Abstract
Back ground
Bronchial asthma is characterized by allergic airway inflammation involving C-C chemokine receptor type 4 (CCR4)-positive Th2 cells. As such, we hypothesize that the disease can be alleviated by targeted-elimination of CCR4+ cells. Thymus and activation-regulated chemokine (TARC)-PE38, a TARC fused the exotoxin fragment PE38 from Pseudomonas aeruginosa, has been shown to efficiently kill CCR4+ cells by delivering the exotoxin fragment PE38 into CCR4+ cells. To test our hypothesis, we examined whether TARC-PE38 could suppress allergic airway inflammation in a mouse model of house dust mite (HDM)-induced allergic airway inflammation.
Methods
We evaluated the effect of TARC-PE38 on the major characteristics of HDM-induced allergic airway inflammation. Airway hyperresponsiveness, lung histopathology, lung Th1/Th2 cell populations, and concentrations of Th1/Th2 cytokines in the lungs were assessed in HDM-sensitized and challenged mice in the presence and absence of TARC-PE38.
Results
TARC-PE38 efficiently suppressed allergic airway inflammation by significantly reducing airway hyperresponsiveness, the overall area of inflammation, and goblet cell hyperplasia. In HDM-sensitized and challenged mice, TARC-PE38 specifically reduced the numbers of CCR4+ cells. This reduction was associated with a significant decrease in the production of Th2 cytokines in the airway,and a decrease in the number of leukocytes, including macrophages, eosinophils and lymphocytes, within the subepithelial area of the lungs and airway lumen. TARC-PE38 had noeffect on Th1 cells.
Conclusion
Our data suggest that the elimination of CCR4+ cells via TARC-PE38 treatment is sufficient to control allergic airway inflammation and airway hyperresponsiveness.
Keywords: CCR4, TARC-PE38, Allergic inflammation, Airway hyperresponsiveness, Bronchial asthma
1. Introduction
Bronchial asthma is a chronic inflammatory disorder of the airways characterized by airway hyperresponsiveness, air-flow obstruction, and chronic inflammation. Chronic inflammation is associated with several types of inflammatory cells, including mast cells, lymphocytes, and eosinophils. Most importantly, Th2 lymphocytes play a critical role in the pathogenesis of bronchial asthma. Following antigen presentation by antigen-presenting cells, Th2 cells produce Th2-cytokines, including IL-4, IL-13, and IL-5, and promote eosinophilic inflammation. Recently, treatments that target IL-4 and IL-5 have shown potential as new therapies for asthma. Pitrakinra, a recombinant human IL-4 and IL-13 antagonist, competitively inhibits the binding IL-4 or IL-13 to the IL-4Rα receptor and improve pulmonary function after allergen challenge in asthmatic patients [1]. A variant human anti-IL-5 monoclonal antibody, mepolizumab, improved symptoms in refractory eosinophilic asthmatic patients [2]. Thus, regulating the action of Th2 cytokines or Th2 cells is a feasible therapeutic strategy to target the pathogenesis of bronchial asthma.
Th2 cells characteristically express C-C chemokine receptor type 4 (CCR4) [3]. Thymus and activation-regulated chemokine (TARC) [4], macrophage-derived chemokine (MDC) [5], and chemokine-like factor 1(CKLF1) [6] are known ligands of CCR4. CCR4-positive cells have been associated with inflammatory diseases. The numbers of CCR4-positive cells are increased in the skin of atopic dermatitis patients [7,8]. Th2 cells in the airways express CCR4 in atopic bronchial asthma patients after allergen challenge [9]. Furthermore, administration of a CCR4 blocking antibody abolished airway eosinophilia, goblet cell hyperplasia, and bronchial hyperreactivity in a humanized model of asthma [10]. Therefore, we focused on exploring molecular targeted drugs that could inhibit CCR4 function or reduce the numbers of CCR4-expressing cells, which could lead to improvement in asthma symptoms or exacerbations.
TARC-PE38 is a chemotoxin that is synthesized by the fusion of the chemokine TARC with the exotoxin fragment PE38 from Pseudomonas aeruginosa. It specifically binds to CCR4-expressing cells and once internalized, activation of the exotoxin fragment PE38 induces cell apoptosis and cell death [11]. TARC-PE38 effectively caused the depletion of human CCR4+ T-cell lymphoma cells [11] and successfully reuced breast cancer metastasis via depleting regulatory T cells [12]. Based on these results, TARC-PE38 may be effective for allergic airway inflammation by reducing the numbers of CCR4-expressing cells.
In this study, we examined whether TARC-PE38 affected allergic airway inflammation, airway hyperresponsiveness, and the production of Th1/Th2 cytokines in a mouse model of HDM-induced allergic airway inflammation.
