Abstract
Transfusion of blood components and factor concentrates is clinically used to replenish clotting factors and treat coagulopathy after injury when bleeding is severe. Alternatively, direct manipulation of fibrin polymerization via synthetic cross-linking agents may also improve clot formation during coagulopathic conditions as a novel way to treat coagulopathy. We recently developed a synthetic hemostatic polymer, PolySTAT, that promotes clot formation and stabilizes fibrin network structure by cross-linking fibrin monomers. In this study, we used rotational thromboelastometry (ROTEM) to monitor the effect of PolySTAT on the mechanical strength of clots during clot formation and breakdown in comparison to replacement clotting factors and antifibrinolytics under conditions of simulated trauma-induced coagulopathy (sTIC). Human recombinant activated Factor VII (rFVIIa) shortened clotting onset time and accelerated clotting rate, while tranexamic acid (TXA) eliminated clot lysis and restored maximal clot firmness.In contrast, fibrinogen and PolySTAT were both able to speed up clot formation, increase maximal firmness, and inhibit clot lysis. Furthermore, PolySTAT acted synergistically with TXA and fibrinogen, enhancing their individual effects on clot formation. Thus, manipulating fibrin clot structure by physical cross-linking with a synthetic polymer has beneficial effects on clot formation and may be a viable transfusion strategy for treatment of coagulopathy.
Keywords: Trauma-induced coagulopathy, Hemostatic polymer, Fibrin cross-linking, ROTEM
TOC image
INTRODUCTION
Hemorrhage is responsible for approximately 40% of deaths within 24 h after traumatic injury.1 Formation of stable blood clots is essential to resolve bleeding. However, at the time of admittance into the hospital, approximately 25% of patients have trauma-induced coagulopathy (TIC),2 a multifactorial condition in which clotting function is impaired due to tissue injury and shock,3 depleted levels of functional clotting factors,4 and activation of fibrinolytic pathways leading to accelerated clot breakdown, or hyperfibrinolysis.5–8 These changes have been linked to activation of the protein C anticoagulant system, inhibition of PAI-1, and release of tPA from shocked endothelium. In addition, dilutional coagulopathy is often present due to the use of crystalloid resuscitation fluids. One of the main goals of bleeding management after trauma is to restore clot formation.
In the past two decades, drugs such as human recombinant activated Factor VII (rFVIIa; MW 50 kDa; Novo Nordisk), tranexamic acid (TXA; MW 157.21 Da; Pfizer), and fibrinogen concentrate (MW 340 kDa; CSL Behring) have been investigated to treat bleeding after traumatic injury. These drugs are able to promote quick and stable fibrin formation by accelerating thrombin generation, inhibiting fibrinolytic enzyme activity, and replenishing depleted fibrinogen levels, respectively.
rFVIIa, currently approved for use in hemophilia A patients with FVIII inhibitors, initiates coagulation through complexation with tissue factor (TF) at sites of tissue injury and is responsible for a thrombin burst at platelet surfaces.9 Case reports and anecdotal evidence of off-label use for trauma have shown instances in which intravenous administration of rFVIIa was able to stop bleeding and correct coagulopathy in trauma patients when all standard measures were exhausted.10–13 However, two clinical trials showed no improvement in survival despite reduced RBC transfusion requirements.14,15 Given the evidence, rFVIIa is currently recommended as a last resort.16,17
In contrast, TXA is recommended for early administration after traumatic injury to inhibit hyperfibrinolysis, which is associated with increased mortality.7,8 TXA is a synthetic analogue of lysine and binds to plasminogen to prevent its conversion into active plasmin. It is currently approved in the United States for treatment of menorrhagia as well as for prevention of peri- and post-operative bleeding in patients with established coagulopathies.18 In 2010, a large multicenter trial (CRASH-2) was conducted which showed a reduced risk of hemorrhage-related mortality in trauma patients administered TXA within 3 h after injury, and this evidence has helped establish TXA as a viable hemostatic agent for use in trauma.19
While regulation of enzymatic activity can in some instances rescue clot formation, adequate levels of substrate are also essential for fibrin formation. Fibrinogen is cleaved by thrombin to generate fibrin monomers for fibrin polymerization and promotes aggregation of activated platelets through binding of GPIIb-IIIa integrin receptors in adjacent platelets. After severe blood loss, fibrinogen reaches critically-low levels earlier than other clotting factors, and concentrations below 2.29 mg/mL are associated with significantly increased mortality rate.4,20 Administration of fibrinogen in the form of concentrates or as a component of blood products is, therefore, recommended should fibrinogen levels fall below 1.5–2.0 mg/mL.17
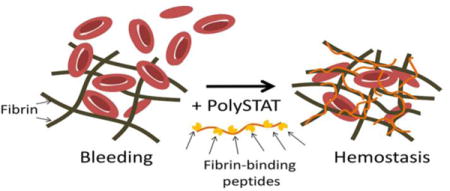
We recently reported the synthesis of PolySTAT, a fibrin cross-linking hemostatic polymer, and demonstrated its efficacy in reducing blood loss in a rat femoral artery injury model.21 In the study, PolySTAT was synthesized with a linear poly(hydroxyethyl methacrylate) backbone grafted with ~16 fibrin-binding peptides22 (Fig. 1A). Multivalent display of fibrin-binding domains along the polymer enables a single polymer to non-covalently bind to multiple fibrin monomers during fibrin polymerization to create a stable, cross-linked fibrin network. In the following work, a functional comparison between PolySTAT and the aforementioned hemostatic agents was completed.
