Abstract
BACKGROUND
Comparative clinical effects of balanced crystalloids and saline are uncertain, particularly in noncritically ill patients cared for outside an intensive care unit (ICU).
METHODS
We conducted a single-center, pragmatic, multiple-crossover trial comparing balanced crystalloids (lactated Ringer’s solution or Plasma-Lyte A) with saline among adults who were treated with intravenous crystalloids in the emergency department and were subsequently hospitalized outside an ICU. The type of crystalloid that was administered in the emergency department was assigned to each patient on the basis of calendar month, with the entire emergency department crossing over between balanced crystalloids and saline monthly during the 16-month trial. The primary outcome was hospital-free days (days alive after discharge before day 28). Secondary outcomes included major adverse kidney events within 30 days — a composite of death from any cause, new renal-replacement therapy, or persistent renal dysfunction (defined as an elevation of the creatinine level to ≥200% of baseline) — all censored at hospital discharge or 30 days, whichever occurred first.
RESULTS
A total of 13,347 patients were enrolled, with a median crystalloid volume administered in the emergency department of 1079 ml and 88.3% of the patients exclusively receiving the assigned crystalloid. The number of hospital-free days did not differ between the balanced-crystalloids and saline groups (median, 25 days in each group; adjusted odds ratio with balanced crystalloids, 0.98; 95% confidence interval [CI], 0.92 to 1.04; P = 0.41). Balanced crystalloids resulted in a lower incidence of major adverse kidney events within 30 days than saline (4.7% vs. 5.6%; adjusted odds ratio, 0.82; 95% CI, 0.70 to 0.95; P = 0.01).
CONCLUSIONS
Among noncritically ill adults treated with intravenous fluids in the emergency department, there was no difference in hospital-free days between treatment with balanced crystalloids and treatment with saline.
Administration of intravenous isotonic crystalloids is one of the most common medical therapies, with routine use in emergency departments, hospital wards, intensive care units (ICUs), and operating rooms.1 However, it is not known whether the composition of isotonic crystalloid fluid has an effect on patient outcomes.1–3 In the United States, saline (0.9% sodium chloride; “normal saline”) is the most commonly used isotonic crystalloid, with more than 200 million liters administered annually. 1 The chloride concentration of saline (154 mmol per liter) is higher than that of human plasma (94 to 111 mmol per liter). Infusion of saline generally causes hyperchloremic metabolic acidosis and may increase renal inflammation and impair renal perfusion.4–8 Although the clinical significance of these physiological effects is incompletely understood, accumulating evidence suggests that the supraphysiologic chloride concentration of saline may contribute to kidney injury and impair a patient’s ability to recover from severe illness.9–15 The chloride concentration in physiologically balanced crystalloids, such as lactated Ringer’s solution (109 mmol per liter) and Plasma-Lyte A (98 mmol per liter), are more similar to that of human plasma.1,2
Previous clinical studies that compared balanced crystalloids and saline have focused on critically ill patients in the ICU and operating room.9–18 Although critically ill patients may be the most vulnerable to potential detrimental effects of saline, acutely ill patients without organ failure or other critical illness comprise a large patient population that is routinely treated with intravenous fluids.1,19 Owing to the vast number of noncritically ill patients exposed to crystalloids, even small differences in the absolute risk of kidney injury or death between balanced crystalloids and saline may have large public health implications. In the present trial, we investigated the clinical effect of balanced crystalloids versus saline for routine intravenous fluid therapy in the emergency department among noncritically ill adults. We hypothesized that balanced crystalloids would result in earlier hospital discharge and a lower incidence of major adverse kidney events than saline.
METHODS
TRIAL DESIGN AND OVERSIGHT
Our trial, the Saline against Lactated Ringer’s or Plasma-Lyte in the Emergency Department (SALT-ED) trial, was a single-center, pragmatic, unblinded, multiple-crossover trial that compared balanced crystalloids and saline among consecutive noncritically ill adults treated with intravenous crystalloids in the emergency department before hospitalization outside the ICU. The rationale, design, and statistical analysis plan were prespecified and have been published.20 The protocol is also available with the full text of this article at NEJM.org. The institutional review board at Vanderbilt University approved the trial with waiver of informed consent. The trial was monitored by an independent data and safety monitoring board.20 The first and fourth authors vouch for the completeness and accuracy of the data and analyses.
