ABSTRACT
In response to hypoxia, plant roots produce very high levels of nitric oxide. Recently, it was demonstrated that NO and ethylene both are essential for development of aerenchyma in wheat roots under hypoxia. Increased NO under hypoxia correlated with induction of NADPH oxidase gene expression, ROS production and lipid peroxidation in cortical cells. Tyrosine nitration was prominent in cells developing aerenchyma suggesting that NO and ROS play a key role in development of aerenchyma. However, the role of antioxidant genes during development of aerenchyma is not known, therefore, we checked gene expression of various antioxidants such as SOD1, AOX1A, APX and MnSOD at different time points after hypoxia treatment and found that expression of these genes elevated in 2 h but downregulated in 24 h where development of aerenchyma is prominent. Further, we found that plants growing under ammonium nutrition displayed delayed aerenchyma development. Taken together, new insights presented in this short communication highlighted additional regulatory role of antioxidants gene expression during aerenchyma development.
KEYWORDS: Aerenchyma, antioxidants, nitric oxide, reactive oxygen species, superoxide
Oxygen is essential for generation of energy as it acts as a terminal electron acceptor in electron transport chain of mitochondria. The situation of hypoxia occurs in plants during various stages of their development (seed germination) and also under flooding or waterlogging (submergence). Oxygen depletion leads to energy crisis which eventually impairs its growth and productivity. To cope up with this hypoxia associated energy crisis, plants have developed several adaptive strategies. Example of one such adaptation is the formation of aerenchyma.1,2 Aerenchyma is a gas-filled tissue which allows exchange of gases between shoot and root. Basically, there are two types of aerenchyma viz., schizogenous and lysigenous. Schizogenous aerenchyma is formed by a process of cell separation at the middle lamella during cell development and lysigenous aerenchyma is formed due to the random death of some cells. Schizogenous aerenchyma is usually constitutive (Example: Saggitarria lancifolia),3 whereas lysigenous aerenchyma can be constitutive as well as induced (rice and maize respectively).4 Hypoxia is one of the prominent physiological inducers of aerenchyma in many plant species.4 Aerenchyma formation is readily induced in the roots of maize under hypoxia (laboratory conditions) and in flooded soils.2 In contrast to hypoxia, anoxia is not an inducer for aerenchyma formation4 which might be due to the requirement of oxygen in ethylene biosynthesis. Hence, low partial pressures of oxygen, initiates the development of the aerenchyma. The formation of lysigenous aerenchyma was studied in various abiotic stress conditions such as hypoxia, high temperature, drought and nutrient deficiency.2 Lysigenous aerenchyma formation is also called as root cortical death (RCD). Cell death during aerenchyma development is associated with electrolyte leakage, low ionic equilibrium, DNA fragmentation and cytoplasmic streaming.5 These events also take place during hypersensitive response.6
Several lines of evidence suggests that ethylene plays important role in the development of aerenchyma in plant roots.7-10. Mechanical impedance and hypoxia act synergistically in ethylene biosynthesis in maize roots.11 Ethylene induced aerenchyma formation is characterized by induction of several signal transduction pathways.2
Nitric oxide (NO) is a free radical signaling molecule that plays a role in wide range of physiological processes such as germination, growth and development of the plant and development of programmed cell death (PCD) during pathogen infection.12 There are several reports which suggests that, plant roots produce high amount of NO under hypoxia using nitrate reductase and the mitochondrial electron transport chain.13,14 The produced NO can be oxidized to nitrate that leads to generation of limited amount of ATP,15 induction of alternative oxidase via inhibition of aconitase16 and protection of mitochondrial structure.17,18
