Abstract
Background
Amlodipine is rarely used in the treatment of pregnant hypertensive women due to limited pharmacokinetic data during pregnancy and the postpartum period.
Objective
To evaluate the pharmacokinetics of amlodipine besylate in the peri-partum period including quantities of placental passage, breast milk excretion and infant exposure.
Study Design
This was a prospective study of pregnant women who were prescribed 5 mg of amlodipine daily for treatment of chronic hypertension and delivered at term. Cord and maternal blood samples were collected at delivery. On postpartum day 2, six paired maternal plasma and breast milk samples were obtained at 4, 6, 8, 12, 15 and 24 hours following amlodipine dosing. Infant plasma samples were collected 24–48 hours after delivery. All samples were analyzed for amlodipine concentration. A one compartment, first-order model was used to calculate pharmacokinetic estimates for maternal plasma.
Results
Of the 16 patients enrolled in the study, 11 had cord blood and maternal serum collected at delivery, of which only 6 produced sufficient breast milk for sampling. Amlodipine was detected in infant cord blood plasma with a mean concentration of 0.49 ± 0.29 ng/mL compared to mean maternal serum level of 1.27 ± 0.84 ng/mL. Amlodipine concentrations in both in breast milk and infant plasma were undetectable at the lower limit of assay detection (<0.1 ng/mL). In the immediate postpartum period, the amlodipine elimination half-life was 13.7 ± 4.9 hours, the area under the curve was 53.4 ± 19.8 ng*hr/mL and the peak concentration was 2.0 ± 1.0 ng/mL.
Conclusions
Amlodipine does cross the placenta in measurable quantities, but is not detected in breast milk or infant plasma at 24 to 48 hours of life indicating that it is likely safe to use during the peripartum period.
Keywords: amlodipine, pharmacokinetics, breast milk, chronic hypertension
INTRODUCTION
Chronic hypertension affects approximately 7% of women of childbearing age and complicates about 5% of all pregnancies in the United States.1,2 Although chronic hypertension is among the most common pregnancy complications, there is significant equipoise related to the clinical management of these complicated pregnancies, particularly with regard to optimal pharmacotherapy in women requiring antihypertensive treatment. While there are many well-studied, efficacious antihypertensive regimens available in the non-pregnant population, only a limited number of such drugs have been well-studied during pregnancy and lactation.
Amlodipine besylate is a long-acting dihydropyridine calcium channel blocker commonly used in the treatment of hypertension. Amlodipine selectively inhibits transmembrane influx of calcium ions into cardiac and vascular smooth muscle, leading to decreased vascular tone, reduced systemic vascular resistance, diminished afterload and coronary vasodilation.3,4 The drug’s long half-life, infrequent dosing, low cost and positive side-effect profile5 make it an ideal candidate for treatment of chronic hypertension in pregnancy. However, there are limited data available on its use during pregnancy and lactation proscribing cautionary usage in pregnant and postpartum women. Specifically, it is not currently known whether amlodipine crosses the placenta and there are few clinical publications regarding drug levels in breast milk or potential infant side effects.6–9 We presume that this paucity of information likely hinders widespread amlodipine use in pregnancy or in the postpartum period despite its many potential benefits.
Our objective was to evaluate the pharmacokinetics of amlodipine besylate at the time of delivery and during postpartum lactation in women being treated for chronic hypertension in pregnancy. Specifically, we were interested in measuring amlodipine concentrations in cord blood, maternal serum and breast milk. Secondarily, we were interested in evaluating infant drug levels as well as any neonatal side effects potentially attributable to amlodipine exposure. We hypothesized that amlodipine crosses the placenta and likely passes into the breast milk in minimal quantities based on its pharmacokinetic properties and comparability to other drugs in its class which are safely used in breastfeeding.10–12
MATERIALS AND METHODS
Between March 2015 and January 2016, we identified and enrolled pregnant women attending the Obstetric Complications clinic at Parkland Hospital with chronic hypertension who were already taking amlodipine 5 mg daily and planned to breastfeed their infant. Additionally, the patient had to be 18 years or older at the time of enrollment with plans to deliver at Parkland Hospital. Women with preexisting kidney or liver disease were excluded. Of the eligible patients enrolled, only those women who delivered beyond 36 weeks gestation, did not require amlodipine doses in excess of 5 mg daily or additional antihypertensive medications in the peri-partum period and did not develop chorioamnionitis, endometritis or suffer postpartum hemorrhage were ultimately eligible for study inclusion. All patients eligible in the study underwent a process of informed consent prior to research participation. This study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center at Dallas. Research reported in the publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105.
