Abstract
In this pilot study, we have evaluated bedside diffuse optical monitoring combining diffuse correlation spectroscopy and near-infrared diffuse optical spectroscopy to assess the effect of thrombolysis with an intravenous recombinant tissue plasminogen activator (rtPA) on cerebral hemodynamics in an acute ischemic stroke. Frontal lobes of five patients with an acute middle cerebral artery occlusion were measured bilaterally during rtPA treatment. Both ipsilesional and contralesional hemispheres showed significant increases in cerebral blood flow, total hemoglobin concentration and oxy-hemoglobin concentration during the first 2.5 hours after rtPA bolus. The increases were faster and higher in the ipsilesional hemisphere. The results show that bedside optical monitoring can detect the effect of reperfusion therapy for ischemic stroke in real-time.
OCIS codes: (170.0170) Medical optics and biotechnology; (170.1470) Blood or tissue constituent monitoring; (170.6510) Spectroscopy, tissue diagnostics
1. Introduction
In acute ischemic stroke (AIS) early reperfusion is a robust predictor of early recovery and favorable functional outcome [1, 2]. In clinical practice, perfusion imaging with magnetic resonance and computed tomography (CT) are increasingly used to triage patients for reperfusion therapy, but, are rarely applied to assess its success due to the associated complexities such as expense and the need to transport the patient. Therefore, the monitoring of the treatment efficacy in AIS has focused primarily on the evidence of structural damage and arterial patency rather than on microvascular cerebral blood flow (CBF) restoration. This is partly due to the unavailability of monitoring techniques suitable for repeated microvascular CBF measurement in the acute period. Instead, the recanalization of the occluded artery is frequently used as a surrogate of reperfusion [3, 4]. However, although both are related and are often interchanged, it is increasingly recognized that the recanalization does not necessarily lead to the reperfusion of the distal region, and, contrarily, reperfusion can sometimes occur despite the persistence of an occlusion [5]. The continuous quantification of microvascular hemodynamic changes, in particular, microvascular CBF, with a noninvasive modality at early stages can improve our understanding of the relationship between the reperfusion, recanalization and clinical recovery due to interventions. It could also allow personalized treatment and management strategies.
Diffuse correlation spectroscopy (DCS) is an emerging method that measures local, microvascular CBF noninvasively using near-infrared light [6]. DCS utilizes, noninvasive, near-infrared light to track rapid temporal fluctuations of light intensity in brain tissue that arise when light is scattered by moving red blood cells, which is then analyzed with a physical model to derive a blood flow index.
Numerous validation studies have shown that the relative changes in CBF (ΔrCBF) over time measured by DCS agree with other techniques [7, 8]. DCS can be combined with near-infrared diffuse optical spectroscopy (NIRS-DOS), a more established technique that uses wavelength-dependent light attenuation to measure oxy-hemoglobin (HbO2), deoxy-hemoglobin (Hb), and total hemoglobin concentration (THC) changes. Prior work has shown that this hybrid DCS/NIRS-DOS optical monitor is capable of detecting cerebral hemodynamic changes [8, 9].
Previously, we have shown in a single-case report that DCS/NIRS-DOS measures correlated with the recanalization of the middle cerebral artery (MCA) measured by transcranial Doppler (TCD) during intravenous thrombolysis [10].
In this follow-up study, we aimed to confirm the feasibility of using DCS/NIRS-DOS as a bedside method for real-time monitoring of local microvascular hemodynamic changes in response to intravenous thrombolysis in a small case series of patients with AIS, and their consistency with the recanalization and the clinical outcome.
2. Methods
2.1. Study design and participants
We have prospectively identified patients with an AIS admitted to the Emergency Department within the first 4.5 hours from symptoms onset who were selected to be treated with intravenous thrombolysis. The protocol was approved by the local Ethics Committee (Hospital de la Santa Creu i Sant Pau, EC/09/066/980). Inclusion criteria were: (1) a well-defined onset of symptoms attributable to MCA territory ischemia affecting the frontal lobe (motor aphasia, contralesional motor deficit or gaze deviation), (2) demonstration of MCA occlusion on angio-CT or TCD prior to treatment with intravenous recombinant tissue plasminogen activator (rtPA), and (3) optical monitoring being able to commence before or within the first fifteen minutes of the start of rtPA infusion. We note that during the study period (2011–2012), mechanical thrombectomy was not yet approved at our institution. Informed consent was obtained from the patients or their relatives.
Baseline examinations included a medical history (demographics and vascular risk factors) and a physical examination. The stroke severity was assessed with the National Institutes of Health Stroke Scale (NIHSS). Clinical outcome at three months was evaluated by means of the modified Rankin Scale (mRS). A score >2 was considered indicative of an unfavorable outcome.
