Abstract
CD4+ follicular regulatory T cells (Tfr cells) suppress B cell responses through modulation of follicular helper T cells (Tfh cells) and germinal center (GC) development. We found that Tfr cells also can promote the GC response through provision of IL-10 following acute infection with lymphocytic choriomeningitis virus (LCMV). Sensing of IL-10 by B cells was necessary for optimal development of the GC response. GC B cells formed in the absence of Treg-cell derived IL-10 displayed an altered dark zone state and decreased expression of the transcription factor FOXO1. IL-10 promoted nuclear translocation of FOXO1 in activated B cells. These data indicate that Tfr cells play a multifaceted role in the fine-tuning of the GC response and identify IL-10 as an important mediator by which Tfr cells support the GC reaction.
INTRODUCTION
The germinal center (GC) response is essential for the production of memory B cells and class-switched, long-lived plasma cells that produce high-affinity antibodies, underlying the immunity induced by effective vaccines (1). Understanding the mechanisms regulating the GC response is of interest because of the potential to harness this knowledge to bolster or, in the cases of autoimmunity and B cell lymphomas, restrain the GC reaction (2). Follicular helper T (Tfh) cells are a specialized subset of effector CD4+ T cells that express the transcription factors Bcl6 and Ascl2, and the B cell follicle-homing chemokine receptor CXCR5, and reside within the GC (3, 4). Tfh cells regulate the GC response through secretion of cytokines [e.g., interleukin-21 (IL-21), IL-4, and interferon-γ (IFN-γ)] and expression of surface ligands such as CD40L that signal to GC B cells and promote their maturation (5). Competition for Tfh cell help regulates B cell selection within the GC because B cells, which present the most antigen, preferentially interact with Tfh cells and receive signals necessary to promote the further proliferation and somatic hypermutation of their immunoglobulin (Ig) genes (6, 7).
A subset of effector Foxp3+ regulatory CD4+ T (Treg) cells that express CXCR5 and Bcl6 were recently described (8–10). These cells, known as follicular regulatory T (Tfr) cells, originate from thymic-derived Foxp3+ cells or naive cells and reside within the follicles and GC in mice and humans where they serve to modulate the magnitude and quality of the GC and Tfh cell responses (8–13). Tfr cells express the inhibitory co-receptor CTLA4, which is essential for their restraint of the GC response (14, 15). CTLA4 suppresses the latter response by modulating B cell expression of B7-2 (CD86) outside GCs (15), or regulating GC cells, either dependently or independently of B7-1 (CD80) and B7-2 (14–16). It may also function to control Tfh-cell generation directly by altering CD28 engagement (17). Although Tfr cells deficient in CTLA4 have impaired suppressive ability in vivo, it is likely that both Tfr cells and follicular nonresident Treg cells act through CTLA4 to restrain the GC response (14, 15).
Tfr cells may also modulate the GC response through pathways independent of CTLA4. Treg cells regulate immune cells through control of IL-2 availability, surface expression of ectoenzymes (CD39 and CD73), inhibitory receptors (for example, CTLA4 and Lag3), and secretion of cytokines such as IL-10, IL-35, and TGFβ (18). During influenza infection, Treg cells indirectly promote Tfh-cell differentiation and subsequently the GC response by limiting T cell exposure to IL-2 (19). IL-2 signaling through STAT5 and via Akt and mechanistic target of rapamycin (mTOR) can impair Tfh cell differentiation by suppressing Bcl6 expression and upregulating that of Blimp1 (20). Whether Tfr cells may directly regulate the GC response through similar mechanisms remains ill-defined..
Treg cell production of IL-10 is essential for regulation of inflammation at environmental interfaces such as the colon and lung, and can enhance the development of functional memory CD8+ T cells during acute lymphocytic choriomeningitis virus (LCMV) infection through suppression of inflammation in the spleen (21–23). IL-10 can also promote B cell proliferation, survival, and differentiation into antibody-secreting plasma cells (24–28), although it is not known if Tfr cell-derived IL-10 can modulate the GC response. We demonstrate herein that indeed it does during acute viral infection. These findings reveal that Tfr cells play a multifaceted role in the fine-tuning of the GC response, and are capable of promoting or suppressing the GC response depending on the signals provided and the context in which these signals are received.
RESULTS
Treg cell-derived IL-10 promotes B cell differentiation and GC development
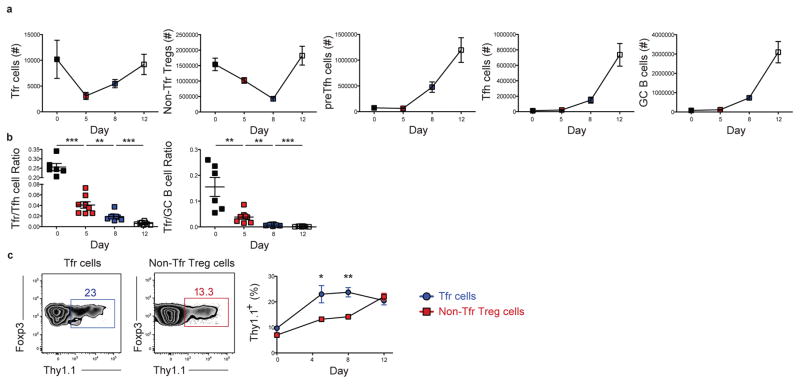
To examine Tfr-derived IL-10 regulation of the GC response, we infected 10BiT IL-10 reporter mice acutely with the Armstrong strain of LCMV and assessed Tfr cell phenotype and kinetics. These reporter mice possess a bacterial artificial chromosome transgene containing the Il10 gene locus, with the Thy1.1 cDNA (containing a stop codon) replacing the endogenous coding segment of exon 1 of the Il10 locus such that cells transcribing its mRNA express Thy1.1 on their cell surface (29). Our prior work validated that Thy1.1+ cells produce IL-10 during LCMV infection (30). While Tfr cell numbers initially declined following LCMV infection, their number increased from day 5 onwards mirroring the kinetics of Tfh and preTfh, and GC B cells (Fig. 1a, gated as in Fig. S1a, b, and as described (31–35)). The ratio of Tfr cells to Tfh or GC B cells peaked at day 5 p.i. and progressively declined at days 8 and 12 as the increase in Tfh and GC B cell numbers outpaced that of Tfr cells (Fig. 1b) (9, 10). There was an increased percentage of Thy1.1+ cells within the Tfr cell population relative to non-Tfr Treg cells at days 5 and 8 p.i. suggesting that IL-10 secretion may be a mechanism by which Tfr cells regulate the emerging GC response within the follicle (Fig. 1c). Detection of Thy1.1+ Treg cells within the GC by immunoflourescence was impeded due to technical complications related to the disrupted splenic architecture at days 5 and 8 p.i. and the dimness of Thy1.1 expression. Only a small percentage of Tfh or GC B cells were competent to express IL-10, with their percentages declining over time, while making less of this cytokine on a per cell basis relative to Tfr and non-Tfr Treg cells (Fig. S1c), suggesting these cells by comparison to Tfrs or Tregs are not an important source of IL-10 for the GC response.
