Lyme disease is an emerging tick-borne disease in the U.S., Europe, and Asia including China, and has become the most common vector-borne disease in both Europe and North America. Infection is caused by the spirochetal pathogen Borrelia burgdorferi sensu lato, transmitted via tick bites. The clinical manifestations of Lyme disease range from fever and skin lesions (erythema migrans) to multisystem disorders such as arthritis, carditis, and neuroborreliosis (Steere et al., 2004). Although treatable with antibiotics, the infection is underdiagnosed, and the late stages of Lyme disease are difficult to treat. No commercial vaccine is available. An alternative strategy is to block the transmission of B. burgdorferi. Understanding how B. burgdorferi is maintained in the enzootic cycle is the key for such strategy.
B. burgdorferi is maintained in an enzootic cycle containing two markedly different hosts, an arthropod vector and a mammalian host (Radolf et al., 2012; Samuels, 2011). B. burgdorferi must be able to adapt to both distinct host environments. On the other hand, as a non-free living pathogen, B. burgdorferi has a dramatically reduced genome. Remarkably, B. burgdorferi has evolved in utilizing its limited genomic capabilities to adapt to and survive in these two host environments. Therefore, B. burgdorferi provides a wonderful system to study mechanism of signal transduction and host adaptation. In this regard, bacterial two-component systems (TCSs) are the main signaling pathways that bacteria utilize to sense and respond to environmental conditions. A typical TCS consists of a histidine kinase as a sensor and a corresponding response regulator that mediates the cellular response (Stock et al., 2000). Most bacteria have many TCS systems. For example, Escherichia coli has over 30 TCSs. In contrast, the B. burgdorferi genome only has two TCSs, (in addition to the chemotactic CheA-CheY system), Hk1-Rrp1 and Hk2-Rrp2. Considering that B. burgdorferi encounters two hosts throughout its life cycle, could B. burgdorferi have evolved to employ these two TCSs to survive in each of the hosts?
Uncovering the function of Hk2-Rrp2 pathway in the enzootic cycle of B. burgdorferi was one of the milestones in the research of molecular biology and pathogenesis of B. burgdorferi (Radolf et al., 2012; Samuels, 2011). In the past decade, we and others have shown that Rrp2 functions as a transcriptional regulator that activates a sigma factor cascade, the σ54-σS cascade. Rrp2 is a NtrC-type bacterial enhancer-binding protein (bEBP) required for activation of the alternative sigma factor σ54. Upon stimulation, Rrp2 becomes phosphorylated at its N-terminal response receiver domain, and phosphorylated Rrp2 and σ54 work together to activate transcription of rpoS from a -24/-12 σ54-type promoter. The sigma factor RpoS (σS) functions as a global regulator, controlling expression of more than 15% of the genes in B. burgdorferi. Many of these genes encode surface lipoproteins (B. burgdorferi does not have lipopolysaccharide on its surface), and these lipoproteins, such as OspC, DbpA/B, and BBK32, have been shown to interact with host molecules and are required for colonization and host immune evasion (Radolf et al., 2012; Samuels, 2011).
Bodies of evidence indicate that Rrp2 is activated during spirochetal transmission from ticks to mammals. Along with other PerR/Fur-like activator BosR, phosphorylated Rrp2 activates the σ54-σS cascade. In addition to Rrp2 and BosR, a small RNA-binding protein DsrA, a ROC-type repressor BadR, and a plasmid-coded protein BBI16, have also been recently shown to be involved in regulation of RpoS levels in B. burgdorferi (Dulebohn et al., 2014; Miller et al., 2013; Radolf et al., 2012; Samuels, 2011). The Rrp2-RpoN-RpoS signaling pathway functions as a gate-keeper, is activated upon tick feeding, and controls production of many virulence factors required for the process of transmission and invasion of the mammalian host (Radolf et al., 2012; Samuels, 2011). Therefore, the Hk2-Rrp2 pathway plays a central role in B. burgdorferi survival in the mammalian host environment.
The function of the second TCS signaling system in B. burgdorferi, Hk1-Rrp1, has begun to be elucidated in the past few years. The response regulator Rrp1 is the sole diguanylatecyclase for synthesis of diguanylate (c-di- GMP). c-di-GMP is a new global second messenger found ubiquitously in the bacterial world (Römling et al., 2013). c-di-GMP is synthesized by diguanylatecyclases (DGCs), a group of GGDEF domain-containing proteins, and is broken down by phosphodiesterases (PDEs) that contain a conserved EAL or HD-GYP domain. Most free-living bacteria have numerous DGCs for synthesis of c-di-GMP, and phosphodiesterases (PDEs) for hydrolysis of c-di-GMP, and in fact, GGDEF, EAL and HD-GYP domains are among the most abundant domains encoded in bacterial genomes. Each DGC regulates its own environmental signal(s). It has been shown to play a key role in controlling the switch between the motile, single-cellular lifestyle and the sessile, multicellular lifestyle (biofilms). It is also involved in regulating virulence, antibiotic production, cell cycle regulation, and host innate immunity.
