753-2/2/5f1478258008f15d3fe981c8929c1052. [DOI] [PubMed] [Google Scholar]
- Hanrahan PL. The Plume Volume Molar Ratio Method for determining NO2/NOX ratios in modeling - Part I: Methodology. Journal of the Air & Waste Management Association. 1999;49:1324–1331. doi: 10.1080/10473289.1999.10463960. http://dx.doi.org/10.1080/10473289.1999.10463960. [DOI] [PubMed] [Google Scholar]
- Härkönen J, Valkonen E, Kukkonen J, Rantakrans E, Lahtinen K, Karppinen A, Jalkanen L. A model for the dispersion of pollution from a road network. Finnish Meteorological Institute, Publications on Air Quality 23; Helsinki: 1996. p. 34. [Google Scholar]
- HEI. Traffic-related air pollution: A critical review of the literature on emissions, exposure, and health effects. Boston, MA: 2010. [Last Accessed: April 13, 2017]. https://www.healtheffects.org/publication/traffic-related-air-pollution-critical-review-literature-emissions-exposure-and-health. [Google Scholar]
- Janssen NA, Brunekreef B, van Vliet P, Aarts F, Meliefste K, Harssema H, Fischer P. The relationship between air pollution from heavy traffic and allergic sensitization, bronchial hyperresponsiveness, and respiratory symptoms in Dutch schoolchildren. Environ Health Perspect. 2003;111:1512–1518. doi: 10.1289/ehp.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez JL, McRae GJ, Nelson DD, Zahniser MS, Kolb CE. Remote Sensing of NO and NO2 Emissions from Heavy-Duty Diesel Trucks Using Tunable Diode Lasers. Environmental Science & Technology. 2000;34:2380–2387. http://dx.doi.org/10.1021/es9911622. [Google Scholar]
- Karner AA, Eisinger DS, Niemeier DA. Near-roadway air quality: Synthesizing the findings from real-world data. Environmental Science & Technology. 2010;44:5334–5344. doi: 10.1021/es100008x. http://dx.doi.org/10.1021/es100008x. [DOI] [PubMed] [Google Scholar]
- Kimbrough ES, Baldauf RW, Watkins N. Seasonal and diurnal analysis of NO2 concentrations from a long-duration study conducted in Las Vegas, Nevada. Journal of the Air & Waste Management Association. 2013a;63:934–942. doi: 10.1080/10962247.2013.795919. http://dx.doi.org/10.1080/10962247.2013.795919. [DOI] [PubMed] [Google Scholar]
- Kimbrough S, Baldauf R, Hagler G, Shores RC, Mitchell W, Whitaker DA, Croghan CW, Vallero DA. Long-term continuous measurement of near-road air pollution in Las Vegas: Seasonal variability in traffic emissions impact on local air quality. Air Quality, Atmosphere & Health. 2013b;6:295–305. http://dx.doi.org/10.1007/s11869-012-0171-x. [Google Scholar]
- Kimbrough S, Palma T, Baldauf RW. Analysis of mobile source air toxics (MSATs)—Near-road VOC and carbonyl concentrations. Journal of the Air & Waste Management Association. 2014;64:349–359. doi: 10.1080/10962247.2013.863814. http://dx.doi.org/10.1080/10962247.2013.863814. [DOI] [PubMed] [Google Scholar]
- Mavroidis I, Chaloulakou A. Long-term trends of primary and secondary NO2 production in the Athens area. Variation of the NO2/NOx ratio. Atmospheric Environment. 2011;45:6872–6879. http://dx.doi.org/10.1016/j.atmosenv.2010.11.006. [Google Scholar]
- McAdam K, Steer P, Perrotta K. Using continuous sampling to examine the distribution of traffic related air pollution in proximity to a major road. Atmospheric Environment. 2011;45:2080–2086. http://dx.doi.org/10.1016/j.atmosenv.2011.01.050. [Google Scholar]
- Mejía JF, Choy SL, Mengersen K, Morawska L. Methodology for assessing exposure and impacts of air pollutants in school children: Data collection, analysis and health effects - A literature review. Atmospheric Environment. 2011;45:813–823. http://dx.doi.org/10.1016/j.atmosenv.2010.11.009. [Google Scholar]
- NAS. Rethinking the Ozone Problem in Urban and Regional Air Pollution. National Academies Press; 1992. [Google Scholar]
- NOAA. Automated Surface Observing System (ASOS) National Centers for Environmental Information; Ashville, NC: 2015. [Last Accessed: October 1, 2015]. https://www.ncdc.noaa.gov/data-access/land-based-station-data/land-based-datasets/automated-surface-observing-system-asos. [Google Scholar]
- Olesen HR, Ketzel M, Jensen SS, Løfstrøm P, Im U, Becker T. A tool for air pollution assessments along highways. Denmark: Technical Report from DCE – Danish Centre for Environment and Energy No. 59; 2015. [Last Accessed: October 19, 2016]. User Guide to OML-Highway. http://dce2.au.dk/pub/TR59.pdf. [Google Scholar]