TRIAL POPULATION
The trial was conducted between January 1, 2016, and April 30, 2017, in the Vanderbilt University Medical Center Adult Emergency Department, a tertiary-care, academic, hospital-based emergency department in the United States with approximately 75,000 visits per year. The trial population consisted of adults (≥18 years old) who received at least 500 ml of intravenous isotonic crystalloids in the emergency department and were subsequently hospitalized outside an ICU. Patients who were admitted to an ICU from the emergency department were defined as critically ill and were enrolled in a separate trial that compared balanced crystalloids and saline among critically ill adults, the Isotonic Solutions and Major Adverse Renal Events Trial (SMART), reported in this issue of the Journal.16 Patients who received less than 500 ml of crystalloids in the emergency department were excluded owing to the low dose of exposure to the intervention.15 The unit of analysis was unique emergency department visit, with individual patients potentially contributing multiple visits. In a sensitivity analysis, we limited the trial population to the first emergency department visit among unique patients.
TREATMENT ASSIGNMENTS
The trial protocol guided the type of isotonic crystalloid that was administered in the emergency department. All other aspects of care were determined by treating clinicians independent of the trial protocol, including whether to treat with crystalloids and the volume of crystalloids administered. Consistent with the concept of a pragmatic clinical trial,21 trial procedures were embedded within routine care and executed by clinical personnel.
The methods of treatment assignment have been described previously.20 In brief, the type of isotonic crystalloid was assigned according to calendar month, with all patients in the trial emergency department during the same month assigned to the same fluid, either balanced crystalloids or saline. During balanced-crystalloids months, clinicians had the option of choosing either lactated Ringer’s solution or Plasma-Lyte A. Clinicians and patients were aware of the treatment assignments. The first trial month was assigned by means of computer-generated simple randomization. Treatment assignments then sequentially crossed over between balanced crystalloids and saline each month for a total of 16 months (Fig. S1 in the Supplementary Appendix, available at NEJM.org). Selection of fluids after the patient’s transfer from the emergency department to a hospital floor was not included as part of the trial intervention.
Electronic advisors within the electronic order-entry system informed providers about the trial, asked about relative contraindications to the assigned crystalloid, and guided them through crystalloid orders.20 Relative contraindications to the use of balanced crystalloids included hyperkalemia and brain injury; the severity of hyperkalemia and brain injury at which saline was used instead of balanced crystalloids was determined by the treating provider. There were no relative contraindications listed for saline in the electronic advisor. Providers had the option of ordering off-protocol crystalloids if they believed an alternative was specifically indicated. Patients who received off-protocol fluids were included in the primary analysis according to intention-to-treat principles. In a secondary per-protocol analysis, the population was limited to patients who received all fluids in accordance with the protocol.
DATA COLLECTION
Data were extracted from the electronic medical record. We have previously validated these data-collection techniques for relevant data points.15,22,23 Coexisting conditions at baseline were summarized with the Elixhauser Comorbidity Index score.24
OUTCOMES
The primary outcome was hospital-free days to day 28, a composite of in-hospital death and hospital length of stay defined as the number of days alive and out of the hospital between the index emergency department visit and 28 days later.20,25 Patients who died during the index hospitalization and those hospitalized for more than 28 days were classified as having zero hospital-free days. For patients discharged alive before day 28, hospital-free days were calculated as 28 minus length of stay.
The trial included three key secondary outcomes: major adverse kidney events within 30 days, acute kidney injury of stage 2 or higher, and in-hospital death. Major adverse kidney events within 30 days was a composite of death, new renal-replacement therapy, or persistent renal dysfunction (final serum creatinine concentration, ≥200% of the baseline value) at the earliest of hospital discharge or 30 days after the index emergency department visit (Table S1 in the Supplementary Appendix).26 Stage 2 or higher acute kidney injury was defined according to Kidney Disease: Improving Global Outcomes (KDIGO) creatinine criteria as a maximum serum creatinine concentration at least 200% of the baseline value, an increase in the serum creatinine concentration to at least 4 mg per deciliter (354 μmol per liter) with an absolute increase of at least 0.5 mg per deciliter (44 μmol per liter), or initiation of new renal-replacement therapy before the earliest of hospital discharge or 30 days after the index emergency department visit.27 In-hospital death was defined as death before hospital discharge, regardless of hospital length of stay.