Figure 1.
Comparison of PolySTAT and rFVIIa in an sTIC plasma model. (A) Schematic of PolySTAT reproduced with permission from21. (B) Representative ROTEM traces of untreated sTIC controls, PolySTAT-treated sTIC plasma, and rFVII-treated sTIC plasma without dilution are shown. (C) Clotting time, (D) α-Angle, (E) MCF, and (F) ML were measured. Data are averages ± SD of experiment completed in triplicate. P-values were determined using one-way ANOVA and Tukey post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001 versus no treatment. Dotted line indicates values in the absence of tPA.
Rotational thromboelastometry (ROTEM, Tem International GmbH, Munich, Germany), is a clinical tool used to monitor clotting in blood samples. ROTEM measurements showing reduced clot strength and fibrinolytic activity are validated indicators of TIC23 and is correlated with higher mortality rates7. In particular, ROTEM assays reporting only the fibrin contribution to clot firmness by inhibiting platelet activity have been shown to be highly sensitive for TIC and the need for massive transfusion of blood products.23 In the following work, ROTEM was used to compare the effects of direct fibrin cross-linking using PolySTAT to the effects of hemostatic agents that work by enzymatic inhibition (TXA) or factor replacement (rFVIIa and fibrinogen concentrate). These experiments were completed in the absence of platelets in a simulated trauma-induced coagulopathy (sTIC) plasma model. Furthermore, we aimed to study the effects on clot formation when PolySTAT is added in combination with the aforementioned drugs.
MATERIALS AND METHODS
Fresh frozen plasma from healthy human donors and rFVIIa (NovoNordisk, Denmark) were purchased from Bloodworks Northwest (Seattle, WA). Human tissue factor (TF; BT-pro-312) was purchased from Biotang Inc. Human tissue plasminogen activator (tPA; T0831) and TXA (1672745) were purchased from Sigma-Aldrich. Plasminogen-depleted human fibrinogen (FIB 1) was purchased from Enzyme Research Laboratories. Fibri-Prest Automate 2 (00613), Unicalibrator (00675), and Owren-Koller buffer (00360) were purchased from Diagnostica Stago. PolySTAT (MW ~45 kDa) was synthesized as previously described.21
Fibrinogen Quantification
Fibrinogen concentration in the human plasma was measured using a standard Fibri-Prest Automate 2 assay in a steel ball Diagnostica Stago Start 4 hemostasis analyzer (Diagnostica Stago, Asnieres, France).
Simulated Trauma-Induced Coagulopathy plasma model
ROTEM was used to measure clotting time (CT), clotting rate (α-angle), maximum clot firmness (MCF), and maximum lysis at t = 1 h (ML). Each ROTEM machine contains four channels for measurement. Access to 2 ROTEM units allowed simultaneous measurements for 8 samples. A plasma model of simulated TIC demonstrating decreased maximum clot firmness and intermediate hyperfibrinolysis was adapted from a published in vitro whole-blood trauma coagulopathy model.24 The ROTEM plasma sTIC model required decreased concentrations of tissue factor and tPA to produce clotting curves representative of clinically-relevant intermediate hyperfibrinolysis.7 Final concentrations were 3 pM TF and 4 nM tPA to reproduce sTIC coagulation profiles. Additional studies were performed using the same activators in diluted plasma (dilPlasma; 60 v/v % plasma diluted with sterile 0.9% saline) to simulate dilutional coagulopathy. Plasma was thawed in a 37˚C water bath and maintained at room temperature during ROTEM studies.
