Abstract
BACKGROUND
Despite current therapies, diffuse cutaneous systemic sclerosis (scleroderma) often has a devastating outcome. We compared myeloablative CD34+ selected autologous hematopoietic stem-cell transplantation with immunosuppression by means of 12 monthly infusions of cyclophosphamide in patients with scleroderma.
METHODS
We randomly assigned adults (18 to 69 years of age) with severe scleroderma to undergo myeloablative autologous stem-cell transplantation (36 participants) or to receive cyclophosphamide (39 participants). The primary end point was a global rank composite score comparing participants with each other on the basis of a hierarchy of disease features assessed at 54 months: death, event-free survival (survival without respiratory, renal, or cardiac failure), forced vital capacity, the score on the Disability Index of the Health Assessment Questionnaire, and the modified Rodnan skin score.
RESULTS
In the intention-to-treat population, global rank composite scores at 54 months showed the superiority of transplantation (67% of 1404 pairwise comparisons favored transplantation and 33% favored cyclophosphamide, P = 0.01). In the per-protocol population (participants who received a transplant or completed ≥9 doses of cyclophosphamide), the rate of event-free survival at 54 months was 79% in the transplantation group and 50% in the cyclophosphamide group (P = 0.02). At 72 months, Kaplan–Meier estimates of event-free survival (74% vs. 47%) and overall survival (86% vs. 51%) also favored transplantation (P = 0.03 and 0.02, respectively). A total of 9% of the participants in the transplantation group had initiated disease-modifying antirheumatic drugs (DMARDs) by 54 months, as compared with 44% of those in the cyclophosphamide group (P = 0.001). Treatment-related mortality in the transplantation group was 3% at 54 months and 6% at 72 months, as compared with 0% in the cyclophosphamide group.
CONCLUSIONS
Myeloablative autologous hematopoietic stem-cell transplantation achieved long-term benefits in patients with scleroderma, including improved event-free and overall survival, at a cost of increased expected toxicity. Rates of treatment-related death and post-transplantation use of DMARDs were lower than those in previous reports of nonmyeloablative transplantation. (Funded by the National Institute of Allergy and Infectious Diseases and the National Institutes of Health; ClinicalTrials.gov number, NCT00114530.)
Scleroderma with internal-organ involvement (diffuse cutaneous systemic sclerosis) is a devastating autoimmune disorder. Despite advances in management, mortality driven by pulmonary involvement has not changed in 40 years.1–3 Although disease-modifying anti-rheumatic drugs (DMARDs) and biologics have been studied, none have shown lasting benefit, and only cyclophosphamide given for 12 months has shown short-term benefit as compared with placebo.4–6 For many patients, scleroderma is a fatal disease.
Pilot studies of autologous hematopoietic stem-cell transplantation in scleroderma showed improvement in skin sclerosis and stabilization of pulmonary function.7–10 Two randomized trials of nonmyeloablative transplantation also showed benefit11,12; however, neither trial changed clinical practice in the United States, in part owing to concerns about durability of response and the safety of transplantation.13 Our trial (Scleroderma: Cyclophosphamide or Transplantation [SCOT]) tests a different therapeutic approach: myeloablation with total-body irradiation followed by reconstitution with a CD34+ selected autograft versus cyclophosphamide. We hypothesized that myeloablative transplantation would result in better long-term outcomes than cyclophosphamide treatment. Participants were followed closely over a period of 4.5 years to assess safety and durability of remission. The primary end point, assessed at 54 months, was a global rank composite score based on a hierarchy of disease features.
METHODS
TRIAL DESIGN, INTERVENTIONS, AND OVERSIGHT
This randomized, open-label, phase 2 trial was conducted at 26 sites (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Randomization was performed on a 1:1 basis and stratified according to site, with the difference in the number of participants in the two treatment groups constrained to two or fewer at each site.14 The trial ended when the last participant completed the month 54 evaluation, with a maximum follow-up to 72 months. After an event of respiratory, renal, or cardiac failure occurred (i.e., event-free survival was not achieved), clinic visits ended but telephone contacts continued to month 54. Death was investigated with the use of public records, as needed.
Hematopoietic progenitors were mobilized with granulocyte colony-stimulating factor (G-CSF); after leukapheresis and CD34+ cell enrichment, the autologous product was cryopreserved.15 Fractionated total-body irradiation (800 cGy), cyclophosphamide (120 mg per kilogram of body weight), and equine antithymocyte globulin (90 mg per kilogram) were given as previously reported.9,16 Pulmonary and renal shields limited organ exposure to a target of 200 cGy.17 After conditioning, participants received CD34+ cells (median, 5.6×106 per kilogram; interquartile range, 3.8 to 6.0) and post-transplantation care with G-CSF, glucocorticoids, lisinopril, and anti-infective agents including acyclovir, which was given for 1 year (Table S2 in the Supplementary Appendix).9,18 In the cyclophosphamide group, an initial intravenous dose of 500 mg per square meter of body-surface area was followed by 11 monthly infusions of 750 mg per square meter with mesna prophylaxis.
A data and safety monitoring board appointed by the National Institute of Allergy and Infectious Diseases, the trial sponsor, provided oversight. An independent end-point review committee whose members were aware of the trial-group assignments verified causes of death and verified events of respiratory, renal, or cardiac failure. Institutional review boards at each site approved the protocol, and Rho (Chapel Hill, NC) held and analyzed the data. Members of the steering committee (Table S1 in the Supplementary Appendix) designed the trial, vouch for its adherence to the protocol, and attest to the accuracy and completeness of the data and analyses as specified in the protocol and statistical analysis plan, which are available at NEJM.org. The trial data are accessible from ImmPort (www.immport.org) in study SDY1039 (DOI: 10.21430/M3SM4LTLH). The first author wrote the initial draft, and all the coauthors reviewed the manuscript and agreed to publication. All the participants in the trial provided written informed consent.