Patients who consented to study participation and met all study criteria had cord blood collected at delivery and a maternal blood sample drawn within 1 hour of delivery. Patients were continued on their amlodipine dosage of 5 mg daily in the postpartum period and paired maternal blood and breast milk samples were collected on postpartum day 2 at 4, 6, 8, 12, 15 and 24 hours following amlodipine administration. In addition, the infants of study patients had study blood drawn at 24–48 hours of life in conjunction with the routine heel stick performed for newborn screening. All blood and breast milk samples were immediately placed on ice after collection. Serum samples were then centrifuged to separate plasma and all samples were frozen at −80°C for batch analysis. After all samples were collected, they were transferred to the Texas Tech School of Pharmacy for further analysis.
Amlodipine was quantitated in both plasma and breast milk using an ultra-high performance liquid chromatography/mass spectrometry (LC-MS/MS) method with a Phenomenex (Luna C8, 5 μ, 50 × 2 mm) column at 40° C. The isocratic mobile phase (0.6 mL/hr) consisted of 30 mM ammonium formate/0.1% formic acid in acetonitrile (40/60). Detection was accomplished using a Sciex 5500 QTRAP that was programmed in the positive, multiple reaction mode with monitoring of transition of the mass/charge ratio from 409.095 m/z for the precursor ion to 238.100 m/z for the product ion for amlodipine and 419.149 m/z to 343.200 m/z for the internal standard (nimodipine), respectively. The total run time was 5 minutes. The calibration curve ranged from 0.1 to 100 ng/mL (r2 > 0.99). The analytical method was demonstrated to be accurate and precise; total analytical variation (RSD%) was less than 8% for amlodipine concentrations measured throughout the range of concentrations.
Amlodipine concentrations (ng/mL) in plasma and breast milk were plotted versus time (hours) for each of the respective study patients. The best fit model was selected using Phoenix® WinNonlin® (Pharsight Corporation) to determine pharmacokinetic estimates of the time to maximum concentration (Tmax) and maximum drug concentration (Cmax), half-life (T1/2), clearance (Cl) and area-under-the-curve (AUC).
RESULTS
We enrolled 16 antepartum patients who met inclusion criteria. However, only 11 of the 16 participants had both cord blood and maternal plasma collected at the time of delivery and ultimately, only 6 of these women were able to produce sufficient breast milk to complete the study and only 8 infants had blood collected at 24–48 hours of life. The mean age of the 11 study participants was 33.2 ± 5.8 years old and on average, the participants were obese with a mean body mass index of 38.5 ± 11.4 kg/m2. The mean gestational age at delivery was 38.2 ± 0.8 weeks with the earliest delivery occurring at 37 weeks. The mean infant birth weight was 3281 ± 525 grams and the average nursery stay 3.6 ± 0.5 days. Additional maternal demographics and delivery data and infant characteristics are shown in table 1. None of the infants exposed to amlodipine required admission to the neonatal intensive care unit and none suffered major neonatal complications including intraventricular hemorrhage, apnea, seizures, periventricular leukomalacia or need for respiratory support.
Table 1.