PolySTAT Comparison to rFVIIa and TXA
In each ROTEM cup, 286 μL diluted and undiluted plasma was mixed with 24 μL activation solution (19 μL 0.2 M CaCl2, 4.5 μL 227 pM TF, 0.48 μL 2.9 μM tPA), and 30 μL solution containing PolySTAT, rFVIIa, or TXA. rFVIIa concentrations (2.0, 4.0, 10, 20 nM) and TXA concentrations (6.4, 64, 640 μM) were chosen based on human plasma concentrations after intravenous administration of the standard recommended dosage.25,26 PolySTAT concentrations were chosen to represent various ratios of polymer to fibrin binding sites; 1.8, 4.4, and 18 μM correspond to polymer to fibrin binding site ratios of 1:10, 1:4, and 1:1, respectively. Measurements were also collected in tissue factor-initiated plasma without tPA-induced lysis and are shown on graphs as dotted lines for reference. Due to the length of these studies, comparison of PolySTAT to rFVIIa and PolySTAT to TXA were completed in separate experiments.
PolySTAT Comparison to Fibrinogen Supplementation
7.8, 16, 23, and 31 μL fibrinogen solution (22 mg/mL) was added to diluted plasma containing 1.5 mg/mL fibrinogen to raise fibrinogen concentration by 0.5, 1.0, 1.5, and 2.0 mg/mL, respectively. Clotting was initiated using the same volume and composition activation solution used in the previous ROTEM studies.
Coagulation Effects of PolySTAT in Combination with rFVIIa, TXA, and Fibrinogen
Activation solution and 4.4 μM PolySTAT was added to undiluted plasma in combination with 4 nM rFVIIa, 6.4 μM TXA, and fibrinogen to raise total fibrinogen concentration by 0.5 mg/mL.
Statistical Analysis
Data was analyzed using GraphPad Prism 5. One-way ANOVAs with Tukey post hoc tests were completed to identify significant differences between treatments.
RESULTS
Comparison of PolySTAT to rFVIIa
ROTEM produces curves of clot firmness over time, with an initial positive slope indicating clot build-up and, in some instances, an inflection point at the maximum clot firmness followed by a subsequent decline indicating clot lysis. Representative curves for untreated sTIC controls and PolySTAT- and rFVIIa- treated sTIC plasma are shown in Fig. 1B. The top curve is characteristic of intermediate hyperfibrinolysis, previously defined as complete clot lysis between 30–60 min.7 Under these conditions, rFVIIa primarily affected clotting kinetics (i.e. CT and clotting rate). A dose-dependent trend was observed, where increasing rFVIIa concentrations led to progressively shorter CT in both undiluted and diluted plasma. (Fig. 1C)). These trends were particularly noticeable when each experimental replicate was plotted separately (Fig. S1). 4.0 and 20 μM rFVIIa significantly increased α-angle 1.2-1.3-fold in plasma, fully restoring clotting rates to those observed in the absence of tPA-induced lysis (55±1.7°)(Fig. 1D). A dose-dependent increase in α-angle was observed in diluted plasma, where the highest concentration rFVIIa also produced α-angles comparable to those of non-lysing controls. rFVIIa did not significantly alter maximum clot firmness (MCF) (Fig. 1E) and significantly reduced maximum lysis (ML) only at the highest concentration tested (Fig. 1F).
PolySTAT exhibited similar coagulation-accelerating effects as rFVIIa (Fig. 1D) while also significantly increasing clot firmness (Fig. 1E) and inhibiting lysis (Fig. 1F). No significant effect was observed for CT (Fig. 1C). However, comparable to 4.0 nM and 20 nM rFVIIa, 1.8–18 μM PolySTAT restored clotting rates to those observed in the absence of tPA-induced lysis in undiluted plasma (Fig. 1D). In addition, PolySTAT treatment fully restored MCF and significantly reduced lysis by 85-91% at the two higher concentrations (Fig. 1E). In diluted plasma, PolySTAT increased α-angles (Fig. 1D) and MCF (Fig. 1E) and completely inhibited lysis (Fig. 1F).