On admission, all patients underwent a cranial CT scan and vascular imaging either by angio-CT or TCD before thrombolysis. Recanalization was assessed with TCD at 24 hours and, in some cases, additionally during the rtPA perfusion according to the thrombolysis in brain ischemia (TIBI) flow grade criteria, as previously described [11].
2.2. Optical methods and instrumentation
Optical monitoring was performed with a compact, user-friendly and portable hybrid instrument of DCS and continuous-wave NIRS-DOS. The DCS consisted of a mode-hope free, long-coherence-length, continuous-wave laser at 785 nm and eight single photon avalanche photodiode detectors whose outputs were fed to a custom-built hardware autocorrelator. The continuous-wave NIRS-DOS module consisted of two continuous-wave lasers at 690 and 830 nm and the diffuse light from each was detected by the same detectors above. Optical switches were used to switch the three measurement wavelengths (one for DCS, and two for NIRS-DOS) as well as the measurements from the two cerebral hemispheres. This has allowed us to use continuous-wave NIRS-DOS to evaluate the changes in HbO2 and Hb concentrations as well as microvascular CBF. The output of four channels (four for each hemisphere) was averaged for each wavelength for improving the signal-to-noise ratio.
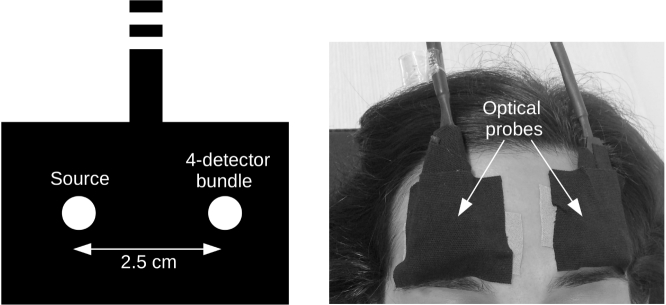
Two optical probes were made of custom built fibers consisting of a source fiber and a detector fiber bundle with four single-mode fibers with 2.5 cm source-detector separation. The use of 2.5 cm was justified by previous validation studies [7, 8] and by the increased sensitivity of DCS to the brain blood flow [12]. The probes were placed bilaterally on the temporal margin of the patient forehead superior to the frontal sinuses as shown in Fig. 1 .
Fig. 1.
The schematic of the source-detector separation of the optical probe (left). The placement of the optical probes bilaterally on the forehead (right).
DCS monitors the light intensity fluctuations of the speckle patterns in order to calculate an intensity autocorrelation function of this detected light. For this study, the reduced scattering coefficient of the tissue at 785 nm was assumed to be constant [13]. The continuous absorption coefficient (µa) changes at 785 nm from their baseline value (µa = 0.14 cm−1) [13] were measured by NIRS-DOS to improve the accuracy of the DCS measurements [8]. The intensity autocorrelation function from the autocorrelator was fitted using the correlation diffusion equation (for a semi-infinite geometry) and also using the measured µa and obtaining a continuous blood flow index (BFI) [6, 14]. The percent relative CBF change (ΔrCBF) was calculated from the BFI as ΔrCBF = ((BFI/BFIbaseline)−1)*100, where BFIbaseline was calculated from the cerebral BFI of the first five minutes of the recording.
A modified Beer-Lambert law [15] was used for the continuous-wave NIRS-DOS analysis with the wavelengths 690 and 830 nm. The data was corrected for pathlength and partial volume effects as explained in previous studies [15, 16]. Changes of oxy-hemoglobin (ΔHbO2) and deoxy-hemoglobin (ΔHb) concentrations were calculated as ΔHbO2(t)=HbO2(t)-HbO2 baseline and ΔHb(t)=Hb(t)-Hbbaseline. Again, the baseline was calculated from the first five minutes of recording. Total hemoglobin concentration changes (ΔTHC) were then calculated as the sum of ΔHbO2 and ΔHb concentration changes. Data was collected from both hemispheres every 14.2 ± 5.7 (mean ± standard deviation) seconds.