Previously, it was thought that ethylene is a precursor for development of aerenchyma but more recently, Wany et al.5 has shown that ethylene alone cannot induce aerenchyma but requires the presence of nitric oxide (NO). Wany et al.5 provided new insights into mechanism of aerenchyma. Wany et al.5 investigated whether hypoxia induced NO is a requirement for ethylene induced aerenchyma. This study revealed that the formation of lysigenous aerenchyma occurs in several stages such as, perceiving hypoxia as a stress signal, release of NO signal in the cortical and vascular region of the plant roots, and thereby transducing signals to induce several other regulatory genes. First, they checked NO production under normoxia and hypoxia by two methods: 1) DAF-FM DA fluorescence and gas phase Griess reagent assay and found that hypoxia treatment for 24 and 48 h leads to production of NO. To find out whether increased NO has any impact on ethylene levels, they checked levels of ethylene by GC-FID and found induction of ethylene in response to hypoxia. However, incubating roots with cPTIO during hypoxia treatment led to reduced levels of ethylene. To further confirm the role of NO in induction of ethylene signaling pathways, they checked expression of ethylene biosynthetic genes ACC oxidase and ACC synthase and ethylene responsive genes (ERF1 and PDF13), suggesting that NO is present upstream to ethylene and NO is indeed a requirement for aerenchyma formation. Comparison of cell death between pathogen infection and aerenchyma suggests that there are common processes between them, hence, they checked various processes that take place during PCD, such as electrolyte leakage, DNA fragmentation and cellulase assay. These findings provided a link between NO and PCD during aerenchyma formation. Both ROS and NO are key players in development of cell death, therefore, authors checked ROS, lipid peroxidation, protein tyrosine nitration and expression of Respiratory burst oxidative homolog/ NADPH oxidase (RBOH/NOX) gene and found that their levels increased under hypoxia. Further, hypoxic-NO induced expression of signal transduction genes such as phospholipase C, G-protein alpha subunit, calcium-dependent protein kinase family genes CDPK, CDPK2, CDPK4, Ca-CamK, inositol 1,4,5-trisphosphate 5-phosphatase 1 and protein kinase C. These results clearly demonstrated that hypoxically-induced NO is essential for the development of ethylene-induced aerenchyma formation. Scavenging NO using cPTIO (2–4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide), and ethylene biosynthetic inhibitor; cobalt chloride (CoCl2) and AVG (Aminoethoxyvinylglycine) led to reduced expression of signal transduction genes involved in aerenchyma. Consequently, this study envisaged the role of hypoxically induced NO in the development of lysigenous aerenchyma in wheat roots.
Inspite of many significant roles of hypoxic-NO elucidated in Wany et al.,5 there are still some unanswered queries which needs further attention. In Wany et al.5 study, it was found that there is increased ROS in the root cortical and stellar region, but superoxides (O2−•) are majorly localized in the stele and pericycle of the tissue but slightly in the cortical region. This is an interesting observation raising further questions about cellular dynamics of O2−• in response to hypoxia. The produced O2−• and NO both, might be responsible for cell death in cortical region. The presence of higher amount of O2−• but lesser amount of NO are probably responsible for non-induction of aerenchyma in stellar region. Another role for produced O2−• in stellar region could be, it can channelize through the vascular system up to the shoots to increase the photosynthetic rate to acclimatize the plant during hypoxia. The localization of superoxides in the vascular region also accounts for many physiological reasons. Firstly, behind the root apex, internal oxygen deficiency is most prominent at the stele19 and it is the first place at which hypoxia is sensed. Secondly, the stellar region of any tissue is the carrier of many nutrients, fluids and also molecular signals perceived during stress. Hypoxic conditions results into a number of important implications. One of them is the production of various by-products of anaerobic metabolism such as alanine and ethanol, found in the stele, but not in the cortex.10 Another is the Crafts-Boyer hypothesis, which postulates that there is a radial passive leakage of ions due to anoxic stele. Further, due to glycolysis or fermentation, there is very slow ATP production in the anoxic core of cells, however, they maintain ATP levels from the normoxic outer cortex. The above statements shed light onto the anoxic stellar part of the tissue enriched with a number of fermentative products and leaked ions.2,10,20