2. Materials and methods
2.1. Preparation of house dust mite antigen
House dust mite antigen, Dermatophagoides pteronyssinus (Dp), was purchased from LSL (Tokyo, Japan). This extract included the major allergens, Der p 1 and Der p 2 and was proteolytically active. Endotoxin removal solution (Sigma-Aldrich, Japan) was used to reduce the endotoxin concentration, after which Dp endotoxin was below 0.5 IU/mg [13].
2.2. Mouse protocols
Five-week-old female BALB/c mice were purchased from CLEA Japan, Inc. (Tokyo, Japan). Mice were maintained in the animal facility at the University of Tokushima under specific pathogen-free conditions according to the guidelines of the university.
The study was approved by the ethics committee of the animal facility of the University of Tokushima (approval number: Toku-doubutu07104; approval date: 22/08/2007).
All mice were sensitized on days 0 and 7 by intraperitoneal injection of Dp mixed with 1 mg of alum (Pierce, Rockford, IL). On days 15, 16, and 17, the mice were challenged with intranasal administration of 10 μg of Dp in 80 μl saline or with saline alone.
2.3. TARC-PE38 treatment
TARC-PE38 was kindly provided by Dr. Arya Biragyn (National Institute on Aging, Baltimore, MD, USA). TARC-PE38 (10 μg) was dissolved in 200 μl of saline. Our treatment experiments included 4 groups of mice (Fig. 1A). In the first group, TARC-PE38 was administered intravenously to Dp-sensitized/challenged mice for 2 days on days 14 and 16 (Dp/Dp/TP38iv2 group). In a second group, TARC-PE38 was administered for 4 days from days 14 to 17 (Dp/Dp/TP38iv4 group). Saline was administered intravenously for 4 days from days 14 to 17 in control mice (Dp/Sal/Sal group) and Dp sensitized/challenged mice (Dp/Dp/Sal group). Mice were sacrificed on day 18 and bronchoalveolar lavage was performed [14]. The lungs were harvested for histopathology and lung homogenization [14].
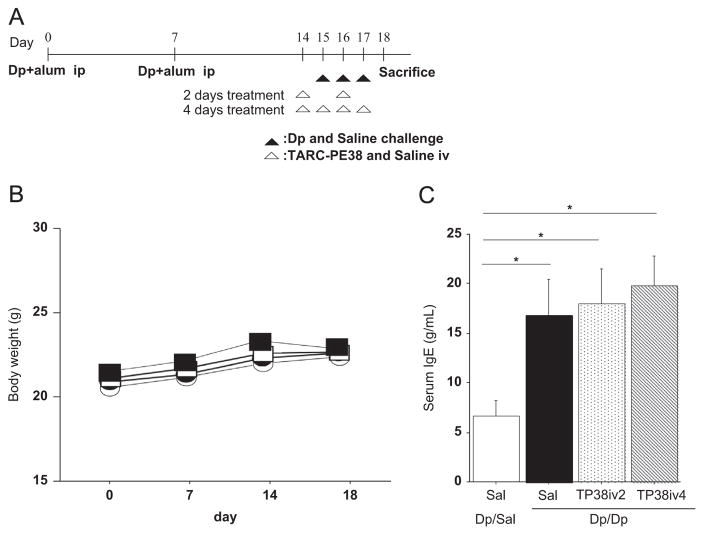
Fig. 1.
(A) Mice protocols. (B) Body weight. (C) Serum IgE levels. Results are means±SE from 3 independent experiments. Each experiment used 4 mice per group. *: p<0.05 compared to control group. †: p<0.05 compared to Dp/Dp/Sal group. Open circles: Dp/Sal/Sal group (control). Closed circles: Dp/Dp/Sal group. Open squares: Dp/TP38iv2 group. Closed squares: Dp/TP38iv4 group.
2.4. Measurement of airway resistance
Lung resistance (RL) was determined by restrained whole-body plethysmography (Buxco Electronics, Troy, NY). On day 18, mice were anesthetized with 6 mg/mL of 2,2,2-tribromoethanol (Sigma, Tokyo, Japan) with 2-methyl-2-butanol (Sigma). After mice were tracheostomized and cannulated with a 19G tracheostomy tube, they were placed in the chamber for plethysmography and mechanically ventilated. Aerosolized methacholine (Mch) was administered for 10 s using an in-line aerosol delivery system with different Mch concentrations (6.25, 12.5, 25 and 37.5 mg/mL). After each Mch challenge, data were continuously collected for 3 min. RL values were calculated to determine changes in functional parameters. Results were expressed as percentages of the baseline RL. The concentration of Mch required to produce a 200% increase in RL, PC200, was calculated by linear interpolation of the dose–response curves [13].
