Abstract
The gut microbiota forms a large community that coexists with all species, including humans and rodents. Genome projects have been conducted by many researchers in nearly every country to better understand and treat diseases that lead to death in humans. However, the gut microbiota is known as a “second genome” because it includes microbes, genomic DNA, proteins, and metabolites. A large number of studies have revealed the importance of the gut microbiota. In elderly people, the diversity of the gut microbiota is reduced and there is an increased incidence of degenerative diseases, including Alzheimer’s and Parkinson’s, and decreased cognitive and memory functions. However, the administration of pre/probiotics can help to improve the symptoms of these diseases. Therefore, we believe that the gut microbiota is important for maintaining homeostasis and diversity, as well as for avoiding gastrointestinal tract-derived diseases and improving health in the elderly population.
Keywords: Gut microbiota, Gastrointestinal tract, Elderly people, Aging, Degenerative diseases
INTRODUCTION
The microbiota of the human gastrointestinal (GI) tract includes colonized microbes and their genes, proteins, and metabolites. The function of the gut microbiota in human health and disease is becoming widely recognized. Many researchers have reported on gut microbiota-related adverse events from development to senescence, including developmental defects [1,2], metabolic diseases [3], and neuro-degenerative diseases [4,5]. Additionally, dysbiosis in the gut has been associated with clinically relevant conditions, such as inflammatory bowel disease (IBD) [6], irritable bowel syndrome (IBS) [7], obesity [8], type 2 diabetes [3], Alzheimer’s disease (AD) [4], and Parkinson’s disease (PD) [5], to name a few. Accordingly, many studies have suggested microbiome-targeted approaches could be promising therapeutic strategies for various diseases through the oral administration of pre/probiotics [9,10,11] and/or application of the fecal microbiota transplantation (FMT) method [12]. However, a change in the gut microbiota related to aging is less understood. Therefore, we discuss the gut microbiota related to aging in this review for a better understanding of the relevance between dysbiosis in the gut and aging in the host.
MICROBIOTA
Many physiological actions occur via trillions of microbes in the human gut. Additionally, the biogeography in the gut is decided through the dominant phylum [13,14], which is affected by the mode of delivery, regional environment [15], diet [13], use of antibiotics [16], and age [17,18]. The gut microbiota is mainly comprised of four phyla (Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria), which occupy more than 90% of the total microbiota population. Composition of the microbiota changes during aging [19]. Gram-negative bacteria increase in number during aging and secrete lipopolysaccharide, which acts as an endotoxin and induces inflammation in the human gut [20]. The gut microbiota also affects various signaling pathways, such as inflammation and oncogenesis, as well as brain function in the hippocampus related to memory and anxiety [21].
METABOLITES
The exact mechanisms by which short chain fatty acids (SCFAs) relate to signaling pathways, including G-protein coupled receptor 41 and 43, and the genesis of obesity, colitis, and asthma, are still under investigation [22]. SCFAs are important metabolites produced by the fermentation of fiber-rich diets or indigestible carbohydrates, and are comprised of three molecules: acetate, butyrate, and propionate [8,23]. Fermentation may lead to the secretion of mucins, which protect the intestinal mucosa from entering pathogens. SCFAs and their receptors also act on the acute immune response to pathogens [24]. Therefore, the synthesis of SCFAs by the gut microbiota is an anti-inflammatory effect to reduce the incidence of diseases such as colorectal cancer [23]. However, the amount of SCFAs is decreased in elderly people. Decreased SCFAs can aggravate the immune system.