2.3. Statistical analysis
The average baseline values for CBF, THC, HbO2 and Hb were obtained by averaging the first five minutes of data, as mentioned, after fifteen minutes from rtPA bolus in each patient. This was used to derive the changes of each variable relative to their baseline values at each time point. The time interval between fifteen minutes from rtPA bolus up to hundred and fifty minutes of monitoring (when available) was used for each patient. We have expressed quantitative variables as a mean and a standard deviation (mean ± standard deviation) and analyzed the data by performing linear mixed-effect models [17] for each individual patient and also for the group. Linear mixed-effect models were needed due to the longitudinal nature of the data (lack of independence of the response variable) when considering each patient independently. When analyzing the patients as a group, these models were needed due to both the longitudinal nature of the data and for correcting for the different patients in the group. The two types of analyses started with a null model that included a continuous dependent variable (CBF, THC, HbO2 or Hb) for each hemisphere (ipsilesional and contralesional). Patients (only when modeling as a group) and time (when modeling as a group and also when considering each patient independently) were the random factors. The predictor variable time was added to the null model as a fixed effect to see whether this new model, with the predictor variable time, improved the null model. The model fit was assessed using chi-square tests on the log-likelihood values to compare the new model versus the null model. If the new model with the predictor variable time (1) improved over the null model significantly, in other words, if the p value of the chi-square test between the two models was <0.05, and (2) the null hypothesis stating that the slope of the new model was equal to zero was rejected, the slope of this model would tell us about the time evolution (rate of increase or decrease over time) of the continuous dependent variable. Residuals plots of this model were tested for the inspection of deviations from normality or homoscedasticity. Instead, if the model with the predictor variable time did not improve the null model significantly, the null hypothesis stating that the slope of the new model was equal to zero was not rejected. Then, the continuous dependent variable was considered constant over time (slope≈0). Wilcoxon rank-sum test was used to assess the difference between the slopes of the two hemispheres with the alternative hypothesis “ipsilesional change > contralesional change”. All analyses were carried out in the R programming language and environment [18] using the “nlme” software package. A p-value <0.05 was considered to be statistically significant.
3. Results
During a 14-month period, we have identified thirteen patients who fulfilled the inclusion criteria. Six were excluded due to late onset of the optical monitoring, mainly due to logistical reasons (late arrival of trained personnel or late consent). Two were excluded due to technical problems with the optical recording. Five patients (three female, mean age of 81 years) were finally included in the analysis (Table 1).
Table 1.
Clinical and optical variables for each individual patient and also for the group.
| Case | 1 | 2 | 3 | 4 | 5 | All | ||
|---|---|---|---|---|---|---|---|---|
| Age (y), Sex(M/F) | 80, F | 58, M | 93, F | 80, M | 92, F | 81 ± 14 | ||
| Arterial occlusion | Left MCA (distal) | Right MCA (prox.) | Left MCA (prox.) | Right MCA (prox.) | Right MCA (prox.) | |||
| NIHSS | Pre-rtPA | 15 | 16 | 22 | 21 | 19 | 19±3 | |
| Post-rtPA | – | 14 | 5 | 13 | 9 | 10±4 | ||
| 24h | 3 | 8 | 2 | 4 | 1 | 4±3 | ||
| Recanalization | Unkn. | Yes | Yes | Yes | Yes | |||
| mRS 90 days | 1 | 2 | 3 | 1 | 1 | 2±1 | ||
| Slope of the linear model | ΔrCBF | Ipsi-side | 0.17† | 0.54† | 0.45† | 0.23† | 0.023 | 0.29†‡ |
| Contra-side | 0.085 | 0.33† | 0.32† | 0.013 | −0.00080 | 0.16† | ||
| ΔTHC | Ipsi-side | 0.023† | 0.035† | 0.063† | 0.030† | 0.0055† | 0.041†‡ | |
| Contra-side | −0.0051 | 0.0023 | 0.030† | 0.029† | 0.041† | 0.019† | ||
| ΔHbO2 | Ipsi-side | 0.046† | 0.053† | 0.059† | 0.026† | 0.050† | 0.047†‡ | |
| Contra-side | 0.00060 | 0.026† | 0.031† | 0.019† | 0.047† | 0.025† | ||
| ΔHb | Ipsi-side | −0.023† | −0.018† | 0.0031† | 0.0042† | 0.0050 | −0.0056 | |
| Contra-side | −0.0057 | −0.024† | −0.0015 | 0.010† | −0.0063† | −0.0053 | ||
(Δ) indicates a change of each optical variable from the baseline (within fifteen minutes from rtPA bolus) to the end of the monitoring (∼2.5 hours). † indicates a statistically significant slope. ‡ indicates a statistically significant difference between ipsi- over contra-lesional side as a group. M=male. F= female. NIHSS=National Institutes of Health Stroke Scale. mRS=modified Rankin scale. rCBF=relative cerebral blood flow. THC=total hemoglobin concentration. HbO2=oxy-hemoglobin. Hb=deoxy-hemoglobin. Ipsi=ipsilesional. Cont.=contralesional. Prox.=proximal. Unkn.=unknown. Mean ± standard deviation.
The mean NIHSS score on admission was 19±3. MCA occlusion (four proximal, one distal) was diagnosed by angio-CT in four patients and by TCD in one. The mean time from symptoms onset to thrombolysis was 144±47 minutes. We note that the rtPA administration was not delayed due to the study. All patients were hemodynamically stable and none received vasoactive drugs.