Maternal and infant characteristics in 11 pregnancies complicated by chronic hypertension treated with amlodipine 5 mg daily.
| Characteristic | Number (%) |
|---|---|
|
| |
| Parity; | |
| 0 | 1 (9) |
| >1 | 10 (91) |
|
| |
| Race/ethnicity; | |
| Black | 3 (27) |
| Hispanic | 8 (73) |
|
| |
| Mode of delivery; | |
| Vaginal | 4 (36) |
| Cesarean | 7 (64) |
|
| |
| Severe preeclampsia* | 3 (27) |
|
| |
| Overt diabetes | 2 (18) |
|
| |
| Nursery; | |
| NICU | 0 |
| Newborn | 11 (100) |
|
| |
| 5 minute Apgar; | |
| 8 | 1 (9) |
| 9 | 10 (91) |
|
| |
| pH; | |
| ≥ 7.2 | 10 (91) |
| < 7.2 | 1 (9) |
| ≤ 7.0 | 0 |
Severe preeclampsia defined as blood pressure elevation ≥ 160 systolic or ≥ 110 diastolic, characteristic symptoms and/or laboratory evidence indicative of severe disease including thrombocytopenia (platelets <100,000/microliter), elevated hepatic transaminase concentrations (twice normal concentration) or increased serum creatinine levels (serum creatinine >1.1 mg/dL in the absence of underlying renal disease).
Amlodipine was found in measurable quantities in cord blood with a mean level of 0.49 ± 0.29 ng/mL compared to a mean maternal serum concentration 1.27 ± 0.84 ng/mL (Figure 1). In contrast, amlodipine concentrations in both maternal breast milk and infant plasma were undetectable at the lower limit of assay detection (<0.1 ng/mL).
Figure 1.
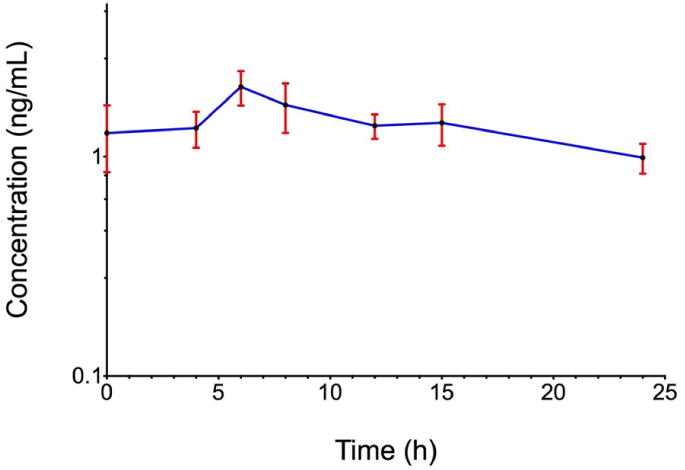
Concentrations of amlodipine besylate in maternal and cord blood plasma at the time of delivery.
First-order pharmacokinetic parameters for amlodipine besylate in maternal plasma are shown in Table 2. However, only 4 of the 6 women who had paired milk and serum samples collected in the postpartum period has results that could be fit to a one-compartment, first-order pharmacokinetic model. For these four patients, the mean peak plasma amlodipine level was 2.0 ng/mL, which was achieved 7.5 hours after dose administration (Figure 2).
Table 2.
Amlodipine pharmacokinetic parameter estimates in maternal plasma after chronic daily dosing of amlodipine 5 mg in the immediate postpartum period.
| Parameter | Plasma |
|---|---|
| AUC (hr*ng/mL) | 53.4 (19.8) |
| Tmax (hr) | 7.5 (1.5) |
| Cmax (ng/mL) | 2.0 (1.0) |
| T½ (hr) | 13.7 (4.9) |
| Cl (L/hr) | 109.7 (58.9) |
Data reported as mean ± standard deviation
AUC, area under the curve; Tmax, time to maximal concentration; Cmax, maximum serum concentration; T½, half-life; Cl, clearance rate
Figure 2.
