Abstract
Photodynamic antimicrobial chemotherapy (PACT) is considered a promising alternative to conventional antibiotic approach. We have previously developed a novel PS containing five lysine amino acids, pentalysine-β-carbonylphthalocyanine Zinc (ZnPc(Lys)5), which in the presence of light, is highly toxic against a range of bacterial strains, including hospital isolated, drug resistant Acinetobacter baumannii. Here, we study the effect of light fluence of the two light sources on the PACT potency of ZnPc(Lys)5. We observed that an exposure of E.coli to a red LED light for only 2 seconds (light fluence of 0.15 J/cm2) in the presence of ZnPc(Lys)5 significantly eradicated 80% of the E.coli. We further demonstrated that a light fluence of 4.5 J/cm2 from a household light source induced a noticeable photodynamic effect in vitro and in vivo animal model. This study points to a new research direction of reducing light illumination time by increasing potency of PS.
OCIS codes: (170.5180) Photodynamic therapy, (230.3670) Light-emitting diodes, (170.3890) Medical optics instrumentation, (170.1870) Dermatology
1. Introduction
Bacterial infection can usually be treated successfully with antibiotics. However, the widespread use of antibiotics has led to the evolution of strains of bacteria that are resistant to many of current antibiotics and thus becomes a significant cause of antibiotic resistance. Nowadays, the increasing rate of antibiotic resistance and slow discovery of novel antibiotic treatments presents a growing threat to public health and is a worldwide challenge. Photosensitizer-mediated photodynamic antimicrobial chemotherapy (PACT) is an innovative method involving the administration of a photosensitizer which, upon photoactivation with visible light of appropriate wavelength, generates reactive oxygen species (ROS), such as singlet oxygen and free radicals, which are cytotoxic to bacteria [1, 2]. This method is now advocated as a promising approach for treating multidrug resistant infection [3, 4].
PACT is a localized therapy, and has numerous advantages over traditional modalities, including efficacy to drug resistant bacteria strain, effectiveness against broad spectrum of bacteria, lower drug resistance and limited systemic effect. There are three reasons that PACT may cause less resistance of bacteria. (1) It is highly effective with reduction of bacterial load up to 6 order of magnitude and less survived bacteria are left [5]. (2) It provides faster onset of action compared to typical antibiotics and can kill bacteria in minutes before the bacterial self-defense mechanism kicks in [6]. (3) It uses reactive oxygen species (ROS) such as singlet oxygen, as cytotoxic agents that can target multiple sites of bacteria, leaving less chance to induce resistance.
PACT depends on localized light delivery to treat infected area. Lasers and red LED light are the common light sources used in PACT to treat many skin disorders [7, 8]. Adverse effects like, mild pain, skin burning sensation or erythema and edema can happen during PACT session, exacerbated by the use of intense light sources or high drug dose [9]. Pain usually disappears right away after treatment, the erythema and edema take a little longer to fade, but both are manageable [10, 11]. These potential adverse effects can be minimized by lowering the drug or light fluence [12]. It has been demonstrated that, ambient light exposure at a dosage of 37 J/cm2 caused less pain and was more convenient for patients [13]. In another comparative study, photodynamic therapy (PDT) using ablative fractional CO2 laser (10 mJ per pulse) was more effective than using red LED light (37 J/cm2) for the treatment of actinic keratosis. This work found that one PDT treatment using this method is effective as two conventional PDT treatment, leading to improved cure rate and to minimize the treatment time needed in PDT to cure the lesions [14]. Studies also reported that low irradiance caused low level of pain without compromising the PACT effect [15, 16]. Thus it is a feasible strategy to optimize light fluence to reduce adverse effects of PACT while maintaining its antimicrobial efficacy.
Photosensitizers (PSs) are critical components of PACT application. PSs including methylene blue, porphyrin, and phthalocyanine are commonly applied in PACT. Every class of PSs has its unique features of molar extinction coefficient, triplet life time, and singlet oxygen quantum yield. Zinc phthalocyanines (ZnPcs) show higher molar extinction coefficients, longer triplet life times and good singlet oxygen quantum yields (0.67) as compared to other PSs, e.g. porphyrin [17] and therefore has strong PACT effect. We have been developing ZnPc-based PSs for PACT applications and studying PACT mechanisms. We conjugated a bacterial targeting moiety (polylysine) to ZnPc and used the conjugate to treat oral periodontitis by killing Porphyromonas gingivalis, which is the main strain of bacteria identified in periodontal plaque [18]. The conjugate reduced the bacterial load by 100-fold on a dog periodontal disease model at a dose of 20 μM after receiving a light fluence of 1.5 J/cm2 [18]. We also reported a series of ZnPc-based cationic PSs viz ZnPc(TAP)4n+, n = 4,8,12, TAP = 2,9(10),16(17),23(24)-tetrakis[2,4,6-tris(N,N-dimethyl aminomethyl)phenoxy] for eradication of common bacteria. The results showed that ZnPc(TAP)48+ reduced the drug-resistant E.coli by 1000 fold at a concentration of 1 µM and a light fluence of 4.5 J/cm2 [6]. We also developed a structurally well-defined pentalysine ZnPc PS (ZnPc(Lys)5) that was also shown to kill P.acnes, the primary pathogen responsible for cystic acne, at concentration of 1μM under a light fluence of 6 J/cm2, causing a 5 to 6 orders of magnitude reduction of bacteria [28]. This PS also effectively killed the multi-drug resistant (MDR) hospital acquired bacteria (Escherichia coli (EC) or Acinetobacter baumannii (AB). The results demonstrated that at a concentration of 2 µM, ZnPc(Lys)5 remarkably eradicated both MDR strains in vitro under a light fluence (12.7 J/cm2 and 25 J/cm2). PACT effect was further seen on an animal model of the MDR strain at a concentration (0.1 mM or 0.5 mM), demonstrating a high antibacterial efficacy in localized infected animal model [19]. The present work aims to study the effect of light fluence on bacterial killing using PS pentalysine-β-carbonylphthalocyanine zinc, ZnPc(Lys)5. We had a surprised finding during previous experiments: ZnPc(Lys)5 effectively killed bacteria (E.coli) under ambient light. We thus explored the effect of light fluence on the PACT potency of ZnPc(Lys)5. We observed that an exposure to a red LED light (70 mW, 660 nm with a light fluence of 0.15 J/cm2) for only 2-sec killed 80% E.coli. We further evaluated the possibility to use a house-hold light for PACT. Our results showed that ZnPc(Lys)5 is a potent PS and requires light fluence that is the lowest among all the previous reported work.