PARTICIPANTS
Adults (18 to 69 years of age) with scleroderma (American College of Rheumatology 1995 criteria) for 5 years or less with pulmonary or renal involvement were eligible. Pulmonary involvement required active interstitial lung disease (as determined by bronchoalveolar cell composition or ground-glass opacities on computed tomography of the chest) plus either a forced vital capacity (FVC) or a diffusing capacity of the lung for carbon monoxide (DLco) of less than 70% of the predicted value. Renal involvement required previous scleroderma-related renal disease. Key exclusion criteria were active gastric antral vascular ectasia, a DLco of less than 40% of the predicted value, an FVC of less than 45% of the predicted value, a left ventricular ejection fraction of less than 50%, a creatinine clearance of less than 40 ml per minute, pulmonary arterial hypertension, or more than 6 months of previous treatment with cyclophosphamide.19,20
EVALUATIONS AND END POINTS
Participants were evaluated monthly through year 1, then approximately quarterly through year 5. Serial pulmonary-function testing was performed in the same laboratory, with DLco (Crapo–Morris calculation) corrected for anemia. Rheumatologists were certified to assess the modified Rodnan skin score (range, 0 [normal] to 51 [severe skin thickening]); minimally important differences are 3.2 to 5.3 points. Scales are more fully detailed in Section S1 of Methods in the Supplementary Appendix.21,22
The primary end point was the global rank composite score at 54 months. The global rank composite score is an analytic tool that accounts for multiple disease manifestations simultaneously but does not measure disease activity or severity. It reflects how participants compare with one another on the basis of a hierarchy of ordered outcomes: death, event-free survival (survival without respiratory, renal, or cardiac failure), FVC, the score on the Disability Index of the Health Assessment Questionnaire (HAQ-DI; range, 0 to 3, with higher scores indicating more disability), and the modified Rodnan skin score (Section S1 of Methods in the Supplementary Appendix). Participants who were alive at 54 months rank higher than those who died; those who survived event-free rank higher than those who had an event, and so forth down the hierarchy (Sections S2 and S3 of Methods in the Supplementary Appendix). With the assumption that transplant recipients would have worse early outcomes but could fare better long-term than participants in the comparison group, the global rank composite score is intentionally constructed to treat early and late deaths (or events of organ failure) as equal, irrespective of timing. Variables that were used to define an event included death, respiratory failure (decrease from baseline of >30% in percent predicted DLco or >20% in percent predicted FVC) (Section S2 of Methods in the Supplementary Appendix), renal failure (long-term dialysis or renal transplantation), or cardiac failure (clinical congestive heart failure or left ventricular ejection fraction <30%).
The lowest three components of the global rank composite score are ordinal. They were defined by improvement (increase of ≥10% in the percentage of the predicted FVC, decrease of >0.4 in the HAQ-DI score, or decrease of ≥25% in the modified Rodnan skin score, as compared with baseline values), no change (neither improvement nor worsening), or worsening (decrease from baseline of ≥10% in the percentage of the predicted FVC, increase of >0.4 in the HAQ-DI score, or increase of ≥25% in the modified Rodnan skin score, as compared with baseline values).
Secondary end points included individual components of the global rank composite score, measures of disease progression, and quality of life. Safety end points included treatment-related death, death from any cause, treatment-related toxic effects, infections, and hematologic engraftment. Deaths, cancers, and disease-progression events that occurred after an event of respiratory, renal, or cardiac failure were tracked as secondary end points but were not reported as adverse events.
STATISTICAL ANALYSIS
The trial was originally designed for 226 participants, with event-free survival as the primary end point. Low accrual prompted amendments, first to broaden entry criteria, then, ultimately, to reduce the sample size by changing the primary end point to the global rank composite score. Power for the new design with 114 participants was estimated by simulations at 93% for a two-sided test with an alpha level of 0.05. Assumptions for the simulations were guided by data on similarly treated patients involved in previous studies.4,9 No SCOT data informed the redesign process (details in Section S3 of Methods in the Supplementary Appendix). With continued slow accrual but without reviewing efficacy results, the data and safety monitoring board recommended stopping randomization at 75 participants.
For ordinal end points, including the global rank composite score, the Wilcoxon signed-rank test was used for comparisons; the van Elteren extension of the Wilcoxon signed-rank test was used for stratified analyses.23,24 The effect size for the Wilcoxon signed-rank test is reported as the percent of all possible pairs between the two groups that favor transplantation (or cyclophosphamide). Fisher’s exact test was used for dichotomous events, including death and event-free survival at 54 and 48 months; the Mantel–Haenszel chi-square test was used for stratified analysis. Kaplan–Meier survival curves were compared with the use of log-rank tests. The data and safety monitoring board reviewed four prespecified futility analyses that included an ability to stop for efficacy with P<0.0001, leaving an alpha level of 0.0496 for the primary intention-to-treat analysis of the global rank composite score at 54 months after randomization. The intention-to-treat population was defined as all the participants who had undergone randomization. Secondary analyses are presented for the per-protocol population, defined as participants who received a transplant or completed nine or more doses of cyclophosphamide. Secondary analyses are supportive; P values were not adjusted for multiple comparisons. Safety results are summarized for all the participants who initiated treatment. Analyses used SAS software, version 9.3 or higher.
RESULTS
TRIAL POPULATION
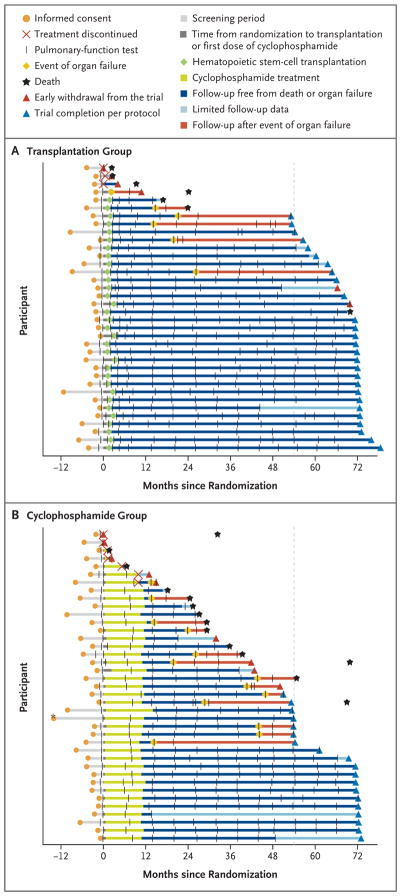
From July 2005 through September 2011, a total of 75 of the 205 screened patients underwent randomization. Patients who did not meet entry criteria or who were denied insurance coverage accounted for the majority of screening failures (Fig. S1 in the Supplementary Appendix). In the transplantation group (36 participants), 34 initiated mobilization and 33 received a transplant. In the cyclophosphamide group (39 participants), 37 initiated treatment; 32 received 12 doses and 34 received 9 or more doses. Of the 75 participants, 46 (27 in the transplantation group and 19 in the cyclophosphamide group) completed the trial, 14 (3 in the transplantation group and 11 in the cyclophosphamide group) died during the trial, and 15 (6 in the transplantation group and 9 in the cyclophosphamide group) withdrew prematurely (Fig. 1, and Fig. S1 in the Supplementary Appendix).
Figure 1. Participant Milestones.