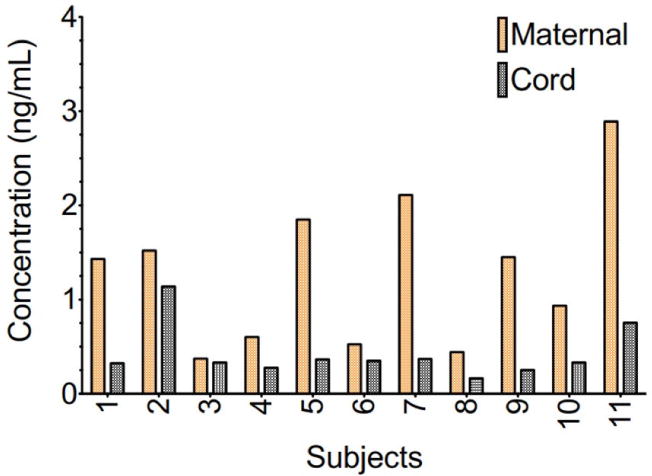
Mean maternal plasma amlodipine concentrations ± standard error of the mean in a 24-hour time period following administration of 5 mg of amlodipine besylate occurring in the immediate postpartum period.
COMMENT
Our data indicate that amlodipine does cross the placenta in measurable quantities in the third trimester of pregnancy with mean concentrations in cord blood that approximate 40% of the maternal serum concentration. Additionally, we found that amlodipine levels were below the limit of detection (<0.1 ng/mL) in breast milk as well as in infant serum collected 24–48 hours following delivery.
There have been only a handful of case reports and one observational study that have reported on amlodipine exposure in breastfeeding women.6–9 The single published clinical study by Naito and colleagues, which examined amlodipine concentrations in breast milk in 31 lactating women, found median milk concentrations of 11.5 ng/mL after a mean maternal amlodipine dose of 6 ± 2.3 mg daily.9 Based on amlodipine plasma and milk concentrations, the authors calculated the relative infant dose of amlodipine to be 4.2% of the total maternal dose, falling below the 10% threshold usually designated for breastfeeding safety.13 These observations are consistent with reports of other calcium channel antagonists, such as nifedipine and verapamil, which are known to cross into breast milk, but at low levels which are considered safe and compatible with breastfeeding.14,15 We also found that amlodipine is likely compatible with breastfeeding although our amlodipine concentrations in breastmilk were all below the limit of assay detection (<0.1 ng/mL) in contrast to the small but detectable mean milk concentration of 11.5 ng/mL cited in the aforementioned study. The discrepancy in the our concentration values may be due to both the higher daily dose used by Naito et al (6.01 ± 2.3 mg versus 5 mg) as well as inclusion of much thinner patients than were examined in our cohort (mean body weight of 61.4 kg versus 101.4 kg). Since obesity is known to significantly lower mean maternal drug concentrations and hence decrease drug crossing to breastmilk, our use of a lower mean amlodipine dose and inclusion of heavier subjects may account for the observed differences in our results. By way of example, the body weight adjusted amlodipine dose was 0.0987 mg/kg in the Naito study, significantly higher than the weight adjusted dose in our study population of 0.0493 mg/kg. Furthermore, the maternal amlodipine concentration reported in Naito and colleagues’ postpartum subjects was notably higher (15.5 ng/mL) than our peak amlodipine concentration (2.0 ng/mL) or the peak concentration reported in a non-pregnant population (2.7 ng/mL)3,17, another possible contributor to the higher amlodipine breast milk concentrations seen in their study compared to ours. With regard to the effect of amlodipine exposure during pregnancy or via breast milk on infant outcomes, the few publications available have noted no significant neonatal effects which could be reasonably attributed to drug contact6–9, which is consistent with our finding of normal newborn outcomes despite amlodipine exposure during pregnancy and breastfeeding.
