Abstract
Background
Sleep disorders can negatively affect quality of life with reduced cognitive function. Since stress and eating behavior are considered crucial factors for sleep, this study’s aim was to compare objective quality of sleep and nutrition between subjects with sleep issues and different stress levels.
Methods
The investigation was performed in adults (≥18 years old) in the Sleep Laboratory between September 2015 and February 2016. Several measurement instruments were utilized, including the Pittsburgh Sleep Quality Index (PSQI), the Korean version of the Brief Encounter Psychosocial Instrument (BEPSI-K), polysomnography, and a food frequency questionnaire (FFQ).
Results
There were no statistical differences in demographic data between the lower and higher stress groups except age, which was adjusted. Sleep quality in the lower stress group was better than the higher stress group in terms of sleep efficiency (90.92 ± 7.72 vs 85.36 ± 10.25%), sleep latency (16.94 ± 20.86 vs 9.42 ± 8.24 min), and WASO result (26 ± 26.13 vs 43.66 ± 41.32 min). There were statistically significant differences in nutritional intake between the groups: the lower stress group consumed more vegetables than the higher stress group while the higher stress group consumed more grains, meat and eggs, soju, and coffee products than the lower stress group.
Conclusion
This study found that stress level and food intake have significant associations with objective sleep quality. Food and stress management for insomnia should be considered for improving not only quantity but also quality of sleep.
Keywords: Sleep disorders, Intrinsic feeding behavior, Stress, Psychological lifestyle
INTRODUCTION
Sleep is a basic physiological function and one of the essential factors that affects immunity, thermoregulation, homeostasis maintenance, and recovery [1]. Sleep disorders, low quality or lack of sleep, result in reduced cognitive functions including a deficiency in concentration or poor decision making; consequently, sleep disorders can have a negative impact on quality of life [2,3].
There are several psychological factors that affect sleep, for example anxiety, depression, and stress [4]. In particular, stress is one direct factor influencing health, while physical exercise, smoking, and eating behaviors can indirectly impact health through the alteration of direct factors [5].
Studies on the relationship between stress and food consumption had shown that chronic life stress may be associated not only with increased amounts of food consumption, but also consumption of greater energy- and nutrient-dense foods that are high in sugar and fat [6,7,8]. To be specific, while excessive acute stress could reduce appetite [7], chronic life stress could lead to increased desire for food with eating patterns of having energy- and nutrient-dense products that might result in obesity [8]. The aim of this study was to compare objective sleep quality and nutritional intake between patients with sleep issues and different stress levels.
MATERIALS AND METHODS
This study was conducted in accordance with the principles of the Declaration of Helsinki and ethical standards of conduct for clinical trials, and approved by the Hannam University Institutional Review Board (No. 15-03-04-0925). The research was performed between September 2015 and February 2016 in the S Sleep Laboratory in Seoul, South Korea. The participants were age 18 years or older and were initially screened with the Pittsburgh Sleep Quality Index (PSQI) [9].
1. Measurements
1) Stress
The Korean translated Brief Encounter Psychosocial Instrument (BEPSI-K) [10], which is the Korean version of the modified BEPSI for evaluating stress levels, consists of five questions. Each question is answered on a 1- to 5-point scale according to the subject’s judgment: 1 to 5 points corresponding to “no event”, “a few times”, “often”, “very often”, and “always” respectively. The points from the five questions are summed, divided by five, and then defined as one of three groups: 1.6 and less is “low stress”, above 1.6 and below 2.2 is “moderate stress”, and 2.2 and above is “high stress” [10]. In the study, the results of the BEPSI-K were defined into one of two groups: a low stress group (lower stress group, ≤ 1.6) and moderate and high stress group (higher stress group > 1.6).
2. Polysomnography
The Embla N7000 (Natus Medical Inc., Pleasanton, CA, USA) was used for polysomnography (PSG).
1) Nutrition assessment
To collect dietary information, 24-hour recall data were obtained by a trained nutritionist. A food diary was collected on a participant’s following visit after having been instructed to write in a food diary for three days total, two weekdays and one weekend day. A food frequency questionnaire (FFQ) from the Korea National Health and Nutrition Examination Survey (KNHANES) [11] was selected and modified by adding questions on 79 foods known to disturb sleep, including coffee, milk-coffee, green tea, black tea, and chocolate. The answer options of intake frequency for food groups were set as follows: three times per day, twice per day, once per day, five to six per week, three to four per week, one to two per week, two to three per month, and less than once per month. A photograph of serving sizes for each food was shown to the participants in order to aid in the calculation of amounts the participants had consumed.