Milestones for each participant assigned to undergo myeloablative hematopoietic stem-cell transplantation (Panel A) or to receive cyclophosphamide (Panel B) are depicted from the time of informed consent. Organ failure refers to respiratory, renal, or cardiac failure. For early withdrawals from the trial, death was investigated with the use of site and public records. In Panels A and B, each black hash mark represents a clinical evaluation with pulmonary-function tests at the transplantation center. In Panel B, the asterisk identifies a participant who gave consent more than 12 months before randomization. The vertical dashed line at 54 months indicates participant status at the time of the primary end point.
Baseline characteristics reflected severe scleroderma: the mean modified Rodnan skin score was 30, the mean DLco was 53% of the predicted value, and 97% of the participants had pulmonary involvement (Table 1, and Table S3 in the Supplementary Appendix). The two groups had similar characteristics except that the cyclophosphamide group had more female participants, participants who had never smoked, and participants who had previously used cyclophosphamide than the transplantation group. Reasons for the imbalance are unknown.
Table 1.
Demographic and Clinical Characteristics at Baseline (Intention-to-Treat Population).*
| Characteristic | Transplantation (N = 36) | Cyclophosphamide (N = 39) | Total (N = 75) |
|---|---|---|---|
| Mean age — yr | 44.9±10.9 | 46.9±10.4 | 45.9±10.6 |
| Female sex — no. (%) | 19 (53) | 29 (74) | 48 (64) |
| Race — no. (%)† | |||
| White | 29 (81) | 31 (79) | 60 (80) |
| Black | 2 (6) | 4 (10) | 6 (8) |
| Other | 5 (14) | 4 (10) | 9 (12) |
| Smoking status — no. (%) | |||
| Current or former smoker | 14 (39) | 10 (26) | 24 (32) |
| Never smoked | 22 (61) | 29 (74) | 51 (68) |
| Mean duration of scleroderma before randomization — mo | 25.1±12.9 | 29.0±16.0 | 27.1±14.6 |
| DMARD use in previous 6 mo — no. (%) | 26 (72) | 25 (64) | 51 (68) |
| Previous use of cyclophosphamide — no. (%) | 8 (22) | 17 (44) | 25 (33) |
| Lung involvement — no. (%) | 36 (100) | 37 (95) | 73 (97) |
| Mean modified Rodnan skin score‡ | 28.5±8.7 | 30.8±10.5 | 29.7±9.7 |
| Mean FVC — % of predicted value | 74.5±14.8 | 73.8±17.0 | 74.1±15.9 |
| Mean DLco — % of predicted value | 53.9±7.6 | 52.7±8.2 | 53.3±7.9 |
| Mean left ventricular ejection fraction — %§ | 61.0±6.1 | 59.9±4.3 | 60.4±5.2 |
| Mean creatinine clearance — ml/min | 122.8±41.7 | 124.9±54.3 | 123.9±48.3 |
| Mean ESR — mm/hr¶ | 29.8±26.5 | 32.2±24.9 | 31.1±25.4 |
| Mean SF-36 physical component score|| | 29.5±9.2 | 28.9±9.5 | 29.2±9.3 |
| Mean SF-36 mental component score|| | 44.7±10.7 | 44.6±9.9 | 44.6±10.2 |
| Mean HAQ-DI score** | 1.2±0.6 | 1.4±0.9 | 1.3±0.8 |
Plus–minus values are means ±SD. Although the between-group differences for sex, smoking status, and previous use of cyclophosphamide appear potentially clinically relevant, no P values for comparisons between the two groups were less than 0.05, on the basis of t-tests for numerical variables and Fisher’s exact test for categorical variables. P = 0.06 for sex, 0.39 for smoking status, and 0.06 for previous use of cyclophosphamide. Additional data on participant characteristics are provided in Table S3 in the Supplementary Appendix. DLco denotes diffusing capacity of the lung for carbon monoxide, DMARD disease-modifying antirheumatic drug, ESR erythrocyte sedimentation rate, and FVC forced vital capacity.
Race was reported by the participant.
Modified Rodnan skin scores range from 0 (normal) to 51 (severe skin thickening).
Data were available for 36 participants in the transplantation group and 37 in the cyclophosphamide group.
Data were available for 29 participants in the transplantation group and 34 in the cyclophosphamide group.
Scores on the physical and mental components of the 36-Item Short Form General Health Survey (SF-36) range from 0 to 100, with higher scores indicating better quality of life. Data were available for 35 participants in the transplantation group and 35 in the cyclophosphamide group.
Scores on the Disability Index of the Health Assessment Questionnaire (HAQ-DI) range from 0 to 3, with higher scores indicating more disability. Data were available for 35 participants in the transplantation group and 38 in the cyclophosphamide group.
EFFICACY
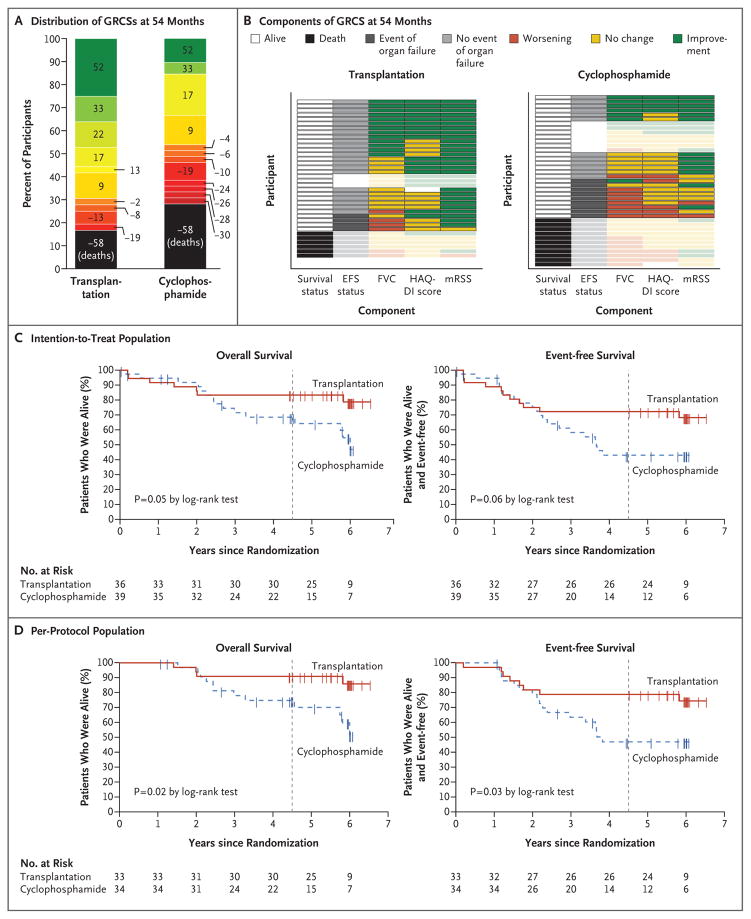
In the intention-to-treat population, the global rank composite score favored transplantation at 54 months (prespecified primary end point) and 48 months (key secondary end point); the percent of 1404 (36 × 39) pairwise comparisons favoring transplantation over cyclophosphamide was 67% versus 33% at 54 months (P= 0.01) and 68% versus 32% at 48 months (P = 0.008) (Table 2 and Fig. 2). In the per-protocol population, the percent of comparisons favoring transplantation on the global rank composite score was 70% versus 30% at 54 months (P = 0.004) and 71% versus 29% at 48 months (P = 0.003) (Table 2).