BRAIN-GUT AXIS
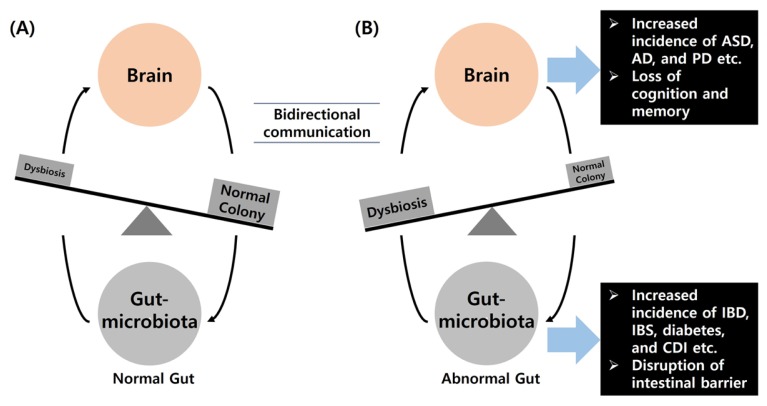
Many researchers have reported a relationship between altered gut microbiota and the brain. There is bidirectional communication between the brain and gut microbiota, which plays an important role in brain activity and the GI tract [25,26,27] (Fig. 1). The gut microbiota is not only essential for normal central nervous system development but it is also related to diseases. The expression of postsynaptic density protein (PSD) 95 is upregulated in germ-free (GF) animals compared to conventional animals. Previous studies have reported that increased expression of PSD95 is related to anxiety-like behavior in GF models [28]. The gut microbiota regulates the maturation and activity of microglia, which are formed by immune cells [29]. Thus, GF animals exhibit an impaired innate immune response. Many researchers have postulated that altered gut microbiota not only affects peripheral inflammation but also neurobehavioral specification in animal models. For example, peripheral inflammation in the gut is related to changes in the brain-gut connection. There is also a close relationship between IBS and neurobehaviors such as anxiety and depression [30,31]. Additionally, adult hippocampal neurogenesis is related to learning and memory functions, and affects neurological diseases, including AD, PD, and epilepsy [32,33]. The gut microbiota regulates the permeability of the blood brain barrier [34], which acts as a mesh in the brain and affects the genesis of vascular malformations resulting in stroke and epilepsy. Tang and colleagues reported that a cerebral cavernous malformation mouse model infected with gram-negative bacteria exhibited more abnormal vessels than the control group and microbes were discovered in their spleens [35], indicating a specific connection between the gut and brain. Investigation of the gut microbiota and their metabolites may lead to the development of new drugs for the treatment of many diseases.
Fig. 1.
Relationship between dysbiosis in the gut microbiota and disease. (A) A bidirectional communication is present between the brain and gut. The gut passes a signal to the brain via the intrinsic primary afferent nerve and the vagus nerve. The brain also regulates the gut through neurologic, immunologic, and hormone messages in various physiopathological conditions. (B) Dysbiosis in the gut microbiota affects several neurological and gut-related diseases. ASD, autism spectrum disorder; AD, Alzheimer’s disease; PD, Parkinson’s disease; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; CDI, Clostridium difficile infection.
AGING AND ITS RELATED DISEASES
Regardless of country, race, and wealth, people all over the world are concerned about well-aging because of increased life expectancy. Aging is accompanied not only by a loss of physiological function and diversity in the gut microbiota but also by reduced physical abilities, including muscle and digestive power [36,37]. Many researchers have indicated that the use of antibiotics affects altered gut microbiota in elderly people. Treatment with antibiotics in the human gut affects genetic changes [38], vitamin production [39], digestion [40], microbe diversity [41–43], the immune system [44], and colonization [45,46].
1. Alzheimer’s disease (AD)
AD is a typical neurodegenerative disease and is the most common cause of dementia in elderly people, appearing as cognitive impairment. The incidence of AD will be sustained in the future because the aging population is increasing in nearly every country [4]. A correlation between AD and the gut microbiota has been investigated; and these articles have shown that brain-derived neurotrophic factor (BDNF), a neurotrophin that plays an important role in synaptic plasticity and cognitive function, is decreased in GF animals [47,48] and in patients with AD. Accordingly, the mRNA expression of N-methyl-D-aspartate (NMDA) receptor 2A and 2B [49], which also contribute to synaptic plasticity and cognitive function, are downregulated in the cortex, hippocampus, and amygdala, respectively. However, the administration of probiotics improves cognitive function, learning, and memory, and restores the expression of BDNF [26,27].