Figure 1. Tfr cells robustly secrete IL-10 following acute viral infection.
Analysis of the Treg cell response post LCMV infection in IL-10 reporter (10BiT Thy1.1) mice. (a) Quantification of the number of Tfr cells, non-Tfr Treg cells, preTfh cells, Tfh cells, and GC B cells at days 0, 5, 8, and 12 following infection. Populations are defined as follows: Tfr cells. CD4+Ly6C− PSGL1loCXCR5hiPD1hiFoxp3+; non-Tfr Treg cells, CD4+CXCR5int− loPD1int− loFoxp3+; preTfh cells, CD4+CD44hiLy6C− PSGL1loCXCR5intPD1intFoxp3−; Tfh cells, CD4+CD44hiLy6C− PSGL1loCXCR5hiPD1hiFoxp3−; and GC B cells, B220+IgDloGL7+CD95+. (b) Quantification of the ratio of Tfr cells to Tfh cells or GC B cells at days 0, 5, 8 and 12 following infection. (c) Representative plot of IL-10 expression (assessed as Thy-1.1) by Tfr cells (left) and Non-Tfr Treg cells (middle) from mice as described in a. Right, frequency of Thy1.1+ cells in Tfr cells and Non-Tfr Treg cells at days 0, 5, 8, and 12 following LCMV Armstrong infection. Statistical analyses were performed using the unpaired two-tailed Student’s t-test. (*, p < 0.05; **, p < 0.01, ***, p < 0.001). Data are from 2 experiments representative of 4 experiments with 3–6 mice per time point post LCMV Armstrong infection.
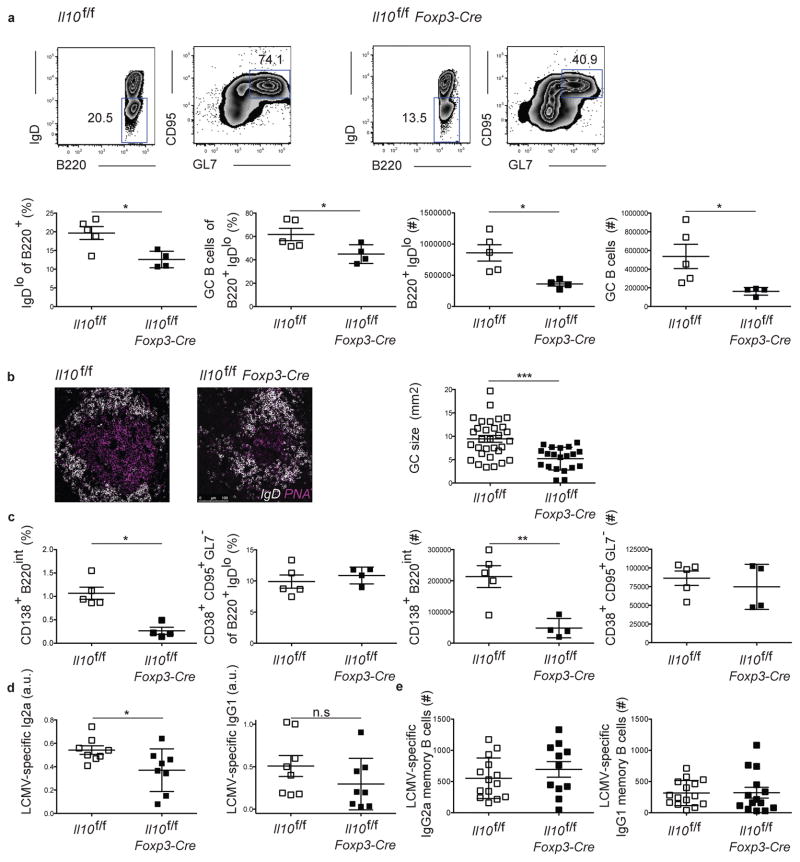
To evaluate the importance of Treg-derived IL-10 in the GC response we generated mice that specifically deleted Il10 in Foxp3-expressing Treg cells, as previously described (29). Il10f/fFoxp3-Cre mice displayed no sign of overt disease at steady state and did not show any consistent trend towards differences in basal percentages of GC B cells, Tfh, or Tfr cells (Fig. S2). Their was also no differences in the Tfr to Tfh and the Tfr to GC B cell ratios, in the spleen, peripheral and mesenteric lymph nodes, and Peyer’s patches. We evaluated the B cell response post acute LCMV infection of these animals in comparison to IL-10 intact ones. While this response appeared similar in the two groups at day 8 p.i., a significant decrease in the percentage and number of B cells with an activated phenotype (IgDlo) and of those cells with a GC phenotype (GL7+CD95+) emerged over time in mice in which Treg cells lacked Il10 expression, with this difference peaking at day 15 p.i. (Fig. 2a, and Fig. S3a). These mutant mice also displayed reduced GC size as determined by confocal imaging of splenic sections (Fig. 2b). We found that mice lacking Treg cell-derived IL-10 had a reduced percentage and number of plasmablasts (CD138+B220int cells) compared to their IL-10 intact counterparts, albeit a similar number of cells with a GC-dependent memory B cell phenotype (B220+IgDloGL7− CD38+CD95+ cells) (Fig. 2c) (36, 37). They also had reduced serum titers of LCMV-specific IgG2a and IgG1 (Fig. 2d), with a similar number of LCMV-specific memory B cells (Fig. 2e). No defects in GC B cell or plasmablast numbers were evident in mice immunized with NP-OVA in complete Freund’s adjuvant (Fig. S3b). There were also no apparent defects in GC B cell or plasmablast numbers in LCMV infected IL-10−/− mice, or in mice treated with αIL-10 suggesting that IL-10 production by different cell types may play opposing roles in regulation of the GC response (Fig. S4a, b) (38). Together, these data indicate that IL-10 specifically produced by Treg cells is important in promoting plasmablast differentiation and the development of the GC following viral infection.
Figure 2. Regulatory CD4+ T cell-derived IL-10 is important for B cell differentiation and the GC response.
