ABSTRACT
Signal modulation is important for the growth and development of plants and this process is mediated by a number of factors including physiological growth regulators and their associated signal transduction pathways. Protein kinases play a central role in signaling, including those involving pathogen response mechanisms. We previously demonstrated an active guanylate cyclase (GC) catalytic center in the brassinosteroid insensitive receptor (AtBRI1) within an active intracellular kinase domain resulting in dual enzymatic activity. Here we propose a novel type of receptor architecture that is characterized by a functional GC catalytic center nested in the cytosolic kinase domain enabling intramolecular crosstalk. This may be through a cGMP-AtBRI1 complex forming that may induce a negative feedback mechanism leading to desensitisation of the receptor, regulated through the cGMP production pathway. We further argue that the comparatively low but highly localized cGMP generated by the GC in response to a ligand is sufficient to modulate the kinase activity. This type of receptor therefore provides a molecular switch that directly and/or indirectly affects ligand dependent phosphorylation of downstream signaling cascades and suggests that subsequent signal transduction and modulation works in conjunction with the kinase in downstream signaling.
KEYWORDS: Auto-regulation, brassinosteroid receptor (BRI1), cyclic GMP, intramolecular crosstalk, phytosulfokine receptor 1 (PSKR1), PeP1 receptor (PEPR1), phosphorylation, receptor kinase, signal transduction
Perception of environmental stimuli and the fine tuning of responses to environmental challenges are vital to maintaining plant growth and development. To achieve this controlled growth and development, plants have repertoires of cellular receptors and signaling pathways that relay information between organs and cells, and within cells and between molecules. All of these signal pathways form complex networks that contain feedback mechanisms to maintain homeostasis and enable appropriate growth and developmental responses. Thus, control points occur at many levels within the signal cascades and involve switches that range from molecular generation (synthesis and processing of both RNA and protein) to molecular destruction (e.g. protein degradation) in response to changes in signal molecule gradients (e.g. various calcium ion profiles). Here we focus on intramolecular signaling and feedback as an example of molecular switching to maintain homeostasis at the subcellular level that enables plant growth and development.
A series of bioinformatic searches identified specific receptor kinases as having a guanylate cyclase (GC) catalytic centre.1-3 The GC catalytic centre is embedded in the cytosolic kinase domain (Fig. 1A). Recombinant studies showed the kinase domain of several of these receptor kinases have GC activity.1,4–7 Combined with observations of increased cGMP production in a time dependent manner in protoplasts treated with the natural ligand to the receptor,4,8,9 it seemed likely that these molecules operate as ligand activated GCs in vivo. As only very low levels of cGMP were generated in the recombinant protein studies we used mass spectrometry analysis of the recombinant kinase domains which indicates that cGMP is indeed produced.4,7 These low cGMP levels raised speculation about the biological role of the GC in these receptors.3,10 At this stage we can only speculate that key in vivo components are missing from the in vitro assays since specific mutations decrease cGMP production as predicted.4,7 One of the missing components may be phosphorylation of specific residues in the kinase domain as certain phosphorylated residues are necessary for GC activity of PSKR1 and BRI1.7,11 Interestingly, some of these residues also contribute to the kinase activity.11–14 Thus phosphorylation status is a component necessary for activation of both the kinase and GC elements of the receptor kinases BRI1 and PSKR1.7,11–15 Both BRI1 and PSKR1 are dual tyrosine and serine/threonine kinases.11,16 This kinase activity is augmented by the additional GC activity creating complex enzymes with dual activity that is potentially important in intracellular control of receptor activation and down-stream signaling.
Figure 1.