2. Materials and methods
2.1 Materials
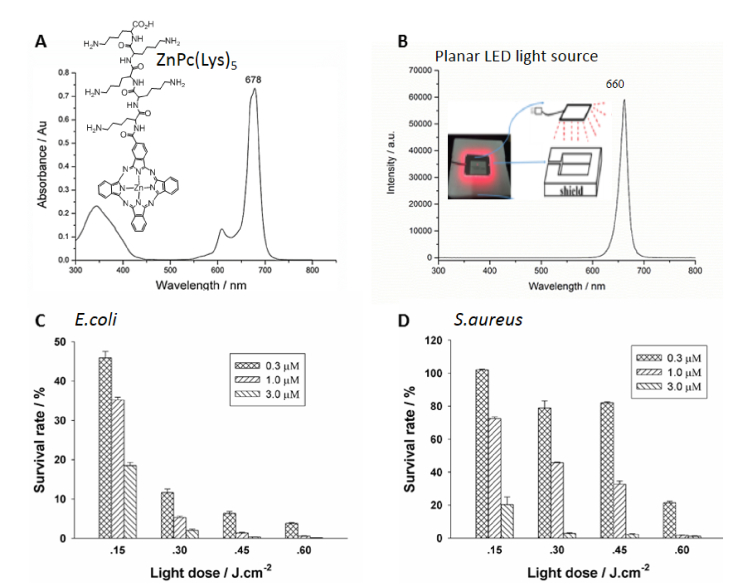
ZnPc(Lys)5, a zinc phthalocyanine derivative, was synthesized via the activation of carboxylic acid group on ZnPc-COOH by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS), followed by its coupling to pentalysine according to our previously published protocols [20, 21]. The synthesized ZnPc(Lys)5 was characterized by 1H NMR, 13C NMR, FTIR, and high-resolution mass spectra. The UV-Vis absorption spectrum of ZnPc(Lys)5 was recorded from 300 to 800 nm using quartz cuvettes with 1 cm path length on a Lambda-35 UV/Vis spectrometer (PerkinElmer, Massachusetts, USA) in DMSO. Analytical high-performance liquid chromatography (HPLC) was carried out on a C-18 reversed-phase HPLC system (Dalian Elite Analytical Instruments Co., Ltd., Dalian, China; Column: SinoChrom ODS-BP 250 × 4.6mm, 5µm), using a linear gradient from 50% to 100% (v/v) of methanol: acetonitrile (1:1) at a flow rate of 1 ml/min. The UV-Visible absorption spectrum of photosensitizer ZnPc(Lys)5 in DMSO (Fig. 1(A)) was typical of ZnPc with the strongest absorption at 678 nm (extinction coefficient of 118380 L·mol−1·cm−1). Furthermore, singlet oxygen (1O2) is believed to be the major cytotoxic agent involved in photodynamic therapy. The quantum yield of singlet oxygen generation of ZnPc(Lys)5 (0.64 ± 0.02) was comparable to ZnPc (0.67), measured in DMSO [22].
Fig. 1.
Ultraviolet-Visible absorption spectrum of photosensitizer ZnPc(Lys)5 in DMSO (A), light intensity distribution of a custom-made planar LED (B) and phototoxicities of ZnPc-(Lys)5 against Escherichia coli DH5α (C) and Staphylococcus aureus Xen29 (D) after receiving 2-sec illumination (light fluence of 0.15 J/cm2) and total of 8 seconds illumination (light fluence of 0.6 J/cm2) from the custom-made planar LED (660 nm). Data represent the mean ± SEM of three independent experiments, each performed in triplicate.
2.2 Microorganisms
A bioluminescent strain of Escherichia coli (E. coli) DH5α was constructed by transformation with the plasmid pAKlux2.1, an expression vector that contains a complete bacterial luciferase operon as described [23]. Bacteria were grown at 37°C using Luria-Bertani broth and agar plates containing ampicillin (100 mg/ml to select for resistance encoded by the plasmid) to an absorbance of 0.6 at 600 nm corresponding to 108 organisms per milliliter. This suspension was centrifuged, washed with phosphate-buffered saline (PBS) and re-suspended in PBS at the same density. Luminescence was routinely measured taking 100 μl aliquots of bacterial suspensions in 96-well black-sided plates, using a Synergy 4 Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT).
The luminescent strain of Staphylococcus aureus Xen29 (NCTC8532), a derivative of the biofilm forming S. aureus that possesses a stable copy of the modified Photorhabdus luminescens luxABCDE operon at a single integration site on the bacterial chromosome, was purchased from Shanghai Biofeng Company (China) and grown at 37°C using Luria-Bertani broth containing kanamycin (200 μg/ml to select for resistance encoded by the plasmid) to an absorbance of 0.5 at 600 nm corresponding to 1.44 x 108 organisms per milliliter.
2.3 Light sources
Two different kinds of light sources were used in current studies. One light source was a custom-made planar LED (Uniglory Electronics (HK) Co. Ltd., Hong Kong, China) containing 24 lamps that emitted a wavelength of 660nm with a power density of 75mW/cm2 on the sample surface. The other one was a house-hold lamp (Osram, Germany) with a power density of 12.5 mW/cm2 at 400-700 nm on the sample surface to simulate ambient light. The light emission spectra of both light sources were measured using a FLS 980 fluorescence spectrometer (Edinburgh Instruments, United Kingdom).
2.4 Antimicrobial studies in cultured bacteria
Antimicrobial assessment using custom-made planar LED: Bacteria suspensions (~106 CFU/ml in Phosphate Buffer Solution, PBS) were incubated in the dark at room temperature for 10 min with ZnPc(Lys)5 (at concentrations of 0.3 μM, 1.0 μM and 3.0 μM respectively) taken in 96-well plate in triplicates. The microplate was subjected to light exposure for 2 seconds from a custom-made planar LED (at 660 nm, light fluence of 0.15J/cm2 per 2 sec) followed by dark interval of 10 minutes before cell counting. Afterwards, cell viability was measured by a Synergy 4 Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT) and the experiment was continued up to 40 minutes measuring four consecutive readings. The percentage of cell survival after each measurement, was determined by comparing the luminescence intensities from treated plates to the luminescence intensities from control plates that were incubated without the PS and were kept in dark for periods equal to the irradiation times.