Table 2.
Efficacy End Points (Intention-to-Treat and Per-Protocol Populations).*
| Variable | Transplantation | Cyclophosphamide | P Value† |
|---|---|---|---|
| Intention-to-treat population | |||
| No. of participants | 36 | 39 | |
| Primary efficacy end point: GRCS at 54 mo‡ | |||
| Median (range) | 17.0 (−58 to 52) | −6.0 (−58 to 52) | 0.01 |
| Percent of favorable pairwise comparisons | 66.6 | 33.4 | |
| GRCS at 48 mo‡ | |||
| Median (range) | 20.0 (−58 to 55) | −8.0 (−58 to 55) | 0.008 |
| Percent of favorable pairwise comparisons | 67.6 | 32.4 | |
| Death or respiratory, renal, or cardiac failure — no. (%) | |||
| By 54 mo | 10 (28) | 20 (51) | 0.06 |
| By 48 mo | 10 (28) | 20 (51) | 0.06 |
| Death from any cause — no. (%) | |||
| By 54 mo | 6 (17) | 11 (28) | 0.28 |
| By 48 mo | 6 (17) | 11 (28) | 0.28 |
| Treatment-related death — no. (%)§ | |||
| By 54 mo | 1 (3) | 0 | 0.48 |
| By 48 mo | 1 (3) | 0 | 0.48 |
| Per-protocol population | |||
| No. of participants | 33 | 34 | |
| GRCS at 54 mo‡ | |||
| Median (range) | 16.0 (−56 to 46) | −11.0 (−56 to 46) | 0.004 |
| Percent of favorable pairwise comparisons | 70.1 | 29.9 | |
| GRCS at 48 mo‡ | |||
| Median (range) | 17 (−56 to 49) | −13.0 (−56 to 49) | 0.003 |
| Percent of favorable pairwise comparisons | 71.3 | 28.7 | |
| Death or respiratory, renal, or cardiac failure — no. (%) | |||
| By 54 mo | 7 (21) | 17 (50) | 0.02 |
| By 48 mo | 7 (21) | 17 (50) | 0.02 |
| Death from any cause — no. (%) | |||
| By 54 mo | 3 (9) | 8 (24) | 0.19 |
| By 48 mo | 3 (9) | 8 (24) | 0.19 |
| Treatment-related death — no. (%)§ | |||
| By 54 mo | 1 (3) | 0 | 0.49 |
| By 48 mo | 1 (3) | 0 | 0.49 |
| Disease-progression event by 54 mo — no. (%) | |||
| Initiated DMARDs¶ | 3 (9) | 15 (44) | 0.001 |
| New or worsening arrhythmia | 6 (18) | 4 (12) | 0.46 |
| Congestive heart failure leading to treatment | 0 | 4 (12) | 0.04 |
| Clinically significant pericardial effusion | 2 (6) | 0 | 0.15 |
| Pulmonary arterial hypertension | 0 | 5 (15) | 0.02 |
| Scleroderma-related renal crisis | 0 | 1 (3) | 0.32 |
| Myositis | 1 (3) | 0 | 0.31 |
The intention-to-treat population was defined as all the participants who had undergone randomization. The per-protocol population was defined as participants who received a transplant or completed nine or more doses of cyclophosphamide. Because pulmonary and renal toxic effects are expected and reversible during the recovery period after autologous stem-cell transplantation, events of respiratory failure or renal failure were not evaluated in either treatment group until month 14 and month 8, respectively.
For the global rank composite score (GRCS), P values are based on the Wilcoxon signed-rank test. For death with or without respiratory, renal, or cardiac failure, P values are based on Fisher’s exact test. For disease-progression events by 54 months, P values are based on Pearson’s chi-square test.
The range for the GRCS is sample specific and depends on the number of participants included in the analysis and the number of ties in score. The higher the score, the better the participant’s performance relative to others in the sample. Percent refers to the percent of pairwise comparisons between participants that favored transplantation or cyclophosphamide. For example, in the intention-to-treat analysis at 54 months, with 36 participants in the transplantation group and 39 in the cyclophosphamide group, there were 1404 possible pairwise comparisons (i.e., 36 × 39). In 935 of the 1404 paired comparisons (66.6%), the global rank composite score favored transplantation.
“Related” includes events that were deemed to be probably or definitely related to the treatment regimen or CD34+ hematopoietic progenitor cells, as reported by the site investigator. By 72 months, there were 2 deaths (6%) in the transplantation group and 0 in the cyclophosphamide group (intention-to-treat and per-protocol populations).
By 24 months, a total of three participants (9%) in the transplantation group and seven participants (21%) in the cyclophosphamide group had initiated disease-modifying antirheumatic drugs (DMARDs).
Figure 2. Primary and Key Secondary Outcomes.
Panel A shows the distribution of global rank composite scores (GRCSs) at month 54 in the intention-to-treat population according to treatment group. Black represents deaths (score, −58). Remaining scores range from −30 to 52 on a red (worst)–yellow–green (best) scale. P = 0.01 for the comparison between treatment groups (Wilcoxon signed-rank test). Panel B shows components of the GRCS at month 54 for participants in the intention-to-treat population. Each row represents an individual participant. In the first column, black indicates death at month 54 and white indicates alive. In the second column, dark gray indicates an event of respiratory, renal, or cardiac failure; light gray indicates no event of respiratory, renal, or cardiac failure; and blank indicates that event-free survival (EFS) status could not be evaluated at month 54. In columns 3 through 5 (percent of predicted forced vital capacity [FVC], score on the Disability Index of the Health Assessment Questionnaire [HAQ-DI], and modified Rodnan skin score [mRSS]), green represents improvement, yellow no change, and red worsening, as compared with baseline values. All deaths (black) are treated equally. The figure shows the last available assessment before death, but boxes are faded to indicate that these steps in the hierarchy were not used for GRCS evaluation. Similarly, if EFS status could not be evaluated (blank), the outcome status for the last available assessments is shown as faded, and participants were ranked on the basis of survival status alone. Panel C shows the Kaplan–Meier estimates of overall survival and event-free survival in the intention-to-treat population (all the participants who had undergone randomization), and Panel D shows such estimates in the per-protocol population (participants who received a transplant or completed ≥9 doses of cyclophosphamide). In Panels C and D, the vertical dashed line represents the 54-month time point.