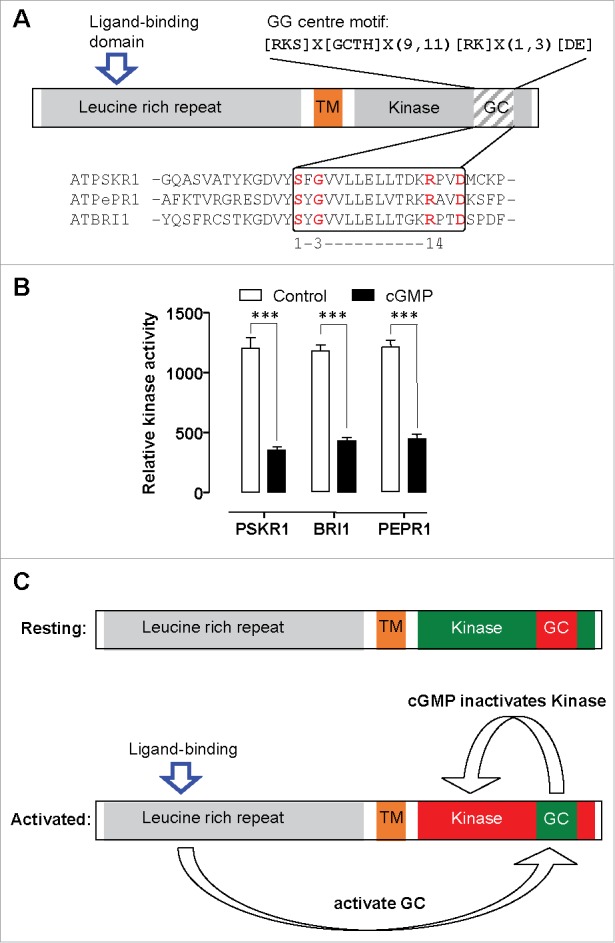
Intramolecular switching in plant receptor-like kinases. A. Schematic showing the domain architecture of receptor like kinases AtBRI1, AtPSKR1 and AtPePR1. These molecules all contain a leucine rich repeat extracellular domain that contains a ligand binding island, a transmembrane (TM) spanning domain and an intracellular kinase domain. Within the intracellular kinase domain is a guanylate cyclase (GC) catalytic center. The diagnostic GC catalytic center core motif shows the functionally assigned amino acids and these are highlighted in the alignment of this region. B. The kinase domain of AtPSKR1, AtBRI1 and AtPePR1 were recombinantly expressed in bacteria and purified using immobilized metal affinity chromatography on nickel-nitrilotriacetic acid agarose. Kinase activity was measured as an increase in fluorescence of Ser/Thr peptide 1 as a substrate in the presence or absence of 0.1 nM cGMP using the Omnia kinase assay. Data is adapted for PSKR1,4 BRI17 and from an unpublished experiment for AtPePR1 and analysed by one-way ANOVA followed by Tukey's multiple comparisons test (*** P < 0.001). C. Model showing the resting state of the receptor with active kinase that can autophosphorylate itself. Following ligand binding further phosphorylation occurs that activates guanylate cyclase center to generate cGMP which in turn inhibits kinase activity.
But what is cGMP doing in this cascade? All the evidence points to some cGMP being generated, but possibly only in comparatively small amounts that are difficult to quantify within a cell. However, even small localized increases of cGMP may modulate activity of other nearby proteins. BRI1 associated kinase 1 (BAK1) is a promiscuous membrane bound kinase that is associated with several of the receptor kinases including BRI1, PSKR1 and PePR1.12,17–20 The receptor complex actually involves other proteins as well. For example, PSKR1 is directly associated with BAK1, H+-ATPase AHA1 and AHA218,20 and also indirectly associated with cyclic nucleotide gated channel 17 (CNGC17) at the plasma membrane.18 Increased levels of cGMP generated via PSKR1 could reach high enough local concentrations to activate CNGC17. Support for a similar pathway in PePR1 signaling exists as PeP ligand activates cGMP dependent calcium influxes.9
Another possibility is that cGMP may be acting autonomously at the site where it is produced by modulating the kinase activity of the receptor. To test this hypothesis, we examined the effect of cGMP at nM levels on recombinant kinase activity using the serine/threonine SOX peptide 1 as the kinase substrate. The kinase activity of recombinant BRI1, PSKR1 and PePR1 are all significantly inhibited by the presence of 0.1 nM cGMP4,7 (Fig. 1B). Thus these dual active molecules appear to be able to regulate their status via modulating the localised molecular environment. A simple model is that in the naïve or first activated state in the absence of cGMP, kinase activity is promoted as depicted by the green kinase domain and red GC centre (Fig. 1C). Once autophosphorylation (and presumably downstream phosphorylation) has occurred, the GC center is primed and generates cGMP. Accumulating cGMP switches off the kinase activity as depicted by the red kinase domain and GC activity may continue as shown by green GC centre (Fig. 1C).