Antimicrobial assay using custom-made planar LED red light and house-hold light sources: Bacteria suspensions (~106 CFU/ml in PBS) were incubated in the dark at room temperature for 10 min with ZnPc(Lys)5 (at concentrations of 0.004 μM, 0.012 μM, 0.036 μM, 0.11 μM, 0.33 μM, 1.0 μM, 3.0 μM and 10 μM, respectively) in triplicates. Each well was exposed to light from a custom-made planar LED (at 660 nm) for 1 min or from a house-hold lamp for 6 min in a parallel experiment, receiving the same light dosage of 4.5 J/cm2. After illumination, luminescence was monitored for up to 40 min on Synergy 4 plate reader, which was used to calculate cell viability. The percentage of cell survival rate was determined by comparing the luminescence intensities from PS treated plates to the luminescence intensities from control plates that were incubated without the PS and were kept in dark for periods equal to the irradiation times. Bacterial suspensions were also exposed to visible light without exogenous PS to investigate the bactericidal effect of the light sources. Both E.coli and S.aureus were irradiated with the same light dosage using house-hold light and LED red light as used for the plates treated with the PS.
The antimicrobial efficacy under house-hold light was also evaluated using colony counting method. Bacterial suspensions (107 CFU/mL) were dispensed in a 96-well plate and incubated with ZnPc(Lys)5 (1.0 μM, 3.0 μM and 10 μM, respectively) at 37°C for 10 min, followed by an illumination with house-hold light for 6 min with a light fluence of 4.5 J/cm2. After illumination, 100 µL solutions from each well were then serially diluted (10−1 to 10−5) in PBS and was plated onto LB agar-plates. The plates were incubated at 37°C in a bacterial culture incubator for 24 h. The bacterial colony forming units (CFUs) were counted and recorded, and thus the antibacterial effect of ZnPc(Lys)5 was investigated.
2.5 Antimicrobial studies in experimental animals
Male Kunming mice (purchased from Shanghai SLAC Laboratory Animal Co. Ltd., Shanghai, China) were maintained and handled in accordance with the recommendations of institutional animal care and use committee (IACUC). The animals were allowed free access to water throughout the course of the experiments. The experimental procedures on mouse were in accordance with Perkin Elmer IACUC guidelines and approved veterinarian requirements for animal care and use. Mice (n = 18) weighing 24.6 ± 0.6 g were anesthetized with an intraperitoneal injection of pentobarbital (30 mg/kg, i.p.) before they were shaved on the back. One excisional wound, measuring 8x8 mm, was then made on the back of each mouse, using surgical scissors, by carefully opening the epidermis layer of the skin with one-side connected. The bottom of the wound was panniculus carnosus, with no visible bleeding. Localized infection on the wound was induced according to our previously published method [24, 25] with slight modification by inoculating a suspension (10 μl) containing 1x106 cells of midlog phase bioluminescent Xen29 S.aureus (~108 cells per ml in PBS). The bacteria were allowed to attach to the tissue for 10 min, then 5 μl of ZnPc(Lys)5 (1 mM in PBS) was added into the wound. Further 10 min were given to the photosensitizer for binding and penetrating the bacteria under subdued room lighting. Wound area in six mice was illuminated by 660 nm custom-made LED red light source (Uniglory Electronics (HK) Co. Ltd., Hong Kong, China) with an irradiance of 75 mW for 10 min, while in other six mice wound area was irradiated by house-hold lamp with an irradiance of 12.5 mW for 10 min. The light dosage in both cases corresponds to 45 J/cm2 and 7.5 J/cm2, respectively. Remaining six mice (without illumination) and kept in their cages in the dark during the whole experimental period.
To measure the bacteria survival after PACT treatment, the epidermis layer of mice skin was cut after anesthesia and washed with PBS. After being immersed in PBS (1 ml) and shake for 1 min, the tissues were washed and the amount of bacteria in the solution was determined by the standard plate count method. In this counting method, dilutions were made of a starting culture and each of the dilutions was plated onto solid agar. The number of colonies were used to back calculate the starting population density in CFU/ ml. The viability of bacteria after PACT treatment was measured by comparing the CFU value to that of a control group treated with saline. All rats were kept in cages after the treatment. Their body weights and their wound areas were measured every three days for 12 days.
3. Results
3.1 Low light fluence (0.15 J/cm2) enables antimicrobial effect of ZnPc(Lys)5
Phthalocyanine-type photosensitizers are very potent among all PSs due to their high molar extinction coefficient and high ROS quantum yield. PACT efficacy is affected by both drug doses and light fluence. Here, we aim to assess the effect of light fluence for the photosensitizer ZnPc(Lys)5. Two bioluminescent strains, i.e., Escherichia coli (E.coli) and Staphylococcus aureus (S.aureus), were used in current studies, where the bioluminescence is proportional to the number of live bacteria [6]. The luminescence signal was proportional to live bacterial CFU from 103 to 107 CFU. We followed the kinetics of the luminescent signal of bacteria using microplate reader for up to 40 min. Typically, the bioluminescent signals of bacteria increased right after the light illumination and then gradually stabilized at 20 min, and thus luminescence signal at 20 min were analyzed to measure the bacterial viability.
Specific wavelength of light emission is required to overlap partly or entirely with the absorption spectrum of PS which subsequently leads to microorganism photoinactivation. A custom-made planar LED (660 ± 25 nm with a power density of 75 mW/cm2) was used in current study, which contains 24 LED units and cover all wells of the standard 96-well microplates. The light emission maximum of this light source (Fig. 1(B)) is within the absorption spectrum (Q-band maximum = 678 nm) of the ZnPc(Lys)5 photosensitizer (Fig. 1(A)). We incubated bacteria suspensions (~106 CFU/ml in PBS) with ZnPc(Lys)5 (at concentrations of 0.3- 3.0 μM) for 10 min followed by exposure to the custom-made planar LED (giving a light fluence of 0.15 J/cm2 per 2 seconds). The bioluminescence intensities of the bacteria were measured using a microplate reader to assess bacterial viabilities. Cell survival rates of E.coli and S. aureus at increasing PS concentration and light dose are shown in Fig. 1(C) and 1(D), respectively.
We observed significant cytotoxicity on E.coli at a low light fluence (0.15 J/cm2), giving a survival of 45% at a low PS concentration (0.3 μM) and more than 80% bacteria were killed at a higher PS concentration (3μM) (Fig. 1(C)). After a higher light fluence (0.3 J/cm2), higher cytotoxicity was observed with cell viability decreased to 10% at 0.3 μM PS concentration and 2% at 3 μM PS concentration. This trend was continued further at increased light fluence and increased PS concentration with optimum effect obtained at light fluence of 0.6 J/cm2 and 3 μM PS concentration when most of the E.coli was eliminated (cell survival rate is 0.001%) (Fig. 1(C)).