Results of prespecified secondary analyses of event-free survival corroborate the composite-score results. In the per-protocol population, the rate of event-free survival at 54 months was 79% in the transplantation group and 50% in the cyclophosphamide group (P = 0.02) (Table 2). Most events of organ failure were respiratory failure (in 5 participants in the transplantation group and 13 in the cyclophosphamide group); renal failure occurred in 1 participant in the transplantation group, and cardiac failure occurred in 1 participant in the cyclophosphamide group. In the per-protocol analysis, Kaplan–Meier estimates for the treatment groups begin to separate in favor of transplantation at approximately 2 years. At 72 months, the rate of event-free survival was 74% in the transplantation group and 47% in the cyclophosphamide group (P = 0.03), and the rate of overall survival was 86% and 51%, respectively (P = 0.02) (Fig. 2). Results of the intention-to-treat analysis were consistent with these findings (Fig. 2).
In the per-protocol population at 54 months, the percentage of participants who had initiated DMARDs was lower in the transplantation group than in the cyclophosphamide group (9% vs. 44%, P = 0.001) (Table 2). No participants in the transplantation group had had congestive heart failure or pulmonary arterial hypertension, as compared with 12% and 15%, respectively, of the participants in the cyclophosphamide group (P = 0.04 and P = 0.02, respectively). Both among participants who had survived event-free (at 54 months) and among those who had died or had had organ failure (at last assessment), the transplantation group was more likely than the cyclophosphamide group to show improvements on prespecified secondary clinical end points, including the modified Rodnan skin score (100% vs. 82% among those who survived event-free and 71% vs. 29% among those who had died or had had organ failure), the HAQ-DI score (65% vs. 35% and 29% vs. 0%), and the score on the physical component of the 36-Item Short Form General Health Survey (SF-36) (73% vs. 35% and 14% vs. 0%) (Fig. S2 in the Supplementary Appendix).
In post hoc analyses controlling individually for between-group imbalances at baseline with respect to sex, smoking status, and previous use of cyclophosphamide, outcomes favored transplantation over cyclophosphamide on the global rank composite score at 54 months. Within individual strata, with the exception of current or former smokers, pairwise comparisons favored transplantation (range, 56 to 77% of comparisons), with the greatest benefit noted for participants who had never smoked (P≤0.04 for all three stratified analyses) (Table S4 in the Supplementary Appendix). Findings for covariate-adjusted analyses of event-free survival at 54 months were consistent with these findings (Table S4 in the Supplementary Appendix). Post hoc sensitivity analyses that used different assumptions to handle missing data on event-free survival at 54 months in computing the global rank composite score also support the primary end-point analysis (Table S5 in the Supplementary Appendix).
SAFETY, INFECTION, AND ENGRAFTMENT
A total of 21 deaths occurred over a period of 72 months (Table 3 and Fig. 1). Deaths after 54 months did not contribute to the primary end point. Of the 7 participants in the transplantation group who died, 3 did not receive the transplant; 2 died of treatment-related causes, at months 17 and 70, after a diagnosis of the myelodysplastic syndrome; and 2 had already had an event of respiratory, renal, or cardiac failure. No transplant recipient died within a year after transplantation. The 14 participants in the cyclophosphamide group who died included 3 who received five or fewer doses and 7 who had already had an event of respiratory, renal, or cardiac failure. No deaths were attributable directly to cyclophosphamide.
Table 3.
Deaths during the 72-Month Trial Period (Intention-to-Treat Population).
| Group and Time from Randomization to Death | Site-Reported Cause of Death | Completed Treatment |
|---|---|---|
| Cyclophosphamide group | ||
| 1.7 Mo | Pulmonary embolism | No (2 doses of cyclophosphamide) |
| 6.6 Mo | Respiratory failure | No (5 doses of cyclophosphamide) |
| 18.2 Mo | Scleroderma | Yes |
| 24.5 Mo | Scleroderma* | Yes |
| 25.4 Mo | Septic shock | Yes |
| 27.2 Mo | Pulmonary hypertension | Yes |
| 29.3 Mo | Respiratory* | Yes |
| 29.3 Mo | Respiratory failure* | Yes |
| 32.3 Mo | Unknown cause† | No (0 doses of cyclophosphamide) |
| 35.7 Mo | Respiratory failure | Yes |
| 39.4 Mo | Arrhythmia* | Yes |
| 54.7 Mo‡ | Infection* | Yes |
| 68.9 Mo‡ | Progression of systemic sclerosis*† | Yes |
| 69.8 Mo‡ | Sepsis*† | Yes |
| Transplantation group | ||
| 2.4 Mo | Unknown cause | No (no mobilization, conditioning, or transplantation) |
| 2.6 Mo | Pulmonary alveolar hemorrhage | No (no conditioning or transplantation) |
| 9.3 Mo | Unknown cause | No (no mobilization, conditioning, or transplantation) |
| 16.9 Mo | Enterococcal meningitis§ | Yes |
| 23.9 Mo | Respiratory* | Yes |
| 24.1 Mo | Metabolic*† | Yes |
| 69.9 Mo‡ | Acute myeloid leukemia§ | Yes |
The participant had had an event of respiratory, renal, or cardiac failure before death.
Death was identified through site contact or public records after participant withdrawal or trial completion.
Deaths after 54 months were not counted in the primary end-point analyses.
Death occurred after a diagnosis of the myelodysplastic syndrome.
Over a period of 72 months, the percentage of participants who had serious adverse events was lower in the cyclophosphamide group than in the transplantation group (51% and 74%, respectively). However, after we accounted for the duration of follow-up, the rate of serious adverse events in person-years was 0.38 in the transplantation group and 0.52 in the cyclophosphamide group (P = 0.08) (Table S6 in the Supplementary Appendix). In the transplantation group, 96% of serious adverse events occurred in the first 26 months, as compared with 71% of serious adverse events in the cyclophosphamide group (Fig. S3 in the Supplementary Appendix). The percentage of participants who had an adverse event of grade 3 or more was higher in the transplantation group than in the cyclophosphamide group (100% vs. 84%); the event rate per person-year was also higher (2.0 vs. 1.2, P<0.001). In the transplantation group, 95% of adverse events of grade 3 or more occurred during the first 26 months, of which 50% were expected cytopenias (Tables S6 and S7 and Fig. S3 in the Supplementary Appendix). We noted cancers in four participants: three in the transplantation group (one had papillary thyroid cancer and two had the myelodysplastic syndrome) and one in the cyclophosphamide group (who had breast cancer).