There could be several advantages in turning down the kinase activity of these receptor like kinases. Obviously, such downregulation will suppress direct ligand activated receptor downstream phosphorylation pathways. The presence of cGMP may also be stimulating localized changes in ions via activation of CNGCs.9,18 If calcium ions are increased (e.g. by activating CNGC dependent calcium influx), they may in turn further down regulate the receptor kinase, ensuring that the phosphorylation switch remains suppressed. Recombinant PSKR1 kinase activity is suppressed by physiological increases in calcium ion concentrations.21 Both BRI1 and PSKR1 contain predicted calmodulin binding sites that are active in vivo and also affect kinase activity.14,22,23 Increases in calcium ions and calmodulin also activate calcium ion and calcium/calmodulin dependent kinases that will relay alternate phosphorylation cascades.
In conclusion, we propose that a novel type of receptor architecture that is characterized by a functional GC nested in the cytosolic kinase domain enables intramolecular crosstalk. We argue that the comparatively low but highly localized cGMP generated by the GC in response to a ligand is sufficient to modulate the kinase activity. This type of receptor therefore provides a molecular switch that directly and/or indirectly affects ligand dependent phosphorylation of downstream signaling cascades.
Funding Statement
Funding for this research was provided by the Australian Research Council's Discovery funding scheme (project numbers DP0878194 and DP110104164) and the National Research Foundation South Africa (grant numbers 78843; IRF2009021800047).
Abbreviations
- BAK1
BRI1 associated kinase
- BRI1
Brassinosteroid insensitive 1
- cGMP
cyclic nucleotide 3´,5´-cyclic guanosine monophosphate
- CNGC
cyclic nucleotide gated channel
- GC
guanylate cyclise
- PePR1
PeP receptor 1
- PSKR1
Phytosulfokine receptor 1
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Kwezi L, Meier S, Mungur L, Ruzvidzo O, Irving H, Gehring C. The Arabidopsis thaliana brassinosteroid receptor (AtBRI1) contains a domain that functions as a guanylyl cyclase in vitro. PLoS ONE. 2007;2:e449. doi: 10.1371/journal.pone.0000449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong A, Gehring C. The Arabidopsis thaliana proteome harbors undiscovered multi-domain molecules with functional guanylyl cyclase catalytic centers. Cell Commun Signal. 2013;11:48. doi: 10.1186/1478-811X-11-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong A, Gehring C, Irving HR. Conserved functional motifs and homology modeling to predict hidden moonlighting functional sites. Front Bioeng Biotech. 2015;3:82. doi: 10.3389/fbioe.2015.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwezi L, Ruzvidzo O, Wheeler JI, Govender K, Iacuone S, Thompson PE, et al.. The phytosulfokine (PSK) receptor is capable of guanylate cyclase activity and enabling cyclic GMP-dependant signaling in plants. J Biol Chem. 2011;286:22580–8. doi: 10.1074/jbc.M110.168823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meier S, Ruzvidzo O, Morse M, Donaldson L, Kwezi L, Gehring C. The Arabidopsis Wall Associated Kinase-Like 10 gene encodes a functional guanylyl cyclase and is co-expressed with pathogen defense related genes. PLoS ONE. 2010;5:e8904. doi: 10.1371/journal.pone.0008904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi Z, Verma R, Gehring C, Yamaguchi Y, Zhao Y, Ryan CA, et al.. Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc Natl Acad Sci USA. 2010;107:21193–8. doi: 10.1073/pnas.1000191107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheeler JI, Wong A, Marondedze C, Groen AJ, Kwezi L, Freihat L, et al.. The brassinosteroid receptor BRI1 can generate cGMP enabling cGMP-dependent downstream signaling. Plant J. 2017;91:590–600. doi: 10.1111/tpj.13589 [DOI] [PubMed] [Google Scholar]