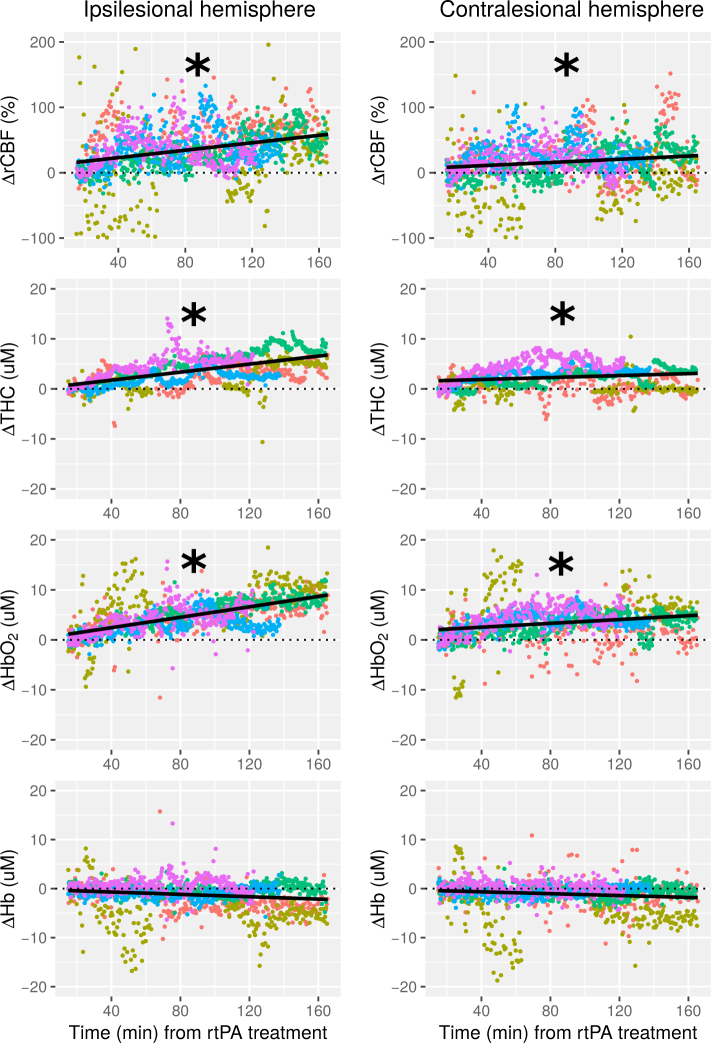
Fig. 2 illustrates the time course of ΔrCBF, ΔTHC, ΔHbO2 and ΔHb of all patients from fifteen minutes of treatment onset in each hemisphere using linear mixed-effect models. The slopes of the change of the optical variables as a function of time in both hemispheres for each individual patient and also for the whole group are shown in Table 1. On a group basis, we have found a statistically significant increase with time for frontal ipsilesional (p<0.001) and contralesional (p=0.018) ΔrCBF, for ipsilesional (p<0.001) and contralesional (p=0.014) ΔTHC and for ipsilesional and contralesional ΔHbO2 (both p<0.001). On the other hand, ΔHb did not change significantly in any hemisphere. We have observed an asymmetrical time evolution of the optical variables between hemispheres. A statistically significant faster increase (greater slope) was observed in the affected hemisphere for ΔrCBF (p=0.031), ΔTHC (p=0.032) and ΔHbO2 (p=0.030) compared to the contralesional hemisphere.
Fig. 2.
Frontal microvascular relative cerebral blood flow (rCBF), total hemoglobin concentration (THC), oxy-hemoglobin (HbO2), and deoxy-hemoglobin (Hb) changes over time are shown for the ipsilesional hemisphere (left column) and the contralesional hemisphere (right column) for all five patients (each patient in different color). (Δ) indicates a change of the optical variable from the baseline. Black line shows the fitted linear model of all the measurement points plotted. The time, x-axis, shows the time elapsed from the rtPA treatment onset. (*) indicates a statistically significant positive slope (p<0.05).
At 24 hours, all patients improved by eight or more points in the NIHSS score. Complete arterial recanalization was documented in all but one patient (due to insufficient acoustic window) at 24 hours. In two patients recanalization was also documented at thirty minutes and at the end of rtPA infusion by continuous TCD.
At three month-follow up, four out of five patients were functionally independent (mRS<3), and one had moderate disability (mRS=3).
4. Discussion
In this pilot study, DCS/NIRS-DOS was able to be deployed at the bedside without any effects on the clinical treatment. On a group basis, it detected an increase in frontal CBF, THC, and HbO2 in both hemispheres which was significantly faster in the ipsilesional side in a series of AIS patients with a clinical improvement after rtPA-associated recanalization. We were able to obtain real-time optical data in five out of thirteen (38%) patients who fulfilled the clinical criteria. The reasons of exclusion were related to the logistics and technical reasons. Although the feasibility of its application in the clinical setting with the current set-up appeared limited (see below), these results support the potential role of diffuse optical technology for continuous cerebral perfusion monitoring during therapeutic interventions in patients with AIS.