Patients with end-stage renal disease who were receiving long-term renal-replacement therapy at presentation were not eligible to meet renal outcomes, including new renal-replacement therapy, persistent renal dysfunction, and acute kidney injury. However, patients with end-stage renal disease could meet the outcome of major adverse kidney events within 30 days through death. The baseline creatinine value was defined as the lowest recorded value within the electronic medical record at the trial institution in the year before presentation in the emergency department. Patients with no recorded creatinine values in the previous year had a baseline creatinine value calculated under the assumption of normal baseline renal function with the use of the following equation: [creatinine (in milligrams per deciliter) = 0.74 – 0.2 (if patient is female) + 0.08 (if patient is black) + 0.003 × age (in years)].28 The serum creatinine concentration in the emergency department was defined as the first recorded value during the index emergency department visit. Creatinine values in the emergency department were considered to be baseline characteristics, whereas creatinine values after hospital admission were considered outcomes. Major adverse kidney events within 30 days and acute kidney injury were calculated on the basis of creatinine values after admission. Patients who presented to the emergency department with a creatinine value that met the criteria for acute kidney injury and who then had a drop in creatinine such that no value after hospital admission met these criteria did not have an outcome of acute kidney injury for the purposes of this trial. Additional, exploratory outcomes are described in Table S2 in the Supplementary Appendix.
STATISTICAL ANALYSIS
A trial duration of 16 months was selected to ensure numerous alternating periods of balanced crystalloids and saline, enrollment throughout the academic and calendar year, coordination with the concomitant trial (SMART),16 and adequate sample size (power) to balance baseline characteristics and detect at least a 0.5-day difference in hospital-free days between groups. Sample size was dependent on the number of patients treated with isotonic crystalloids in the trial emergency department and hospitalized outside an ICU during the 16-month trial period. All the patients who met these criteria were enrolled. On the basis of historical data from the trial emergency department, we estimated that approximately 14,000 patients would be enrolled in 16 months, with the saline group having a mean (±SD) of 24±4 hospital-free days. Under these assumptions, 14,000 patients would provide more than 90% power to detect a difference of 0.5 hospital-free days between groups with a type I error rate of 0.05. One interim analysis was completed by the data and safety monitoring board at the midpoint of enrollment, which resulted in a recommendation to continue enrollment for the planned 16 months.20
An intention-to-treat analysis of eligible patients who were assigned to balanced crystalloids or saline was completed for the primary and secondary outcomes. Hospital-free days were analyzed with a multivariable proportional-odds model. Major adverse kidney events within 30 days, acute kidney injury, and in-hospital death were analyzed with multivariable logistic-regression models. Each model was adjusted for the following baseline characteristics: age, sex, race, admitting inpatient service, and days elapsed since the initiation of the trial.20
Heterogeneity of treatment effect was evaluated by adding an interaction term29 to the models between trial-group assignment and each of the following prespecified baseline characteristics: serum creatinine, chloride, and bicarbonate concentrations in the emergency department; age; hospital admission service; and volume of crystalloid administered in the emergency department. A per-protocol secondary analysis was performed that included patients treated exclusively with the assigned crystalloid in the emergency department (100% adherence to trial treatment assignments).
A two-sided P value of less than 0.049 was considered to indicate statistical significance for the primary outcome after we accounted for one interim analysis with a Haybittle–Peto boundary of less than 0.001. With the use of the Bonferroni approach, a two-sided P value of less than 0.017 was considered to indicate statistical significance for the three key secondary outcomes: major adverse kidney events within 30 days, acute kidney injury, and in-hospital death. Analyses were conducted with R software, version 3.2.0 (R Foundation for Statistical Computing), and STATA software, version 14 (StataCorp).
RESULTS
PATIENTS
During the 16-month trial, 19,949 patients were treated with isotonic crystalloids in the emergency department and hospitalized; 3689 patients received less than 500 ml of crystalloids and were excluded, whereas 2913 patients were admitted from the emergency department to an ICU and enrolled in SMART16 (Fig. S2 in the Supplementary Appendix). The final sample size was 13,347 patients, including 6708 (50.3%) assigned to balanced crystalloids and 6639 (49.7%) assigned to saline. Baseline creatinine values were calculated for 4666 patients (35.0%) who did not have an available measured value. Baseline characteristics were similar between the two groups, including demographic characteristics, burden of coexisting conditions, admitting service, and renal function (Table 1).
Table 1.