In response to stress, plants generate ROS. Hypoxia is an inducer for ROS.21 At the molecular level, increased ROS levels are key signs of stress and as they can interact with a number of cellular molecules and metabolites, leads to irreparable metabolic dysfunction and death.22 Plants have well-developed detoxification systems to counter the deleterious effects of ROS. Therefore, there is always a coordinated control in the ROS production and scavenging which protects the plants from severe damage but during the process such as PCD, plant cells need to keep ROS levels high. Hence, in this study, we checked expression of antioxidant genes at various time points (2 h, 6 h and 24 h) of hypoxia treatment. Further, evidence is the increased RBOH/NOX gene expression that leads to a respiratory oxidative burst directing to PCD and aerenchyma formation.23
In order to check, whether there is the decreased expression of antioxidant genes causing the accumulation of ROS levels mediated by hypoxic-NO, we checked the expression of antioxidant genes (Table 1). There was a gradual reduction in SOD1 expression levels in due course of time. At 2 h of hypoxia, there was 4-fold increase in SOD1 transcript levels, which continued to decrease further i.e., 2-fold reduction in 6 h and 1.2-fold reduction in 24 h of hypoxia (Fig. 1A). A similar trend was observed in AOX1a (Fig. 1B) gene expression levels, where initially at 2 h, there was ∼5-fold increased expression levels, which later decreased to 2-fold in 6 h and sharply fall at 24 h of hypoxia. APX gene expression was increased gradually from 2-fold in 2 h to 3-fold in 6 h of hypoxia, then it decreased significantly in 24 h of hypoxia (Fig. 1C). MnSOD expression levels also shows similar pattern i.e., after a 4-fold induction at 2 h, it gradually decreased to 1.5-fold at 6 h, which further decreased sharply at 24 h (Fig. 1D). This suggests that due to increased ROS levels, plants simultaneously produce antioxidants only within few hours of hypoxia. But, later (24 h or more), the levels declines significantly to cause the accumulation of ROS in the tissues. Therefore, it was evidenced that there is accumulation of ROS and superoxides in the cell layer (cortex, endodermis and pericycle) surrounding aerenchyma in wheat roots under hypoxia5 and Fig. 2). Further, in order to find out whether hypoxia-induced NO play a role in induction of NADPH oxidase (NOX), we checked the expression of RBOH/NOX gene and observed 2.5 fold to 3 fold induction in 2 h and 6 h respectively and a 4-fold induction in 24 h of hypoxia (Fig. 1E). This clearly indicates that NOX gene is induced strongly at 24h. The sole involvement of hypoxia-induced NO in NOX induction was earlier evidenced in the presence of cPTIO pre-treated roots under hypoxia and no changes were observed in NOX/RBOH expression levels in the presence of NO scavenger suggesting that NO can be directly indirectly responsible for induction of NOX gene under hypoxia.5
Table 1.
List of gene-specific primers.
| S.No. | Gene | Accession Id | Orientation | Sequence | Product length |
|---|---|---|---|---|---|
| 1 | SOD1 | FJ890986.1 | Forward | CTTCCATGTGCACGCTCTTG | 151 |
| Reverse | AACACCATCCACTCCAGCTG | ||||
| 2 | AOX1A | AB078882.1 | Forward | GACGGGGAGAAGAAGGAGGT | 156 |
| Reverse | GTGGTGCTTGGTCAGATCGA | ||||
| 3 | APX | EF555121.1 | Forward | ATTCGTCAGTTTGTCCCCGT | 189 |
| Reverse | TCAGAGGGTCACGAGTCCAT | ||||
| 4 | MnSOD | AF092524.1 | Forward | GCGAAGAAAACCCTAGGCCT | 166 |
| Reverse | TGTTGTAGTGGGCGACGTAG | ||||
| 5 | Nox | AY561153.1 | Forward | TTGGTGACTGGACACGAGAG | 156 |
| Reverse | AATCCTGAGCAGGAGAACCA | ||||
| 6 | Actin | KC775780.1 | Forward | TGCCAAGAACAGCTCCTCAG | 216 |
| Reverse | CCACTGAGCACAATGTTGCC |
Figure 1.