2.5. Histopathology and immunohistochemistry
Following sacrifice, lung tissue was harvested, fixed in 10% formalin, and embedded in paraffin. Tissue sections (3 μm thick) were stained with hematoxylin and eosin (H&E) and Periodic acid Schiff (PAS) stain. The areas with infiltrating inflammatory cells around the bronchus and the percentage of PAS-positive cells in the bronchial epithelium were measured using an Olympus BX61 microscope (Tokyo, Japan) with Scion Image software (National Institution of Health). To assess infiltrating eosinophils in the subepithelium, the number of eosinophils was counted in a section stained with Luna-modified stain [14]. The data of inflammatory area and numbers of eosinophils were adjusted by the length of the basement membrane of the target bronchus.
For immunohistochemistry, paraffin sections (3 μm thick) were heated in a microwave oven for 10 min for antigen retrieval. CSA II kit (Dako Japan, Tokyo, Japan) and primary antibodies against CCR4 (Capralogics, Inc. Hardwick, MA, USA), CCR5, CCR3 and CD68 (Abcam, Cambridge, MA, USA) were used according to the manufacturer’s instructions.
2.6. Total protein and cytokine concentrations
Protein concentrations were determined by the BCA method using bovine serum albumin (BSA) standards. Interleukin (IL)-4, 5, 12, 13, interferon (IFN)-γ, and eotaxin were determined with commercial ELISA kits (R&D Systems, Minneapolis, MN). Cytokine concentrations in supernatant of lung homogenate were adjusted by total protein of whole lungs. Serum IgE levels were determined using a commercial ELISA kit (Morinaga, Tokyo, Japan). The sensitivity limits for the ELISA kits were: IL-4= 2 pg/mL; IL-5=7 pg/mL; IL-12 (p70)=4 pg/mL; IL-13=1.5 pg/mL; IFN-γ=2 pg/mL; eotaxin=3 pg/mL and serum IgE=0.5 μg/mL.
2.7. Flow cytometric analysis
Cells in Bronchial alveolar lavage fluid (BALF) cells were stained with PE-labeled anti-mouse CCR4 (BioLegend, San Diego, CA, USA) and FITC-labeled anti-mouse CD4 (BD Pharmingen, San Diego, CA, USA) antibodies for 30 min. Flow cytometric analysis was performed using a FACS Calibur, Cell Quest pro (BD Biosciences San Jose, CA, USA). FITC-conjugated or PE-conjugated immunoglobulins were used as controls.
2.8. Statistical analysis
Data analysis was performed using the Stat-View 5.0 software (Abacus Concept, Inc., Berkeley, CA). Results are presented as means±SEs. Results from different experimental groups were compared using one way analysis of variance (ANOVA). If significant differences were found, the Tukey–Kramer test was used to correct for multiple comparisons. Differences were considered statistically significant for p-values ≤0.05.
3. Results
3.1. Body weight changes after TARC-PE38 administration
We previously demonstrated that TARC-PE38 only specifically killed CCR4-expressing cells in vivo [11,12,15]. To rule out any possible adverse events due to the systemic use of TARC-PE38, we monitored the changes in body weight for the 4 groups. Body weight was measured on days 0, 7, 14, and 18. During the experimental period, no significant difference in body weight among the 4 groups was observed (Fig. 1B), suggesting the absence of obvious signs of adverse events.
3.2. Effect of TARC-PE38 on serum IgE levels
In our model, Dp sensitization and challenge resulted in increase of serum IgE levels [14]. To examine the effects of TARC-PE38 on IgE production, serum IgE levels were measured by ELISA. Dp challenge caused an increase in serum IgE levels in Dp-sensitized mice (Fig. 1C). However, TARC-PE38 treatment did not affect serum IgE levels (Fig. 1C).
3.3. Effect of TARC-PE38 on allergy-induced airway hyperresponsiveness
To examine the effects of TARC-PE38 on airway hyperresponsiveness, we assessed RL values by restrained plethysmography as described in Section 2.
The RL values for the Dp/Dp/Sal group were significantly increased at methacholine (Mch) doses of 12.5–37.5 mg/mL compared to those of Dp/Sal/Sal group. However, the RL values were significantly lower in mice treated with TARC-PE38 for 4 days compared to that observed in the Dp/Dp/Sal group at a Mch dose of 12.5 mg/mL and higher doses (Fig. 2A). The PC200 values of the Dp/Dp/Sal group were also substantially reversed by treatment with TARC-PE38. In particular, after 4 injections of TARC-PE38, the PC200 values were significantly higher than that observed in the Dp/Dp/Sal group (Fig. 2B).