B. burgdorferi has a streamlined c-di-GMP signaling cascade that involves a single DGC, Rrp1, and two PDEs. Three independent groups, using either the hk1 or rrp1 mutant, demonstrated that, unlike the Hk2-Rrp2 pathway which is essential for mammalian infection, both the hk1andrrp1 mutants were still capable of infecting the mice. However, upon acquired by ticks, neither mutant was able to survive in the tick midgut (Caimano et al., 2011; He et al., 2011; Kostick et al., 2011). Thus, the Hk1-Rrp1 pathway is dispensable for mammalian infection, but is essential for B. burgdorferi to survive in the tick vector.
How does Hk1-Rrp1 contribute to spirochetal adaptation to the tick host environment? It appears that one of the defects of the hk1 or rrp1 mutant in ticks is, in part, due to a defect in its inability to utilize glycerol, chitobiose and N-acetylglucosamine (He et al., 2011; Sze et al., 2013). Glycerol is produced by certain insects as well as arthropods as cryoprotective molecule. It was found that expression of the glycerol uptake/metabolism operon glpFKD is important to the fitness of spirochetes in ticks, and the hk1 or rrp1 mutant is defective in glpFKD expression (He et al., 2011; Pappas et al., 2011). Constitutive expression of glpFKD in the rrp1 mutant can partially rescue the rrp1 mutant’s survival in ticks or its transmission to the mammalian host. Chitobiose is a major component of the tick cuticle and an important source of N-acetylglucosamine for cell wall synthesis in B. burgdorferi. One of the chitobiose transporter gene chbC was shown to be defective in the rrp1 mutant, and supplementing N-acetylglucosamine in tick midguts partially rescued the rrp1 mutant defect in ticks (Sze et al., 2013). In addition to nutrient utilization, c-di-MGP appears to also affect the motility of the spirochetes, since the plzA mutant, which encodes PlzA, the only c-di-GMP binding protein identified in the B. burgdorferi, had abnormal swarming ability and reduced survival in ticks (Pitzer et al., 2011). More recently, Caimano et al. employed the live imaging technology and showed that the protective response mediated by c-di-GMP is multifactorial to contribute spirochetal survivals during tick feeding (Caimano et al., 2015). Further studies are warranted on how c-di-GMP modulates these diverse functions.
All evidences available thus far indicate that the two TCS systems in B. burgdorferi, Hk1-Rrp1 and Hk2-Rrp2, each controls spirochetal adaptation to one host environment (Figure 1). It makes biological sense that through genome reduction, only two TCSs were kept, given that B. burgdorferi is an obligated pathogen, not a free-living bacterium that needs to survive in many environmental conditions: ticks and mammals are the only two habitats B. burgdorferi has to deal with. Although each TCS is required for survival in each host, there is an interplay between the two systems. This is not surprising considering that when spirochetes are migrating from ticks to mammals during tick feeding, both Hk1-Rrp1 and Hk2-Rrp2 are activated. A common mechanism of cross-talk between TCS systems in other bacteria is that the histidine kinase sensor of one TCS phosphorylates a response regulator of the other TCS. This appears not to be the case for B. burgdorferi. We recent reported that the interplay between Hk1-Rrp1 and Hk2-Rrp2 is mediated through the c-di-GMP receptor PlzA (He et al., 2014). This is yet another example how B. burgdorferi with a streamlined genome continues unveiling its uniqueness. Further study on how the downstream targets of these two signaling systems function in each host is warranted, which will undoubtedly shed light on our understanding of vector- pathogen-host interactions.
Figure 1.