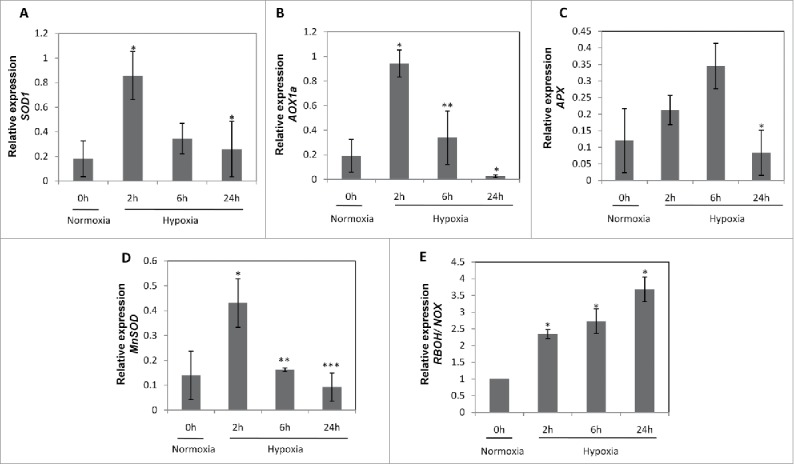
Expression analysis of antioxidant genes (A) Triticum aestivum superoxide dismutase (TaSOD1;FJ890986.1) (B) Triticum aestivum alternative oxidase (TaAOX1a; AB078882.1) (C) Triticum aestivum ascorbate peroxidase (TaAPX; EF555121.1) (D) Triticum aestivum manganese superoxide dismutase (TaMnSOD; AF092524.1) (E) Triticum aestivum respiratory burst oxidase homolog/ NADPH oxidase (TaRBOH/NOX; AY561153.1) under normoxia and hypoxia in the presence of nitrate. Actin was used as internal reference gene for normalization of data. Values are means (n = 3 ± SE). A significant difference between normoxia and hypoxia is analyzed by t-test at P < 0.001 (***), P < 0.01 (**) and P < 0.05 (*).
Figure 2.
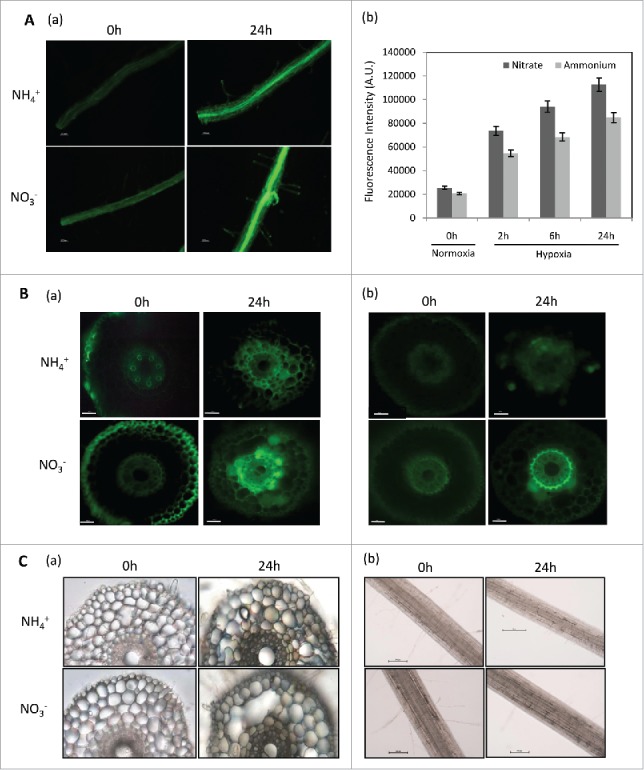
ROS, NO production and superoxide levels in wheat cross-sections and root segments. (A) Total ROS levels in wheat roots grown in NO3− and NH4+ measured by dichlorodihydro-fluorescein diacetate (H2-DCF-DA) by determining production of dichlorofluorescein (DCF) (n = 5). Roots were incubated in 10 µM dichlorodihydro-fluorescein diacetate (H2DCF-DA), 10 mM Tris-HCl buffer, pH-7.2 for 5 minutes in dark, and then washed with buffer three times. Images were recorded with fluorescence microscope (Nikon80i, Japan) at 495 nm excitation and 515 nm emission wavelength; (a) DCF fluorescence in root segments grown in nitrate and ammonium under normoxia and hypoxia-24 h; (b) DCF fluorescence intensities measured by ImageJ in root segments grown in nitrate and ammonium nutrition under normoxia and hypoxia-24 h. Values are (n = 5 ± SE). Scale bar = 250 µm. (B) ROS and NO production in root cross-sections measured by H2DCF-DA and DAF-FM-DA respectively (a) DCF fluorescence in root cross-sections (n = 5) grown in nitrate and ammonium nutrition under normoxia and hypoxia-24 h; (b) DAF-FM fluorescence in root cross-sections (n = 5) grown in nitrate and ammonium nutrition under normoxia and hypoxia-24 h. Scale bar = 250 µm. (C) Aerenchyma development and superoxide (O2−•) production in wheat roots (n = 3) grown in nitrate and ammonium nutrition measured by in vivo staining with nitroblue tetrazolium chloride; NBT as a substrate by measuring the formation of formazan complex. The roots were visualized and photographed under bright field microscope (Nikon 80i, Japan) for the presence of dark blue-violet spots (formazan precipitates); (a) Localization of O2−• in root cross-sections under normoxia and hypoxia-24 h; (b) Localization of O2−• in root segments under normoxia and hypoxia-24 h. Scale bar = 250 µm.