To analyze the data from the questionnaire, the Computer-Aided Nutritional analysis program (CAN Pro 4.0, Korean Nutrition Society, Seoul, Korea), which is based on the Dietary Reference Intakes for Koreans (KDRIs) [12], was utilized.
3. Statistics
In this study, descriptive statistics of continuous data were presented as the mean, standard deviation (SD), and the 95% confidence interval of the mean. For categorical data, frequency and percent were used for analysis. The statistical significance of data, including demographic data, polysomnography findings, and investigational group results were tested by the Student’s t-test and adjusting age with Analysis of Covariance (ANCOVA). The Pearson’s correlation was used to determine correlations. Statistical significance was determined at the level of p < 0.05. All data analyses were performed with SPSS, Version 23.0 (IBM, New York, NY, USA).
RESULTS
1. Participant demographics
The demographics of the subjects are shown in Table 1. There were no statistical differences in attributes between the lower and higher stress groups except for age (lower stress group, 40.7 ± 14.8 years; higher stress group, 29.0 ± 10.1 years; p < 0.001). In the lower stress group, 26 males and 31 females participated, and the mean body mass index (BMI) of subjects in the group was 22.7 ± 3.1 kg/m2. The higher stress group consisted of 21 men and 11 women with a mean BMI of 23.4 ± 3.2 kg/m2. BMI and other variables including smoking, drinking, and physical activity were not significantly different between the groups.
Table 1.
General characteristics of the study subjects
| Variables | Category | Low Stress† (n = 57) | High Stress† (n = 32) | p-value* |
|---|---|---|---|---|
| Age | 40.7 ± 14.8 | 29.0 ± 10.1 | 0.000 | |
| Gender (n) | Total | 57 | 32 | 0.069 |
| Male | 26 | 21 | ||
| Female | 31 | 11 | ||
| BMI (kg/m2)‡ | 22.7 ± 3.1 | 23.4 ± 3.2 | 0.315 | |
| Smoking (n, %) | 0.368 | |||
| none | 39 (78.0) | 15 (62.5) | ||
| Smoking (past) | 4 (8.0) | 3 (12.5) | ||
| Smoking (current) | 7 (14.0) | 6 (25.0) | ||
| Drinking (n, %) | 0.092 | |||
| none | 16 (32.0) | 3 (12.5) | ||
| 1 time/month | 12 (24.0) | 6 (25.0) | ||
| 2–4 times/month | 18 (36.0) | 8 (33.3) | ||
| 2–4 times/week | 4 (8.0) | 6 (25.0) | ||
| >4 times/week | 0 (0.0) | 1 (4.2) | ||
| Physical activity (n, %) | 0.655 | |||
| Sedentary | 0 (0.0) | 0 (0.0) | ||
| Low exercise level | 20 (40.0) | 7 (29.2) | ||
| Moderate exercise level | 26 (52.0) | 15 (62.5) | ||
| High exercise level | 4 (8.0) | 2 (8.3) |
p-values were calculated using a t-test, Chi-squared test.
BEPSI-K, Korean version of the Brief Encounter Psychosocial Instrument; Low stress ≤ 1.6, High Stress > 1.6.
BMI, body mass index.
2. Polysomnography
In general, the lower stress group showed better quality of sleep than the higher stress group (Table 2). There were statistically significant observations from the sleep assessment, including wake after sleep onset (WASO), sleep efficiency, sleep latency, total wake time (TWT), and the percentage of sleep stage 2.
Table 2.