- Podrez M. An update to the ambient ratio method for 1-h NO2 air quality standards dispersion modeling. Atmospheric Environment. 2015;103:163–170. http://dx.doi.org/http://dx.doi.org/10.1016/j.atmosenv.2014.12.021. [Google Scholar]
- Richmond-Bryant J, Chris Owen R, Graham S, Snyder M, McDow S, Oakes M, Kimbrough S. Estimation of on-road NO2 concentrations, NO2/NOX ratios, and related roadway gradients from near-road monitoring data. Air Quality, Atmosphere & Health. 2017:1–15. doi: 10.1007/s11869-016-0455-7. http://dx.doi.org/10.1007/s11869-016-0455-7. [DOI] [PMC free article] [PubMed]
- Roorda-Knape MC, Janssen NAH, De Hartog JJ, Van Vliet PHN, Harssema H, Brunekreef B. Air pollution from traffic in city districts near major motorways. Atmospheric Environment. 1998;32:1921–1930. doi: 10.1016/s1352-2310(97)00496-2. http://dx.doi.org/Doi. [DOI] [Google Scholar]
- Seinfeld JH, Pandis SN. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. Second. John Wiley and Sons, Inc.; Hoboken, NJ: 2012. [Google Scholar]
- Turner DB. Atmospheric Dispersion Modeling - Critical Review. Journal of the Air Pollution Control Association. 1979;29:502–519. <Go to ISI>://WOS:A1979GV61700002. [Google Scholar]
- U.S. EPA. User’s Guide for the AERMOD Meteorological Preprocessor (AERMET) US EPA. Research Triangle Park; North Carolina: 2004. [Last Accessed: October 19, 2016]. EPA-454/B-03-002. [Google Scholar]
- U.S. EPA. [Last Accessed: April 13, 2017];Our Nation's Air - Status and Trends through 2010. 2010a EPA-454/R-12-001. http://www.epa.gov/airtrends/2011/report/fullreport.pdf.
- U.S. EPA. [Last Accessed: April 8, 2017];Primary National Ambient Air Quality Standards for Nitrogen Dioxide (75 FR 6474, February 9, 2010) codified in 40 CFR parts 50 and 58. 2010b https://www3.epa.gov/ttn/naaqs/standards/nox/fr/20100209.pdf.
- U.S. EPA. [Last Accessed: October 19, 2016];Appendix W to Part 51—Guideline on Air Quality Models. 2011a http://www.gpo.gov/fdsys/pkg/CFR-2011-title40-vol2/pdf/CFR-2011-title40-vol2-part51-appW.pdf. Appendix W to Part 51.
- U.S. EPA. Near-Road NO2 Monitoring Technical Assistance Document. Durham, NC: 2011b. [Last Accessed: July 1, 2013]. http://www.epa.gov/ttnamti1/files/nearroad/NearRoadTAD.pdf. [Google Scholar]
- U.S. EPA. [Last Accessed: February 13, 2012];Preferred/Recommended Models AERMOD Modeling System. 2012 http://www.epa.gov/ttn/scram/dispersion_prefrec.htm#aermod.
- U.S. EPA. Clarification on the Use of AERMOD Dispersion Modeling for Demonstrating Compliance with the N02 National Ambient Air Quality Standard. Research Triangle Park, NC: 2014a. [Last Accessed: May 14, 2015]. http://www.epa.gov/scram001/guidance/clarification/NO2_Clarification_Memo-20140930.pdf. [Google Scholar]
- U.S. EPA. [Last Accessed: June 1, 2015];Preferred/Recommended Models. 2014b http://www.epa.gov/ttn/scram/dispersion_prefrec.htm#rec.
- U.S. EPA. [Last Accessed: April 8, 2017];Air Pollutant Emission Trends Data. 2016 https://www.epa.gov/air-emissions-inventories/air-pollutant-emissions-trends-data.
- Van Roosbroeck S, Jacobs J, Janssen NAH, Oldenwening M, Hoek G, Brunekreef B. Long-term personal exposure to PM2.5, soot and NOx in children attending schools located near busy roads, a validation study. Atmospheric Environment. 2007;41:3381–3394. doi: 10.1016/j.atmosenv.2006.12.023. http://dx.doi.org/DOI. [DOI] [Google Scholar]
- Venkatram A, Karamchandani P, Pai P, Goldstein R. The development and application of a simplified ozone modeling system (SOMS) Atmospheric Environment. 1994;28:3665–3678. http://dx.doi.org/http://dx.doi.org/10.1016/1352-2310(94)00190-V. [Google Scholar]
- Wang YJ, DenBleyker A, McDonald-Buller E, Allen D, Zhang KM. Modeling the chemical evolution of nitrogen oxides near roadways. Atmospheric Environment. 2011;45:43–52. http://dx.doi.org/http://dx.doi.org/10.1016/j.atmosenv.2010.09.050. [Google Scholar]
- Wild RJ, Dubé WP, Aikin KC, Eilerman SJ, Neuman JA, Peischl J, Ryerson TB, Brown SS. On-road measurements of vehicle NO2/NOx emission ratios in Denver, Colorado, USA. Atmospheric Environment. 2017;148:182–189. http://dx.doi.org/http://dx.doi.org/10.1016/j.atmosenv.2016.10.039. [Google Scholar]
- Yasuyuki I, Makiko Y, Toshimasa O. Estimation of Primary NO2/NOX Emission Ratio from Road Vehicles Using Ambient Monitoring Data. Studies in Atmospheric Science. 2014;1:1–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.