The rate of infections (of any grade) per person-year were similar in the two groups (0.75 in the transplantation group and 0.79 in the cyclophosphamide group) (Tables S8 through S10 in the Supplementary Appendix). However, the rate of infections of grade 3 or more per person-year was higher in the transplantation group than in the cyclophosphamide group (0.21 vs. 0.13, P = 0.09) (Table S6 in the Supplementary Appendix), with 92% of the events occurring in the first 26 months (Table S7 in the Supplementary Appendix). Varicella zoster infection occurred in 13 participants (12 in the transplantation group and 1 in the cyclophosphamide group, P<0.001). All but two zoster infections occurring in transplant recipients developed 1 to 4 years after transplantation; one episode was disseminated and none were life-threatening. Five cases of cytomegalovirus reactivation occurred, all more than 2.5 months after transplantation, with no herpes simplex or Epstein–Barr virus infections noted.
One transplant recipient had inadequate stem-cell mobilization with G-CSF and required cyclophosphamide. All transplant recipients had had sustained engraftment with neutrophil recovery by days 8 to 12 and final platelet transfusion by days 6 to 23.
DISCUSSION
In the SCOT trial, treatment of severe scleroderma with myeloablative therapy and CD34+ selected autologous hematopoietic stem-cell transplantation led to superior long-term outcomes as compared with cyclophosphamide. The result of the intention-to-treat analysis of the global rank composite score at 54 months (the primary end point) was conclusive even after accounting for between-group imbalances at baseline. Secondary per-protocol analyses of global rank composite scores and traditional end points (overall survival, event-free survival, modified Rodnan skin score, and DMARD use) further corroborated this result.
Our results confirm the findings of the Autologous Stem Cell Transplantation International Scleroderma (ASTIS) trial, including the advantage for patients who had never smoked.12 In the ASTIS trial, scleroderma relapse (defined as the need for DMARD therapy) was observed in 22% of the participants at 12 to 24 months after non-myeloablative transplantation. In the SCOT trial, only 9% of transplant recipients had initiated DMARDs by 24 months (the rate was also 9% at 54 months). Evidence that patients with scleroderma could have significant improvement and remain free of DMARDs supports transplantation as a treatment for this serious disease. After transplantation, the burden of severe adverse events was considerable but limited in onset, with 96% of serious adverse events occurring in the first 26 months after transplantation. Overall infection rates were similar in the two groups with one exception: varicella zoster infection developed in 12 of 33 transplant recipients (36%). Other toxic effects were consistent with those in studies of transplantation in other diseases.
Although the global rank composite score has not been used in studies of scleroderma, similar approaches have gained acceptance in trials of other diseases.25–29 The global rank composite score is not a clinical measure but rather an analytic approach that compares every participant in a study with every other participant on the basis of a predefined hierarchy of outcomes — in this case, outcomes specific for severe scleroderma.1,4,9,12 Objective components (death, event-free survival, and FVC) were scored at a higher priority than more subjective measures (HAQ-DI score and modified Rodnan skin score). The validity of this approach is supported by the more traditional secondary analyses. Use of the global rank composite score permitted the simultaneous assessment of multiple disease features as part of an efficient trial design.
Complications of autologous transplantation for autoimmune disease include treatment-related deaths, cancers, and infections.30 Historically, infections are the most common causes of death; infections tend to cluster in the first month after transplantation and decrease thereafter.31,32 Although scleroderma is associated with an increased risk of cancer, chemotherapy and irradiation are also associated with increased cancer risk.33,34 Patients who are exposed to total-body irradiation are at increased risk for secondary cancers over a lifetime. Additional follow-up will be needed to quantify the risk of late cancer in patients with scleroderma who are treated with total-body irradiation as well as long-term outcomes of the trial.
Transplant-related mortality of 3% at 54 months and 6% at 72 months in the SCOT trial was lower than that previously reported.12,35 No deaths were observed during the first year (95% confidence interval [CI], 0% to 9.7%). In the ASTIS trial, treatment-related mortality among 75 non-myeloablative transplant recipients was 10.7% (95% CI, 4.5 to 19.0) during the first year.12 Differences in treatment-related mortality could be due to differences in disease characteristics at trial entry: none of the SCOT participants had cardiac involvement or pulmonary arterial hypertension, and fewer had ever smoked. The non-myeloablative ASTIS trial used high doses of cyclophosphamide for both mobilization (4 g per square meter, or approximately 100 mg per kilogram) and transplantation (200 mg per kilogram). A similar high-dose regimen of cyclophosphamide in conjunction with transplantation was given to 90 patients with scleroderma and resulted in a 6% treatment-related mortality, with four of the five deaths during the peritransplantation period attributable to cardiac complications.35 These data suggest that high-dose cyclophosphamide may be too toxic for some patients with severe scleroderma, particularly in the presence of heart disease.
In contrast to nonmyeloablative regimens, the conditioning regimen in the SCOT trial was designed to maximally deplete T cells in both the graft and the host before progenitor-cell immune reconstitution. The unique property of total-body irradiation to ablate dividing and resting autoreactive clones probably contributed to the durable remissions that we observed, findings that mirror those of preclinical transplantation studies in autoimmune disease.36 However, the mechanisms whereby immune homeostasis leads to control of autoimmune disease after autologous transplantation are yet to be fully elucidated.37–40
Our trial has limitations. We enrolled patients with severe internal organ disease and not solely skin disease, so results may not be generalizable to all patients with scleroderma. The components of the global rank composite score and the hierarchy were chosen for this severely affected cohort and may not be generalizable to other populations. Although blinding is not possible in a transplantation trial, objective outcomes were placed higher in the global rank composite score hierarchy to mitigate this limitation.
In conclusion, at 54 months after randomization, myeloablative CD34+ selected autologous hematopoietic stem-cell transplantation resulted in significantly better clinical outcomes than 12 months of cyclophosphamide. Although there was greater hematopoietic toxicity and an unquantified risk of second cancers from exposure to total-body irradiation, toxic effects should be weighed against the beneficial results of treatment and the seriousness of the underlying disease.
Supplementary Material
Acknowledgments
Supported by awards from the National Institute of Allergy and Infectious Diseases and the National Institutes of Health to Duke University, the trial contract holder (N01-AI05419 and HHSN 272201100025C), and to Rho, the statistical and clinical coordinating center (N01-AI25481, HHSN272200900057C, and 1UMZAI117870).