Baseline Characteristics of the Patients.*
| Characteristic | Balanced Crystalloids (N = 6708) | Saline (N = 6639) |
|---|---|---|
| Median age (IQR) — yr | 54 (37–67) | 53 (37–67) |
| Female sex — no. (%) | 3507 (52.3) | 3379 (50.9) |
| Race — no. (%)† | ||
| White | 5159 (76.9) | 5189 (78.2) |
| Black | 1335 (19.9) | 1251 (18.8) |
| Other | 214 (3.2) | 199 (3.0) |
| Median Elixhauser Comorbidity Index score (IQR)‡ | 7 (3–14) | 7 (3–14) |
| Admission service — no. (%) | ||
| Medicine services | ||
| General medicine | 4747 (70.8) | 4687 (70.6) |
| Cardiology | 303 (4.5) | 321 (4.8) |
| Neurology | 117 (1.7) | 144 (2.2) |
| Surgery services | ||
| General surgery | 1278 (19.1) | 1211 (18.2) |
| Trauma | 263 (3.9) | 276 (4.2) |
| Median baseline serum creatinine (IQR) — mg/dl | 0.84 (0.71–0.95) | 0.85 (0.71–0.94) |
| Source of baseline creatinine — no. (%) | ||
| Measured value in medical record | 4405 (65.7) | 4276 (64.4) |
| Calculated value by equation | 2303 (34.3) | 2363 (35.6) |
| Initial kidney function in ED | ||
| Serum creatinine | ||
| Mean — mg/dl | 1.32±1.42 | 1.31±1.36 |
| Median (IQR) — mg/dl | 0.93 (0.77–1.33) | 0.93 (0.77–1.32) |
| ≥1.5 mg/dl — no. (%) | 1246 (18.6) | 1240 (18.7) |
| End-stage renal disease with long-term renal-replacement therapy — no. (%) | 126 (1.9) | 109 (1.6) |
| Stage 2 or higher acute kidney injury — no./total no. (%)§ | 643/6582 (9.8) | 631/6530 (9.7) |
| Initial serum electrolytes in ED | ||
| Sodium — mmol/liter | 137.2±4.2 | 137.4±4.3 |
| Chloride — mmol/liter | 102.8±5.4 | 103.1±5.6 |
| Potassium — mmol/liter | 4.1±0.7 | 4.1±0.7 |
| Bicarbonate — mmol/liter | 22.7±3.8 | 22.8±3.7 |
| Blood urea nitrogen — mg/dl | 20±16 | 20±16 |
Plus–minus values are means ±SD. There were no significant differences in baseline characteristics between the two groups, except for initial serum sodium (P = 0.006) and chloride (P = 0.003). To convert the values for creatinine to micromoles per liter, multiply by 88.4. To convert the values for blood urea nitrogen to millimoles per liter, multiply by 0.357. ED denotes emergency department, and IQR interquartile range.
Race was reported by patients or their surrogates and recorded in the electronic health record as a part of routine clinical care.
The Elixhauser Comorbidity Index score summarizes the burden of a patient’s coexisting conditions. Scores range from −19 to 89, with higher scores indicating a profile of coexisting conditions that is more strongly associated with in-hospital death.24
Acute kidney injury was defined according to the Kidney Disease: Improving Global Outcomes creatinine criteria. Patients with end-stage renal disease who were receiving long-term renal-replacement therapy at the time of ED arrival were not eligible for the outcome of acute kidney injury.
CRYSTALLOID TREATMENT
Patients received a median crystalloid volume of 1079 ml (interquartile range, 1000 to 2000). Most balanced crystalloids were administered as lactated Ringer’s solution (95.3%), with a small percentage administered as Plasma-Lyte A (4.7%). Overall, 88.3% of the patients received only the assigned crystalloid in the emergency department with no use of off-protocol crystalloids. The volume of crystalloid that was administered and the adherence to crystalloid assignment were similar in the balanced-crystalloids and saline groups (Table 2, and Fig. S3 in the Supplementary Appendix).
Table 2.
Crystalloids Received in the Emergency Department According to Assigned Treatment Group.*
| Variable | Balanced Crystalloids (N = 6708) | Saline (N = 6639) |
|---|---|---|
| Total crystalloid volume | ||
| Mean — ml | 1608±1095 | 1597±1105 |
| Median (IQR) — ml | 1089 (1000–2000) | 1071 (1000–2000) |
| ≥2000 ml — no. (%) | 2207 (32.9) | 2150 (32.4) |
| Median volume of balanced crystalloids (IQR) — ml | 1000 (1000–2000) | 0 |
| Median volume of saline (IQR) — ml | 0 | 1000 (1000–2000) |
| Percentage of crystalloid volume consistent with assigned group — no. (%) | ||
| 100%: per-protocol population | 5620 (83.8) | 6160 (92.8) |
| 51–99% | 514 (7.7) | 270 (4.1) |
| 1–50% | 254 (3.8) | 131 (2.0) |
| 0% | 320 (4.8) | 78 (1.2) |
Plus–minus values are means ±SD. Percentages may not sum to 100 because of rounding.