Fig. 2.
Effects of TARC-PE38 on the physiological status of mouse in the HDM mouse model of allergic airway inflammation. (A) Lung resistance. (B) Log PC200. Results are means±SE from 3 independent experiments. Each experiment used 4 mice per group. *: p<0.05 compared to Dp/Sal/Sal group (control); †: p<0.05 compared to Dp/Dp/Sal group. Open circles and white bar: Dp/Sal/Sal group (control). Closed circles and black bar: Dp/Dp/Sal group. Open squares and dotted bar: Dp/Dp/TP38iv2 group. Closed squares and shaded bar: Dp/Dp/TP38iv4 group.
3.4. Effect of TARC-PE38 on pathological inflammation in lungs
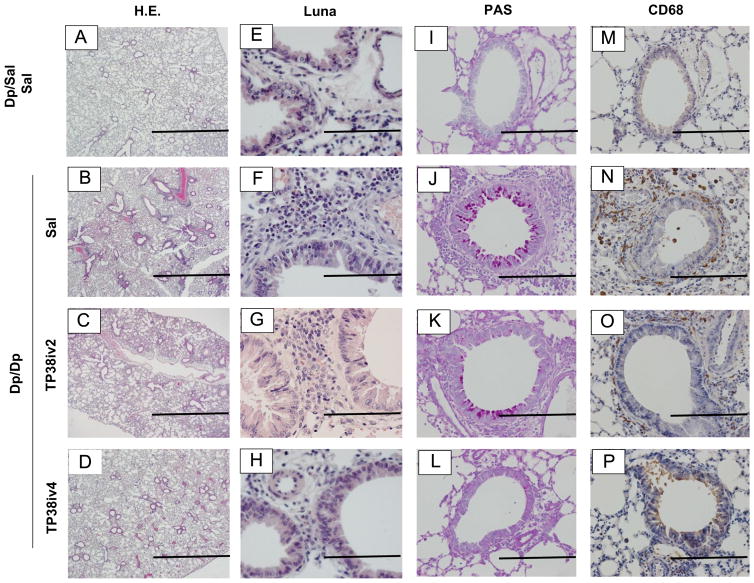
We assessed airway inflammation based on the area of inflammation, eosinophils, goblet cells (PAS+ cells) and CD68+ cells observed within the airway (Fig. 3A–P). Dp/Dp/Sal group showed marked infiltration of inflammatory cells, eosinophils and CD68+ cells around the bronchus compared to Dp/Sal/Sal group (Fig. 3A, B, E, F, M, N and Table 1). TARC-PE38 treatment, in particular after 4 injections, significantly reduced all of these Dp challenge-derived morphological changes (Fig. 3C, D, G, H, K, L, O, P and Table 1). Goblet cells among bronchial epithelial cells were increased by Dp challenge (Fig. 3I and J). The percentages of PAS positive cells in the Dp/Dp/Sal and Dp/Dp/Tp38iv2 groups were significantly higher than that in the Dp/Sal/Sal group (Table 1). However, there was no difference in the number of PAS positive cells between the Dp/Sal/Sal and Dp/Dp/TP38iv4 groups (Table 1).
Fig. 3.
Effects of TARC-PE38 on the hallmarks of allergic airway inflammation in the HDM mouse model of allergic airway inflammation. A–D: H&E sections (original magnification: ×40; bars=1 mm). E–H: Luna-modified method sections (original magnification: ×400; bars=100 μm). I–L: PAS stained sections (original magnification: ×200; bars=200 μm). M–P: CD68 immunohistochemical sections (original magnification: ×200; bars=200 μm). Results are representative of 3 experiments. Each experiment used 4 mice per group.
Table 1.
Morphological analysis.
| Group | Area of inflammation μm2/mm | Eosinophils No./mm | PAS+ cells % | CD68+ cells No./HPF |
|---|---|---|---|---|
| Dp/Sal/Sal | ND | ND | ND | 4.8±1.3 |
| Dp/Dp/Sal | 11456.3±1276.8a | 28.4±2.8a | 38.6±8.3a | 22.6±3.7a |
| Dp/Dp/TP38iv2 | 8602.4±1057.3a | 37.3±5.2a | 26.7±7.6a | 11.4±4.4b |
| Dp/Dp/TP38iv4 | 6309.0±783.5ab | 17.1±2.1a,b | 21.2±3.5 | 9.1±1.3b |
Morphological analysis was performed using histology of all groups described in Section 2. Results are means±SE from 3 independent experiments. Each experiment used 4 mice per group. ND: not detected.
p<0.05 compared to control group.
p<0.05 compared to Dp/Dp/Sal group.