- 8.Irving HR, Kwezi L, Wheeler JI, Gehring C. Moonlighting kinases with guanylate cyclase activity can tune regulatory signal networks. Plant Signal Behav. 2012;7:201–4. doi: 10.4161/psb.18891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y, Walker RK, Zhao Y, Berkowitz GA. Linking ligand perception by PEPR pattern recognition receptors to cytosolic Ca2+ elevation and downstream immune signaling in plants. Proc Natl Acad Sci USA. 2012;109:19852–7. doi: 10.1073/pnas.1205448109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bojar D, Martinez J, Santiago J, Rybin V, Bayliss R, Hothorn M. Crystal structures of the phosphorylated BRI1 kinase domain and implications for brassinosteroid signal initiation. Plant J. 2014;78:31–43. doi: 10.1111/tpj.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muleya V, Marondedze C, Wheeler JI, Thomas L, Mok YF, Griffin MWD, et al.. Phosphorylation of the dimeric cytoplasmic domain of the phytosulfokine receptor, PSKR1. Biochem J. 2016;473:3081–98. doi: 10.1042/BCJ20160593 [DOI] [PubMed] [Google Scholar]
- 12.Clouse SD. Brassinosteroid signal transduction: from receptor kinase activation to transcriptional networks regulating plant development. Plant Cell. 2011;23:1219–30. doi: 10.1105/tpc.111.084475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartmann J, Linke D, Bönniger C, Tholey A, Sauter M. Conserved phosphorylation sites in the activation loop of Arabidopsis phytosulokine receptor PSKR1 differentially affect kinase and receptor activity. Biochem J. 2015;472:379–91. doi: 10.1042/BJ20150147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann C, Motzkus M, Sauter M. Phosphorylation of the phytosulokine peptide receptor PSKR1 controls receptor activity. J Exp Bot. 2017;68:1411–23. doi: 10.1093/jxb/erx030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauter M. Phytosulfokine peptide signaling. J Exp Bot. 2015;66:5161–9. doi: 10.1093/jxb/erv071 [DOI] [PubMed] [Google Scholar]
- 16.Oh M-H, Wang X, Kota U, Goshe MB, Clouse SD, Huber SC. Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a component of brassinosteroid signaling in Arabidopsis. Proc Natl Acad Sci USA. 2009;106:658–63. doi: 10.1073/pnas.0810249106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinchilla D, Shan L, He P, de Vries S, Kemmerling B. One for all: the receptor-associated kinase BAK1. Trends Plant Sci. 2009;14:535–41. doi: 10.1016/j.tplants.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ladwig F, Dahlke RI, Stuhrwohdldt N, Hartmann J, Harter K, Sauter M. Phytosulfokine regulates growth in Arabidopsis through a response module at the plasma membrane that includes CYCLIC NUCLEOTIDE-GATED CHANNEL17, H-ATPase, and BAK1. Plant Cell. 2015;27:1718–29. doi: 10.1105/tpc.15.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang J, Han Z, Sun Y, Zhang H, Gong X, Chai J. Structural basis for recognition of an endogenous peptide by the pareceptor kinase PEPR1. Cell Res. 2015;25:110–20. doi: 10.1038/cr.2014.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Li H, Han Z, Zhang H, Wang T, Lin G, et al.. Allosteric receptor activation by the plant peptide hormone phytosulfokine. Nature. 2015;525:265–8. doi: 10.1038/nature14858 [DOI] [PubMed] [Google Scholar]
- 21.Muleya V, Wheeler JI, Ruzvidzo O, Freihat L, Manallack DT, Gehring C, Irving HR. Calcium is the switch in the moonlighting dual function of the ligand-activated receptor kinase phytosulfokine receptor 1. Cell Commun Signal. 2014;12:60 (1-5). doi: 10.1186/s12964-014-0060-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartmann J, Fischer C, Dietrich P, Sauter M. Kinase activity and calmodulin binding are essential for growth signaling by the phytosulfokine receptor PSKR1. Plant J. 2014;78:192–202. doi: 10.1111/tpj.12460 [DOI] [PubMed] [Google Scholar]
- 23.Oh M-H, Kim HS, Wu X, Clouse SD, Zielinski RE, Huber SC. Calcium/calmodulin inhibition of the Arabidopsis BRASSINOSTEROID-INSENSITIVE 1 receptor kinase provides a possible link between calcium and brassinosteroid signalling. Biochem J. 2012;443:515–23. doi: 10.1042/BJ20111871 [DOI] [PMC free article] [PubMed] [Google Scholar]