SERUM ELECTROLYTE CONCENTRATIONS
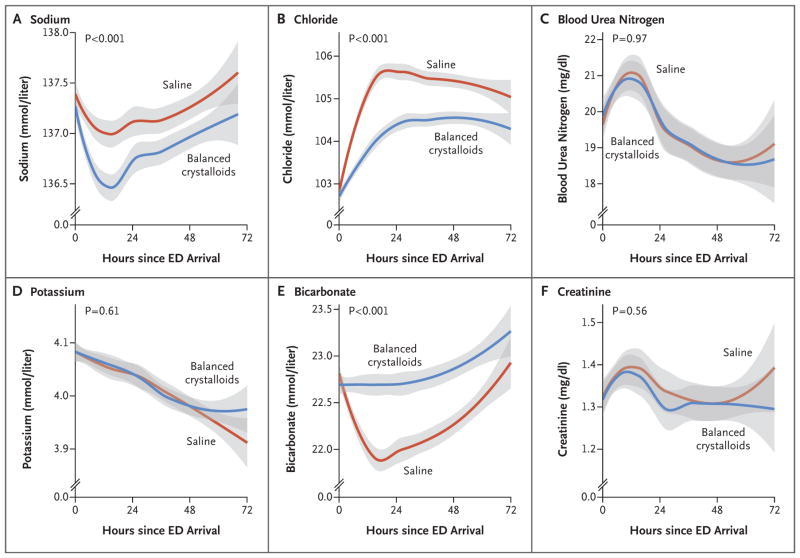
After treatment with intravenous fluids in the emergency department, patients in the balanced-crystalloids group had lower chloride and higher bicarbonate concentrations than those in the saline group; these differences persisted for several days into the hospitalization (Fig. 1). Hyperchloremia (serum chloride concentration, >110 mmol per liter) and acidemia (serum bicarbonate concentration, <20 mmol per liter) were less common after treatment with balanced crystalloids than with saline (Table S3 in the Supplementary Appendix).
Figure 1. Serum Electrolyte Concentrations in the First 72 Hours after Arrival in the Emergency Department (ED).
Lines and bands represent means and 95% confidence intervals, respectively. Plots were generated with the use of locally weighted scatterplot smoothing. The P values in the figure represent the overall difference between groups, calculated with the use of proportional-odds models. Over time, the separation between groups increased for chloride (P<0.001 for interaction) and bicarbonate (P<0.001 for interaction); interaction terms for the other variables were not significant. To convert the values for blood urea nitrogen to millimoles per liter, multiply by 0.357. To convert the values for creatinine to micromoles per liter, multiply by 88.4.
INTENTION-TO-TREAT ANALYSIS
There was no difference in the number of hospital-free days between patients in the balanced-crystalloids and saline groups (median, 25 days in each group; adjusted odds ratio with balanced crystalloids, 0.98; 95% confidence interval [CI], 0.92 to 1.04; P = 0.41) (Table 3, and Fig. S4 in the Supplementary Appendix). Patients in the balanced-crystalloids group had a lower incidence of major adverse kidney events within 30 days than those in the saline group (4.7% vs. 5.6%; adjusted odds ratio, 0.82; 95% CI, 0.70 to 0.95; P = 0.01). A lower count for each component of major adverse kidney events — death, renal-replacement therapy, and persistent renal dysfunction — in the balanced-crystalloids group contributed to the lower incidence of the composite outcome (Table 3, and Fig. S5 in the Supplementary Appendix). Stage 2 or higher acute kidney injury occurred in 8.0% of patients in the balanced-crystalloids group and 8.6% of patients in the saline group (adjusted odds ratio, 0.91; 95% CI, 0.80 to 1.03; P = 0.14). Other clinical outcomes did not differ significantly between the two groups (Table S3 in the Supplementary Appendix).
Table 3.
Clinical Outcomes According to Assigned Treatment Group in the Intention-to-Treat Analysis.
| Outcome | Balanced Crystalloids (N = 6708) | Saline (N = 6639) | Adjusted Odds Ratio (95% CI)* | Adjusted P Value |
|---|---|---|---|---|
| Median hospital-free days to day 28 (IQR) | 25 (22–26) | 25 (22–26) | 0.98 (0.92–1.04) | 0.41 |
| Major adverse kidney event within 30 days — no. (%) | 315 (4.7) | 370 (5.6) | 0.82 (0.70–0.95) | 0.01 |
| Death — no. (%) | 94 (1.4) | 102 (1.5) | 0.89 | |
| New renal-replacement therapy — no./total no. (%)† | 18/6582 (0.3) | 31/6530 (0.5) | 0.56 | |
| Final serum creatinine ≥200% of baseline — no./total no. (%)† | 253/6582 (3.8) | 293/6530 (4.5) | 0.84 | |
| Stage 2 or higher acute kidney injury — no./total no. (%)† | 528/6582 (8.0) | 560/6530 (8.6) | 0.91 (0.80–1.03) | 0.14 |
| In-hospital death — no. (%) | 95 (1.4) | 105 (1.6) | 0.88 (0.66–1.16) | 0.36 |
Multivariable models were adjusted for age, sex, race, admitting service, and time (days since trial initiation).