For Gram positive S.aureus, no cytotoxicity was clearly observed after receiving same light fluence of 0.15 J/cm2 at low concentration (0.3 μM) of ZnPc(Lys)5. Higher light fluence (0.3 or 0.45 J/cm2) at this PS concentration did not show significant bacterial killing effect either. However, at higher PS concentration (1 μM), notable cytotoxicity was observed where 30% of bacteria were killed, followed 80% killing at 3 μM PS (Fig. 1(D)). At higher light fluence of 0.30 J/cm2 and 0.45 J/cm2, further reduction in cell viability was observed. At 0.45 J/cm2, significant decrease in cell viability was observed with survival rate down to 3% at higher PS concentration (3 μM). A higher light fluence of 0.6 J/cm2 led to significant bacterial killing (20% survival) at a low PS concentration (0.3 μM), and at 3 μM almost all bacteria were cleared off. Thus, light fluence is a key factor in antimicrobial efficacy for S.aureus.
We conclude that E.coli is more susceptible to photodynamic inactivation compared to S.aureus. Resistance shown by S.aureus at lower PS concentration and low light fluence is mostly due to the structural variation in cell membrane of both E.coli and S.aureus. High susceptibility of E.coli towards PS is due to the cationic nature of ZnPc (Lys)5 which can bind to bacterial cell wall that carries net negative charges on the surface.
3.2 Antimicrobial effect using house-hold light and custom-made red LED light sources
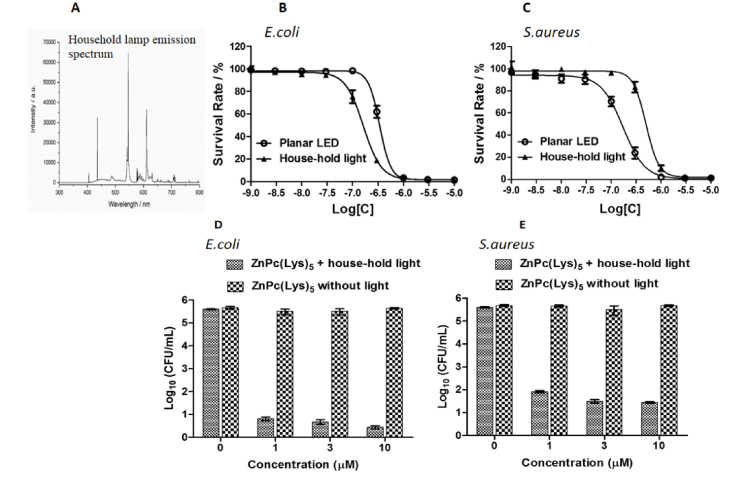
Encouraged by the significant antimicrobial effect of low light fluence of 0.15 J/cm2, emitted from the custom-made LED (red light) for a short time of 2 seconds, we launched a study to assess antimicrobial effect using house-hold lamp (white light mimicking the ambient light) under the same experimental conditions. We used a house-hold lamp with a power density of 12.5 mW/cm2 radiating from 400 to 700 nm. The emission spectrum of the house-hold lamp, as shown by its measured emission spectrum (Fig. 2(A)), overlaps the absorption band of the PS ranging from 650 to 700 nm. Using this house-hold lamp, bacterial strains were incubated with PS for 2 seconds (to a light fluence of 0.025 J/cm2) followed by cell survival measurement. No decrease in cell survival was observed at this condition. The experiment was then carried several times with higher light fluence till a decline in cell survival was observed (6 min irradiation). We thus chose 6-min as the irradiation time period, giving a light fluence of 4.5 J/cm2, for the house-hold lamp. In subsequent comparative experiments, bacterial strains incubated with variable PS concentrations were illuminated with custom-made LED light for 1 min and house-hold lamp for 6 minutes amounting to equal light fluence from both sources. The survived bacteria were measured by monitoring relative luminescent unit (RLU) and the results are shown in Fig. 2(B) and 2(C).
Fig. 2.
Light intensity distribution of a house-hold lamp (A) and antibacterial effects of ZnPc(Lys)5 against Gram-negative bacteria E.coli (B) and Gram-positive bacteria S.aureus (C) under light illumination from either the custom-made planar LED (4.5 J/cm2, open circles) or a house-hold lamp (4.5 J/cm2, black triangles). Antibacterial effects of ZnPc(Lys)5 against E.coli (D) and S.aureus (E) were also measured by colony counting method under light illumination from a house-hold lamp (4.5 J/cm2).
We found that ZnPc(Lys)5 significantly inactivated both bacterial strains in dose dependent manner irrespective of the light source. IC50 value for E.coli was calculated to be 197 nM under illumination from house-hold lamp and that of from LED light was 424 nM (Fig. 2(B)). This shows that E.coli are more susceptible to the light from a house-hold lamp (Fig. 2(B)). Opposite results were obtained for S.aureus with IC50 value of 192 nM for LED light and 650 nM for that of house-hold lamp, respectively (Fig. 2(C)).
To validate the antibacterial effect of ZnPc(Lys)5, we used a different assay method, – colony counting assay at higher concentrations (1.0 μM, 3.0 μM and 10 μM) under house-hold light at a light fluence of 4.5 J/cm2. ZnPc(Lys)5 (at concentrations 3.0 μM and 10 μM) caused a 5 log decrease (99.999%) in CFU of E.coli strain (Fig. 2(D)). On the other hand, 4 log reduction (99.99%) in CFU of S. aureus were observed at higher concentration of PS (Fig. 2(E)). These results are consistent with the results obtained using bioluminescence assays.
3.3 In vivo PACT in animal model with house-hold light source
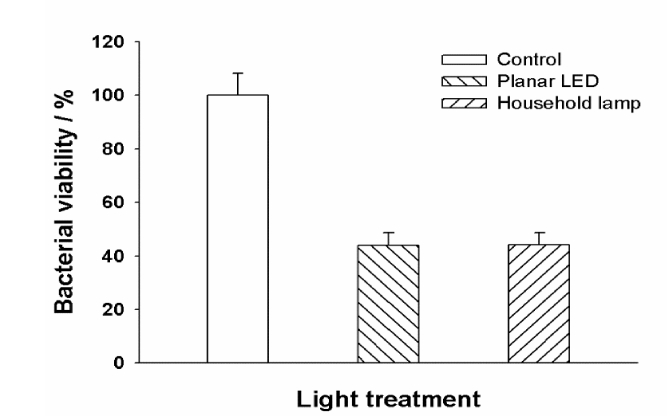
To study the antimicrobial effect of the house-hold light source in vivo, we established a mouse superficial and localized skin infection model by inoculating the genetically engineered bioluminescent S.aureus into an excisional wound on mouse. In our experiments, the wound was infected by 106 CFU of the luminescent S. aureus from a mid log culture. The photosensitizer (ZnPc(Lys)5 5 μl at1 mM) was applied directly onto the wound followed by photo illumination to treat the infection. The efficacy of PACT with ZnPc(Lys)5 were determined by bacterial load determination of viable S.aureus in infected wounds (Fig. 3) and wound area measurements (Fig. 4). The determination of viable S.aureus in infected wounds on mice using colony forming unit (CFU) Our results confirmed that ZnPc(Lys)5 inactivated the bacteria in the wound using either LED or house-hold light source (Fig. 3). Quantitatively, the bacteria load of S.aureus was reduced to 44.1% using the house-hold lamp at a light fluence of 7.5 J/cm2, and to 43.9% using the LED light source at a fluence of 45 J/cm2. It is not clear why the lower light fluence of household light gave the comparable effect to the LED light at a higher light fluence. One potential reason is the effect of other wavelengths, e.g., blue light at 400-500nm [26], of household light, which have emission intensity much stronger than at the PS wavelength (680 nm) (Fig. 2(A)). Also, heating can be another potential reason to affect PACT efficacy. This was concern because the total power of house-hold light (11Watt) is much higher than the LED light (70 mW). We have limited the light illumination time (10 min) in order to avoid the potential heating effect from the light sources. Although further experiments are needed to understand this observation, it is clear that household light can be effective to activate the ZnPc(Lys)5.