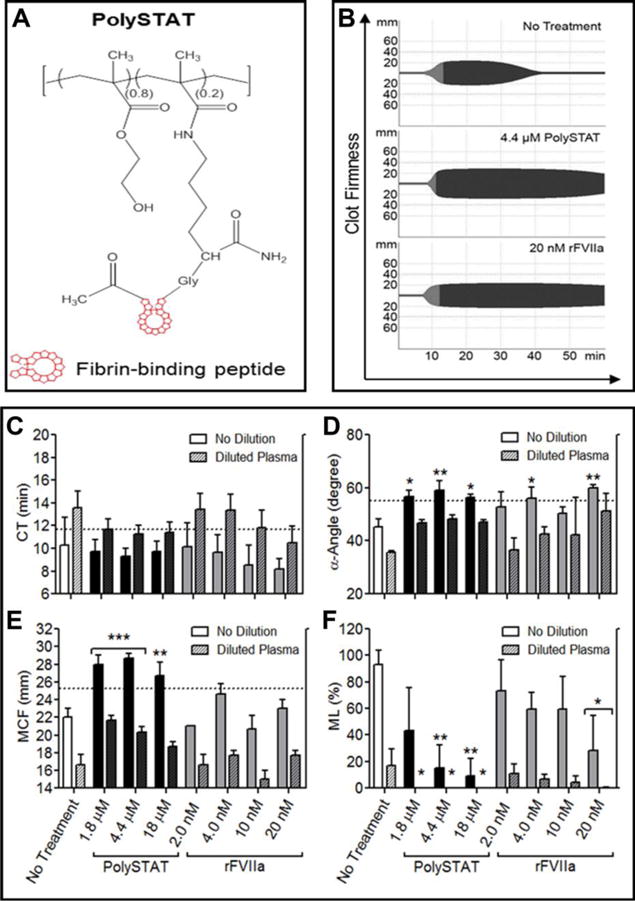
Comparison of PolySTAT to TXA
In the same sTIC plasma model, TXA showed improvements in MCF and ML compared to untreated controls (Fig. 2A, D, E) while having no effect on CT (Fig. 2B) or α-angle (Fig. 2C). In the absence of plasma dilution, 6.4 μM and 640 μM TXA increased MCF 1.2-fold, resulting in a full recovery of clot firmness(Fig. 2D). Clot lysis was completely eliminated at all TXA concentrations in both diluted and undiluted plasma (Fig. 2E).
Figure 2.
Comparison of PolySTAT and rFVIIa in an sTIC plasma model. (A) Representative ROTEM traces of untreated sTIC controls, PolySTAT-treated sTIC plasma, and TXA-treated sTIC plasma without dilution are shown. (B) Clotting time, (C) α-Angle, (D) MCF, and (E) ML were measured. Data are averages ± SD of experiment completed in triplicate. P-values were determined using one-way ANOVA and Tukey post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001 versus no treatment.
Consistent with Fig. 1, PolySTAT significantly improved clotting rate (Fig. 2C), MCF (Fig. 2D), and ML (Fig. 2E). Similar to 6.4 μM and 640 μM TXA, 1.8 μM and 4.4 μM PolySTAT significantly increased MCF 1.3-fold in undiluted plasma and 1.2-1.3-fold in diluted plasma (Fig, 2D) and reduced lysis by 91-100% (Fig. 2E).
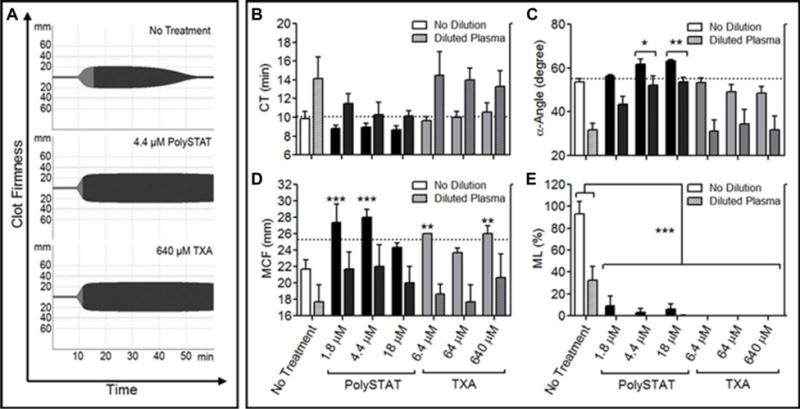
Comparison of PolySTAT to Fibrinogen Supplementation
Fibrinogen concentration in diluted plasma containing 1.5 mg/mL fibrinogen was raised by 0.5, 1.0, 1.5, and 2.0 mg/mL by adding fibrinogen (Fig. 3A). CT was shortened by 2.2 min with each additional mg/mL fibrinogen up to an additional 1.5 mg/mL (3.0 mg/mL total), beyond which, no further reduction in CT was observed (Fig. 3B). α-Angle increased by 11˚ (Fig. 3C) and MCF increased by 11 mm (Fig. 3D) with each mg/mL fibrinogen added. Fibrinogen supplementation reduced average ML (Fig. 3E). However, these changes were insignificant due to large standard deviations. 1.8 μM and 4.4 μM PolySTAT produced similar α-angles (Fig. 3C) and MCF (Fig. 3D) as 0.5 mg/mL supplemented fibrinogen. At the highest dose of supplemented fibrinogen (4.5 mg/mL total), clot lysis was comparable to that of PolySTAT-treated plasma.