The role of nitrate in nitrogen assimilation and NO signalling, their cross-talk under hypoxic stress is largely unknown,24 but in agricultural soils, nitrogen is mainly supplied as either NO3− or NH4+, hence it will be interesting to know, under which nutrient conditions aerenchyma formation is prominent. First, we checked ROS levels using the 2'-7'-dichlorodihydro-fluorescein diacetate (H2DCFDA) dye and superoxide levels by NBT staining method in ammonium and nitrate grown roots (Fig. 2 A, B). Under both conditions, there was an increase in ROS but it was slightly higher in NO3− (Fig. 2A–a and b) in comparison to NH4+. In order to localize the ROS, freshly cut root cross-sections were stained with DCF-DA dye and we found a marked increase in DCF fluorescence in both nitrate and ammonium root cortical cells in 24 h of hypoxia (Fig. 2B–a). This suggests that NO3− and NH4+, has no major impact on hypoxia induced ROS. Then, NO production was checked by using DAF-FM diacetate fluorescence method. Strikingly, we found an elevated DAF-FM fluorescence in the root cross-sections in nitrate grown roots in comparison to ammonium grown roots (Fig. 2B–b) suggesting that increased ROS and NO both are responsible for aerenchyma formation in 24 h of hypoxia in NO3− and decreased NO in NH4+ is responsible for delay of aerenchyma in NH4+ grown plants. Therefore, under hypoxia, wheat roots exhibit a rapid synthesis of nitric oxide (NO) and a parallel accumulation of reactive oxygen species (ROS) under NO3− nutrition. This conjoint responses may trigger PCD process faster,25 on the other hand, reduced levels of NO might be responsible for delay in aerenchyma formation in NH4+ grown plants. Hence, these accumulating evidence suggests that both NO and ROS play key roles in PCD.
NO3− supplemented roots showed slightly higher O2−• levels and more aerenchyma forming cells than NH4+ supplemented roots under 24 h of hypoxia (Fig. 2C–a and b). Freshly cut cross-sections of nitrate grown roots revealed that O2−• formation is preferentially localized in the pericycle lining the vascular region under normoxia and showed increased O2−• levels in 24 h of hypoxia. This increase in the formazan complex within few hours of hypoxia revealed that, the superoxides are produced in very less time and acts as signals for accumulation of other ROS compounds in the cortical and epidermal region of the root tissue. We found similar results under normoxia conditions which suggests that epidermis and the vascular region is the centre for O2−• production. This suggests that there is a functional role of apoplastic O2−• generation by plasma membrane NOX gene in cell elongation in the growing zone of the root.26
Taken together, this study uncovered some interesting aspects which were not discussed in Wany et al.,5 firstly, we found negative correlation between antioxidants and increased ROS at 24 h of hypoxia which can support hypothesis that suppression of antioxidant genes in needed for acceleration of cell death and secondly, we found that NO3− nutrition has positive effect in flooding tolerance via aerenchyma formation.