General polysomnography characteristics
| Variables | Category | Low Stress† (n = 57) | High Stress† (n = 32) | p-value* |
|---|---|---|---|---|
| Total sleep time (min) | 359.9 ± 29.0 | 357.3 ± 41.9 | 0.287 | |
| WASO (min) | 26.6 ± 26.1 | 43.6 ± 41.3 | 0.041 | |
| Sleep Efficiency (%) | 90.9 ± 7.7 | 85.3 ± 10.2 | 0.004 | |
| Sleep Latency (min) | 9.4 ± 8.2 | 16.9 ± 20.8 | 0.049 | |
| REM Latency (min) | 96.3 ± 45.2 | 100.2 ± 59.4 | 0.917 | |
| REM (%) | 18.7 ± 5.4 | 18.7 ± 5.6 | 0.535 | |
| TWT (%) | 6.6 ± 6.4 | 10.6 ± 9.9 | 0.013 | |
| Stage 1 (%) | 7.3 ± 3.7 | 7.3 ± 4.8 | 0.777 | |
| Stage 2 (%) | 63.7 ± 8.5 | 54.0 ± 11.8 | 0.002 | |
| Stage 3 (%) | 1.0 ± 3.6 | 5.1 ± 6.2 | 0.026 | |
| AHI | 23.1 ± 17.7 | 10.9 ± 14.3 | 0.230 | |
| Obs AI | 3.2 ± 6.7 | 2.9 ± 7.0 | 0.104 | |
| Cent AI | 1.3 ± 4.2 | 0.8 ± 1.2 | 0.846 | |
| HI | 18.5 ± 13.2 | 7.1 ± 9.0 | 0.015 |
WASO, wake after sleep onset; REM, rapid eye movement sleep; TWT, total wake time; Stage 1 (%), Stage 2 (%), or Stage 3 (%), non-rapid eye movement sleep stage; AHI, apnea-hypopnea index (Obs AI + Cent AI + HI); Obs AI, obstructive apnea arousal index; Cent AI, central apnea arousal index; HI, hypopnea index.
p-values were calculated by an independent t-test, polysomnographic variables were adjusted for age.
BEPSI-K, Korean version of the Brief Encounter Psychosocial Instrument; Low stress ≤ 1.6, High Stress > 1.6.
The participants in the lower stress group showed less time in WASO (26.6 ± 26.1 min) than those in the higher stress group (43.6 ± 41.3 min) (p = 0.041). Greater sleep efficiency (90.9 ± 7.7%) was observed in the lower stress group than was observed in the higher stress group (85.3 ± 10.2%) (p = 0.004). Shorter sleep latency was observed in the lower group (9.4 ± 8.2) than was observed in the higher stress group (16.9 ± 20.8; p = 0.049). The mean value of TWT in the higher stress group was 10.6 ± 9.9%, which was greater than the value in the lower stress group (6.6 ± 6.4%) (p = 0.013).
Total sleep times in the lower and higher stress groups were 359.9 ± 29.0 min and 357.3 ± 41.9 min, respectively (p = 0.287).
3. Nutrition
The average nutritional intake between the lower (1735.2 ± 394.3 kcal) and the higher (1662.9 ± 459.8 kcal) stress groups did not significantly differ (Table 3). The intake of carbohydrates, fats, and protein in the lower stress group was 250.5 ± 59.2 g, 51.2 ± 21.6 g, 68.1 ± 20.6 g, respectively; and intake in the higher stress group was 231.8 ± 57.3 g, 50.8 ± 21.1 g, 62.6 ± 19.1 g, respectively (p > 0.05). The proportion of nutritional intake in both groups met the nutrient recommendations according to the Dietary Reference Intake for Koreans (2015) (carbohydrates, 55–65%; fats, 15–30%; protein, 7–20%) [12] showing the figures of 58.5 ± 10.1% (carbohydrate), 26.0 ± 7.8% (fat), and 15.6 ± 2.6% (protein) in the lower stress group; the figures of 56.8 ± 9.0%, 26.8 ± 7.0%, 15.0 ± 2.4% in the higher group (p > 0.05).
Table 3.