Fig. 3.
Reduction of viable S.aureus in infected incision wounds on mice upon incubation with 5 μl of ZnPc(Lys)5 (1 mM) followed by a 10-min light exposure from either the custom-made planar LED (75 mW/cm2) or a house-hold lamp (12.5 mW/cm2). Values of colony forming units obtained from defined infected wounds. Data points are means of values from the wounds on six mice per group and bars are SEM.
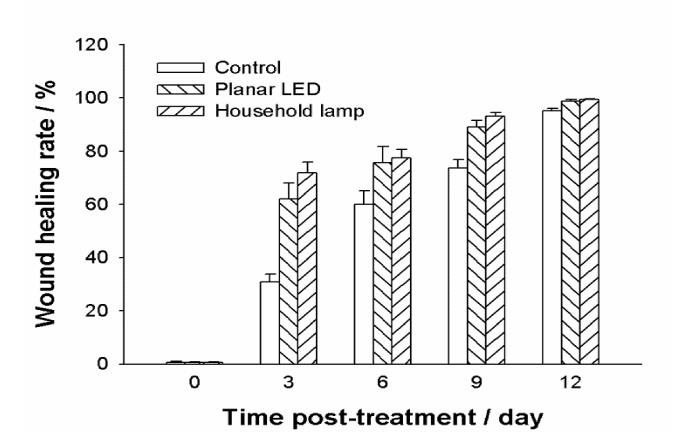
Fig. 4.
Effects of ZnPc(Lys)5 on the healing of S.aureus infected excisional wounds in mice under the light exposure from different light sources. Each wound was infected with 1x106 cells of S. aureus 10 min before the photodynamic treatment with ZnPc(Lys)5. Wounds were measured in two dimensions every three days after the infection, and the areas were calculated. Data points are means of values from the corresponding wound on six mice and bars are SEM.
To further evaluate the effects of ZnPc(Lys)5 on the healing of S. aureus infected excisional wounds in mice, we monitored the wound healing process in living animals by measuring the wound areas every 3 days. We observed that ZnPc(Lys)5 exhibited a dramatic promotion of wound healing process with either the house-hold lamp or the LED light (Fig. 4). The wound recovery rate was significantly enhanced from 1st to 3rd day after PACT treatment. By contrast, the wound healing rate of living mice in the absence of light were lot more slower, especially during the first 3 days after PACT.
4. Discussion
PACT has been considered to be promising and an alternative method in addition to conventional antibiotics to treat multi-drug resistant bacterial infections. Many PSs have been shown to photoinactivate bacteria with light in the visible wavelength range and exhibited significant photosensitizing activity gainst broad range of pathogens in the presence of light (Table 1), but these studies typically required high light fluence (from 20.4 J/cm2 to 2430 J/cm2, Table 1) [27, 28]. High light fluence together with significant PS concentration consume oxygen rapidly, causing oxygen depletion and low PACT efficacy. High light fluence can also lead to increased pain during clinical treatment [29]. In present study, we aimed at using very low dose of light to investigate the antibacterial efficacy of ZnPc(Lys)5 in vitro as well as in vivo animal model. We demonstrate it is possible to achieve the effective phodynamic killing of bacteria under ambient light. In addition, we investigated the effect of fractional illumination of red light separated by dark intervals, instead of continueous illumination, using LED to achieve the targeted minimal effective light fluence.
Table 1. Summary of PACT efficacy using white light as reported in literature.
| Studies | Pathogens | Photosensitizer | Light source | Light fluence | Assay method | Efficacy | Note |
|---|---|---|---|---|---|---|---|
| Pereira, et al. [41], 2012 |
E.coli | Cationic- Thiopyridinium ZincPhthalocynine (20µM) |
White light (150mW/cm2) Red light (150mW/cm2) |
270 J/cm2 | Colony counting | 105 reduction | Bacterial cells were washed twice with PBS |
| Alves, et al. [37], 2009. |
E.coli and E.faecalis | Cationicmeso- substitutedporphyrin (5µM) | White light (40 W/m2) |
64.8 J/cm2 | Colony counting | 107 reduction | No washing |
| Wood, et al. [38], 2006 |
S. mutans deposited biofilm | Erythrosine (22 µM) |
White light (22.5mW/cm2) |
20.4 J/cm2 | To evaluate the clinical plaque disclosing agent erythrosine | 102.2 reduction biofilm | 200 µm thickness were grown in a constant-depth film fermenter |
| Paschoal, et al. [39], 2014 |
S.mutans | Curcumin (7.5 µM) and Toluidine blue (25 µM) |
White light (3410mW/cm2) |
42 J/cm2 | Colony counting | 105 reduction | No washing |
| Marciel, L.,et al. [40] 2017 |
E.coli and
S.aureus |
Zn(MeOPy + )8Pc and Tri-Py + -Me-PF (5-10 µM) |
White light (150 mW/cm2) |
2430 J/cm2 |
Colony counting | 106 reduction | No washing |
| Rubel et.al [34], 2014 |
Actinic keratosis | Methyl aminolevulinate | Daylight PDT | 22 J/cm2 | Lesion treatment | 89% lesion treated response rate | Demonstrated the efficacy and safety of DL-PDT vs C-PDT in treating mild facial/scalp AKs. |
| Neittaanmäki et.al [35], 2014 |
Actinic keratosis | 5-aminolaevulinic acid 5-Methyl aminolevulinate |
Daylight PDT and c-PDT | 2 hrs a day sun exposure | Lesion treatment | 84.5% and 74.2% treated |
Compared the efficacy and adverse effect of 5-aminolaevulinicacid (nanoemulsion) with MAL in DL-PDT |
| Present work | E.coli | Pentalysine Zinc Phthalocynine (10 µM) |
White light (12.5 mW/cm2) |
4.5 J/cm2 | Colony counting | 105 reduction |
4.1 Fractionation of red LED light against bacterial strains
The current study investigated the antibacterial efficacy of water soluble ZnPc(Lys)5 at low power density. Our results showed that bacterial strains illuminated with short red light (2 to 8 seconds) using low PS concentration (3µM) caused a significant photokilling efficacy against E.coli and S.aureus strains. Efficacy of ZnPc(Lys)5 towards bacteria by red light fractionation (2 sec) are in agreement with the results reported for other photosensitizers. For example, Maisch et.al. Investigated photodynamic efficacy of porphyrin derivative using laser pulse (100 millisecond) up to 10 sec (light fluence of 20 to 80 J/cm2) against broad range of pathogens. A photodynamic killing efficacy up to 6 log reduction was achieved using a concentration range of 1-100 µM [30]. In another study, erythrosine meditated PDT of S.mutans biofilm resulted into an improvement of 1.7 orders of magnitude reduction when the light fluence was supplied in fractions of 0.675 J/cm2 compared to the continuous irradiation [31]. Pulse light is more effective and found to be less painful than conventional photodynamic therapy when a total 355 milliseconds of laser pulse was applied using a PS (MAL) in photodynamic therapy treatment [32].