Patients with end-stage renal disease who were receiving long-term renal-replacement therapy at the time of emergency department arrival (126 in the balanced-crystalloids group and 109 in the saline group) were not eligible for the following outcomes: new renal-replacement therapy within 30 days, final serum creatinine concentration within 30 days at least 200% of the baseline value, and stage 2 or higher acute kidney injury.
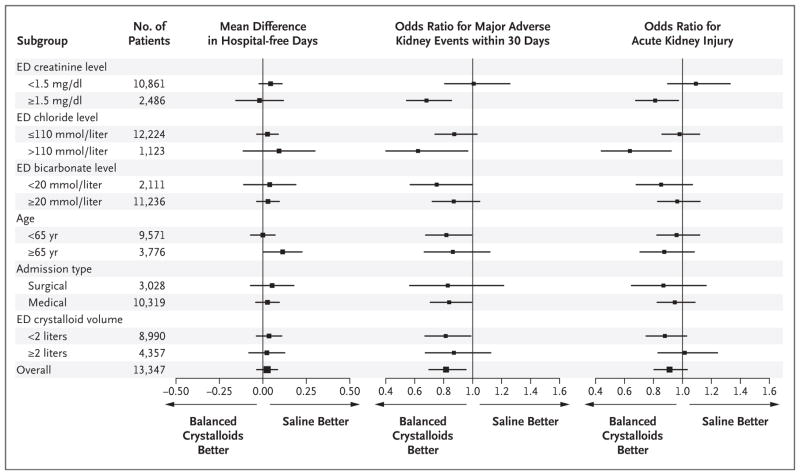
HETEROGENEITY OF TREATMENT EFFECT
Hospital-free days were similar for patients in the balanced-crystalloids and saline groups across a broad range of baseline characteristics (Fig. 2). Patients who presented to the emergency department with renal dysfunction (serum creatinine concentration, ≥1.5 mg per deciliter [133 μmol per liter]) or hyperchloremia (serum chloride concentration, >110 mmol per liter) appeared to have the largest benefit from balanced crystalloids for avoiding major adverse kidney events within 30 days and acute kidney injury. Among patients who presented to the emergency department meeting KDIGO criteria for stage 2 or higher acute kidney injury (1274 patients), resolution of acute kidney injury during hospitalization was more common with balanced crystalloids, as shown by a lower incidence of major adverse kidney events within 30 days in the balanced-crystalloids group (28.0%) than in the saline group (37.6%) (P<0.001).
Figure 2. Heterogeneity of Treatment Effect.
Shown are forest plots for hospital-free days to day 28, major adverse kidney events within 30 days, and acute kidney injury of stage 2 or higher according to Kidney Disease: Improving Global Outcomes creatinine criteria. The outcome of major adverse kidney events within 30 days was a composite of death from any cause, new renal-replacement therapy, or persistent renal dysfunction (defined as an elevation of the creatinine level to ≥200% of baseline) — all censored at hospital discharge or 30 days, whichever occurred first. Patients with end-stage renal disease who were receiving long-term renal-replacement therapy at the time of arrival in the emergency department (235 patients) were not eligible for the outcome of acute kidney injury; hence, the total sample size for the analysis of acute kidney injury was 13,112.
SENSITIVITY AND PER-PROTOCOL ANALYSES
Sensitivity analyses that were adjusted for period effect and that limited the trial population to patients without end-stage renal disease at presentation in the emergency department (13,112 patients), to patients with a measured baseline serum creatinine value (8681 patients), and to the first emergency department visit among unique patients in the trial (10,573 patients) all produced results similar to those of the primary analysis (Table S4 in the Supplementary Appendix). The per-protocol analysis (11,780 patients) also produced similar results (Tables S5 and S6 in the Supplementary Appendix).