Figure 3.
Comparison of PolySTAT and fibrinogen concentrate in an sTIC plasma model. Fibrinogen concentration of diluted plasma containing 1.5 mg/mL fibrinogen was raised by 0.5, 1.0, 1.5, and 2.0 mg/mL for functional comparison to PolySTAT. (A) Representative ROTEM traces are shown. (B) Clotting time, (C) α-Angle, (D) MCF, and (E) ML were measured. Data are averages ± SD of experiment completed in triplicate. P-values were determined using one-way ANOVA and Tukey post hoc test. n.s. = no significance.
Effects of PolySTAT in Combination with rFVIIa, TXA, and Fibrinogen
While neither PolySTAT alone nor fibrinogen alone significantly shortened CT, the combination of PolySTAT with fibrinogen resulted in a significant 0.4-fold shortening in CT (10±0.50 min in untreated controls to 5.9±0.84 min in combination group) (Fig. 4A). Fibrinogen alone significantly accelerated clotting rate 1.6-fold which did not increase with the addition of PolySTAT (Fig. 4B). Consistent with previous experiments, all treatments with the exception of rFVIIa produced greater average MCF compared to untreated controls (Fig. 4C). Significant differences, however, were only observed for PolySTAT alone and PolySTAT in combination with rFVIIa, TXA, and fibrinogen. rFVIIa with PolySTAT did not increase MCF above PolySTAT alone. In contrast, when combined with either TXA or fibrinogen (which each alone produced 1.3-fold greater MCF), the resulting average MCF was 1.5- and 1.7-fold greater than untreated controls. As shown before in Fig. 2E, TXA and PolySTAT were able to inhibit—if not completely eliminate—clot lysis while clots continued to break down completely when either rFVIIa or fibrinogen alone were added (Fig. 4D). PolySTAT in combination with rFVIIa, TXA, and fibrinogen retained its ability to minimize ML.
Figure 4.
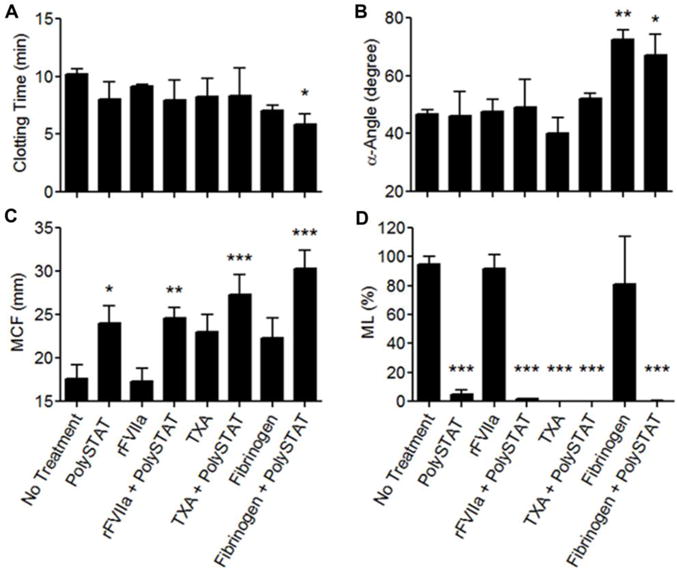
Effects of combining PolySTAT with rFVIIa, TXA, and fibrinogen on coagulation in an sTIC plasma model. (A) Clotting time, (B) α-angle, (C) MCF, and (D) ML were measured. Data are averages ± SD of experiment completed in triplicate. P-values were determined using one-way ANOVA and Tukey post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001 versus no treatment.
DISCUSSION
Multiple pathological processes interact during TIC, manifesting as prolonged clotting onset times, decreased clot firmness, and increased clot lysiswhen measured using ROTEM.27 As such, multiple blood components and/or factor concentrates are often used algorithmically in combinations to reverse coagulopathy after trauma.28 In this study, we compare a new way to restore clot formation by directly affecting fibrin polymerization using PolySTAT—both individually and in combination with several well-studied hemostatic agents.