Analysis of the B cell response in Il10f/f and Il10f/f Foxp3-Cre mice 15 days following LCMV infection. (a) Representative plots of the B cell responses in Il10f/f or Il10f/fFoxp3-Cre mice (top). Bottom: frequency and number of cells as indicated by the gates shown above. (b) Representative confocal images of the GC from Il10f/f or Il10f/f Foxp3-Cre mice. Right, GC sizes as measured by imageJ. The sections were taken from four mice of each genotype. Scale bar=100um. (c) Frequency of plasmablasts and memory B cells defined by the expression of surface markers (left). Right, absolute numbers of plasma cells and memory B cells. (d) ELISA quantification of LCMV-specific IgG2a and IgG1 antibody levels, indicated by arbitrary unit (a.u.). All the analyses of the GC response were performed 15 days post acute LCMV Armstrong infection. (e) ELISPOT quantification of the number of LCMV-specific IgG2a and IgG1 memory B cells. All the analyses of the GC response were performed 60 days post acute LCMV Armstrong infection. Statistical analyses were performed using the unpaired two-tailed Student’s t-test. (*, p < 0.05; **, p < 0.01). Data for panels a-c are from one experiment representative of 3 experiments with at 3–5 mice per group carried out at 15 days and 4 experiments with 3–5 mice per group carried out 12 days following LCMV Armstrong infection. Data for panel d are pooled from 2 experiments carried out at days 12 or 15 post infection. Data for panel e are pooled from 2 experiments carried out day 60 post infection with 5–7 mice per group.
Tfr-derived IL-10 promotes the GC response
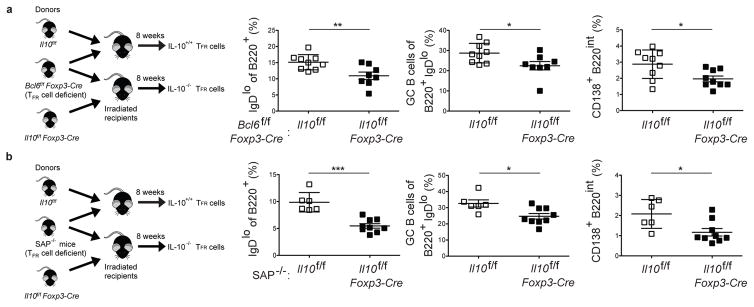
These findings did not distinguish between the role of Tfr cell-derived IL-10 and IL-10 produced by Bcl6− Treg cells in regulating the GC response. Follicular nonresident Treg cells can modulate the GC response as early as day 3 post immunization and likely serve as precursors for Tfr cells (14). To discriminate between these possibilities, mixed bone marrow chimeras (mBMCs) were generated in which Il10 was specifically ablated in Bcl6-expressing Tfr cells via generation of 50:50 Il10f/fFoxp3-Cre:Bcl6f/fFoxp3-Cre and control 50:50 Il10f/f:Bcl6f/fFoxp3-Cre chimeras (Fig. 3a) (12). In the absence of Tfr cell-derived IL-10, there was a significantly reduced percentage of IgDlo B cells at day 15 post LCMV infection and reduced percentages of GC B cells and plasmablasts (CD138+B220int) in comparison to control chimeras. The importance of Tfr cell-derived IL-10 was further tested using another approach in which mBMCs were generated using SAP-deficient (SAP−/−) mice in place of Bcl6f/fFoxp3-Cre (Fig. 3b). SAP is required for Tfr cell differentiation (8, 9). Again we found that loss of Tfr cell-derived IL-10 resulted in decreased percentages of IgDlo B cells, GC B cells and plasmablasts. These data indicate that Tfr cell-derived IL-10 supports B cell differentiation and the GC response.
Figure 3. Tfr cell-derived IL-10 is important for B cell differentiation and the GC response.
(a) Analysis of the B cell response day 15 post acute LCMV-Armstrong infection in 50:50 Il10f/f: Bcl6f/fFoxp3-Cre or Il10f/fFoxp3-Cre: Bcl6f/fFoxp3-Cre mixed BMCs. Schematic for experiment (left). Data are pooled from 2 experiments with 4–5 mice per group carried out 15 days following LCMV Armstrong infection (right). (b) Analysis of the B cell response at day 15 post acute LCMV-Armstrong infection in 50:50 Il10f/f: SAP−/− or Il10f/fFoxp3-Cre: SAP−/− mixed BMCs. Schematic for experiment (left). Data are from one experiment representative of 2 experiments with 4–9 mice per group carried out 15 days following LCMV Armstrong infection (right). Statistical analyses were performed using the unpaired two-tailed Student’s t-test. (*, p < 0.05; **, p < 0.01).
IL-10 signals via B cells to promote the GC response
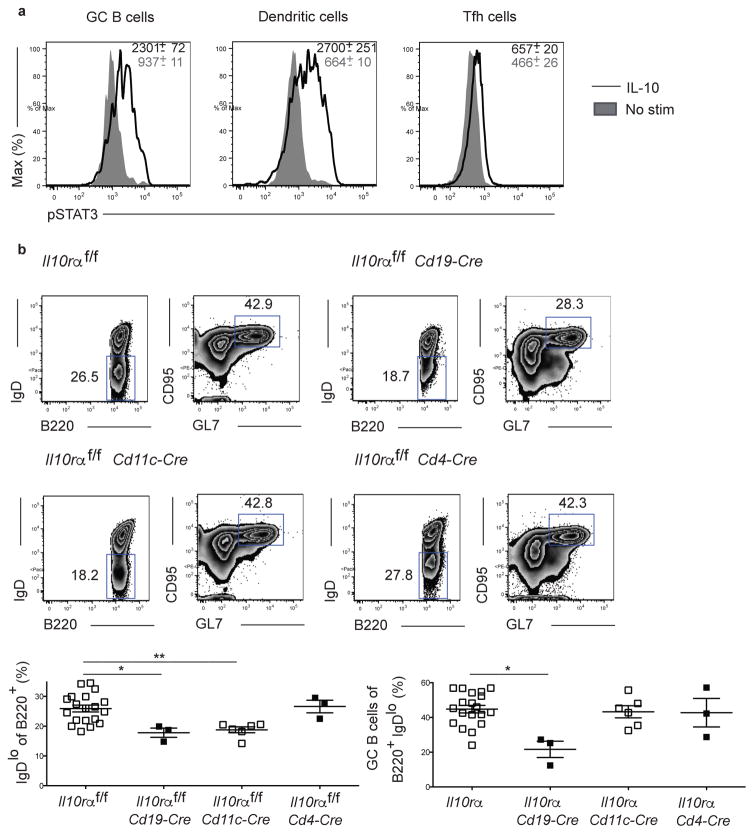
There are multiple pathways by which Treg cell derived IL-10 could modulate the GC response including acting directly on B cells or indirectly through Tfh cells or dendritic cells (DCs) (39, 40). To assess which cell populations were responsive to IL-10 signaling, we stimulated splenocytes from LCMV-infected mice with IL-10 and determined phospho-STAT3 (pSTAT3) in GC B cells, DCs, and Tfh cells. While both GC B cells and DCs displayed robust pSTAT3 expression relative to unstimulated controls, Tfh cells were poorly responsive suggesting that IL-10 does not act directly upon them to regulate the GC response by acting directly on Tfh cells (Fig. 4a). Consistent with this finding, we determined that the LCMV-specific CD4+ T cell response against the immunodominant GP66-77 epitope was similar between control mice and those lacking Treg cell-derived IL-10 at day 8 p.i. (Fig. S5a). The LCMV-specific Th1 (CD44hiPSGLlhiLy6C+ cells) and Tfh (CD44hiPSGLlloLy6C− CXCR5+PD1+ cells) responses, as well as the percentages of Treg (Foxp3+CD4+ cells) and Tfr cells, were also similar between the groups (Fig. S5b) (31).