4.2 House-hold lamp in vitro and in vivo animal model
We also report that house-hold white light (4.5 J/cm2) achieved a substantial photokilling effect on E.coli strain. We compared red light of the same light fluence (4.5 J/cm2) to photoinactivate the bacterial strain and results indicated that E.coli strain was more sensitive to house-hold lamp compared to LED light source. Although the current finding is not the first report on white light photoinactivation, the light fluence (4.5 J/cm2) of white light using ZnPc(Lys)5 is much lower than the one reported (Table 1). Short illumination time are found to be more efficient in reactive oxygen generation and associated to low photodegradation of PS [33]. Literature survey revealed that conventional PDT has certain degree of limitations such as pain during treatment or on subsequent light exposure. Daylight PDT is an alternative to minimize this adverse effect and provides pain relief with better cure rates [34–36].
We further evaluated the efficacy of house-hold lamp in vivo, in localized skin infection animal model. Our finding showed that significant bacterial inhibition occurred after 10-min ambient light illumination using the house-hold lamp (at a total light fluence of 7.5 J/cm2), which was comparable to that of PACT using the planar LED light source to a total light fluence of 45 J/cm2. These data indicated that using a house-hold lamp in PACT is indeed an effective approach for antibacterial treatment. The study was further evaluated to wound healing of S.aureus infected excisional wounds, which shows that ZnPc(Lys)5 exhibited a similar dramatic promotion of wound healing process in the presence of light either from a house-hold lamp or from LED. By contrast, the antibacterial effects of ZnPc(Lys)5 without light exposure on the infected wounds in living mice were limited as the evidence of a low wound healing rate during the observation period, especially the first 3 days after PACT. This fast recovery in wound healing in mice under house-hold lamp is comparable to LED light and much stronger than the one treated without light.
The current finding using house-hold white light are supported by the previous reports that attained a significant photokilling efficacy using the same broad visible spectrum of white light of wavelength 400-700 nm (Table 1). A high light fluence of 270 J/cm2 (150 mW/cm2 of white light) was needed to achieve PDT effect (5 log) using thio-pyridinium ZnPc (20 µM) as PS [40]. Cationic meso substituted porphyrin (5 µM) reported by Alves et al. showed an efficient photosensitizing effect against the bacterial strains at a total light fluence of 64.8 J cm−2 of white light (40 mW/cm2) [37]. Erythrosine (22 µM) showed a significant bactericidal efficacy in the treatment of dental plaque biofilm compared to methylene blue and photofrin after a light fluence (20.3 J/cm2) of 400W white light (22.5-22.7 mW/cm2) [38]. Another study involving curcumin and toluidine demonstrated the bacterial reduction up to 5 log against S. mutans after short exposure of white light (3410 mW/cm2) to 42 J/cm2 [39]. A very recent study described by Marciel et al. concluded that their PSs (Zn(MeOPy + )8Pc and Tri-Py + -Me-PF) photoinactivated E. coli and S. aureus at 5 µM and total light fluence of 2.43 KJ/cm2 using white light (400-800 nm, 150 mW/cm2, to 270 min) [40].
Conclusion
The present work is the first report to demonstrate the bactericidal effect of a potent photosensitizer, ZnPc(Lys)5, under fractional illumination of red light for only 2 seconds with minimal light dosage of 0.15 J/cm2. Our in vitro studies using the same PS showed that the ambient white light of 4.5 J/cm2 is fairly enough to eliminate the E. coli and S. aureus with IC50 values of 197 nM and 424 nM, respectively. In vivo studies using S. aureus infected mouse model showed significant bacterial inhibition with a house-hold light source of 7.5 J/cm2 light flux and a remarkable improvement in wound healing process. These results suggest that PACT using a house-hold light source is a promising antimicrobial treatment, which is time and cost effective, as well as more convenient for infectious skin diseases
Acknowledgment
AU would like to thank the University of Chinese Academy of Science and Fujian Institute of Research on the Structure Matter for the support and the research facilities provided at the Institute.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding
CAS/SAFEA International Partnership Program for Creative Research Teams; National Natural Science Foundation (81171634, 81572944, U1405229); Ministry of Science and Technology (2017YFE0103200); Strategic Priority Research Program of the CAS (XDA09030307); Fujian Natural Science Foundation (2013J01387 and 2013J01066).
Disclosures
The authors declares no conflicts of interest related to this article.