2. Parkinson’s disease (PD)
PD is characterized both clinically and neuropathologically by neurodegenerative changes. One of the most important clinical signs of PD is that individuals with this disease are accompanied by GI symptoms [5]. Many researchers have reported that dysregulation of the brain-gut axis may affect the pathogenesis of PD [25]. Dysbiosis in the gut disrupts the epithelial barrier and alters intestinal permeability [50], which affects the immune system. Also, dysbiosis in the gut has an effect on occurrence of abnormal brain-gut axis, which builds a hierarchic four levels: enteric nerve system – prevertebral ganglia – autonomic nervous system in spinal cord – brain center including basal ganglia, results in pathogenesis of PD [5]. Additionally, intestinal inflammation may induce misfolding of α-synuclein, which plays a major role in the development of PD [51]. Inflammation in the GI tract may also lead to brain inflammation and dopaminergic neuron damage.
INTERVENTIONS
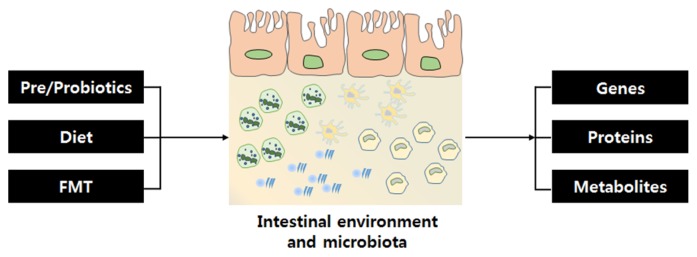
Many approaches have been recommended to maintain homeostasis and diversity of the gut microbiota in humans. Genes, proteins, and metabolites of the gut microbiota provide energy and nutrients to the host organism through fermentation and affect the integrity of the epithelial layer and offer protection from an inflammatory reaction in the GI tract [52]. One of the most important things that affects the gut is the diet (e.g., consuming a Western diet, such as high-fat, sugar-rich foods can aggravate dysbiosis) [13]. Currently, most people take prebiotics and probiotics for a healthy gut. Doctors also perform FMT for patients with IBD, IBS, chronic colitis, and Clostridium difficile infection [53] (Fig. 2).
Fig. 2.
Factors that affect the gut microbiota and gut microbiotaderived materials. Various factors, including pre/probiotics, diet, and FMT, affect homeostasis and diversity of the gut microbiota. Trillions of microbes are present in the gut along with their genes, proteins, and metabolites. Metabolites, such as SCFAs, are present due to fermentation. SCFAs, short chain fatty acids.
1. Prebiotics and probiotics
Prebiotics are selectively fermented ingredients that activate specific microbes, such as Bifidobacterium and Lactobacillus, and benefit healthy individuals as well as those with GI tract diseases [54]. A previous study reported that taking prebiotics resulted in increased Bifidobacterium, anti-inflammatory cytokines, and decreased pro-inflammatory cytokines, which may lead to reduced gut inflammation in elderly people [55,56]. The administration of prebiotics also affects the growth of beneficial microbes, such as Lactobacillus, and facilitates the synthesis of metabolites, such as SCFAs, which hamper the growth of intestinal tumors and promotes the absorption of minerals, such as calcium and magnesium, in the host gut [57].
Probiotics are defined as live microorganisms that are advantageous for a healthy gut [58]. Probiotics are used to improve or restore dysbiosis, GI tract diseases, and antibiotic treatment-related symptoms. A recent study described the benefits of probiotics in old rats. Long-term potentiation (LTP) was decreased in the hippocampus of old rats. The same researchers investigated a relationship between altered gut microbiota and reduced LTP in an aging rat model [59]. The administration of probiotics altered colonization of the gut microbiota and increased the expression of neural plasticity-related genes, such as BDNF and synapsin, and improved memory function [59,60].
2. Diet
Consuming fiber-rich diets or indigestible carbohydrates promotes the production of SCFAs via fermentation by microbes and maintains a healthy gut environment [19]. Moreover, vitamin D, which is affected by sunlight, is another important factor in the aging process. Levels of 7-dehydrocholesterol in the skin of older people are decreased compared to younger people [61]. A deficiency in vitamin D affects homeostasis and calcium absorption [62]. Elderly people do not consume as much vitamin D as younger people because of a decreased sense of taste and smell [19]. Additionally, a lack of vitamin D may alter the gut microbiota, which weakens the immune system [62]. Therefore, it is important for elderly people to consume a fiber-rich diet since these foods act as prebiotics and exert a positive effect on the gut microbiota.