We thank the members of the data and safety monitoring board and the end-point review committee for their service; Erica H. Brittain, Ph.D., who provided statistical input; Derek Brown, Ph.D., Christopher Kelsey, M.D., and Bouthaina Dabaja, M.D., for assistance with radiation therapy; Heidi Oehme, who prepared an earlier version of the manuscript; and the patients and families who participated in this trial.
APPENDIX
The authors’ full names and academic degrees are as follows: Keith M. Sullivan, M.D., Ellen A. Goldmuntz, M.D., Ph.D., Lynette Keyes-Elstein, Dr. P.H., Peter A. McSweeney, M.B., Ch.B., Ashley Pinckney, M.S., Beverly Welch, R.N., M.S.N., Maureen D. Mayes, M.D., M.P.H., Richard A. Nash, M.D., Leslie J. Crofford, M.D., Barry Eggleston, M.S., Sharon Castina, R.N., M.S.N., Linda M. Griffith, M.D., M.H.S., Ph.D., Julia S. Goldstein, M.D., Dennis Wallace, Ph.D., Oana Craciunescu, Ph.D., Dinesh Khanna, M.D., Rodney J. Folz, M.D., Ph.D., Jonathan Goldin, M.D., E. William St. Clair, M.D., James R. Seibold, M.D., Kristine Phillips, M.D., Ph.D., Shin Mineishi, M.D., Robert W. Simms, M.D., Karen Ballen, M.D., Mark H. Wener, M.D., George E. Georges, M.D., Shelly Heimfeld, Ph.D., Chitra Hosing, M.D., Stephen Forman, M.D., Suzanne Kafaja, M.D., Richard M. Silver, M.D., Leroy Griffing, M.D., Jan Storek, M.D., Ph.D., Sharon LeClercq, M.D., Richard Brasington, M.D., Mary E. Csuka, M.D., Christopher Bredeson, M.D., Carolyn Keever-Taylor, Ph.D., Robyn T. Domsic, M.D., M.P.H., M. Bashar Kahaleh, M.D., Thomas Medsger, M.D., and Daniel E. Furst, M.D.
The authors’ affiliations are as follows: the Duke University Medical Center (K.M.S., O.C., E.W.S.C.) and RTI International (D.W.), Durham, and Rho Federal Systems Division, Chapel Hill (L.K.-E., A.P., B.E., S.C.) — all in North Carolina; National Institute of Allergy and Infectious Diseases, Bethesda, MD (E.A.G., B.W., L.M.G., J.S.G.); Colorado Blood Cancer Institute, Denver (P.A.M., R.A.N.); University of Texas McGovern Medical School (M.D.M.) and M.D. Anderson Cancer Center (C.H.) — both in Houston; Vanderbilt University, Nashville (L.J.C., K.P.); University of Michigan, Ann Arbor (D.K., J.R.S.); Case Western Reserve University and University Hospitals, Cleveland (R.J.F.); University of Alabama, Birmingham (S.M.); Boston University, Boston (R.W.S.); University of Virginia, Charlottesville (K.B.); University of Washington (M.H.W., D.E.F.) and the Fred Hutchinson Cancer Research Center (G.E.G., S.H.) — both in Seattle; University of California, Los Angeles, Los Angeles (J.G., S.K., D.E.F.); City of Hope National Medical Center, Duarte, CA (S.F.); Medical University of South Carolina, Charleston (R.M.S.); Mayo Clinic, Scottsdale, AZ (L.G.); University of Calgary, Calgary, AB, Canada (J.S., S.L.); Washington University, St. Louis (R.B.); Medical College of Wisconsin, Milwaukee (M.E.C., C.K.-T.); Ottawa Hospital Research Institute, Ottawa (C.B.); University of Pittsburgh, Pittsburgh (T.M., R.T.D.); and University of Toledo Medical Center, Toledo, OH (M.B.K.).
Footnotes
Disclosure forms provided by the authors are available with full text of this article at NEJM.org.
References
- 1.Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–55. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 2.Elhai M, Meune C, Avouac J, Kahan A, Allanore Y. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2012;51:1017–26. doi: 10.1093/rheumatology/ker269. [DOI] [PubMed] [Google Scholar]
- 3.Nihtyanova SI, Schreiber BE, Ong VH, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol. 2014;66:1625–35. doi: 10.1002/art.38390. [DOI] [PubMed] [Google Scholar]
- 4.Tashkin DP, Elashoff R, Clements PJ, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med. 2007;176:1026–34. doi: 10.1164/rccm.200702-326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanna D, Denton CP, Jahreis A, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet. 2016;387:2630–40. doi: 10.1016/S0140-6736(16)00232-4. [DOI] [PubMed] [Google Scholar]
- 6.Tashkin DP, Roth MD, Clements PJ, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4:708–19. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyndall A, Black C, Finke J, et al. Treatment of systemic sclerosis with autologous haemopoietic stem cell transplantation. Lancet. 1997;349:254. doi: 10.1016/s0140-6736(05)64864-7. [DOI] [PubMed] [Google Scholar]
- 8.Binks M, Passweg JR, Furst D, et al. Phase I/II trial of autologous stem cell transplantation in systemic sclerosis: procedure related mortality and impact on skin disease. Ann Rheum Dis. 2001;60:577–84. doi: 10.1136/ard.60.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nash RA, McSweeney PA, Crofford LJ, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe systemic sclerosis: long-term follow-up of the US multicenter pilot study. Blood. 2007;110:1388–96. doi: 10.1182/blood-2007-02-072389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan KM, Muraro P, Tyndall A. Hematopoietic cell transplantation for auto-immune disease: updates from Europe and the United States. Biol Blood Marrow Transplant. 2010;16(Suppl):S48–S56. doi: 10.1016/j.bbmt.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burt RK, Shah SJ, Dill K, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet. 2011;378:498–506. doi: 10.1016/S0140-6736(11)60982-3. [DOI] [PubMed] [Google Scholar]
- 12.van Laar JM, Farge D, Sont JK, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA. 2014;311:2490–8. doi: 10.1001/jama.2014.6368. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan KM, Shah A, Sarantopoulos S, Furst DE. Hematopoietic stem cell transplantation for scleroderma: effective immunomodulatory therapy for patients with pulmonary involvement. Arthritis Rheumatol. 2016;68:2361–71. doi: 10.1002/art.39748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger VW, Ivanova A, Knoll MD. Minimizing predictability while retaining balance through the use of less restrictive randomization procedures. Stat Med. 2003;22:3017–28. doi: 10.1002/sim.1538. [DOI] [PubMed] [Google Scholar]