Figure 4. IL-10 acts on B cells to regulate the GC response.
(a) GC B cells and dendritic cells but not Tfh cells are responsive to IL-10. Analysis of IL-10 responsiveness in GC B cells, dendritic cells (CD11chiMHCII+), and Tfh cells. Splenocytes isolated from mice at day 12 post LCMV infection were stimulated for 30 minutes with IL-10 and phospo-STAT3 levels determined. Data are from one experiment representative of 3 experiments with 4 mice per group. (b) Analysis of the B cell response in Il10rα f/f, Il10rαf/fCd19-Cre, Il10rαf/fCd11c-Cre, and Il10rαf/fCd4-Cre mice 15 days following LCMV infection. Top: Representative plots of B cell response. Bottom: quantification of B cell response shown above. Statistical analyses were performed using the unpaired two-tailed Student’s t-test (*, p < 0.05; **, p < 0.01). Data are from one experiment representative of 2–3 experiments with 3–7 mice per group carried out 15 days following LCMV Armstrong infection. Mice for the control Il10rαf/f group were pooled from littermate controls produced from the independent crosses used to generate the experimental groups.
To identify the cell population that IL-10 was acting upon to regulate the GC response we generated mice in which the Il10ra gene was deleted specifically on B cells, DCs, or T cells (22). The B cell response in these mice was then assessed at day 15 post LCMV infection. While the B cell response in mice in which T cells could not sense IL-10 was comparable to control mice, Il10rαf/f Cd19-Cre mice displayed a significant decrease in the percentages of activated and GC B cells compared to Il10raαf/f controls (Fig. 4b). Il10raαf/f Cd11c-Cre mice also had a reduced percentage of IgDlo B cells but an equivalent fraction of these cells with a GC phenotype indicating that while IL-10 may partially act through DCs to promote B cell activation, this pathway does not account for the defect in the GC response (Fig. 4b). Together, these findings suggest that Treg cell-derived IL-10 can act directly on B cells to promote their activation and differentiation into GC B cells.
Treg cell-derived IL-10 drives GC B cells to adopt a dark zone phenotype
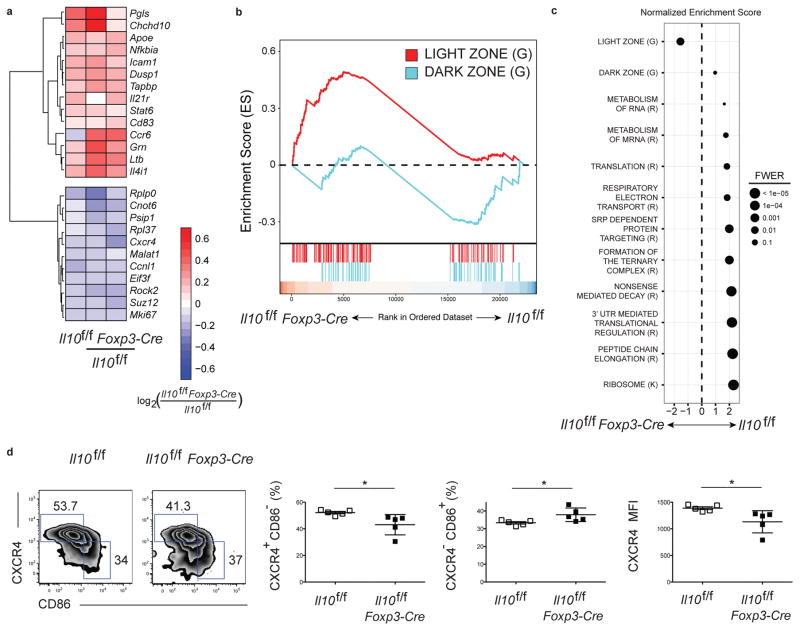
To help understand how Treg-derived IL-10 influenced the fate of GC B cells, we performed RNA-seq analysis to evaluate the gene expression profile of GC B cells isolated from Il10f/f Foxp3-Cre and control Il10f/f mice at day 12 post LCMV infection. There were 138 differentially expressed genes (DEGs) in GC B cells between the two groups with 84 genes upregulated and 54 genes downregulated in the Il10f/f Foxp3-Cre mice compared to controls (padj < 0.1) (Fig. 5a and Fig. S6a). Among the DEGs were a number of genes used to distinguish dark zone and light zone GC B cells including Cxcr4 and Cd83 (6, 41). Gene Set Enrichment Analysis (GSEA) was used to detect genome-wide changes, and identified a significant enrichment for a light zone GC B cell signature in GC B cells isolated from Il10f/f Foxp3-Cre mice (Fig. 5b, c and Fig. S6b). The dark zone is the proliferative compartment of the GC and the predominant site of Ig gene somatic hypermutation, in contrast to the light zone where GC B cells have a largely nonproliferative state and compete for the T cell help necessary to induce dark zone reentry (42–44). GC B cells isolated from Il10f/f Foxp3-Cre compared to control mice also were highly impaired in gene sets associated with translational activity (Fig. 5c). Together, these findings suggest that Treg cell-derived IL-10 promotes GC B cells to adopt a dark zone phenotype.
Figure 5. GC B cells in mice lacking regulatory CD4+ T cell-derived IL-10 display an enhanced light zone GC B cell gene signature.
(a) RNA-seq analysis of select differentially expressed genes among mRNA isolated from GC B cells pooled from Il10f/f and Il10f/fFoxp3-Cre mice 12 days after infection with LCMV Armstrong presented as expression (log2) in Il10f/f Foxp3-Cre cells relative to that in Il10f/f cells (key below; columns indicate paired replicates). (b) GSEA of light zone and dark zone signatures in GC B cells, based on published gene sets (6, 41). A positive ES signifies enrichment in the Il10f/f Foxp3-Cre sample relative to the Il10f/f condition of a given gene set; i.e., more highly expressed. (c) Normalized enrichment score (NES) for select pathways identified from the Reactome Pathway Database (R), Kegg Pathway Database (K) and published gene sets (G), where dot size represents p-values adjusted by the family-wise error rate (FWER). All gene sets attain significant enrichment (FDR<.0.001), with the exception of the dark zone gene set (FDR=0.5). Data are from three independent experiments with three mice per group pooled for each sample. (d) Analysis of the light zone and dark zone GC B cell response in Il10f/f and Il10f/f Foxp3-Cre mice 15 days post LCMV infection. Representative plots (left) of GC B cells as gated in Fig S1b. The numbers of the outlined area indicate dark zone (top left) and light zone (bottom right) based on the expression of surface markers CXCR4 and CD86. Middle, frequency of GC B cells from light zone gate defined by CXCR4+CD86− and dark zone gate defined by CXCR4− CD86+ from Il10f/f or Il10</i>f/f Foxp3-Cre mice. Right, expression of CXCR4 in GC B cells. Statistical analyses were performed using the unpaired two-tailed Student’s t-test. (*, p < 0.05). Data are from one experiment representative of 2 experiments with at least 4 mice per group carried out 15 days following LCMV Armstrong infection.