3. Fecal microbiota transplantation (FMT)
FMT transfers the gut microbiota from a healthy donor to a recipient with intestinal disease. The fecal microbiota in the donor can be separated from stool and cryopreserved [53]. Transplantation of stored healthy microbiota can be transferred by various methods, including enema and oral administration. FMT normalizes the colonization and action of the gut microbiota in patients with Clostridium difficile infection [63]. Moreover, restored intestinal communities of the microbiota can suppress pathogens by various routes, such as activation of the immune system and metabolite synthesis. Elderly people want longevity without disease, and many researchers have found evidence that the gut microbiota may be involved. Smith et al. reported that the life span of older turquoise killifish was extended by recolonizing the gut microbiota by feeding them the feces of younger killifish [64].
CONCLUSION
The number of elderly people continues to increase and many elderly people are concerned with well-dying and want a high quality of life. Although the gut microbiota affects processes ranging from development to aging, we do not understand its underlying mechanisms. Previous studies have suggested that pre/probiotics exert beneficial effects in elderly people and have identified a correlation between degenerative diseases and the gut microbiota. Additionally, the administration of pre/probiotics and intake of a fiber-rich diet and indigestible carbohydrates may reduce the socio-economic cost of healthcare in the aging population. We conclude that maintaining a healthy gut microbiota may lead to a reduced incidence of disease and result in longevity.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Research Foundation (NRF-2013R1A2A2A01067169 to Y.H., NRF-2017R1D1A1B03029565 to Y.H.), and by the KRIBB Research Initiative Program (KGM6411821 to Y.H.). This work was also supported by the 2016 Creative Research Program of Inje University.
REFERENCES
- 1.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–36. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q, Han Y, Dy ABC, Hagerman RJ. The gut microbiota and autism spectrum disorders. Front Cell Neurosci. 2017;11:120. doi: 10.3389/fncel.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 4.Jiang C, Li G, Huang P, Liu Z, Zhao B. The gut microbiota and alzheimer’s disease. J Alzheimers Dis. 2017;58:1–15. doi: 10.3233/JAD-161141. [DOI] [PubMed] [Google Scholar]
- 5.Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol. 2015;21:10609–20. doi: 10.3748/wjg.v21.i37.10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes care. 2010;33:2277–84. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nobutani K, Sawada D, Fujiwara S, Kuwano Y, Nishida K, Kutsumi H, Azuma T, Rokutan K. The effects of administration of the Lactobacillus gasseri strain CP2305 on quality of life, clinical symptoms and changes in gene expression in patients with irritable bowel syndrome. J Appl Microbiol. 2017;122:212–24. doi: 10.1111/jam.13329. [DOI] [PubMed] [Google Scholar]
- 10.Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539–46. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 11.Lambert JE, Parnell JA, Eksteen B, Raman M, Bomhof MR, Rioux KP, Madsen KL, Reimer RA. Gut microbiota manipulation with prebiotics in patients with non-alcoholic fatty liver disease: a randomized controlled trial protocol. BMC Gastroenterol. 2015;15:169. doi: 10.1186/s12876-015-0400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, Weese JS, Collins S, Moayyedi P, Crowther M, Ropeleski MJ, Jayaratne P, Higgins D, Li Y, Rau NV, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent clostridium difficile infection: A randomized clinical trial. JAMA. 2016;315:142–9. doi: 10.1001/jama.2015.18098. [DOI] [PubMed] [Google Scholar]
- 13.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015;350:1214–5. doi: 10.1126/science.aac8469. [DOI] [PubMed] [Google Scholar]
- 15.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336:1255–62. doi: 10.1126/science.1224203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodmansey EJ. Intestinal bacteria and ageing. J Appl Microbiol. 2007;102:1178–86. doi: 10.1111/j.1365-2672.2007.03400.x. [DOI] [PubMed] [Google Scholar]