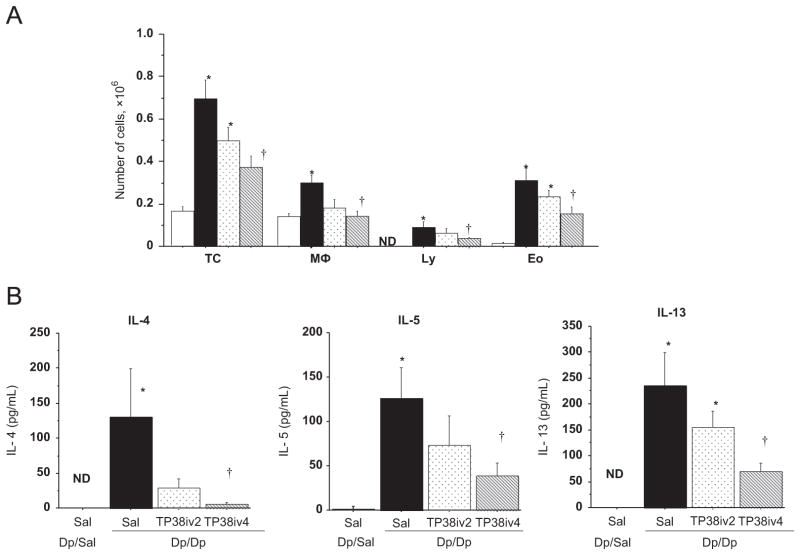
In BALF, the total number of cells (TC), macrophages (Mϕ), lymphocytes (Ly), and eosinophils (Eo) were significantly increased in BALF of the Dp/Dp/Sal group compared to the those in the BALF of Dp/Sal/Sal group (Fig. 4A). TARC-PE38 treatment for 4 days significantly reduced the numbers of all these cell types (Fig. 4A). Treatment with TARC-PE38 for 2 days was less effective and did not result in a significant decrease in the numbers of all cell types (Fig. 4A).
Fig. 4.
Effect of TARC-PE38 on BALF cells and Th2 cytokine concentration in BALF in the HDM mouse model of allergic airway inflammation. (A) BALF cell analysis. TC: total cells; Mφ: macrophages; Ly: lymphocytes; Eo: eosinophils. (B) Th2 cytokine concentrations in BALF. Results are means±SE from 3 independent experiments. Each experiment used 4 mice per group. *: p<0.05 compared to Dp/Sal/Sal group (control). †: p<0.05 compared to Dp/Dp/Sal group. White bars: Dp/Sal/Sal group (control). Black bars: Dp/Dp/Sal group. Dotted bars: Dp/Dp/TP38iv2 group. Shaded bars: Dp/Dp/TP38iv4 group.
3.5. TARC-PE38 decreases Th2 cytokine production in the inflamed lungs
Airway inflammation is associated with a pronounced production of Th2-type cytokines expressed from CCR4+ T cells. Thus, to test if TARC-PE38 could block their production by eliminating CCR4+ T cells, we measured cytokine concentrations in BALF. In Dp/Dp/Sal mice, we observed significantly increased amounts of IL-4, IL-5, and IL-13 as compared to Dp/Sal/Sal mice. However, the levels of these Th2-type cytokines was significantly reduced in Dp/Dp/TP38iv4 mice (Fig. 4B), suggesting that TARC-PE38 indeed lowered their production. As expected, TARC-PE38 treatment for 2 days also reduced the concentrations of these Th2 cytokine, although to a lesser degree (Fig. 4B). We did not detect eotaxin, IL-12, or IFN-γ in the BALF of any of the mice (data not shown).
To further confirm these results, we also examined cytokine concentrations in lung homogenates (Fig. 5). Enhanced levels of IL-4, IL-5, and IL-13 in the lung homogenates from the Dp/Dp/Sal group were significantly reduced by TARC-PE38 treatment (Fig. 5). Dp/Dp/Sal mice had higher levels of eotaxin than those observed in the Dp/Sal/Sal group. However, there was no significant difference in the eotaxin concentration observed in the Dp/Dp/Sal, Dp/Dp/TP38iv2, and Dp/Dp/TP38iv4 groups (Fig. 5). Although Dp challenge did not affect the levels of Th1 cytokines, including IL-12 and IFN-γ, TARC-PE38 treatment did appear to decrease IFN-γ production without affecting IL-12 (Fig. 5).
Fig. 5.