DISCUSSION
In this pragmatic trial of noncritically ill adults treated with intravenous fluid in the emergency department, treatment with balanced crystalloids did not result in a shorter time to hospital discharge (hospital-free days) than treatment with saline but did result in a lower incidence of the composite of death, new renal-replacement therapy, and persistent renal dysfunction (major adverse kidney events within 30 days), which was a secondary outcome. The lower incidence of major adverse kidney events within 30 days in the balanced-crystalloids group is consistent with the results of SMART, which was conducted concurrently in critically ill adults.16
Patients in the present trial had lower risks of renal outcomes and death overall than critically ill adults requiring ICU admission.10,15,16,30 Despite these lower risks, there was an absolute difference of 0.9 percentage points in the risk of major adverse kidney events within 30 days in favor of the balanced-crystalloids group, corresponding to a number needed to treat of 111. Although this risk difference is modest for each patient, implications on a population level may be substantial owing to the millions of patients who receive isotonic crystalloids annually.1,19 Operationally, lactated Ringer’s solution and saline are similar in terms of cost, availability, and procedures for administration.2,31
A strength of our trial was high adherence to the assigned crystalloid group. Use of an unblinded, pragmatic design in a learning health care system32 facilitated incorporation of the trial into routine practice, allowing the assigned crystalloid to be systematically used for early fluid resuscitation immediately after arrival in the emergency department.
Limitations of the trial include its single-center setting, unblinded design, and outcome ascertainment that was limited to the index hospitalization. Owing to the pragmatic design that used data collection from the electronic medical record, more detailed information about patient characteristics was not available. In addition, crystalloids used for intravenous fluid therapy in the emergency department were included in the trial intervention, but fluids administered after hospital admission and those used as medication carriers were not controlled. Lactated Ringer’s solution represented more than 95% of the balanced crystalloids used in the trial; additional study is required to compare Plasma-Lyte A with both saline and lactated Ringer’s solution. Last, this trial evaluated balanced crystalloids versus saline as the routine, first-line isotonic fluid in a broad patient population; fluid selection that is tailored to specific patient characteristics is an alternative approach that was not evaluated in this trial.
In conclusion, in this pragmatic clinical trial involving noncritically ill adults treated with intravenous fluids in the emergency department, the number of hospital-free days, the primary outcome of the trial, did not differ between patients assigned to balanced crystalloids and those assigned to saline.
Supplementary Material
Acknowledgments
Funded by the Vanderbilt Institute for Clinical and Translational Research and others; SALT-ED ClinicalTrials.gov number, NCT02614040.
Supported by the Vanderbilt Institute for Clinical and Translational Research through a Clinical and Translational Science Award (UL1 TR000445) from the National Institutes of Health (NIH) National Center for Advancing Translational Sciences and by grants from the NIH National Institute of General Medical Sciences (K23GM110469, to Dr. Self), the NIH National Heart, Lung, and Blood Institute (HL08773809 and K12HL133117, to Dr. Semler; and R34HL105869, to Dr. Rice), the Vanderbilt Center for Kidney Disease (to Dr. Siew), and the Department of Veterans Affairs Health Services Research and Development Service (IIR 13-073, to Dr. Siew).
We thank the Vanderbilt University Medical Center Department of Emergency Medicine nurses, physicians, paramedics, pharmacists, and staff for their dedicated effort required to conduct this trial.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Dr. Self reports receiving advisory board fees from Venaxis, Cempra Pharmaceuticals, Ferring Pharmaceuticals, and Biotest, consulting fees from Abbott Point of Care, and travel support from Gilead Sciences; and Dr. Rice, receiving consulting fees from Cumberland Pharmaceuticals and Avisa Pharma. No other potential conflict of interest relevant to this article was reported.