- 18.Saraswati S, Sitaraman R. Aging and the human gut microbiota-from correlation to causality. Front Microbiol. 2015;5:764. doi: 10.3389/fmicb.2014.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar M, Babaei P, Ji B, Nielsen J. Human gut microbiota and healthy aging: Recent developments and future prospective. Nutr Healthy Aging. 2016;4:3–16. doi: 10.3233/NHA-150002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiffrin EJ, Morley JE, Donnet-Hughes A, Guigoz Y. The inflammatory status of the elderly: the intestinal contribution. Mutat Res. 2010;690:50–6. doi: 10.1016/j.mrfmmm.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Murphy T, Dias GP, Thuret S. Effects of diet on brain plasticity in animal and human studies: mind the gap. Neural Plast. 2014;2014:563160. doi: 10.1155/2014/563160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ang Z, Ding JL. GPR41 and GPR43 in obesity and inflammation – protective or causative? Front Immunol. 2016;7:28. doi: 10.3389/fimmu.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661–72. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 24.Corrêa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology. 2016;5:e73. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu X, Han Y, Du J, Liu R, Jin K, Yi W. Microbiotagut-brain axis and the central nervous system. Oncotarget. 2017;8:53829–38. doi: 10.18632/oncotarget.17754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–17. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 27.Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y, Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–77. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 28.Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–52. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iadecola C. Dangerous leaks: blood-brain barrier woes in the aging hippocampus. Neuron. 2015;85:231–3. doi: 10.1016/j.neuron.2014.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan WI, Corthesy-Theulaz I, Cherbut C, Collins SM, et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–12. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 31.O’Mahony SM, Felice VD, Nally K, Savignac HM, Claesson MJ, Scully P, Woznicki J, Hyland NP, Shanahan F, Quigley EM, Marchesi JR, O’Toole PW, Dinan TG, Cryan JF. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience. 2014;277:885–901. doi: 10.1016/j.neuroscience.2014.07.054. [DOI] [PubMed] [Google Scholar]
- 32.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 33.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–60. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 34.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Kundu P, Gulyás B, Halldin C, Hultenby K, Nilsson H, Hebert H, et al. The gut microbiota infulences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang AT, Choi JP, Kotzin JJ, Yang Y, Hong CC, Hobson N, Girard R, Zeineddine HA, Lightle R, Moore T, Cao Y, Shenkar R, Chen M, Mericko P, Kahn ML, et al. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature. 2017;545:305–10. doi: 10.1038/nature22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langille MG, Meehan CJ, Koenig JE, Dhanani AS, Rose RA, Howlett SE, Beiko RG. Microbial shifts in the aging mouse gut. Microbiome. 2014;2:50. doi: 10.1186/s40168-014-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211:144–56. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prudhomme M, Attaiech L, Sanchez G, Martin B, Claverys JP. Antibiotic stress induces genetic trans-formability in the human pathogen streptococcus pneumonia. Science. 2006;313:89–92. doi: 10.1126/science.1127912. [DOI] [PubMed] [Google Scholar]
- 39.Tran B, Armstrong BK, Ebeling PR, English DR, Kimlin MG, van der Pols JC, Venn A, Gebski V, Whiteman DC, Webb PM, Neale RE. Effect of vitamin D supplementation on antibiotic use: a randomized controlled trial. Am J Clin Nutr. 2014;99:156–61. doi: 10.3945/ajcn.113.063271. [DOI] [PubMed] [Google Scholar]