Currently, TCD is the only available tool for routine clinical use for the noninvasive and real-time monitoring of cerebral hemodynamics after reperfusion therapy [19]. TCD measures blood flow velocities in the major intracranial arteries, which correlates with CBF only as long as the vessel diameter remains constant [20]. Its main limitations are that it is operator dependent and requires training and experience to perform and interpret the results [19]. Also, an insufficient acoustic bone window limits its application in 20% to 30% of the patients [21]. Furthermore, TCD can directly insonate only proximal vascular segments of large arteries and only indirectly provides information about more distal vascularity [22]. Therefore, TCD is suitable for the monitoring of the large artery recanalization, but not for the microcirculatory reperfusion.
In contrast, diffuse optical methods provide real time information of the microvascular CBF and microvascular blood oxygenation. Diffuse optical instrumentation is in general portable and, in principle, can be easy to apply without the need of extended technical expertise [8, 23]. Despite this, the clinical application of diffuse optical techniques in the context of cerebrovascular disease is limited. Many reasons can be speculated, including the concern about the potential contamination of the signal by superficial tissues, the problems of motion artifacts and its limited spatial resolution. This is bound to change since more advanced instrumentation and algorithms are being introduced.
Previously, anecdotal studies of single case reports or small series have evaluated the yield of NIRS-DOS-based cerebral oximetry to estimate the adequacy of cerebral perfusion in AIS [24–26]. Recently, Hametner et al. reported a larger series of sixty-three patients monitored with commercial NIRS-DOS during mechanical thrombectomy, of whom forty-three had valid data [27]. During the procedure, regional oxygen saturation peaks were observed in fourteen (32.6%) patients and a sustained increase after recanalization in only two patients (4.7%). Although some regional oxygen saturation indices were reported to be associated with the reperfusion status and clinical outcome, the authors concluded that the ability of the current commercial NIRS-DOS monitors to probe local reperfusion is limited. Others have used indocyanine green as a tracer to demonstrate CBF reductions with NIRS-DOS in patients with AIS, showing a good correlation with perfusion weighted imaging measurements [25, 28]. However, the need of an exogenous contrast agent limits their use for continuous monitoring purposes.
Previous studies have shown that DCS can successfully track autoregulatory changes of CBF without the need of contrast agents in patients with cerebrovascular disease by using vasoactive stimuli [29, 30]. In this report, we have described a real-time sustained ΔrCBF increase measured by DCS after intravenous thrombolysis. DCS-derived ΔrCBF measures were in agreement with the simultaneously NIRS-DOS-measured THC and HbO2, as we have previously suggested in a single case report [10]. Furthermore, DCS parameters showed a different response between the ipsilesional and contralesional hemispheres in agreement with NIRS-DOS, demonstrating the potential of DCS to detect early changes in cerebral perfusion caused by the rtPA treatment. This asymmetric response found between both hemispheres also adjusts for possible systemic circulatory or respiratory disorders as potential confounders.
The main difference of DCS over NIRS-DOS is that it provides a direct measure of cerebral perfusion, which is the goal of most interventions in AIS. In contrast, NIRS-DOS is used as an indirect measure of cerebral perfusion based on changes in blood oxygenation or volume which has been shown to differ from CBF in brain-injured patients [31]. Furthermore, the combination of both techniques can be used to assess the cerebral metabolic rate of oxygen extraction (CMRO2), a potentially sensitive biomarker of brain injury [8, 32]. Thus, the combination of DCS and NIRS-DOS offers a more complete view of brain health than any of the techniques alone.
The increase in CBF during thrombolysis may reflect the restoration of downstream flow not only by the recanalization of the occluded artery, but also by the increase of collateral circulation. We have documented arterial recanalization on TCD in four patients at 24 hours. In two of them, by using continuous TCD monitoring, we have showed that the arterial recanalization coincided with CBF increase during rtPA infusion. We note that leptomeningeal collateral flow may also contribute to cortical CBF changes detected by DCS, as suggested by previous studies, in which CBF variations correlated with changes in MCA velocities on TCD in healthy volunteers but diverged in patients with severe carotid artery stenosis who had activated compensatory flow sources [30, 33]. Unfortunately, our neuroimaging protocol did not include the assessment of the collateral circulation to support this hypothesis in order to avoid delays in the administration of intravenous rtPA.