To more directly test this hypothesis, the dark zone and light zone phenotypes of GC B cells from control mice and those lacking Treg cell-derived IL-10 were determined. In agreement with our RNA-seq data, there was a small but significant decrease in the percentage of dark zone GC B cells and increase in that of light zone GC B cells in GC B cells from Il10f/f Foxp3-Cre mice (Fig. 5d). Aligning with previous results, we did not detect a difference in GC B cell proliferation or survival between the groups (43, 44) (Fig. S7). These data support a model in which Treg cell-derived IL-10 acts on light zone GC B cells to skew them towards a dark zone phenotype. These data do not discount the possibility that Treg cell-derived IL-10 acts on B cells during the early stages of the GC response to influence their ability to later adopt a dark zone phenotype.
Treg cell-derived IL-10 promotes dark zone phenotype through induction of nuclear FOXO1
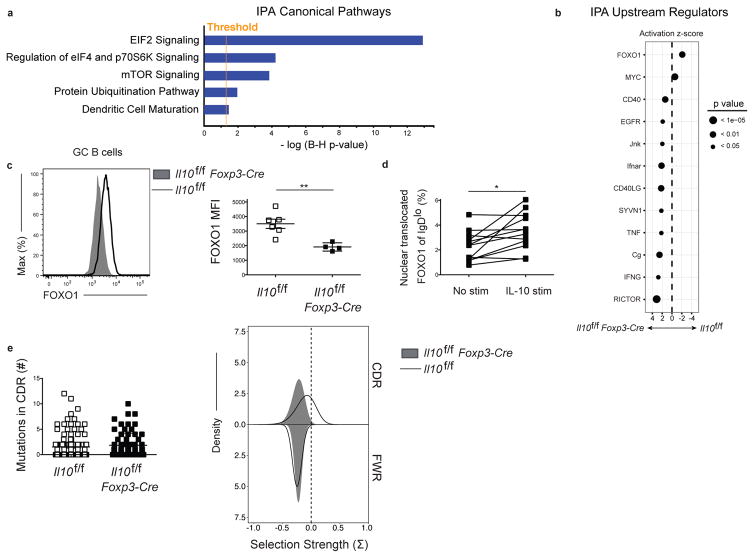
We next analyzed our RNA-seq data using Integrated Pathway Analysis (IPA, Ingenuity Systems, www.ingenuity.com). This analysis revealed that Eukaryotic Initiation Factor 2 (eIF2) signaling was the most significantly dysregulated pathway in Il10f/f Foxp3-Cre mice compared to controls (Fig. 6a), in agreement with our GSEA analysis indicating impaired translation. mTOR signaling was also significantly upregulated (Fig. 6a). Analysis of potential upstream regulators identified impaired Forkhead box protein 1 (FOXO1) and enhanced RICTOR signaling, a mTOR signaling component, as the most significant regulators of the gene signature (Fig. 6b). The transcription factor FOXO1 and PI3K signaling, an inducer of mTOR, execute opposing roles in the regulation of the dark zone and light zone GC B cell fate, with ablation of FOXO1 or induction of PI3K activity resulting in a loss of the dark zone phenotype cells (42, 43, 45). Thus, regulation of FOXO1 activity by Treg cell-derived IL-10 could modulate the dark zone phenotype of GC B cells. To test this hypothesis we determined total FOXO1 expression at day 15 post LCMV infection in GC B cells from Il10f/f Foxp3-Cre mice and identified decreased protein levels relative to controls (Fig. 6c). This decrease was evident in in both dark zone and light zone GC B cells (Fig. S8a). We also found that GC B cells from Il10f/f Foxp3-Cre mice displayed increased pS6 and pAktS473 expression (Fig. S8b). Collectively, these data indicated that Treg cell-derived IL-10 is necessary for maximal FOXO1 induction in GC B cells.
Figure 6. Reduced FOXO1 expression GC B cells from mice lacking regulatory CD4+ T cell-derived IL-10.
(a) Ingenuity Pathway Analysis (IPA) canonical pathway analysis. The number on the x-axis indicates the negative log p-values adjusted by the Benjamini-Hochberg (B–H) procedure, which is the significance of the probability that the genes in the dataset described in Figure 6 are associated with the canonical pathway listed. The orange line represents the threshold of significance for a B–H multiple testing correction p-value of 0.05. (b) IPA upstream regulator analysis. The top upstream regulators that are predicted to be responsible for the gene expression changes observed in the Il10f/fFoxp3-Cre sample relative to the Il10f/f sample are shown. A negative activation z-score indicates that the upstream regulator is predicted to be inhibited in the Il10f/f Foxp3-Cre sample relative to the Il10f/f sample and a positive score indicates that the upstream regulator is predicted to be activated. (c) Expression of FOXO1 in GC B cells from Il10f/f or Il10f/f Foxp3-Cre mice 15 days after infection with LCMV. Data are from one experiment representative of 2 experiments with at least 4 mice per group carried out 15 days following LCMV Armstrong infection. (d) Assessment of the percentage of nuclear translocated FOXO1 following IL-10 stimulation in IgDlo B cells 5 days post LCMV infection as determined by Amnis Imagestream. Statistical analyses were performed using the paired two-tailed Student’s t-test (*, p < 0.05; **, p < 0.01). Data are pooled from 3 experiments with 4 mice per group carried out 5 days following LCMV Armstrong infection. (e) Assessment of the number of mutations in the CDR region (left) and selection strength (right) in both the CDR region and framework region of a large number of the heavy chain VH genes of GC B cells from Il10f/f or Il10f/f Foxp3-Cre mice 15 days after infection with LCMV. Data are pooled from 2 experiments with 3–4 mice per group carried out 15 days following LCMV Armstrong infection.