- 15.Keever-Taylor CA, Heimfeld S, Steinmiller KC, et al. Manufacture of autologous CD34+ selected grafts in the NIAID-sponsored HALT-MS and SCOT multicenter clinical trials for autoimmune diseases. Biol Blood Marrow Transplant. 2017;23:1463–72. doi: 10.1016/j.bbmt.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McSweeney PA, Nash RA, Sullivan KM, et al. High-dose immunosuppressive therapy for severe systemic sclerosis: initial outcomes. Blood. 2002;100:1602–10. [PMC free article] [PubMed] [Google Scholar]
- 17.Craciunescu OI, Steffey BA, Kelsey CR, et al. Renal shielding and dosimetry for patients with severe systemic sclerosis receiving immunoablation with total body irradiation in the Scleroderma: Cyclophosphamide or Transplantation trial. Int J Radiat Oncol Biol Phys. 2011;79:1248–55. doi: 10.1016/j.ijrobp.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosing C, Nash R, McSweeney P, et al. Acute kidney injury in patients with systemic sclerosis participating in hematopoietic cell transplantation trials in the United States. Biol Blood Marrow Transplant. 2011;17:674–81. doi: 10.1016/j.bbmt.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung EW, Mayes MD, Sharif R, et al. Gastric antral vascular ectasia and its clinical correlates in patients with early diffuse systemic sclerosis in the SCOT trial. J Rheumatol. 2013;40:455–60. doi: 10.3899/jrheum.121087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan KM, McSweeney PA, Nash RA. Cyclophosphamide in scleroderma lung disease. N Engl J Med. 2006;355:1173–4. [PubMed] [Google Scholar]
- 21.Clements PJ, Lachenbruch PA, Seibold JR, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol. 1993;20:1892–6. [PubMed] [Google Scholar]
- 22.Khanna D, Furst DE, Hays RD, et al. Minimally important difference in diffuse systemic sclerosis: results from the D-penicillamine study. Ann Rheum Dis. 2006;65:1325–9. doi: 10.1136/ard.2005.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finkelstein DM, Schoenfeld DA. Combining mortality and longitudinal measures in clinical trials. Stat Med. 1999;18:1341–54. doi: 10.1002/(sici)1097-0258(19990615)18:11<1341::aid-sim129>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Stokes ME, Davis CS, Koch GG. Categorical data analysis using the SAS system. 2. Cary, NC: SAS Institute; 2009. [Google Scholar]
- 25.Bartunek J, Terzic A, Davison BA, et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J. 2017;38:648–60. doi: 10.1093/eurheartj/ehw543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cudkowicz ME, van den Berg LH, Shefner JM, et al. Dexpramipexole versus placebo for patients with amyotrophic lateral sclerosis (EMPOWER): a randomised, double-blind, phase 3 trial. Lancet Neurol. 2013;12:1059–67. doi: 10.1016/S1474-4422(13)70221-7. [DOI] [PubMed] [Google Scholar]
- 27.Packer M. Development and evolution of a hierarchical clinical composite end point for the evaluation of drugs and devices for acute and chronic heart failure: a 20-year perspective. Circulation. 2016;134:1664–78. doi: 10.1161/CIRCULATIONAHA.116.023538. [DOI] [PubMed] [Google Scholar]
- 28.Berry JD, Miller R, Moore DH, et al. The Combined Assessment of Function and Survival (CAFS): a new endpoint for ALS clinical trials. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:162–8. doi: 10.3109/21678421.2012.762930. [DOI] [PubMed] [Google Scholar]
- 29.Wittkop L, Smith C, Fox Z, et al. Methodological issues in the use of composite endpoints in clinical trials: examples from the HIV field. Clin Trials. 2010;7:19–35. doi: 10.1177/1740774509356117. [DOI] [PubMed] [Google Scholar]
- 30.Daikeler T, Tichelli A, Passweg J. Complications of autologous hematopoietic stem cell transplantation for patients with autoimmune diseases. Pediatr Res. 2012;71:439–44. doi: 10.1038/pr.2011.57. [DOI] [PubMed] [Google Scholar]
- 31.Farge D, Labopin M, Tyndall A, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years’ experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica. 2010;95:284–92. doi: 10.3324/haematol.2009.013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storek J, Zhao Z, Lin E, et al. Recovery from and consequences of severe iatrogenic lymphopenia (induced to treat auto-immune diseases) Clin Immunol. 2004;113:285–98. doi: 10.1016/j.clim.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onishi A, Sugiyama D, Kumagai S, Morinobu A. Cancer incidence in systemic sclerosis: meta-analysis of population-based cohort studies. Arthritis Rheum. 2013;65:1913–21. doi: 10.1002/art.37969. [DOI] [PubMed] [Google Scholar]
- 34.Vaxman I, Ram R, Gafter-Gvili A, et al. Secondary malignancies following high dose therapy and autologous hematopoietic cell transplantation — systematic review and meta-analysis. Bone Marrow Transplant. 2015;50:706–14. doi: 10.1038/bmt.2014.325. [DOI] [PubMed] [Google Scholar]
- 35.Burt RK, Oliveira MC, Shah SJ, et al. Cardiac involvement and treatment-related mortality after non-myeloablative haemopoietic stem-cell transplantation with unselected autologous peripheral blood for patients with systemic sclerosis: a retrospective analysis. Lancet. 2013;381:1116–24. doi: 10.1016/S0140-6736(12)62114-X. [DOI] [PubMed] [Google Scholar]
- 36.van Bekkum DW. Conditioning regimens for the treatment of experimental arthritis with autologous bone marrow transplantation. Bone Marrow Transplant. 2000;25:357–64. doi: 10.1038/sj.bmt.1702153. [DOI] [PubMed] [Google Scholar]
- 37.Muraro PA, Robins H, Malhotra S, et al. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J Clin Invest. 2014;124:1168–72. doi: 10.1172/JCI71691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Kleer I, Vastert B, Klein M, et al. Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood. 2006;107:1696–702. doi: 10.1182/blood-2005-07-2800. [DOI] [PubMed] [Google Scholar]
- 39.Delemarre EM, van den Broek T, Mijnheer G, et al. Autologous stem cell transplantation aids autoimmune patients by functional renewal and TCR diversification of regulatory T cells. Blood. 2016;127:91–101. doi: 10.1182/blood-2015-06-649145. [DOI] [PubMed] [Google Scholar]
- 40.Snowden JA. Rebooting autoimmunity with autologous HSCT. Blood. 2016;127:8–10. doi: 10.1182/blood-2015-11-678607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.