Wheat (Triticum aestivum L.) genotype cv. HD 3086 was used for all experiments. Seeds were initially surface sterilized with 4% sodium hypochlorite for 10 minutes and washed five times with distilled water and subsequently germinated on moist filter paper in petridishes at 23°C under dark conditions in growth chamber. The growth conditions were followed according to Wany et al.5 The hypoxic conditions were maintained by intermittently flushing the chamber with 0.4% oxygen. Storage and harvesting of samples was performed according to Wany et al.5 Similarly, methods for RNA isolation, cDNA synthesis and quantitative real time PCR was performed with the set of primers given in Table 1 as described in Wany et al.5 The meth`ods for ROS levels, NO production and superoxide levels were also followed according to Wany et al.5
Funding Statement
Science and Engineering Research Board (SERB), Department of Science and Technology, New Delhi and Department of Biotechnology, New Delhi.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by Ramalingaswami re-entry fellowship and IYBA from (KJG);SERB National Postdoctoral Fellowship to (AW). The Central Instrumentation Facility (CIF) of NIPGR, New Delhi is greatly acknowledged.
References
- 1.Bailey-Serres J, Lee SC, Brinton E. Waterproofing crops: Effective flooding survival strategies. Plant Physiol. 2012;160:1698–709. doi: 10.1104/pp.112.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drew MC, He C, Morgan PW. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000;3:123–27. doi: 10.1016/S1360-1385(00)01570-3. [DOI] [PubMed] [Google Scholar]
- 3.Shussler E, Longstreth DJ. Aerenchyma develops by cell lysis in roots and cell separation in leaf petioles in Sagittaria lancifolia (Alismataceae). Am J Bot. 1996;83:1266–73. doi: 10.2307/2446110. [DOI] [Google Scholar]
- 4.Evans DE. Aerenchyma formation. New Phytol. 2003;161:35–49. doi: 10.1046/j.1469-8137.2003.00907.x. [DOI] [Google Scholar]
- 5.Wany A, Kumari A, Gupta KJ. Nitric oxide is essential for the development of aerenchyma in wheat roots under hypoxic stress. Plant Cell Environ. 2017;40:3002–17. doi: 10.1111/pce.13061. [DOI] [PubMed] [Google Scholar]
- 6.Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;6394:585–8. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi T, Watanabe K, Fukazawa A, Mori H, Abe F, Kawaguchi K, Oyanagi A, Nakazono M. Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J Exp Bot. 2014;65:261–73. doi: 10.1093/jxb/ert371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson MB, Armstrong W. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol. 1999;1:274–87. doi: 10.1111/j.1438-8677.1999.tb00253.x. [DOI] [Google Scholar]
- 9.Gunawardena AHLAN, Pearce DM, Jackson MB, Hawes CR, Evans DE. Characterization of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta. 2001;212:205–14. doi: 10.1007/s004250000381. [DOI] [PubMed] [Google Scholar]
- 10.Drew MC. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:223–50. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- 11.He CJ, Drew MC, Morgan PW. Induction of enzymes associated with lysigenous aerenchyma formation in roots of Zea mays during hypoxia or nitrogen starvation. Plant Physiol. 1994;112:1679–85. doi: 10.1104/pp.105.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mur LA, Mandon J, Persijn S, Cristescu SM, Moshkov IE, Novikova GV, Hall MA, Harren FJ, Hebelstrup KH, Gupta KJ. Nitric oxide in plants: an assessment of the current state of knowledge. AoB Plants. 2013;5:pls052. doi: 10.1093/aobpla/pls052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta KJ, Stoimenova M, Kaiser WM. In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J Exp Bot. 2005;56:2601–09. doi: 10.1093/jxb/eri252. [DOI] [PubMed] [Google Scholar]