We next asked if IL-10 promotes FOXO1 nuclear translocation in activated B cells. Active FOXO1 localizes to the nucleus where it can instruct a specific gene program (46). To mirror the in vivo context in which B cells are exposed to IL-10, cells were isolated from mice at day 5 post LCMV infection and cultured with or without IL-10. Day 5 coincides with the peak expression of IL-10 by Tfr cells, a major source of IL-10 within the inner follicle (Fig. 1c, S1c), and allowed us to assess FOXO1 nuclear translocation in cells that had not yet had prolonged exposure to IL-10. Using Amnis Imagestream analysis, we observed an increase in the percent of nuclear translocated FOXO1 in activated B cells (Fig. 6d). IL-10 did not induce nuclear translocation of FOXO1 in follicular B cells, despite robust pSTAT3 signaling (Fig. S8c), suggesting that IL-10 acts in concert with other signals received by activated B cells, such as BCR, costimulation, and cytokines, to promote FOXO1 activity.
In addition to regulating dark zone reentry, FOXO1 is also important in promoting Ig affinity maturation (42, 43). To assess affinity maturation, GC B cells from control Il10f/f and experimental Il10f/f Foxp3-Cre mice were sorted at day 15 post LCMV infection and a large number of the immunoglobulin heavy chain variable region (VH) genes were cloned and sequenced using a pooled primer approach as previously described (47). The overall mutation frequency in the complementarity determining region (CDR) was similar between groups (Fig. 6e). However, we identified a reduction in the degree of positive selection within the CDR in the Il10f/f Foxp3-Cre mice, which was absent in the framework region (FWR) (Fig. 6e). The trend was observed in two independent experiments, but did not reach statistical significance due to the limitation of the number of clones obtained. We further examined the amino acid physicochemical properties of CDR3, as diversity in this region is a key determinant of antigen binding. Mice that lacked Treg-derived IL-10 displayed a trend towards higher grand average of hydrophobicity (GRAVY) score, mean side-chain bulkiness and abundance of aromatic residues in this region, consistent with the notion that less selection is occurring in these cells as positively selected cells typically display reduction in these metrics (Fig. S9) (48).
DISCUSSION
Understanding the signals regulating GC B cell differentiation is critical for the development of targeted therapies that can modulate GC output. Here, we describe a role for Tfr cells in promoting the GC response through local production of IL-10 during viral infection. IL-10 secreting Treg cells act on B cells to drive their differentiation into plasmablasts and GC B cells. Both IL-10 expression and the Tfr to GC B cell ratio peak at day 5 post infection, suggesting that Tfr cell-derived IL-10 may be predominantly acting early to drive B cell differentiation. Tfr cell-derived IL-10 promotes expression and activity of FOXO1, thereby facilitating adoption of a dark zone phenotype by GC B cells and potentially enhancing affinity maturation. These results illustrate the complex role of Tfr cells in the regulation of the GC response, and the need to consider both the suppressive and stimulatory roles of Treg cells in control of humoral immunity.
How do Tfr cells balance their suppression and promotion of GC output? IL-10 competent Tfr cells express high levels of CTLA4 so it is unlikely that IL-10 secreting and CTLA4 expressing Tfr cells represent unique populations. One possible explanation is that suppression by Tfr cells is reliant on cell contact (e.g., CTLA4-CD80/86 interactions). In such a scenario, light zone GC B cells engaged in productive MHC-peptide TCR exchange with Tfh cells would be less available for this type of interaction, thus allowing IL-10 secretion by nearby Tfr cells to stimulate GC B cell differentiation and dark zone reentry. However, GC B cells not engaged with Tfh cells would be available to interact with Tfr cells, where the suppressive function of CTLA4 could dominate the stimulatory role of IL-10. In this manner, Tfr cells could promote the continued differentiation of affinity-matured, antigen-bearing GC B cells while suppressing those cells with low antigen affinity. Alternatively, B cells engaged in productive interactions with Tfh cells, including before GC formation, might be rendered more sensitive to IL-10 signaling (or less susceptible to inhibition by CTLA4), providing signals that initiate dark zone polarization of these cells or their growth advantage. The finding that Tfr cells restrict the outgrowth of non-antigen specific GC B cells supports these models, and suggests an important role for Tfr cells in restraining the expansion of autoreactive GC B cells (9). It will be important for future work to more directly test these ideas and explore other mechanisms by which Tfr cells may modulate the GC response.
Distinguishing between the function of Tfr cells and follicular nonresident Treg cells in modulating the GC response is critical. Ablation of CTLA4 expression on Tregs impairs CD86 expression on B cells prior to the formation of Tfr cells indicating that follicular nonresident Treg cells influence the GC response (14). It is likely there are multiple mechanisms by which Treg cells could exert this effect including regulation of T or B cell avidity and the activation state of DCs (22, 23, 49). Here, we found that depletion of Tfr cell-derived IL-10 led to an impaired GC response. This finding does not rule out a contribution of IL-10 producing follicular nonresident Treg cells on regulation of the GC response. It also does not distinguish between the roles of Tfr cells inside and outside of the GC, as well as those cells present within the follicle prior to GC development.
Tfr cell-derived IL-10 likely contributes to the GC B-cell response through multiple mechanisms. Our work reveals its promotion of FOXO1 expression, which is required for dark zone formation due to its essential role in gene-program instruction, including upregulation of the chemokine receptor CXCR4 required for GC B cell migration into the dark zone (42–45). However, while regulation of FOXO1 activity may explain the dsyregulated dark zone phenotype and affinity maturation of GC B cells from mice lacking Treg cell-derived IL-10, it does not explain the decrease in GC B cell number and output. GC B cell proliferation and somatic hypermutation typically occur in the dark zone, but these processes are maintained even in cells unable to access the dark zone (42–44). Therefore, it is likely that Tfr cell-derived IL-10 functions in a FOXO1-independent manner to regulate B cell differentiation and GC development.
The IL-10-STAT3 pathway has been implicated as a promoter of diffuse large B-cell lymphoma (DLBCL) and systemic lupus erthematosus (SLE) (50–53). A high level of circulating IL-10 and active intracellular STAT3 are associated with clinically aggressive cases of DLBCL (51, 52). Constitutively active STAT3 promotes cell proliferation and survival, and anti-IL-10R antibody treatment induces cell cycle arrest and apoptosis in DLBCL cell lines (50, 52). Among known STAT3 targets that are inhibited by IL-10R blockade are genes involved in cell cycle progress, antiapoptotic factors, and proto-oncogenes including Ccnd1, Mcl1, Junb, and cMyc (50, 52). We find a robust impairment in pathways associated with RNA translation in mice lacking Treg cell-derived IL-10 suggesting that IL-10-driven STAT3 may regulate GC B cell proliferation and differentiation by inducing the translation of mRNAs encoding proliferation-promoting proteins or proteins involved in differentiation (54). It will be important to elucidate which genes IL-10 driven STAT3 is acting upon to drive B cell proliferation and differentiation during viral infection and the extent that this pathway overlaps with disease progression in individuals with DLBCL. Therapies designed to modulate the stimulatory potential of Tfr cells could prove an effective method to bolster humoral immunity or restrain the expansion of autoreactive or malignant B cell clones.