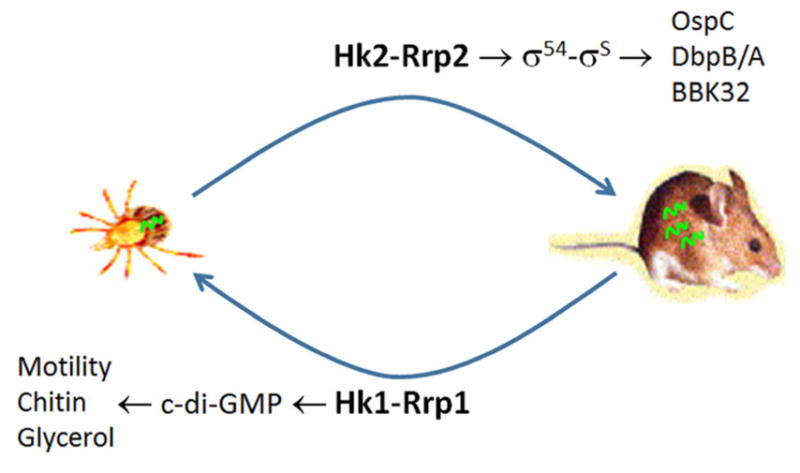
The Lyme disease pathogen B. burgdorferi has only two TCSs (in addition to chemotaxis systems). One system, Hk2-Rrp2, upon sensing the signals, activates genes required for mammalian infection. The other system, Hk1-Rrp1, governs multiple activities that are essential for the pathogen’s survival in the tick vector.
Acknowledgments
This work was supported by National Institute of Health grants (AI083640, AI117198 to X. Frank Yang), the National Science Foundation of China (81428015, 81171611 to Yongliang Lou) and (81501772 to MeipingYe), Zhejiang Science Foundation (Y15H190025 to Yan Zhou).
Footnotes
Compliance and ethics The author(s) declare that they have no conflict of interest.
References
- Caimano MJ, Dunham-Ems S, Allard AM, Cassera MB, Kenedy M, Radolf JD. Cyclic di-GMP modulates gene expression in Lyme disease spirochetes at the tick-mammal interface to promote spirochete survival during the blood meal and tick-to-mammal transmission. Infect Immun. 2015;83:3043–3060. doi: 10.1128/IAI.00315-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Kenedy MR, Kairu T, Desrosiers DC, Harman M, Dunham-Ems S, Akins DR, Pal U, Radolf JD. The hybrid histidine kinase Hk1 is part of a two-component system that is essential for survival of Borrelia burgdorferi in feeding Ixodes scapularis ticks. Infect Immun. 2011;79:3117–3130. doi: 10.1128/IAI.05136-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulebohn DP, Hayes BM, Rosa PA. Global repression of host-associated genes of the lyme disease spirochete through post-transcriptional modulation of the alternative sigma factor RpoS. PLoS One. 2014;9:e93141. doi: 10.1371/journal.pone.0093141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Ouyang Z, Troxell B, Xu H, Moh A, Piesman J, Norgard MV, Gomelsky M, Yang XF. Cyclic di-GMP is essential for the survival of the Lyme disease spirochete in ticks. PLoS Pathog. 2011;7:e1002133. doi: 10.1371/journal.ppat.1002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Zhang JJ, Ye M, Lou Y, Yang XF. The cyclic di-GMP receptor PlzA controls virulence gene expression through RpoS in Borrelia burgdorferi. Infect Immun. 2014;82:445–452. doi: 10.1128/IAI.01238-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostick JL, Szkotnicki LT, Rogers EA, Bocci P, Raffaelli N, Marconi RT. The diguanylate cyclase, Rrp1, regulates critical steps in the enzootic cycle of the Lyme disease spirochetes. Mol Microbiol. 2011;81:219–231. doi: 10.1111/j.1365-2958.2011.07687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Karna SLR, Seshu J. Borrelia host adaptation Regulator (BadR) regulates rpoS to modulate host adaptation and virulence factors in Borrelia burgdorferi. Mol Microbiol. 2013;88:105–124. doi: 10.1111/mmi.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas CJ, Iyer R, Petzke MM, Caimano MJ, Radolf JD, Schwartz I. Borrelia burgdorferi requires glycerol for maximum fitness during the tick phase of the enzootic cycle. PLoS Pathog. 2011;7:e1002102. doi: 10.1371/journal.ppat.1002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzer JE, Sultan SZ, Hayakawa Y, Hobbs G, Miller MR, Motaleb MA. Analysis of the Borrelia burgdorferi cyclic-di-GMP-binding protein PlzA reveals a role in motility and virulence. Infect Immun. 2011;79:1815–1825. doi: 10.1128/IAI.00075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Micro. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS. Gene regulation in Borrelia burgdorferi. Annu Rev Microbiol. 2011;65:479–499. doi: 10.1146/annurev.micro.112408.134040. [DOI] [PubMed] [Google Scholar]
- Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113:1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock AM, Robinson VL, Goudreau PN. Two component signal transduction. Ann Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- Sze CW, Smith A, Choi YH, Yang X, Pal U, Yu A, Li C. Study of the response regulator Rrp1 reveals its regulatory role in chitobiose utilization and virulence of Borrelia burgdorferi. Infect Immun. 2013;81:1775–1787. doi: 10.1128/IAI.00050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