Macronutrient and micronutrient intake per calorie status as recorded by a 24-hr food diary
| Variables | Low Stress† (n = 57) | High Stress† (n = 32) | p-value* |
|---|---|---|---|
| Total energy intake (kcal) | 1735.2 ± 394.32) | 1662.9 ± 459.8 | 0.286 |
| Carbohydrate (g) | 250.5 ± 59.2 | 231.8 ± 57.3 | 0.344 |
| Fat (g) | 51.2 ± 21.6 | 50.8 ± 21.1 | 0.237 |
| Protein (g) | 68.1 ± 20.6 | 62.6 ± 19.1 | 0.236 |
| Carbohydrates, % of E‡ | 58.5 ± 10.1 | 56.8 ± 9.0 | 0.793 |
| Fat, % of E‡ | 26.0± 7.8 | 26.8 ± 7.0 | 0.420 |
| Protein, % of E‡ | 15.6 ± 2.6 | 15.0 ± 2.4 | 0.662 |
| Dietary fiber (g) | 18.4 ± 6.02) | 14.0 ± 4.1 | 0.092 |
| Vitamin A (ug RE) | 1698 ± 1158.2 | 1236.6 ± 537.7 | 0.035 |
| Vitamin D (ug) | 2.8 ± 2.6 | 3.50 ± 2.9 | 0.429 |
| Vitamin E (mg) | 15.9 ± 4.9 | 15.0 ± 8.4 | 0.542 |
| Vitamin K (ug) | 175.9 ± 87.8 | 129.3 ± 71.6 | 0.211 |
| Vitamin C (mg) | 93.2 ± 52.3 | 62.3 ± 31.9 | 0.260 |
| Thiamin (mg) | 1.2 ± 0.3 | 1.1 ± 0.4 | 0.227 |
| Riboflavin (mg) | 1.1 ± 0.4 | 0.9 ± 0.3 | 0.208 |
| Niacin (mg) | 14.4 ± 4.4 | 14.1 ± 4.7 | 0.781 |
| Vitamin B6 (mg) | 1.5 ± 0.4 | 1.3 ± 0.5 | 0.418 |
| Folate (ug) | 435.1 ± 135.1 | 363.3 ± 128.2 | 0.371 |
| Vitamin B12 (ug) | 8.0 ± 5.8 | 5.3 ± 2.6 | 0.210 |
| Pantothennic acid (mg) | 4.3 ± 1.3 | 4.2 ± 1.4 | 0.727 |
| Biotin (ug) | 18.0 ± 8.2 | 17.8 ± 8.8 | 0.970 |
| Total Ca (mg) | 492.9 ± 227.4 | 368.6 ± 114.1 | 0.135 |
| P (mg) | 986.0 ± 278.4 | 872.0 ± 221.8 | 0.237 |
| Na (mg) | 3773.9 ± 1353.4 | 3482.9 ± 1151.4 | 0.559 |
| K (mg) | 2488.6 ± 852.3 | 1914.4 ± 501.8 | 0.183 |
| Mg (mg) | 82.7 ± 42.2 | 63.2 ± 29.5 | 0.257 |
| Total iron (mg) | 14.1 ± 4.1 | 11.4 ± 3.9 | 0.129 |
| Zn (mg) | 9.7 ± 3.0 | 8.6 ± 2.2 | 0.203 |
| Cu (mg) | 1.0 ± 0.3 | 0.9 ± 0.2 | 0.423 |
| Cholesterol (mg) | 343.1 ± 165.0 | 366.7 ± 168.6 | 0.983 |
| Total fatty acid (g) | 27.7 ± 14.4 | 28.7 ± 16.1 | 0.517 |
| Saturated fatty acid (g) | 8.8 ± 5.3 | 9.3 ± 5.7 | 0.841 |
| Monounsaturated fatty acid(g) | 11.6 ± 6.6 | 11.8 ± 6.9 | 0.692 |
| Polyunsaturated fatty acid (g) | 8.9 ± 3.9 | 8.9 ± 5.1 | 0.482 |
p-values were calculated using an independent t-test, variables were adjusted for age.
BEPSI-K, Korean version of the Brief Encounter Psychosocial Instrument; Low stress ≤ 1.6, High Stress > 1.6.
Total energy intake (kcal)/Nutrient (kcal) × 100.
According to the reported food frequency questionnaire (FFQ), there were significant differences in the intake of grains, meat and eggs, and vegetables (Table 4). Participants in the higher stress group consumed about twice as many grains (939.1 ± 581.5 g/day) as the lower stress group (492.5 ± 589.6 g/day) (p = 0.041). Furthermore, the mean amount of meat and eggs consumed by the higher stress group (217.4 ± 253 g/day) was more than twice the mean of the lower stress group (92.6 ± 78.5 g/day) (p = 0.023). Vegetable intake was also significantly different; the higher stress group (124.1 ± 104.7 g/day) consumed approximately half the amount the lower stress group consumed (239.5 ± 164.0 g/day) (p = 0.005). Specifically, intake of white rice and rice mixed with grain in the higher stress group was greater than the lower stress group (p < 0.05). Consumption of pork in the meat and eggs category was similarly skewed (p < 0.05). In the fish and shellfish category, salted seafood was consumed more in the lower stress group than in the higher stress group (p < 0.05). There was less consumption of vegetables including radish, lettuce, cabbage and tomato among the higher stress subjects than the lower stress subjects. Banana consumption was lesser in the lower stress group than in the higher stress group (p < 0.05).
Table 4.