- 40.Pérez-Cobas AE, Gosalbes MJ, Friedrichs A, Knecht H, Artacho A, Eismann K, Otto W, Rojo D, Bargiela R, von Bergen M, Neulinger SC, Däumer C, Heinsen FA, Latorre A, Moya A, et al. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut. 2013;62:1591–601. doi: 10.1136/gutjnl-2012-303184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sekirov I, Tam NM, Jogova M, Robertson ML, Li Y, Lupp C, Finlay BB. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect Immun. 2008;76:4726–36. doi: 10.1128/IAI.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, Young VB. Decreased diversity of the fecal microbiome in recurrent clostridium difficile-associated diarrhea. J Infect Dis. 2008;197:435–8. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 43.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boursi B, Mamtani R, Haynes K, Yang YX. The effect of past antibiotic exposure on diabetes risk. Eur J Endocrinol. 2015;172:639–48. doi: 10.1530/EJE-14-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, Yu Z, Newburg DS, Ward DV, Schibler KR. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of enterobacter. J Pediatr. 2014;165:23–9. doi: 10.1016/j.jpeds.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39–50. doi: 10.1016/j.cell.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leung K, Thuret S. Gut microbiota: A modulator of brain plasticity and cognitive function in ageing. Healthcare (Basel) 2015;3:898–916. doi: 10.3390/healthcare3040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li F, Tsien JZ. Memory and the NMDA receptors. N Engl J Med. 2009;361:302–3. doi: 10.1056/NEJMcibr0902052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galland L. The gut microbiome and the brain. J Med Food. 2014;17:1261–72. doi: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H, Coron E, Bruley des Varannes S, Naveilhan P, Nguyen JM, Neunlist M, Derkinderen P. Colonic inflammation in Parkinson’s disease. Neurobiol Dis. 2013;50:42–8. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Chambers ES, Morrison DJ, Frost G. Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms? Proc Nutr Soc. 2015;74:328–36. doi: 10.1017/S0029665114001657. [DOI] [PubMed] [Google Scholar]
- 53.Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13:508–16. doi: 10.1038/nrgastro.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson PC, Klinder A, O’Riordan M, O’Sullivan GC, Pool-Zobel B, Rechkemmer G, Roller M, Rowland I, Salvadori M, Collins JK, et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr. 2007;85:488–96. doi: 10.1093/ajcn/85.2.488. [DOI] [PubMed] [Google Scholar]
- 55.Vulevic J, Drakoularakou A, Yaqoob P, Tzortzis G, Gibson GR. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr. 2008;88:1438–46. doi: 10.3945/ajcn.2008.26242. [DOI] [PubMed] [Google Scholar]
- 56.Schiffrin EJ, Thomas DR, Kumar VB, Brown C, Hager C, Van’t Hof MA, Morley JE, Guigoz Y. Systemic inflammatory markers in older persons: the effect of oral nutritional supplementation with prebiotics. J Nutr Health Aging. 2007;11:475–9. [PubMed] [Google Scholar]
- 57.De Preter V, Hamer HM, Windey K, Verbeke K. The impact of pre- and/or probiotics on human colonic metabolism: does it affect human health? Mol Nutr Food Res. 2011;55:46–57. doi: 10.1002/mnfr.201000451. [DOI] [PubMed] [Google Scholar]
- 58.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 59.Distrutti E, O’Reilly JA, McDonald C, Cipriani S, Renga B, Lynch MA, Fiorucci S. Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age-related deficit in LTP. PLoS One. 2014;9:e106503. doi: 10.1371/journal.pone.0106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Magsood R, Stone TW. The gut-brain axis, BDNF, NMDA and CNS disorders. Neurochem Res. 2016;41:2819–35. doi: 10.1007/s11064-016-2039-1. [DOI] [PubMed] [Google Scholar]
- 61.Oudshoorn C, van der Cammen TJ, McMurdo ME, van Leeuwen JP, Colin EM. Ageing and vitamin D deficiency: effects on calcium homeostasis and considerations for vitamin D supplementation. Br J Nutr. 2009;101:1597–606. doi: 10.1017/S0007114509338842. [DOI] [PubMed] [Google Scholar]
- 62.Ly NP, Litonjua A, Gold DR, Celedón JC. Gut microbiota, probiotics, and vitamin D: interrelated exposures influencing allergy, asthma, and obesity? J Allergy Clin Immunol. 2011;127:1087–94. doi: 10.1016/j.jaci.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weingarden A, González A, Vázquez-Baeza Y, Weiss S, Humphry G, Berg-Lyons D, Knights D, Unno T, Bobr A, Kang J, Khoruts A, Knight R, Sadowsky MJ. Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome. 2015;3:10. doi: 10.1186/s40168-015-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith P, Willemsen D, Popkes M, Metge F, Gandiwa E, Reichard M, Valenzano DR. Regulation of life span by the gut microbiota in the short-lived African turquoise killifish. Elife. 2017:6. doi: 10.7554/eLife.27014. [DOI] [PMC free article] [PubMed] [Google Scholar]