Effects of TARC-PE38 on cytokine concentrations in lung tissue homogenates in the HDM mouse model of allergic airway inflammation. Results are means±SE from 3 independent experiments. Each experiment used 4mice per group. *: p<0.05 compared to Dp/Sal/Sal group (control). †: p<0.05 compared to Dp/Dp/Sal group. White bars: Dp/Sal/Sal group (control). Black bars: Dp/Dp/Sal group. Dotted bars: Dp/Dp/TP38iv2 group. Shaded bars: Dp/Dp/TP38iv4 group.
3.6. Effect of TARC-PE38 on CCR4, CCR5, and CCR3-expressing cells
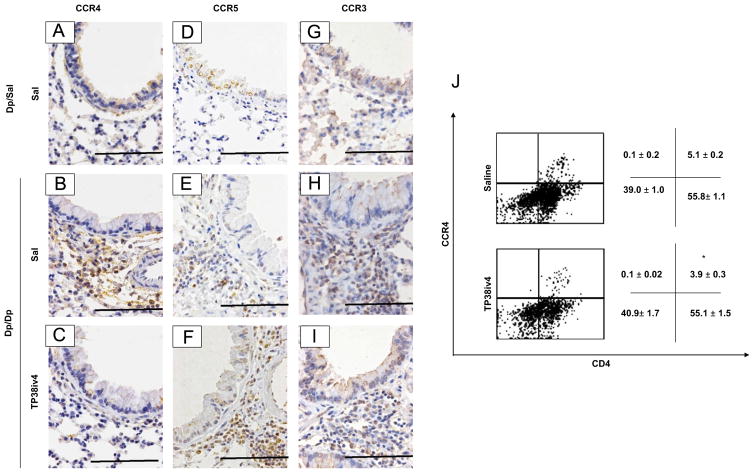
Since TARC-PE38 specifically eliminates CCR4-expressing cells in vivo [11,12,15], we tested whether the reduction in airway inflammation was due to decreased infiltration of CCR4+ cells using immunohistochemical staining and flow cytometric analysis. Airway inflammation in the Dp/Dp/Sal group was associated with increased infiltration of CCR4, CCR5, and CCR3-expressing cells (Fig. 6A, B, D, E, G and H). As expected, TARC-PE38 treatment decreased the numbers of CCR4-expressing cells in the lung, while the presence of CCR5-expressing cells was not affected (Fig. 6C and F). The levels of CCR3+ polymorphonuclear cells and CCR3+ small mononuclear cells were decreased by TARC-PE38 treatment, however some CCR3+ cells remained in inflammatory sites around the bronchus (Fig. 6I).
Fig. 6.
Effects of TARC-PE38 on CCR4+, CCR5+ and CCR3+ cells in the HDM mouse model of allergic airway inflammation. (A–C) Immunohistochemistry for CCR4+ cells (original magnification: ×400; bars=100 μm). (D–F) Immunohistochemistry for CCR5+ cells (original magnification: ×400; bars=100 μm). (G–I) Immunohistochemistry forCCR3+ cells (original magnification: ×400; bars=100 μm). Results are representative of 3 experiments. (J) Flow cytometric analysis of CD4+CCR4+ cells in BALF. Results are representative of 3 experiments. The numbers are means±SE for the percentages of cells obtained by flow cytometry quadrant analysis from 3 independent experiments. *: p<0.05 compared to Dp/Dp/Sal group.
Using flow cytometric analysis, we detected significant reduction of CD4+CCR4+cells in the Dp/Dp/Tp38iv4 group (3.9±0.3%) compared to the Dp/Dp/Sal group (5.1±0.2%; Fig. 6G).
4. Discussion
In this study, we demonstrated that TARC-PE38 administration effectively reduced airway hyperresponsiveness, allergic airway inflammation, and Th2 cytokine production in a mouse model of HDM-induced allergic airway inflammation.
CCR4-expressing Th2 cells play a critical role in the pathogenesis of bronchial asthma by producing Th2 cytokines. CCR4-positive T cells in the serum are increased in proportion to the severity of asthma [16]. IL-4-expressing blood and BALF T cells also express significant levels of CCR3 and CCR4 [17]. IL-4 and IL-13 also activate eosinophils, contribute to the development of airway hyperresponsiveness, increase mucus cell hyperplasia (as an index of airway remodeling), and induce the expression of TARC by murine macrophages, inducing allergic inflammation [18].