The sTIC plasma model used in these studies is most comparable to the conditions in FIBTEM, a type of ROTEM assay which reports only the fibrin contribution to clot firmness by inhibiting platelet activity.29 Decreased clot firmness and evidence of hyperfibrinolysis on FIBTEM are strong and clinically-relevant predictors of TIC and the need for blood transfusion after trauma.23 Therefore, our fibrin-specific plasma model with tPA-induced intermediate hyperfibrinolysis (corresponding to 91% mortality7) provides a stringent method to study the effects of hemostatic agents to determine their clinical relevance. Using this model, we evaluated PolySTAT’s ability to improve fibrin-specific ROTEM parameters in comparison to rFVIIa, TXA, and fibrinogen supplementation. Additionally, we evaluated PolySTAT’s ability to restore ROTEM parameters to values generated without tPA-induced hyperfibrinolysis (indicated by dotted lines in Fig. 1–3), which would presumably restore the normal clotting phenotype.
We found that PolySTAT improved clotting two-fold—by accelerating clotting and by stabilizing the fibrin network (as evidenced by significantly reduced lysis and increased clot firmness). In contrast, rFVIIa and TXA promoted clotting by only one of the two mechanisms.
rFVIIa primarily shortened clotting onset time and increased clotting rate as expected due to faster thrombin generation. In a previous ROTEM study, rFVIIa demonstrated the same improvements while also increasing MCF.30 Increased MCF is most likely due to platelet aggregation and thrombin burst at platelet surfaces initiated by rFVIIa-TF complexation. Since platelets were absent from our system, we did not observe this effect.
In contrast to rFVIIa, TXA improved clot stability with no effect on CT or α-angle. Increased MCF was coupled with decreased ML and is likely due to TXA’s protective anti-fibrin/fibrinogenolytic effects during the clotting process which leaves more fibrinogen available to polymerize. In the CRASH-2 clinical trial, a 1 g infusion of TXA over a 10 min period followed by 1 g infusion over the subsequent 8 h was shown to improve survival rate.19 TXA is cleared renally, and over the course of an hour after a 1 g injection, TXA plasma concentrations drop from 570±35 μM (90±5.4 μg/mL) to 180±3.1 μM (28±0.49 μg/mL).31 Our results show that fibrinolytic activity is completely inhibited by 6.4 μM TXA during the 1 h clotting time. Therefore, it is not surprising that plasma concentrations 1–2 orders of magnitude greater would demonstrate hemostatic efficacy in humans.
Stepwise supplementation of fibrinogen caused an expected increase in clotting rate and maximum clot firmness with reductions in lysis that was most evident at 2.0 mg/mL supplemented fibrinogen. Fibrinogen deficiency is recognized as a critical component of TIC, and fibrinogen is typically supplemented in the form of blood products (fresh frozen plasma or cryoprecipitate) given in fixed ratios with packed red blood cells (PRBC), or by using fibrinogen concentrates. Unfortunately, ROTEM measurements taken during fixed ratio blood product resuscitation of trauma have not demonstrated improvement clot firmness that would suggest adequate fibrinogen repletion.32 Furthermore, high-dose fibrinogen concentrate or high-dose cryoprecipitate are needed to restore ROTEM MCF in blood from trauma patients with TIC.29 Our results demonstrating synergy of fibrinogen supplementation with PolySTAT suggest that fibrinogen replacement therapy may be enhanced when fibrinogen is co-administered with a fibrin cross-linker.
The multiple effects of PolySTAT on clot formation are presumably due to its cross-linking of adjacent fibrin monomers. These crosslinks increase overall clot firmness and renders the clot resistant to fibrinolysis by decreasing clot porosity and creating a semi-synthetic fibrin network bridged by plasmin-resistant polymers. Previous studies in pure fibrin systems showed that PolySTAT simultaneously accelerates clotting rate, increases clot firmness, and inhibits fibrinolysis.21 In the present study, PolySTAT was evaluated in a more complex system (human plasma) and maintained the same effects, resulting in ROTEM parameters comparable to current therapies used to treat TIC. While whole blood studies would be more relevant for in vivo translation, fibrin-specific clot formation is an important clinical parameter used to detect coagulopathy, guide therapy, and predict outcomes after trauma.29,23 The predictive value of the fibrin-specific component of clot firmness actually demonstrates better predictive value for the need for massive transfusion compared to its whole-blood equivalent that includes platelet action.33 In whole blood, it is anticipated that PolySTAT and fibrinogen effects on clot firmness would be muted due to platelet activity, and clotting kinetics accelerated due to thrombin burst generation at platelet surfaces. Therefore, our reductionist view of fibrin-specific clot formation allows us to parse out the hemostatic effects of PolySTAT and other hemostatic agents while remaining clinically relevant.
The alteration in fibrin formation and breakdown that we are able to see in the current studies suggest that fibrin cross-linking agents, such as PolySTAT, may add benefit to current hemostatic treatment approaches.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health 1R21EB018637; the Washington Research Foundation; the National Institutes of Health National Center for Advancing Translational Sciences KL2 TR00042 (NJW); and the National Institutes of Health Bioengineering Cardiovascular Training grant 2T32EB001650-06A2 (LWC).