We found that amlodipine is indeed detected in cord blood at delivery and these concentrations are 38% of the maternal serum amlodipine level. Despite amlodipine crossing the placenta, serum newborn amlodipine levels were below the limit of detection (<0.1 ng/mL) within 48 hours of birth. This is consistent with the findings from a single case report in which amlodipine levels in a breastfeeding infant were reported. These authors also noted an undetectable serum amlodipine concentration in a preterm infant being exclusively breastfed four days following exposure.6
When considering the pharmacokinetics of amlodipine in the puerperium, significant differences were noted in comparison to the non-pregnant population. The area under the curve (AUC), a measure of drug exposure14, was found to be 53.4 ± 19.8 ng*hr/mL in our cohort of peri-partum patients, which is approximately 45% lower than the AUC reported for non-pregnant individuals.17 The plasma half-life of amlodipine was also more than 50% shorter than noted in non-pregnant subjects, 13.7 hours compared to 33 to 35 hours.17,18 The time to maximum concentration of 7.5 hours, however, is comparable with the previously reported 6 to 12 hours.3,17 The maximum serum amlodipine concentration measured in our study was also on par with that of non-pregnant individuals, measuring 2.0 ng/mL in our pregnant patients compared to mean concentrations of 2.7 ng/mL described in non-pregnant subjects given 5 mg of amlodipine daily.3,17 While Naito et al9 noted a much higher median plasma concentration of 15.5 ng/mL in their cohort of 31 postpartum women, their study population was on average receiving a two-fold higher weight adjusted amlodipine dose compared to our study population. Overall, the noted differences in important pharmacokinetic parameters in our study subjects compared to the non-pregnant population suggest that infant exposure in pregnancy is likely reduced compared to estimates based on non-pregnant pharmacokinetic measures.
There are several limitations to our study. First, we were limited by the sensitivity of the drug assay, which only allowed detection of amlodipine concentrations to a level of ≥ 0.1 ng/mL, so while we were unable to detect amlodipine in breast milk or infant plasma, this may be a function of assay limitation rather than biologic reality. Second, the study was performed on lactating women in the immediate postpartum period. Colostrum, which is produced directly following delivery, has a higher protein concentration with lower quantities of glucose and fat compared to milk produced after established breastfeeding. These changes in milk composition directly influence the extent of drug transfer into the milk compartment and drug concentrations measured in breast milk.19 In general, these time-dependent alterations in milk composition lead to higher drug concentrations in colostrum compared to established breast milk in highly protein-bound drugs, as would be the case for amlodipine which is 93% bound to plasma proteins.4 Theoretically, this would decrease the amlodipine levels found in established breast milk compared to colostrum, which already did not have detectable amlodipine quantities in our study. Nonetheless, we cannot exclude a potential impact related to time-dependent changes in breast milk composition. Third and finally, our study population consisted almost entirely of obese women. While obesity does not appear to have a clear or consistent impact on drug volume of distribution or drug clearance, two important variables affecting drug concentrations in human plasma, increased body weight does generally lower drug concentrations in plasma. Therefore, it is possible that our results are not transferable to normal weight individuals.20
In summary, we found that amlodipine does cross the placenta in measurable quantities, but is below the limit of detection in both breast milk in the first 48 hours and infant blood measured 24 to 48 hours following delivery. Additionally, the pharmacokinetics of amlodipine are differ in this peri-partum population, with lower levels of drug exposure and a shorter half-life compared to non-pregnant individuals. Together, these findings suggest that amlodipine use during pregnancy is likely to be safe and should be considered in hypertensive women requiring treatment during pregnancy and/or postpartum.
CONDENSATION.
Amlodipine does cross the placenta in measurable quantities, but is not detected in breast milk or infant blood at 24 to 48 hours of life.