Our report has some limitations including the availability of only relative data, the small number of patients recruited and the lack of a control group (patients without recanalization). Instead, we have used the contralesional hemisphere as a control. The recent emergence of commercial hybrid systems (e.g. HemoPhotonics S.L. and ISS) and European projects (BabyLux [34]) where user-friendly neuromonitors combining both methods have been developed should allow us to increase the yield of transcranial optical monitoring in the clinical settings which was lacking in our case. Moreover, the use of time resolved methods (e.g. in Babylux) should allow for absolute measurements even in the adult head [35]. Due to the small number of subjects in this proof-of-concept study, we have avoided a discussion of each individual’s data. Also, our measurements were limited to one single location in the forehead. We could have measured on different cortical areas; however, we prioritized simplicity to avoid delays on the treatment. As with other modalities based on diffuse light in the near-infrared, DCS has a relatively poor spatial resolution and the light penetration depth is restricted to a few millimeters of the superficial cortex (total depth of penetration of ∼1.5 cm). Also, diffuse optical spectroscopy techniques measure information from the brain, but also contain scalp and skull contributions [8, 35]. In this work, instead of including also a small source-detector separation (∼1 cm) to study the superficial effects, we chose to keep all eight detectors (four for each side) for large separations to focus on the signal-to-noise ratio. However, a source-detector separation of 2.5 cm is a good compromise as shown by other earlier studies [7, 8, 12]. Future implementations could utilize more source-detector pairs and pressure modulation [36]. Finally, DCS results show a higher relative noise than NIRS-DOS results which is well known with the current technology. This is partly remedied since the increased contrast in CBF amounts to a comparable contrast-to-noise-ratio [12].
5. Conclusion
In conclusion, we have shown that continuous diffuse optical monitoring of cerebral hemodynamics at the bedside in AIS patients treated with intravenous thrombolysis is feasible. Our study supports further research in larger populations with the emerging improved set-ups of optical technology for the clinical environment.
A typographical correction was made to the author listing.
Funding
Fondo de Investigaciones Sanitarias PI09/0557, Redes Temáticas de Investigación Cooperativa (RETICS-INVICTUS RD012/0014 and RETICS-INVICTUS PLUS RD16/0019/0010); Fundació CELLEX Barcelona, Ministerio de Economía y Competitividad/FEDER (PHOTODEMENTIA, DPI2015-64358-C2-1-R); Instituto de Salud Carlos III/FEDER (MEDPHOTAGE, DTS16/00087); the “Severo Ochoa” Programme for Centres of Excellence in R&D (SEV-2015-0522); the Obra Social “la Caixa” Foundation (LlumMedBcn), Institució CERCA, AGAUR-Generalitat (2014SGR-1555); LASERLAB-EUROPE IV (EU-H2020 654148); Marie Curie initial training network (OILTEBIA 317526).
Disclosures
We disclose that ICFO has equity ownership in the spin-off company HemoPhotonics S.L. in a related technology. Potential financial conflicts of interest and objectivity of research have been monitored by ICFO’s Knowledge & Technology Transfer Department and none was identified.
References and links
- 1.De Silva D. A., Fink J. N., Christensen S., Ebinger M., Bladin C., Levi C. R., Parsons M., Butcher K., Barber P. A., Donnan G. A., Davis S. M., “Assessing reperfusion and recanalization as markers of clinical outcomes after intravenous thrombolysis in the echoplanar imaging thrombolytic evaluation trial (EPITHET),” Stroke 40, 2872–2874 (2009). 10.1161/STROKEAHA.108.543595 [DOI] [PubMed] [Google Scholar]
- 2.Cho T. H., Nighoghossian N., Mikkelsen I. K., Derex L., Hermier M., Pedraza S., Fiehler J., Østergaard L., Berthezène Y., Baron J. C., “Reperfusion Within 6 Hours Outperforms Recanalization in Predicting Penumbra Salvage, Lesion Growth, Final Infarct, and Clinical Outcome,” Stroke 46, 1582–1589 (2015). 10.1161/STROKEAHA.114.007964 [DOI] [PubMed] [Google Scholar]
- 3.Christou I., Alexandrov A. V., Burgin W. S., Wojner A. W., Felberg R. A., Malkoff M., Grotta J. C., Felberg A., Malkoff M., Grotta J. C., Christou I., Alexandrov A. V., Burgin W. S., “Timing of recanalization after tissue plasminogen activator therapy determined by transcranial doppler correlates with clinical recovery from ischemic stroke,” Stroke. 31, 1812–1816 (2000). 10.1161/01.STR.31.8.1812 [DOI] [PubMed] [Google Scholar]