Thus, the management of CCR4-expressing Th2 cells may be an attractive therapeutic strategy for the treatment of bronchial asthma. From this point of view, a novel chemotoxin, TARC-PE38, may be useful for specifically eliminating CCR4 positive cells. TARC is a strongly binding ligand for CCR4 and a chemoattractant for CCR4-expressing cells [4,19,20], and as such, TARC-PE38 delivers the PE38 toxin directly CCR4-expressing cells. TARC-PE38 binds to cell surface CCR4 and is internalized into the cytosol [11]. The exotoxin, PE38 appears to be non-toxic, unless delivered into the cell cytosol [11]. Internalization of TARC-PE38 by CCR4-expressing human T-cell lymphoma cells, TARC-PE38 induced their early apoptosis and increased cell death [11]. In the present study, CCR4-expressing cells and Th2 cytokine production were decreased after the TARC-PE38 administration. These previous reports and our present findings suggest that TARC-PE-mediated killing of CCR4+ cells reverses the enhanced production of Th2 cytokines in the lungs, leading to suppression of allergic airway inflammation. This is the first report to demonstrate that TARC-PE38 could be effective for managing bronchial asthma by reducing the numbers of Th2 cells.
Interestingly, in addition to decrease allergen induced eosinophilia and Th2 cell infiltration into airway, we also demonstrated that macrophage infiltration and IFN-γ production were decreased by TARC-PE38 treatment in our model of allergic airway inflammation. Importantly, CCR4 is expressed on the macrophage cell surface. Th2-mediated responses differentiated macrophages into alternative alveolar macrophages (AAM) which are characterized by the expression of mannose receptors, arginase, chitinases and fibrotic factors as well as CD68 [21]. AAMs stimulate the migration of Th2 memory cells and eosinophils, leading to Th2 inflammatory environment within the lung [21]. In addition, CCR4 is expressed by invariant natural killer T (iNKT) cells [22]. iNKT cells, which have an invariant TCR repertoire, recognize bacterial and endogenous glycolipid antigens presented by CD1d [23]. When activated, iNKT cells rapidly produce large amounts of cytokines, including IL-4 and IFN-γ [23]. Thus, it is likely that depleting iNKT cells, via TARC-PE38 treatment causes a reduction in IFN-γ production. In a NKT-knockout mouse model airway hyperresponsiveness failed to develop and was reversed by the adoptive transfer of NKT cells [24]. iNKT cells require CCR4 in order to localize to the airways and induce airway hyperreactivity [25]. Although we did not assess NKT cells in our study, as their numbers were quite low in our model, it is likely that inhibition of other CCR4+ cells, including macrophages and NKT cells, synergistically contributed to the observed inhibitory effects of TARC-PE38.
Interestingly, TARC-PE38 administration affected neither the serum IgE levels nor eotaxin levels in the lungs in our model. Moreover, CCR3+ cells were present within inflammatory sites around the bronchus following TARC-PE38 treatment. CCR3+ and CCR4− cells, including mast cells and basophils play critical roles in the response to parasite infection. The present findings suggest that protective effects provided by these cells against parasite infection is not completely inhibited by TARC-PE38. In addition, since CCR4+ depletion reduced the eosinophils and macrophages that are known to be involved in the pathogenesis of airway remodeling, TARC-PE38 may be effective for managing chronic asthma symptoms, including airway remodeling. Our findings that TARC-PE38 inhibited goblet cells hyperplasia support this hypothesis. Taken together, additional studies on TARC-PE38-associated adverse events and its effects on airway remodeling will be necessary to establish whether this agent may be a useful and safe therapy for targeting asthma and allergic airway disease.
In summary, we demonstrated that TARC-PE38 could effectively control allergic airway inflammation by selectively depleting CCR4+ cells. These findings suggest that depletion of CCR4+ cells using TARC-PE38 may be a useful strategy to manage allergic airway inflammation.
Acknowledgments
We thank Mrs. Megumi Kume and Miss Hitomi Umemoto (Department of Molecular and Environmental Pathology, Institute of Health Biosciences, the University of Tokushima Graduate School) for preparing the histological sections.
Abbreviations
- CCR4
C-C chemokine receptor type 4
- TARC
thymus and activation-regulated chemokine
- HDM
house dust mite
- MDC
macrophage-derived chemokine
- CKLF1
chemokine-like factor 1
- Dp
Dermatophagoides pteronyssinus
- RL
Lung resistance
- Mch
methacholine
- H&E
hematoxylin and eosin
- PAS
periodic acid Schiff
- IFN
interferon
- BSA
bovine serum albumin
- BALF
bronchial alveolar lavage fluid
Footnotes
Conflict of interest
The authors have no conflicts of interest.