References and links
- 1.Denis T. G. S., Hamblin M. R., “An introduction to photoantimicrobials: photodynamic therapy as a novel method of microbial pathogen eradication,” Science against microbial pathogens: communicating current research and technological advances, Méndez-Vilas A. (Ed.), 675–683 (2011). [Google Scholar]
- 2.Karimi M., Sahandi Zangabad P., Baghaee-Ravari S., Ghazadeh M., Mirshekari H., Hamblin M. R., “Smart nanostructures for cargo delivery: Uncaging and activating by light,” J. Am. Chem. Soc. 139(13), 4584–4610 (2017). 10.1021/jacs.6b08313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maisch T., Eichner A., Späth A., Gollmer A., König B., Regensburger J., Bäumler W., “Fast and effective photodynamic inactivation of multiresistant bacteria by cationic riboflavin derivatives,” PLoS One 9(12), e111792 (2014). 10.1371/journal.pone.0111792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Z., Gao Y., Meng S., Yang B., Pang L., Wang C., Liu T., “Mechanism and In Vivo Evaluation: Photodynamic Antibacterial Chemotherapy of Lysine-Porphyrin Conjugate,” Front. Microbiol. 7, 242 (2016). 10.3389/fmicb.2016.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamblin M. R., Hasan T., “Photodynamic therapy: a new antimicrobial approach to infectious disease?” Photochem. Photobiol. Sci. 3(5), 436–450 (2004). 10.1039/b311900a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y., Zheng K., Chen Z., Chen J., Hu P., Cai L., Iqbal Z., Huang M., “Rapid killing of bacteria by a new type of photosensitizer,” Appl. Microbiol. Biotechnol. 101(11), 4691–4700 (2017). 10.1007/s00253-017-8133-8 [DOI] [PubMed] [Google Scholar]
- 7.Halldin C. B., Gillstedt M., Paoli J., Wennberg A.-M., Gonzalez H., “Predictors of pain associated with photodynamic therapy: a retrospective study of 658 treatments,” Acta Derm. Venereol. 91(5), 545–551 (2011). 10.2340/00015555-1101 [DOI] [PubMed] [Google Scholar]
- 8.Ericson M. B., Wennberg A. M., Larkö O., “Review of photodynamic therapy in actinic keratosis and basal cell carcinoma,” Ther. Clin. Risk Manag. 4(1), 1–9 (2008). [PMC free article] [PubMed] [Google Scholar]
- 9.Ang J. M., Riaz I. B., Kamal M. U., Paragh G., Zeitouni N. C., “Photodynamic therapy and pain: A systematic review,” Photodiagn. Photodyn. Ther. 19, 308–344 (2017). 10.1016/j.pdpdt.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 10.Morton C. A., Szeimies R. M., Sidoroff A., Braathen L. R., “European guidelines for topical photodynamic therapy part 1: treatment delivery and current indications - actinic keratoses, Bowen’s disease, basal cell carcinoma,” J. Eur. Acad. Dermatol. Venereol. 27(5), 536–544 (2013). 10.1111/jdv.12031 [DOI] [PubMed] [Google Scholar]
- 11.Chaves Y. N., Torezan L. A., Niwa A. B. M., Sanches Junior J. A., Festa Neto C., “Pain in photodynamic therapy: mechanism of action and management strategies,” An. Bras. Dermatol. 87(4), 521–529 (2012). 10.1590/S0365-05962012000400001 [DOI] [PubMed] [Google Scholar]
- 12.Boen M., Brownell J., Patel P., Tsoukas M. M., “The Role of Photodynamic Therapy in Acne: An Evidence-Based Review,” Am. J. Clin. Dermatol. 18(3), 311–321 (2017). 10.1007/s40257-017-0255-3 [DOI] [PubMed] [Google Scholar]
- 13.Wiegell S. R., Haedersdal M., Philipsen P. A., Eriksen P., Enk C. D., Wulf H. C., “Continuous activation of PpIX by daylight is as effective as and less painful than conventional photodynamic therapy for actinic keratoses; a randomized, controlled, single-blinded study,” Br. J. Dermatol. 158(4), 740–746 (2008). 10.1111/j.1365-2133.2008.08450.x [DOI] [PubMed] [Google Scholar]
- 14.Togsverd-Bo K., Haak C. S., Thaysen-Petersen D., Wulf H. C., Anderson R. R., Hædersdal M., “Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial,” Br. J. Dermatol. 166(6), 1262–1269 (2012). 10.1111/j.1365-2133.2012.10893.x [DOI] [PubMed] [Google Scholar]
- 15.Mandadi S., Tominaga T., Numazaki M., Murayama N., Saito N., Armati P. J., Roufogalis B. D., Tominaga M., “Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCε-mediated phosphorylation at S800,” Pain 123(1), 106–116 (2006). 10.1016/j.pain.2006.02.016 [DOI] [PubMed] [Google Scholar]
- 16.Morton C. A., Whitehurst C., Moore J. V., MacKie R. M., “Comparison of red and green light in the treatment of Bowen’s disease by photodynamic therapy,” Br. J. Dermatol. 143(4), 767–772 (2000). 10.1046/j.1365-2133.2000.03773.x [DOI] [PubMed] [Google Scholar]
- 17.Nyokong T., “Effects of substituents on the photochemical and photophysical properties of main group metal phthalocyanines,” Coord. Chem. Rev. 251(13-14), 1707–1722 (2007). 10.1016/j.ccr.2006.11.011 [DOI] [Google Scholar]
- 18.Chen J., Chen Z., Zheng Y., Zhou S., Wang J., Chen N., Huang J., Yan F., Huang M., “Substituted zinc phthalocyanine as an antimicrobial photosensitizer for periodontitis treatment,” J. Porphyr. Phthalocyanines 15(04), 293–299 (2011). 10.1142/S1088424611003276 [DOI] [Google Scholar]
- 19.Wang D., Zhang Y., Yan S., Chen Z., Deng Y., Xu P., Chen J., Liu W., Hu P., Huang M., “An effective zinc phthalocyanine derivative against multidrug-resistant bacterial infection,” J. Porphyr. Phthalocyanines 21, 205–210 (2017). [Google Scholar]
- 20.Chen J., Chen N., Huang J., Wang J., Huang M., “Derivatizable phthalocyanine with single carboxyl group: synthesis and purification,” Inorg. Chem. Commun. 9(3), 313–315 (2006). 10.1016/j.inoche.2005.12.002 [DOI] [Google Scholar]
- 21.Chen Z., Zhou S., Chen J., Deng Y., Luo Z., Chen H., Hamblin M. R., Huang M., “Pentalysine beta-carbonylphthalocyanine zinc: an effective tumor-targeting photosensitizer for photodynamic therapy,” ChemMedChem 5(6), 890–898 (2010). 10.1002/cmdc.201000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuznetsova N. A., Gretsova N. S., Kalmykova E. A., Makarova E. A., Dashkevich S. N., Negrimovskii V. M., Kaliya O. L., Luk’yanets E. A., “Relationship between the photochemical properties and structure of pophyrins and related compounds,” Russ. J. Gen. Chem. 70, 133–140 (2000). [Google Scholar]