- 4.Delgado-Mederos R., Rovira A., Alvarez-Sabín J., Ribó M., Munuera J., Rubiera M., Santamarina E., Maisterra O., Delgado P., Montaner J., Molina C. A., “Speed of tPA-induced clot lysis predicts DWI lesion evolution in acute stroke,” Stroke 38, 955–960 (2007). 10.1161/01.STR.0000257977.32525.6e [DOI] [PubMed] [Google Scholar]
- 5.Soares B. P., Chien J. D., Wintermark M., “MR and CT monitoring of recanalization, reperfusion, and penumbra salvage: Everything that recanalizes does not necessarily reperfuse!” Stroke 40, 24–28 (2009). 10.1161/STROKEAHA.108.526814 [DOI] [PubMed] [Google Scholar]
- 6.Durduran T., Choe R., Baker W. B., Yodh A. G., “Diffuse optics for tissue monitoring and tomography,” Rep. Prog. Phys. 73, 76701 (2010). 10.1088/0034-4885/73/7/076701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesquita R. C., Durduran T., Yu G., Buckley E. M., Kim M. N., Zhou C., Choe R., Sunar U., Yodh A. G., “Direct measurement of tissue blood flow and metabolism with diffuse optics,” Philos. Trans. A. Math. Phys. Eng. Sci. 369, 4390–4406 (2011). 10.1098/rsta.2011.0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durduran T., Yodh A. G., “Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement,” Neuroimage 85, 51–63 (2014). 10.1016/j.neuroimage.2013.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durduran T., Yu G., Burnett M. G., Detre J. A., Greenberg J. H., Wang J., Zhou C., Yodh A. G., “Diffuse optical measurement of blood flow, blood oxygenation, and metabolism in a human brain during sensorimotor cortex activation,” Opt. Lett. 29, 1766–1768 (2004). 10.1364/OL.29.001766 [DOI] [PubMed] [Google Scholar]
- 10.Zirak P., Delgado-Mederos R., Dinia L., Carrera D., Martí-Fàbregas J., Durduran T., “Transcranial diffuse optical monitoring of microvascular cerebral hemodynamics after thrombolysis in ischemic stroke,” J. Biomed. Opt. 19, 18002 (2014). 10.1117/1.JBO.19.1.018002 [DOI] [PubMed] [Google Scholar]
- 11.Burgin W. S., Malkoff M., Felberg R. A., Demchuk A. M., Christou I., Grotta J. C., Alexandrov A. V., “Transcranial doppler ultrasound criteria for recanalization after thrombolysis for middle cerebral artery stroke,” Stroke. 31, 1128–1132 (2000). 10.1161/01.STR.31.5.1128 [DOI] [PubMed] [Google Scholar]
- 12.Selb J., Boas D. A., Chan S.-T., Evans K. C., Buckley E. M., Carp S. A., “Sensitivity of near-infrared spectroscopy and diffuse correlation spectroscopy to brain hemodynamics: simulations and experimental findings during hypercapnia,” Neurophotonics 1, 015005 (2014). 10.1117/1.NPh.1.1.015005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comelli D., Bassi A., Pifferi A., Taroni P., Torricelli A., Cubeddu R., Martelli F., Zaccanti G., “In vivo time-resolved reflectance spectroscopy of the human forehead,” Appl. Opt. 46, 1717–1725 (2007). 10.1364/AO.46.001717 [DOI] [PubMed] [Google Scholar]
- 14.Boas D. A., Campbell L. E., Yodh A. G., “Scattering and imaging with diffusing temporal field correlations,” Phys. Rev. Lett. 75, 1855 (1995). 10.1103/PhysRevLett.75.1855 [DOI] [PubMed] [Google Scholar]
- 15.Duncan A., Meek J. H., Clemence M., Elwell C. E., Fallon P., Tyszczuk L., Cope M., Delpy D. T., “Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy,” Pediatr. Res. 39, 889–894 (1996). 10.1203/00006450-199605000-00025 [DOI] [PubMed] [Google Scholar]
- 16.Kohri S., Hoshi Y., Tamura M., Kato C., Kuge Y., Tamaki N., “Quantitative evaluation of the relative contribution ratio of cerebral tissue to near-infrared signals in the adult human head: a preliminary study,” Physiol. Meas. 23, 301–312 (2002). 10.1088/0967-3334/23/2/306 [DOI] [PubMed] [Google Scholar]
- 17.Pinheiro J. C., Bates D. M., Mixed-effects models in S and S-PLUS (2000). 10.1007/978-1-4419-0318-1 [DOI]
- 18.R Core Team , R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria: (2015). [Google Scholar]
- 19.Sloan M., Alexandrov A., Tegeler C., Spencer M., Caplan L., Feldmann E., Wechsler L., Newell D., Gomez C., Babikian V., Lefkowitz D., Goldman R., Armon C., Hsu C., Goodin D., “Assessment: transcranial Doppler ultrasonography: report of the T therapeutics and technology assessment subcommittee of the american academy of neurology,” Neurology 62, 1468–1481 (2004). 10.1212/WNL.62.9.1468 [DOI] [PubMed] [Google Scholar]
- 20.Kirkham F., Padayachee T., Parsons S., Seargeant L., House F., Gosling R., “Transcranial measurement of blood velocities in the basal cerebral arteries using pulsed Doppler ultrasound: velocity as an index of flow,” Ultrasound Med Biol. 12, 15–21 (1986). 10.1016/0301-5629(86)90139-0 [DOI] [PubMed] [Google Scholar]