Footnotes
Supporting Information
The dose-dependent relationship of rFVIIa and clotting time is further supported in graphs of each experimental replicate provided in the supporting information. Each replicate contains samples that were measured simultaneously with an hour time-lapse between replicates due to limited channels on the ROTEM machines. Changes in plasma enzymatic activity over time (that would partially account for standard deviations across different replicates) do not affect the trends observed within each replicate.
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of Hemorrhage on Trauma Outcome: An Overview of Epidemiology, Clinical Presentations, and Therapeutic Considerations. J Trauma Inj Infect Crit Care. 2006;60(Supplement):S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–1130. doi: 10.1097/01.ta.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 3.Frith D, Davenport R, Brohi K. Acute traumatic coagulopathy. Curr Opin Anaesthesiol. 2012;25(2):229–234. doi: 10.1097/ACO.0b013e3283509675. [DOI] [PubMed] [Google Scholar]
- 4.Fries D, Martini WZ. Role of fibrinogen in trauma-induced coagulopathy. Br J Anaesth. 2010;105(2):116–121. doi: 10.1093/bja/aeq161. [DOI] [PubMed] [Google Scholar]
- 5.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, Pittet J-F. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64(5):1211–1217. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 6.Raza I, Davenport R, Rourke C, Platton S, Manson J, Spoors C, Khan S, De’ath HD, Allard S, Hart DP, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013;11(2):307–314. doi: 10.1111/jth.12078. [DOI] [PubMed] [Google Scholar]
- 7.Schöchl H, Frietsch T, Pavelka M, Jambor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma. 2009;67(1):125–131. doi: 10.1097/TA.0b013e31818b2483. [DOI] [PubMed] [Google Scholar]
- 8.Schöchl H, Voelckel W, Maegele M, Solomon C. Trauma-associated hyperfibrinolysis. Hamostaseologie. 2012;32(1):22–27. doi: 10.5482/ha-1178. [DOI] [PubMed] [Google Scholar]
- 9.Monroe DM, Hoffman M, Roberts HR. Platelets and Thrombin Generation. Arterioscler Thromb Vasc Biol. 2002;22(9):1381–1389. doi: 10.1161/01.ATV.0000031340.68494.34. [DOI] [PubMed] [Google Scholar]
- 10.Vlachos K, Archontovasilis F, Papadima A, Maragiannis D, Aloizos S, Lagoudianakis E, Dalianoudis IG, Koronakis N, Chrysikos J, Zaravinos S, et al. Successful use of recombinant activated factor VII for postoperative associated haemorrhage: a case report. Cases J. 2008;1(1):361. doi: 10.1186/1757-1626-1-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutton RP, Parr M, Tortella BJ, Champion HR, Bernard GR, Boffard K, Bouillon B, Croce MA, Dimsits J, Holcomb JB, et al. Recombinant activated factor VII safety in trauma patients: results from the CONTROL trial. J Trauma. 2011;71(1):12–19. doi: 10.1097/TA.0b013e31821a42cf. [DOI] [PubMed] [Google Scholar]
- 12.Martinowitz U, Kenet G, Segal E, Luboshitz J, Lubetsky A, Ingerslev J, Lynn M. Recombinant Activated Factor VII for Adjunctive Hemorrhage Control in Trauma. J Trauma Inj Infect Crit Care. 2001;51(3):431–439. doi: 10.1097/00005373-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Kenet G, Walden R, Eldad A, Martinowitz U. Treatment of traumatic bleeding with recombinant factor VIIa. Lancet. 1999;354(9193):1879. doi: 10.1016/S0140-6736(99)05155-7. [DOI] [PubMed] [Google Scholar]
- 14.Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, Axelsen M, Kluger Y. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005;59(1):8–18. doi: 10.1097/01.ta.0000171453.37949.b7. [DOI] [PubMed] [Google Scholar]
- 15.Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, Leppaniemi A, Parr M, Vincent J-L, Tortella BJ, et al. Results of the CONTROL trial: efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69(3):489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- 16.Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Hunt BJ, Komadina R, Nardi G, Neugebauer E, et al. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14(2):R52. doi: 10.1186/cc8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Filipescu D, Hunt BJ, Komadina R, Nardi G, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17(2):R76. doi: 10.1186/cc12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Napolitano LM, Cohen MJ, Cotton BA, Schreiber MA, Moore EE. Tranexamic acid in trauma: How should we use it? J Trauma Acute Care Surg. 2013;74(6):1575–1586. doi: 10.1097/TA.0b013e318292cc54. [DOI] [PubMed] [Google Scholar]