- 23.Karsi A., Lawrence M. L., “Broad host range fluorescence and bioluminescence expression vectors for Gram-negative bacteria,” Plasmid 57(3), 286–295 (2007). 10.1016/j.plasmid.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 24.Chen Z., Zhou S., Chen J., Li L., Hu P., Chen S., Huang M., “An effective zinc phthalocyanine derivative for photodynamic antimicrobial chemotherapy,” J. Lumin. 152, 103–107 (2014). 10.1016/j.jlumin.2013.10.067 [DOI] [Google Scholar]
- 25.Chen Z., Zhang Y., Wang D., Li L., Zhou S., Huang J. H., Chen J., Hu P., Huang M., “Photodynamic antimicrobial chemotherapy using zinc phthalocyanine derivatives in treatment of bacterial skin infection,” J. Biomed. Opt. 21(1), 018001 (2016). 10.1117/1.JBO.21.1.018001 [DOI] [PubMed] [Google Scholar]
- 26.Yin R., Dai T., Avci P., Jorge A. E., de Melo W. C., Vecchio D., Huang Y. Y., Gupta A., Hamblin M. R., “Light based anti-infectives: ultraviolet C irradiation, photodynamic therapy, blue light, and beyond,” Curr. Opin. Pharmacol. 13(5), 731–762 (2013). 10.1016/j.coph.2013.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komagoe K., Kato H., Inoue T., Katsu T., “Continuous real-time monitoring of cationic porphyrin-induced photodynamic inactivation of bacterial membrane functions using electrochemical sensors,” Photochem. Photobiol. Sci. 10(7), 1181–1188 (2011). 10.1039/c0pp00376j [DOI] [PubMed] [Google Scholar]
- 28.Maisch T., Wagner J., Papastamou V., Nerl H. J., Hiller K. A., Szeimies R. M., Schmalz G., “Combination of 10% EDTA, Photosan, and a blue light hand-held photopolymerizer to inactivate leading oral bacteria in dentistry in vitro,” J. Appl. Microbiol. 107(5), 1569–1578 (2009). 10.1111/j.1365-2672.2009.04342.x [DOI] [PubMed] [Google Scholar]
- 29.Cottrell W. J., Paquette A. D., Keymel K. R., Foster T. H., Oseroff A. R., “Irradiance-dependent photobleaching and pain in δ-aminolevulinic acid-photodynamic therapy of superficial basal cell carcinomas,” Clin. Cancer Res. 14(14), 4475–4483 (2008). 10.1158/1078-0432.CCR-07-5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maisch T., Spannberger F., Regensburger J., Felgenträger A., Bäumler W., “Fast and effective: intense pulse light photodynamic inactivation of bacteria,” J. Ind. Microbiol. Biotechnol. 39(7), 1013–1021 (2012). 10.1007/s10295-012-1103-3 [DOI] [PubMed] [Google Scholar]
- 31.Metcalf D., Robinson C., Devine D., Wood S., “Enhancement of erythrosine-mediated photodynamic therapy of Streptococcus mutans biofilms by light fractionation,” J. Antimicrob. Chemother. 58(1), 190–192 (2006). 10.1093/jac/dkl205 [DOI] [PubMed] [Google Scholar]
- 32.Dysart J. S., Singh G., Patterson M. S., “Calculation of singlet oxygen dose from photosensitizer fluorescence and photobleaching during mTHPC photodynamic therapy of MLL cells,” Photochem. Photobiol. 81(1), 196–205 (2005). 10.1562/2004-07-23-RA-244.1 [DOI] [PubMed] [Google Scholar]
- 33.Dovigo L. N., Pavarina A. C., Ribeiro A. P. D., Brunetti I. L., Costa C. A. S., Jacomassi D. P., Bagnato V. S., Kurachi C., “Investigation of the photodynamic effects of curcumin against Candida albicans,” Photochem. Photobiol. 87(4), 895–903 (2011). 10.1111/j.1751-1097.2011.00937.x [DOI] [PubMed] [Google Scholar]
- 34.Rubel D. M., Spelman L., Murrell D. F., See J. A., Hewitt D., Foley P., Bosc C., Kerob D., Kerrouche N., Wulf H. C., Shumack S., “Daylight photodynamic therapy with methyl aminolevulinate cream as a convenient, similarly effective, nearly painless alternative to conventional photodynamic therapy in actinic keratosis treatment: a randomized controlled trial,” Br. J. Dermatol. 171(5), 1164–1171 (2014). 10.1111/bjd.13138 [DOI] [PubMed] [Google Scholar]
- 35.Neittaanmäki-Perttu N., Karppinen T. T., Grönroos M., Tani T. T., Snellman E., “Daylight photodynamic therapy for actinic keratoses: a randomized double-blinded nonsponsored prospective study comparing 5-aminolaevulinic acid nanoemulsion (BF-200) with methyl-5-aminolaevulinate,” Br. J. Dermatol. 171(5), 1172–1180 (2014). 10.1111/bjd.13326 [DOI] [PubMed] [Google Scholar]
- 36.Wiegell S. R., Fabricius S., Heydenreich J., Enk C. D., Rosso S., Bäumler W., Baldursson B. T., Wulf H. C., “Weather conditions and daylight-mediated photodynamic therapy: protoporphyrin IX-weighted daylight doses measured in six geographical locations,” Br. J. Dermatol. 168(1), 186–191 (2013). 10.1111/j.1365-2133.2012.11200.x [DOI] [PubMed] [Google Scholar]
- 37.Alves E., Costa L., Carvalho C. M., Tomé J. P., Faustino M. A., Neves M. G., Tomé A. C., Cavaleiro J. A., Cunha A., Almeida A., “Charge effect on the photoinactivation of Gram-negative and Gram-positive bacteria by cationic meso-substituted porphyrins,” BMC Microbiol. 9(1), 70 (2009). 10.1186/1471-2180-9-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wood S., Metcalf D., Devine D., Robinson C., “Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms,” J. Antimicrob. Chemother. 57(4), 680–684 (2006). 10.1093/jac/dkl021 [DOI] [PubMed] [Google Scholar]
- 39.Paschoal M. A., Santos-Pinto L., Lin M., Duarte S., “Streptococcus mutans photoinactivation by combination of short exposure of a broad-spectrum visible light and low concentrations of photosensitizers,” Photomed. Laser Surg. 32(3), 175–180 (2014). 10.1089/pho.2013.3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marciel L., Teles L., Moreira B., Pacheco M., Lourenço L. M., Neves M. G., Tomé J. P., Faustino M. A., Almeida A., “An effective and potentially safe blood disinfection protocol using tetrapyrrolic photosensitizers,” Future Med. Chem. 9(4), 365–379 (2017). 10.4155/fmc-2016-0217 [DOI] [PubMed] [Google Scholar]
- 41.Pereira J. B., Carvalho E. F., Faustino M. A., Fernandes R., Neves M. G., Cavaleiro J. A., Gomes N. C., Cunha A., Almeida A., Tomé J. P., “Phthalocyanine Thio-Pyridinium Derivatives as Antibacterial Photosensitizers,” Photochem. Photobiol. 88(3), 537–547 (2012). 10.1111/j.1751-1097.2012.01113.x [DOI] [PubMed] [Google Scholar]