References
- 1.Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369:1243–51. doi: 10.1056/NEJMra1208627. [DOI] [PubMed] [Google Scholar]
- 2.Semler MW, Rice TW. Saline is not the first choice for crystalloid resuscitation fluids. Crit Care Med. 2016;44:1541–4. doi: 10.1097/CCM.0000000000001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young P. Saline is the solution for crystalloid resuscitation. Crit Care Med. 2016;44:1538–40. doi: 10.1097/CCM.0000000000001844. [DOI] [PubMed] [Google Scholar]
- 4.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–35. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullivant EM, Wilcox CS, Welch WJ. Intrarenal vasoconstriction during hyperchloremia: role of thromboxane. Am J Physiol. 1989;256:F152–F157. doi: 10.1152/ajprenal.1989.256.1.F152. [DOI] [PubMed] [Google Scholar]
- 6.Kellum JA, Bellomo R, Kramer DJ, Pinsky MR. Etiology of metabolic acidosis during saline resuscitation in endotoxemia. Shock. 1998;9:364–8. doi: 10.1097/00024382-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Yunos NM, Kim IB, Bellomo R, et al. The biochemical effects of restricting chloride-rich fluids in intensive care. Crit Care Med. 2011;39:2419–24. doi: 10.1097/CCM.0b013e31822571e5. [DOI] [PubMed] [Google Scholar]
- 8.Zhou F, Peng ZY, Bishop JV, Cove ME, Singbartl K, Kellum JA. Effects of fluid resuscitation with 0. 9% saline versus a balanced electrolyte solution on acute kidney injury in a rat model of sepsis. Crit Care Med. 2014;42(4):e270–e278. doi: 10.1097/CCM.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0. 9% saline compared to Plasma-Lyte. Ann Surg. 2012;255:821–9. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 10.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–72. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 11.Yunos NM, Bellomo R, Glassford N, Sutcliffe H, Lam Q, Bailey M. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intensive Care Med. 2015;41:257–64. doi: 10.1007/s00134-014-3593-0. [DOI] [PubMed] [Google Scholar]
- 12.Raghunathan K, Shaw A, Nathanson B, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med. 2014;42:1585–91. doi: 10.1097/CCM.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 13.Shaw AD, Raghunathan K, Peyerl FW, Munson SH, Paluszkiewicz SM, Schermer CR. Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive Care Med. 2014;40:1897–905. doi: 10.1007/s00134-014-3505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rochwerg B, Alhazzani W, Sindi A, et al. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med. 2014;161:347–55. doi: 10.7326/M14-0178. [DOI] [PubMed] [Google Scholar]
- 15.Semler MW, Wanderer JP, Ehrenfeld JM, et al. Balanced crystalloids versus saline in the intensive care unit: the SALT randomized trial. Am J Respir Crit Care Med. 2017;195:1362–72. doi: 10.1164/rccm.201607-1345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378:829–39. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammond NE, Bellomo R, Gallagher M, et al. The Plasma-Lyte 148 v Saline (PLUS) study protocol: a multicentre, randomised controlled trial of the effect of intensive care fluid therapy on mortality. Crit Care Resusc. 2017;19:239–46. [PubMed] [Google Scholar]
- 18.Zampieri FG, Azevedo LCP, Corrêa TD, et al. Study protocol for the Balanced Solution versus Saline in Intensive Care Study (BaSICS): a factorial randomised trial. Crit Care Resusc. 2017;19:175–82. [PubMed] [Google Scholar]
- 19.Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373:1350–60. doi: 10.1056/NEJMra1412877. [DOI] [PubMed] [Google Scholar]
- 20.Self WH, Semler MW, Wanderer JP, et al. Saline versus balanced crystalloids for intravenous fluid therapy in the emergency department: study protocol for a cluster-randomized, multiple-crossover trial. Trials. 2017;18:178. doi: 10.1186/s13063-017-1923-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ware JH, Hamel MB. Pragmatic trials — guides to better patient care? N Engl J Med. 2011;364:1685–7. doi: 10.1056/NEJMp1103502. [DOI] [PubMed] [Google Scholar]
- 22.Semler MW, Rice TW, Shaw AD, et al. Identification of major adverse kidney events within the electronic health record. J Med Syst. 2016;40:167. doi: 10.1007/s10916-016-0528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upchurch CP, Grijalva CG, Russ S, et al. Comparison of etomidate and ketamine for induction during rapid sequence intubation of adult trauma patients. Ann Emerg Med. 2017;69(1):24–33. e2. doi: 10.1016/j.annemergmed.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–33. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 25.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–7. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Palevsky PM, Molitoris BA, Okusa MD, et al. Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol. 2012;7:844–50. doi: 10.2215/CJN.12791211. [DOI] [PubMed] [Google Scholar]
- 27.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(Suppl):1–138. [Google Scholar]
- 28.Závada J, Hoste E, Cartin-Ceba R, et al. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant. 2010;25:3911–8. doi: 10.1093/ndt/gfp766. [DOI] [PubMed] [Google Scholar]
- 29.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine — reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–94. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 30.Young P, Bailey M, Beasley R, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015;314:1701–10. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- 31.Laplante S, Makhija DU, Munson SH, et al. Impact of fluid choice in systemic inflammatory response syndrome patients on hospital cost savings. PharmacoEconomics Open. 2017 Sep 4; doi: 10.1007/s41669-017-0055-y. ( https://link.springer.com/article/10.1007/s41669-017-0055-y) [DOI] [PMC free article] [PubMed]
- 32.Califf RM, Robb MA, Bindman AB, et al. Transforming evidence generation to support health and health care decisions. N Engl J Med. 2016;375:2395–400. doi: 10.1056/NEJMsb1610128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.