- 21.Seidel G., Kaps M., Gerriets T., “Potential and limitations of transcranial color-coded sonography in stroke patients,” Stroke 26, 2061–2066 (1995). 10.1161/01.STR.26.11.2061 [DOI] [PubMed] [Google Scholar]
- 22.Aries M. J. H., Elting J. W., De Keyser J., Kremer B. P. H., Vroomen P. C. A. J., “Cerebral autoregulation in stroke: A review of transcranial doppler studies,” Stroke 41, 2697–2704 (2010). 10.1161/STROKEAHA.110.594168 [DOI] [PubMed] [Google Scholar]
- 23.Ferrari M., Quaresima V., “A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application,” Neuroimage 63, 921–935 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Nagashima H., Okudera H., Kobayashi S., Iwashita T., “Monitoring of cerebral hemodynamics using near-infrared spectroscopy during local intraarterial thrombolysis: case report,” Surg. Neurol. 49, 420–424 (1998). 10.1016/S0090-3019(97)00199-7 [DOI] [PubMed] [Google Scholar]
- 25.Liebert A., Wabnitz H., Steinbrink J., Möller M., Macdonald R., Rinneberg H., Villringer A., Obrig H., “Bed-side assessment of cerebral perfusion in stroke patients based on optical monitoring of a dye bolus by time-resolved diffuse reflectance,” Neuroimage 24, 426–435 (2005). 10.1016/j.neuroimage.2004.08.046 [DOI] [PubMed] [Google Scholar]
- 26.Ritzenthaler T., Cho T. H., Luis D., Berthezene Y., Nighoghossian N., “Usefulness of near-infrared spectroscopy in thrombectomy monitoring,” J. Clin. Monit. Comput. 29, 585–589 (2015). 10.1007/s10877-014-9636-9 [DOI] [PubMed] [Google Scholar]
- 27.Hametner C., Stanarcevic P., Stampfl S., Rohde S., Veltkamp R., Bösel J., “Noninvasive cerebral oximetry during endovascular therapy for acute ischemic stroke: an observational study,” J. Cereb. Blood Flow Metab. 35, 1722–1728 (2015). 10.1038/jcbfm.2015.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terborg C., Gröschel K., Petrovitch A., Ringer T., Schnaudigel S., Witte O. W., Kastrup A., “Noninvasive assessment of cerebral perfusion and oxygenation in acute ischemic stroke by near-infrared spectroscopy,” Eur. Neurol. 62, 338–343 (2009). 10.1159/000239794 [DOI] [PubMed] [Google Scholar]
- 29.Favilla C. G., Mesquita R. C., Mullen M., Durduran T., Lu X., Kim M. N., Minkoff D. L., Kasner S. E., Greenberg J. H., Yodh A. G., Detre J. A., “Optical bedside monitoring of cerebral blood flow in acute ischemic stroke patients during head-of-bed manipulation,” Stroke 45, 1269–1274 (2014). 10.1161/STROKEAHA.113.004116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zirak P., Delgado-Mederos R., Dinia L., Martí-Fàbregas J., Durduran T., “Microvascular versus macrovascular cerebral vasomotor reactivity in patients with severe internal carotid artery stenosis or occlusion,” Acad. Radiol. 21, 168–174 (2014). 10.1016/j.acra.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 31.Kim M. N., Edlow B. L., Durduran T., Frangos S., Mesquita R. C., Levine J. M., Greenberg J. H., Arjun G., “Continuous optical monitoring of cerebral hemodynamics during head-of-bed manipulation in brain-injured adults,” Neurocrit. Care 20, 443–453 (2014). 10.1007/s12028-013-9849-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung C., Culver J. P., Takahashi K., Greenberg J. H., Yodh A. G., “In vivo cerebrovascular measurement combining diffuse near-infrared absorption and correlation spectroscopies,” Phys. Med. Biol. 46, 2053–2065 (2001). 10.1088/0031-9155/46/8/302 [DOI] [PubMed] [Google Scholar]
- 33.Zirak P., Delgado-Mederos R., Martí-Fàbregas J., Durduran T., “Effects of acetazolamide on the micro- and macro-vascular cerebral hemodynamics: a diffuse optical and transcranial doppler ultrasound study,” Biomed. Opt. Express 1, 1443–1459 (2010). 10.1364/BOE.1.001443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.“Babylux; an optical neuro-monitor of cerebral oxygen metabolism and blood flow for neonatology,” http://www.babylux-project.eu/.
- 35.Torricelli A., Contini D., Pifferi A., Caffini M., Re R., Zucchelli L., Spinelli L., “Time domain functional NIRS imaging for human brain mapping,” Neuroimage 85, 28–50 (2014). 10.1016/j.neuroimage.2013.05.106 [DOI] [PubMed] [Google Scholar]
- 36.Baker W. B., Parthasarathy A. B., Ko T. S., Busch D. R., Abramson K., Tzeng S.-Y., Mesquita R. C., Durduran T., Greenberg J. H., Kung D. K., Yodh A. G., “Pressure modulation algorithm to separate cerebral hemodynamic signals from extracerebral artifacts,” Neurophotonics 2, 035004 (2015). 10.1117/1.NPh.2.3.035004 [DOI] [PMC free article] [PubMed] [Google Scholar]