Acknowledgments
National Center for Advancing Translational Sciences of the National Institutes of Health, award number UL1TR001105
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors report no conflicts of interest
Clinical Trial ID NCT02353806; https://clinicaltrials.gov/ct2/show/NCT02353806
Presented at the 37th Annual Pregnancy Meeting, Society for Maternal-Fetal Medicine, Las Vegas NV, January 23–28, 2017
References
- 1.Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy: Executive Summary. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 2.National Health and Nutrition Examination Survey (NHANES) Centers for Disease Control and Prevention. 2011 Accessed May 21, 2014. [Google Scholar]
- 3.Norvasc Product Monograph. Pfizer Canada, Inc; Kirkland, Quebec: Available at http://www.pfizer.ca/sites/g/files/g10017036/f/201505/Norvasc_PM_E_177714_30_Jan_2015.pdf. Accessed May 24, 2014. [Google Scholar]
- 4.Burnett A. Norvasc Drug Monograph. University of New Mexico College of Pharmacy; Albuquerque, New Mexico: Accessed May 23, 2014. [Google Scholar]
- 5.Consumer Reports Health Best Buy Drugs, “Using Calcium Channel Blockers to treat high blood pressure and heart disease: comparing effectiveness, safety and price”. Best Buy Drugs (Consumer Reports) accessed May 11, 2015. [Google Scholar]
- 6.Vasa R, Martha Ramirez M. Amlodipine exposure through breastfeeding in a 32 week preterm newborn. Breastfeeding Med. 2013;8(suppl 1):S15. [Google Scholar]
- 7.Ahn HK, Nava-Ocampo AA, Han JY, et al. Exposure to amlodipine in the first trimester of pregnancy and during breastfeeding. Hypertens Pregnancy. 2007;26:179–87. doi: 10.1080/10641950701204554. [DOI] [PubMed] [Google Scholar]
- 8.Szucs KA, Axline SE, Rosenman MB. Maternal membranous glomerulonephritis and successful exclusive breastfeeding. Breastfeed Med. 2010;5:123–6. doi: 10.1089/bfm.2009.0076. [DOI] [PubMed] [Google Scholar]
- 9.Naito T, Kubono N, Deguchi S, et al. Amlodipine passage into breast milk in lactating women with pregnancy-induced hypertension and its estimation of infant risk for breastfeeding. J Hum Lact. 2015;31:301–6. doi: 10.1177/0890334414560195. [DOI] [PubMed] [Google Scholar]
- 10.Norvasc (Amlodipine besylate) tablets label. US Food and Drug Administration website. Http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019787s047lbl.pdf. Accessed May 24, 2014.
- 11.Burkey BW, Holmes AP. Evaluating medication use in pregnancy and lactation: what every pharmacist should know. J Pediatr Pharmacol Ther. 2015;18:274–58. doi: 10.5863/1551-6776-18.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berlin C, Briggs G. Drugs and chemicals in human milk. Semin Fetal Neonatal Med. 2005;10:149–59. doi: 10.1016/j.siny.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Nice FJ, Luo AC. Medications and breast-feeding: current concepts. J Am Pharm Assoc. 2012;52:86–94. doi: 10.1331/JAPhA.2012.10139. [DOI] [PubMed] [Google Scholar]
- 14.Shannon ME, Malecha SE, Cha AJ. Calcium channel antagonists and lactation: an update. J Hum Lac. 2000;16(1):60–64. doi: 10.1177/089033440001600113. [DOI] [PubMed] [Google Scholar]
- 15.Committee on Drugs, American Academy of Pediatrics. The transfer of drugs and chemicals into human milk. Pediatrics. 1994;93:137–150. [PubMed] [Google Scholar]
- 16.Twitchett H, Grimsey P. (PhUSE 2012, paper IS05).A peak at PK – an introduction to pharmacokinetics. Available from: http://www.lexjansen.com/phuse/2012/is/IS05.pdf. Accessed February 8, 2017.
- 17.Williams DM, Cubeddu LX. Amlodipine pharmacokinetics in healthy volunteers. J Clin Pharmacol. 1988;28:990–994. doi: 10.1002/j.1552-4604.1988.tb03119.x. [DOI] [PubMed] [Google Scholar]
- 18.Burges RA, Dodd MG, Gardiner DG. Pharmacologic profile of amlodipine. Am J Cardiol. 1989;64:10I–20I. doi: 10.1016/0002-9149(89)90956-9. [DOI] [PubMed] [Google Scholar]
- 19.Begg EJ, Duffull SB, Hackett LP, Ilett KF. Studying drugs in human milk: time to unify the approach. J Hum Lact. 2002;18:323–32. doi: 10.1177/089033402237904. [DOI] [PubMed] [Google Scholar]
- 20.Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49:71–87. doi: 10.2165/11318100-000000000-00000. [DOI] [PubMed] [Google Scholar]