References
- 1.Antoniu SA. Pitrakinra, a dual IL-4/IL-13 antagonist for the potential treatment of asthma and eczema. Curr Opin Invest Drugs. 2010;11:1286–94. [PubMed] [Google Scholar]
- 2.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai T, Baba M, Nishimura M, et al. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997;272:15036–42. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- 5.Imai T, Chantry D, Raport CJ, et al. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem. 1998;273:1764–8. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Zhang Y, Yang X, et al. Chemokine-like factor 1 is a functional ligand for CC chemokine receptor 4 (CCR4) Life Sci. 2006;78:614–21. doi: 10.1016/j.lfs.2005.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakatani T, Kaburagi Y, Shimada Y, et al. CCR4 memory CD4+ T lymphocytes are increased in peripheral blood and lesional skin from patients with atopic dermatitis. J Allergy Clin Immunol. 2001;107:353–8. doi: 10.1067/mai.2001.112601. [DOI] [PubMed] [Google Scholar]
- 8.Wakugawa M, Nakamura K, Kakinuma T, et al. CC chemokine receptor 4 expression on peripheral blood CD4+ T cells reflects disease activity of atopic dermatitis. J Invest Dermatol. 2001;117:188–96. doi: 10.1046/j.0022-202x.2001.01430.x. [DOI] [PubMed] [Google Scholar]
- 9.Panina-Bordignon P, Papi A, Mariani M, et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107:1357–64. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perros F, Hoogsteden HC, Coyle AJ, et al. Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy. 2009;64:995–1002. doi: 10.1111/j.1398-9995.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- 11.Baatar D, Olkhanud P, Newton D, et al. CCR4-expressing T cell tumors can be specifically controlled via delivery of toxins to chemokine receptors. J Immunol. 2007;179:1996–2004. doi: 10.4049/jimmunol.179.3.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olkhanud PB, Baatar D, Bodogai M, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa H, Azuma M, Muto S, et al. IkappaB kinase beta inhibitor IMD-0354 suppresses airway remodelling in a dermatophagoides pteronyssinus-sensitized mouse model of chronic asthma. Clin Exp Allergy. 2011;41:104–15. doi: 10.1111/j.1365-2222.2010.03564.x. [DOI] [PubMed] [Google Scholar]
- 14.Sugita A, Ogawa H, Azuma M, et al. Antiallergic and anti-inflammatory effects of a novel I kappaB kinase beta inhibitor, IMD-0354, in a mouse model of allergic inflammation. Int Arch Allergy Immunol. 2009;148:186–98. doi: 10.1159/000161579. [DOI] [PubMed] [Google Scholar]
- 15.Baatar D, Olkhanud P, Sumitomo K, et al. Human peripheral blood T regulatory cells (Tregs), functionally primed CCR4+ Tregs and unprimed CCR4− Tregs, regulate effector T cells using FasL. J Immunol. 2007;178:4891–900. doi: 10.4049/jimmunol.178.8.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurashima K, Fujimura M, Myou S, et al. Asthma severity is associated with an increase in both blood CXCR3+ and CCR4+ T cells. Respirology. 2006;11:152–7. doi: 10.1111/j.1440-1843.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- 17.Morgan AJ, Symon FA, Berry MA, et al. IL-4-expressing bronchoalveolar T cells from asthmatic and healthy subjects preferentially express CCR 3 and CCR 4. J Allergy Clin Immunol. 2005;116:594–600. doi: 10.1016/j.jaci.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 18.Liddiard K, Welch JS, Lozach J, et al. Interleukin-4 induction of the CC chemokine TARC (CCL17) in murine macrophages is mediated by multiple STAT6 sites in the TARC gene promoter. BMC Mol Biol. 2006;7:45. doi: 10.1186/1471-2199-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikhak Z, Fukui M, Farsidjani A, et al. Contribution of CCR4 and CCR8 to antigen-specific T(H)2 cell trafficking in allergic pulmonary inflammation. J Allergy Clin Immunol. 2009;123:67–73. e3. doi: 10.1016/j.jaci.2008.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsunemi Y, Saeki H, Nakamura K, et al. CCL17 transgenic mice show an enhanced Th2-type response to both allergic and non-allergic stimuli. Eur J Immunol. 2006;36:2116–27. doi: 10.1002/eji.200535564. [DOI] [PubMed] [Google Scholar]
- 21.Dasgupta P, Keegan AD. Contribution of alternatively activated macrophages to allergic lung inflammation: a tale of mice and men. J Innate Immun. 2012;4:478–88. doi: 10.1159/000336025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehner T. Special regulatory T cell review: the resurgence of the concept of contrasuppression in immunoregulation. Immunology. 2008;123:40–4. doi: 10.1111/j.1365-2567.2007.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taniguchi M, Harada M, Kojo S, et al. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 24.Akbari O, Stock P, Meyer E, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–8. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 25.Meyer EH, Wurbel MA, Staton TL, et al. iNKT cells require CCR4 to localize to the airways and to induce airway hyperreactivity. J Immunol. 2007;179:4661–71. doi: 10.4049/jimmunol.179.7.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]