- 19.Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, Herrera J, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. doi: 10.1016/s0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 20.Hagemo JS, Stanworth S, Juffermans NP, Brohi K, Cohen M, Johansson PI, Røislien J, Eken T, Næss PA, Gaarder C. Prevalence, predictors and outcome of hypofibrinogenaemia in trauma: a multicentre observational study. Crit Care. 2014;18(2):R52. doi: 10.1186/cc13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan LW, Wang X, Wei H, Pozzo LD, White NJ, Pun SH. A synthetic fibrin cross-linking polymer for modulating clot properties and inducing hemostasis. Sci Transl Med. 2015;7(277):277ra29. doi: 10.1126/scitranslmed.3010383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolodziej AF, Nair SA, Graham P, McMurry TJ, Ladner RC, Wescott C, Sexton DJ, Caravan P. Fibrin specific peptides derived by phage display: characterization of peptides and conjugates for imaging. Bioconjug Chem. 2012;23(3):548–556. doi: 10.1021/bc200613e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagemo JS, Christiaans SC, Stanworth SJ, Brohi K, Johansson PI, Goslings JC, Naess PA, Gaarder C. Detection of acute traumatic coagulopathy and massive transfusion requirements by means of rotational thromboelastometry: an international prospective validation study. Crit Care. 2015;19:97. doi: 10.1186/s13054-015-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostousov V, Wang Y, Cotton B, Wade C, Holcomb J, Matijevic N. Influence of resuscitation fluids, fresh frozen plasma and antifibrinolytics on fibrinolysis in a thrombelastography-based, in-vitro, whole-blood model. Blood Coagul Fibrinolysis. 2013;24(5):489–497. doi: 10.1097/MBC.0b013e32835e4246. [DOI] [PubMed] [Google Scholar]
- 25.Villar A, Aronis S, Morfini M, Santagostino E, Auerswald G, Thomsen HF, Erhardtsen E, Giangrande PLF. Pharmacokinetics of activated recombinant coagulation factor VII (NovoSeven) in children vs adults with haemophilia A. Haemophilia. 2004;10(4):352–359. doi: 10.1111/j.1365-2516.2004.00925.x. [DOI] [PubMed] [Google Scholar]
- 26.Klitgaard T, Tabanera y Palacios R, Boffard KD, Iau PTC, Warren B, Rizoli S, Rossaint R, Kluger Y, Riou B. Pharmacokinetics of recombinant activated factor VII in trauma patients with severe bleeding. Crit care. 2006;10(4):R104. doi: 10.1186/cc4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davenport R, Manson J, De’Ath H, Platton S, Coates A, Allard S, Hart D, Pearse R, Pasi KJ, MacCallum P, et al. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med. 2011;39(12):2652–2658. doi: 10.1097/CCM.0b013e3182281af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schöchl H, Maegele M, Solomon C, Görlinger K, Voelckel W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand J Trauma Resusc Emerg Med. 2012;20(1):15. doi: 10.1186/1757-7241-20-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport R, Stanworth S, Brohi K. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost. 2012;10(7):1342–1351. doi: 10.1111/j.1538-7836.2012.04752.x. [DOI] [PubMed] [Google Scholar]
- 30.Rea CJ, Foley JH, Ingerslev J, Sorensen B. Factor XIII combined with recombinant factor VIIa: a new means of treating severe hemophilia A. J Thromb Haemost. 2011;9(3):510–516. doi: 10.1111/j.1538-7836.2010.04171.x. [DOI] [PubMed] [Google Scholar]
- 31.Eriksson O, Kjellman H, Pilbrant A, Schannong M. Pharmacokinetics of Tranexamic Acid after Intravenous Administration to Normal Volunteers. Eur J Clin Pharmacol. 1974;7(5):375–380. doi: 10.1007/BF00558210. [DOI] [PubMed] [Google Scholar]
- 32.Khan S, Brohi K, Chana M, Raza I, Stanworth S, Gaarder C, Davenport R. Hemostatic resuscitation is neither hemostatic nor resuscitative in trauma hemorrhage. J Trauma Acute Care Surg. 2014;76(3):561–567. doi: 10.1097/TA.0000000000000146. discussion 567–568. [DOI] [PubMed] [Google Scholar]
- 33.Schöchl H, Cotton B, Inaba K, Nienaber U, Fischer H, Voelckel W, Solomon C. FIBTEM provides early prediction of massive transfusion in trauma. Crit Care. 2011;15(6):R265. doi: 10.1186/cc10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