- 14.Planchet E, Gupta KJ, Sonoda M, Kaiser WM. Nitric oxide emission from tobacco leaves and cell suspensions: Rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J. 2005;41:732–43. doi: 10.1111/j.1365-313X.2005.02335.x. [DOI] [PubMed] [Google Scholar]
- 15.Stoimenova M, Igamberdiev AU, Gupta KJ, Hill RD. Nitrite driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta. 2007;226:465–74. doi: 10.1007/s00425-007-0496-0. [DOI] [PubMed] [Google Scholar]
- 16.Gupta KJ, Shah JK, Brotman Y, Jahnke K, Willmitzer L, Kaiser WM, Bauwe H, Igamberdiev AU. Inhibition of aconitase by nitric oxide leads to induction of the alternative oxidase and to a shift of metabolism towards biosynthesis of amino acids. J Exp Bot. 2012;63:1773–84. doi: 10.1093/jxb/ers053. [DOI] [PubMed] [Google Scholar]
- 17.Gupta AK, Kumari A, Mishra S, Wany A, Gupta KJ. The functional role of nitric oxide in plant mitochondrial metabolism. Adv Bot Res. 2016;77:145–63. doi: 10.1016/bs.abr.2015.10.007. [DOI] [Google Scholar]
- 18.Gupta KJ, Lee CP, Ratcliffe RG. Nitrite protects mitochondrial structure and function under hypoxia. Plant Cell Physiol. 2017;58:175–83. doi: 10.1093/pcp/pcw174. [DOI] [PubMed] [Google Scholar]
- 19.Gibbs J, Turner DW, Armstrong W, Darwent MJ, Greenway H. Response to oxygen deficiency in primary maize roots. I. Development of oxygen deficiency in the stele reduces radial solute transport to the xylem. Aust J Plant Physiol. 1998;25:745–58. doi: 10.1071/PP97135. [DOI] [Google Scholar]
- 20.Thomson CJ, Atwell BJ, Greenway H. Response of wheat seedlings to low O2 concentrations in nutrient solution. I. Growth, O2 uptake and synthesis of fermentative end-products by root segments. J Exp Bot. 1989;40:985–91. doi: 10.1093/jxb/40.9.985. [DOI] [Google Scholar]
- 21.Vergara R, Parada F, Rubio S, Perez FJ. Hypoxia induces H2O2 production and activates antioxidant defence system in grapevine buds through mediation of H2O2 and ethylene. J Exp Bot. 2012;63:4123–31. doi: 10.1093/jxb/ers094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasanuzzaman M, Hossain MA, Jaime A, da Silva T, Fujita M. Plant Response and Tolerance to Abiotic Oxidative Stress: Antioxidant Defense Is a Key Factor. In: Venkateswarlu B., Shanker A., Shanker C., Maheswari M. (eds) Crop Stress and its Management: Perspectives and Strategies. Springer, Dordrecht. 2012. doi: 10.1007/978-94-007-2220-0_8. [DOI] [Google Scholar]
- 23.Yun BW, Feechan A, Yin M, Saidi NB, Le Bihan T, Yu M, Moore JW, Kang JG, Kwon E, Spoel SH, et al.. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature. 2011;478:264–8. doi: 10.1038/nature10427. [DOI] [PubMed] [Google Scholar]
- 24.Frungillo L, Skelly MJ, Loake GJ, Spoel SH, Salgado I. S-nitrosothiols regulate nitric oxide production and storage in plants through the nitrogen assimilation pathway. Nat Commun. 2014;5:5401. doi: 10.1038/ncomms6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Loake GJ, Chu C. Cross-talk of nitric oxide and reactive oxygen species in plant programmed cell death. Front Plant Sci. 2013;4:314. doi: 10.3389/fpls.2013.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liszkay A, van der Zalm E, Schopfer P. Production of reactive oxygen intermediates (O2˙−, H2O2, and ˙OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 2004;136:3114–23. 10.1104/pp.104.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]