MATERIALS AND METHODS
Study design
The aim of this study was to characterize the role of Tfr-cell derived IL-10 in the regulation of the germinal center response following acute viral infection. Most of the experiments consisted of enumerating population frequencies by flow cytometry in different genetic mouse models, analysis of germinal centers by immunofluorescence, analysis of RNAseq data, and quantification of nuclear localized transcription factors. Littermate comparisons were used for all experiments where possible. Control and experimental groups were age and sex-matched. The investigators were not blinded. Experimental replications are indicated in the figure legends.
Mice
C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD) or the Jackson Laboratory (Bar Harbor, ME). B6.129P2 Il10tm1Cgn/J (Il10−/−), B6.129(cg)-Foxp3tm3(DTR/GFP)Ayr/J (Foxp3GFP− DTR), B6.129S(FVB)-Bcl6tm1.1Dent/J (Bcl6f/f), B6.129S6-Sh2d1atm1Pls/J (SAP−/−), and B6.129 (Cg)-Foxp3tm4(YFP/cre)Ayr/J (Foxp3-Cre) mice were purchased from the Jackson Laboratory. 10BiT mice (29), Il10f/f mice (55) and Cd4-Cre, Cd19-Cre and Cd11c-Cre mice have been described. Il10raf/f mice were generated by the Flavell lab as previously described (22). All animal experiments were done with approval of the Yale Institutional Animal Care and Use Committee.
Infection and treatments
Mice were given intraperitoneal (i.p.) administration of 2× 105 plaque-forming units of LCMV Armstrong. Diphtheria toxin was reconstituted according to manufacturer’s instructions (Sigma). Mice were given diphtheria toxin at a dose of 50 μg per kg body weight on day 4 and 5 (2 doses) following infection, as described (56). For IL-10 blockade, αIL-10 mAb antibody (JES5-2A5 clone, kindly provided by J.M.M. den Haan, UV University Medical Center, Amsterdam, Netherland) was administered 0.25 mg/ml i.p. every other day. For NP-OVA in complete Freund’s adjuvant immunization, mice were given 100ug NP-OVA mixed with an equal volume of complete Freund’s adjuvant.
Statistical analysis
Results represent the mean ± SEM unless indicated otherwise. Statistical significance was determined by the paired or unpaired Student’s t-test. Statistical analyses were performed using Prism GraphPad software v6.0. (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Supplementary Material
Summary.
Follicular regulatory T cells promote the germinal center response following viral infection through provision of IL-10
Acknowledgments
We thank all members of the Craft labs for helpful discussions and critical reading of the manuscript.
FUNDING:
Supported by grants from the NIH (R37AR40072 [J.C.], R01AR068994 [J.C.], P30AR053495 [J.C.], R21AR063942 [J.C.], T32AI07019 [B.L.], F31AG07777 [B.L])), K01AR067892 [J.S.W.], and Gruber Science Fellowship [Y.L.].
Footnotes
Supplemental Figure 1. Flow cytometric gating strategy for Tfh, preTfh, GC B, Treg, and Tfr cells, with IL-10 expression by these populations.
Supplemental Figure 2. Mice lacking regulatory CD4+ T cell-derived IL-10 do not have defects in steady state lymphoid cell populations.
Supplemental Figure 3. Temporal development of the germinal center B cell response in mice lacking regulatory CD4+ T cell-derived IL-10.
Supplemental Figure 4. Systemic IL-10 is not required for the germinal center response.
Supplemental Figure 5. Regulatory CD4+ T cell-derived IL-10 is not required for effector CD4+ T cell differentiation.
Supplemental Figure 6. Heat map of differentially expressed genes based on RNA-seq.
Supplemental Figure 7. Germinal center B cells in mice lacking regulatory CD4+ T cell-derived IL-10 display similar levels of proliferation and death.
Supplemental Figure 8. IL-10 does not induce FOXO1 nuclear translocation in IgDhi B cells.
Supplemental Figure 9. The VH CDR3 region of GC B cells in mice lacking regulatory CD4+ T cell-derived IL-10 displays altered amino acid physiochemical properties.
Table S1. Tabulated data for Figs. 1–6 and Supplemental Figs. 1–9.
AUTHOR CONTRIBUTIONS:
B.J.L., Y.L., S.M.K., J.C. conceived and designed the experiments. B.J.L., Y.L., J.S.W. performed the experiments. B.J.L., Y.L., R.A.A, J.AV.H., N.T.G, S.H.K. analyzed the data. B.J.L., Y.L., J.C. wrote the manuscript.
COMPETING INTERESTS:
The authors declare no competing financial interests.
DATA AVAILABILITY:
The RNA-seq data reported in this paper are tabulated in the Supporting Online Material and archived at NCBI Geo (Accession code pending).
References
- 1.De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol. 2015;15:137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basso K, Dalla-Favera R. Germinal centres and B cell lymphomagenesis. Nat Rev Immunol. 2015;15:172–184. doi: 10.1038/nri3814. [DOI] [PubMed] [Google Scholar]
- 3.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature. 2014;507:513–518. doi: 10.1038/nature12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly - TFH cells in human health and disease. Nat Rev Immunol. 2013;13:412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 6.Victora GD, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gitlin AD, Shulman Z, Nussenzweig MC. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 2014;509:637–640. doi: 10.1038/nature13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linterman MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollenberg I, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 11.Aloulou M, et al. Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nat Commun. 2016;7:10579. doi: 10.1038/ncomms10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H, et al. Follicular regulatory T cells repress cytokine production by follicular helper T cells and optimize IgG responses in mice. Eur J Immunol. 2016;46:1152–1161. doi: 10.1002/eji.201546094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sage PT, et al. Suppression by TFR cells leads to durable and selective inhibition of B cell effector function. Nat Immunol. 2016;17:1436–1446. doi: 10.1038/ni.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T Cells Control Antigen-Specific Expansion of Tfh Cell Number and Humoral Immune Responses via the Coreceptor CTLA-4. Immunity. 2014;41:1013–1025. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor ctla-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41:1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sage PT, Sharpe AH. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol. 2015;36:410–418. doi: 10.1016/j.it.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang CJ, et al. CTLA-4 controls follicular helper T-cell differentiation by regulating the strength of CD28 engagement. Proc Natl Acad Sci USA. 2015;112:524–529. doi: 10.1073/pnas.1414576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.León B, Bradley JE, Lund FE, Randall TD, Ballesteros-Tato A. FoxP3+ regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability. Nat Commun. 2014;5:3495. doi: 10.1038/ncomms4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray JP, et al. The Interleukin-2-mTORc1 Kinase Axis Defines the Signaling, Differentiation, and Metabolism of T Helper 1 and Follicular B Helper T Cells. Immunity. 2015;43:690–702. doi: 10.1016/j.immuni.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Laidlaw BJ, et al. Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat Immunol. 2015;16:871–879. doi: 10.1038/ni.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laidlaw BJ, Craft JE, Kaech SM. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat Rev Immunol. 2016;16:102–111. doi: 10.1038/nri.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rousset F, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choe J, Choi YS. IL-10 interrupts memory B cell expansion in the germinal center by inducing differentiation into plasma cells. Eur J Immunol. 1998;28:508–515. doi: 10.1002/(SICI)1521-4141(199802)28:02<508::AID-IMMU508>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 26.Yoon S-O, Zhang X, Berner P, Choi YS. IL-21 and IL-10 have redundant roles but differential capacities at different stages of Plasma Cell generation from human Germinal Center B cells. J Leukoc Biol. 2009;86:1311–1318. doi: 10.1189/jlb.0409268. [DOI] [PubMed] [Google Scholar]
- 27.Cai G, et al. A regulatory role for IL-10 receptor signaling in development and B cell help of T follicular helper cells in mice. J Immunol. 2012;189:1294–1302. doi: 10.4049/jimmunol.1102948. [DOI] [PubMed] [Google Scholar]
- 28.Guthmiller JJ, Graham AC, Zander RA, Pope RL, Butler NS. Cutting Edge: IL-10 Is Essential for the Generation of Germinal Center B Cell Responses and Anti-Plasmodium Humoral Immunity. J Immunol. 2017;198:617–622. doi: 10.4049/jimmunol.1601762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maynard CL, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 30.Parish IA, et al. Chronic viral infection promotes sustained Th1-derived immunoregulatory IL-10 via BLIMP-1. J Clin Invest. 2014;124:3455–3468. doi: 10.1172/JCI66108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall HD, et al. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity. 2011;35:633–646. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poholek AC, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odegard JM, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linterman MA, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hale JS, et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38:805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007;204:2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dogan I, et al. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 38.Tian Y, Mollo SB, Harrington LE, Zajac AJ. IL-10 Regulates Memory T Cell Development and the Balance between Th1 and Follicular Th Cell Responses during an Acute Viral Infection. J Immunol. 2016;197:1308–1321. doi: 10.4049/jimmunol.1502481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shulman Z, et al. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science. 2014;345:1058–1062. doi: 10.1126/science.1257861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi YS, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Victora GD, et al. Identification of human germinal center light and dark zone cells and their relationship to human B-cell lymphomas. Blood. 2012;120:2240–2248. doi: 10.1182/blood-2012-03-415380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dominguez-Sola D, et al. The FOXO1 Transcription Factor Instructs the Germinal Center Dark Zone Program. Immunity. 2015;43:1064–1074. doi: 10.1016/j.immuni.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Sander S, et al. PI3 Kinase and FOXO1 Transcription Factor Activity Differentially Control B Cells in the Germinal Center Light and Dark Zones. Immunity. 2015;43:1075–1086. doi: 10.1016/j.immuni.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 44.Bannard O, et al. Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity. 2013;39:912–924. doi: 10.1016/j.immuni.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue T, et al. The transcription factor Foxo1 controls germinal center B cell proliferation in response to T cell help. J Exp Med. 2017;214:1181–1198. doi: 10.1084/jem.20161263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staron MM, et al. The Transcription Factor FoxO1 Sustains Expression of the Inhibitory Receptor PD-1 and Survival of Antiviral CD8(+) T Cells during Chronic Infection. Immunity. 2014;41:802–814. doi: 10.1016/j.immuni.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White H, Gray D. Analysis of immunoglobulin (Ig) isotype diversity and IgM/D memory in the response to phenyl-oxazolone. J Exp Med. 2000;191:2209–2220. doi: 10.1084/jem.191.12.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vander Heiden JA, et al. Dysregulation of B Cell Repertoire Formation in Myasthenia Gravis Patients Revealed through Deep Sequencing. J Immunol. 2017;198:1460–1473. doi: 10.4049/jimmunol.1601415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pace L, et al. Regulatory T cells increase the avidity of primary CD8+ T cell responses and promote memory. Science. 2012;338:532–536. doi: 10.1126/science.1227049. [DOI] [PubMed] [Google Scholar]
- 50.Ding BB, et al. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood. 2008;111:1515–1523. doi: 10.1182/blood-2007-04-087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta M, et al. Elevated serum IL-10 levels in diffuse large B-cell lymphoma: a mechanism of aberrant JAK2 activation. Blood. 2012;119:2844–2853. doi: 10.1182/blood-2011-10-388538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Béguelin W, et al. IL10 receptor is a novel therapeutic target in DLBCLs. Leukemia. 2015;29:1684–1694. doi: 10.1038/leu.2015.57. [DOI] [PubMed] [Google Scholar]
- 53.Chun H-Y, et al. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol. 2007;27:461–466. doi: 10.1007/s10875-007-9104-0. [DOI] [PubMed] [Google Scholar]
- 54.Dowling RJO, et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roers A, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200:1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 57.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ray JP, et al. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. 2014;40:367–377. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anders S, et al. Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nat Protoc. 2013;8:1765–1786. doi: 10.1038/nprot.2013.099. [DOI] [PubMed] [Google Scholar]
- 60.McHeyzer-Williams MG, McLean MJ, Lalor PA, Nossal GJ. Antigen-driven B cell differentiation in vivo. J Exp Med. 1993;178:295–307. doi: 10.1084/jem.178.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sastry L, et al. Cloning of the immunological repertoire in Escherichia coli for generation of monoclonal catalytic antibodies: Construction of a heavy chain variable region-specific cDNA library. Proc Natl Acad Sci USA. 1989;86:5728–5732. doi: 10.1073/pnas.86.15.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye J, Ma N, Madden TL, Ostell JM. IgBLAST: an immunoglobulin variable domain sequence analysis tool. Nucleic Acids Research. 2013;41:W34–40. doi: 10.1093/nar/gkt382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alamyar E, Giudicelli V, Li S, Duroux P, Lefranc M-P. IMGT/HighV-QUEST: The IMGT® web portal for immunoglobulin (IG) or antibody and T cell receptor (TR) analysis from NGS high throughput and deep sequencing. Immunome Res. 2012:1–15. [Google Scholar]
- 64.Gupta NT, et al. Change-O: a toolkit for analyzing large-scale B cell immunoglobulin repertoire sequencing data. Bioinformatics. 2015;31:3356–3358. doi: 10.1093/bioinformatics/btv359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yaari G, Uduman M, Kleinstein SH. Quantifying selection in high-throughput Immunoglobulin sequencing data sets. Nucleic Acids Research. 2012;40:e134. doi: 10.1093/nar/gks457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yaari G, Kleinstein SH. Practical guidelines for B-cell receptor repertoire sequencing analysis. Genome Med. 2015;7:121. doi: 10.1186/s13073-015-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stern JNH, et al. B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci Transl Med. 2014;6:248ra107. doi: 10.1126/scitranslmed.3008879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.