Intake of grains, beans, meat and eggs, and fish and shellfish per day as recorded by the food frequency questionnaire
| Variables | Low Stress† (n = 57) | High Stress† (n = 32) | p-value* |
|---|---|---|---|
| Grains (g/day)‡ | 492.5 ± 589.6 | 939.1 ± 581.5 | 0.041 |
| Beans (g/day)‡ | 55.4 ± 101.6 | 66.4 ± 83.0 | 0.911 |
| Meat and Eggs (g/day)‡ | 92.6 ± 78.5 | 217.4 ± 253.0 | 0.023 |
| Fish and Shellfish (g/day)‡ | 52.9 ± 59.1 | 46.6 ± 73.3 | 0.246 |
| Vegetables (g/day)‡ | 239.5 ± 164.0 | 124.1 ± 104.7 | 0.005 |
| Seaweed (g/day)‡ | 7.5 ± 10.0 | 9.0 ± 13.4 | 0.352 |
| Fruit s (g/day)‡ | 383.1 ± 490.6 | 171.3 ± 196.7 | 0.409 |
p-values were calculated by an independent t-test, variables were adjusted for age.
BEPSI-K, Korean version of the Brief Encounter Psychosocial Instrument; Low stress ≤ 1.6, High Stress > 1.6.
Grains: white rice, rice mixed with grain, ramyun, noodle, bread and cake, rice cake, potato, sweet potato, and snack;
Beans: soybean curd, braised bean, and soybean milk (begimill); Meat and Eggs: beef, pork, chicken, ham, sausage, and Egg; Fish and Sellfish: mackerel, tuna, croaker, alaska pollock, dried anchovy, common squid, fish cake, shellfish, and salted seafood; Vegetables: chiness cabbage, radish, radish leaves, soybean sprout, spinach, cucumber, red pepper, carrot, pumkin, lettuce, cabbage, tomato, and mushroom; Seeweed: sea mustard, and laver; Fruits: citrus, persimmon, pear, watermelon, oriental melon, strawberry, grape, peach, apple, and banana.
For drink intake, milk consumption in the higher stress group was less than in the lower stress group. However, drinks that contained alcohol and general beverages were more than twice that in the higher stress group (drink and liquor, beverages (g/day): 131.4 ± 227.5, 561.1 ± 894.1) than in the lower stress group (49.6 ± 77.4, 225.5 ± 286.3) (p = 0.008 and p = 0.005, respectively). In particular, soju (Korean liquor) intake in the higher stress group (87.8 ± 167.5 g/day) was more than four times than was observed in the lower stress group (19.7 ± 59.9 g/day) (p = 0.004). The consumption of coffee and tea also showed statistically significant differences. The amount of coffee mix consumed by the higher stress group (20.5 ± 35.6 g/day) was three times more than that in the lower stress group (6.1 ± 6.8 g/day); brewed coffee was nearly 13 times greater in the higher stress group than in the lower stress group (440.8 ± 887.2 g/day and 34.7 ± 221.3 g/day, respectively) (p = 0.000). The higher stress group consumed approximately 10 times less tea (1.2 ± 2.8 g/day) than the lower stress group consumed (13.0 ± 22.2 g/day) (p = 0.045).
DISCUSSION
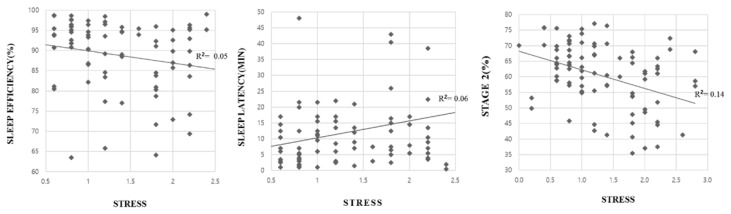
We examined polysomnography and nutritional intake between lower and higher stress groups. The results from polysomnography showed a total sleep time of 359.9 ± 29.0 min in the lower stress group and 357.3 ± 41.9 min in the higher stress group. Both groups showed a lack of total sleep time as compared to the sleep recommendations (7–9 hours) from the National Sleep Foundation [13]; although, this difference was not statistically significant (p = 0.287). Generally, quality of sleep in the lower stress group was better than in the higher stress group. Sleep efficiency was also lower in the higher stress group (85.36 ± 10.25%) than in the lower stress group (90.92 ± 7.72%). Furthermore, sleep latency was longer in the higher stress group (16.94 ± 20.86 min) as compared to the lower stress group (9.42 ± 8.24 min), which indicated that the time to get to sleep was more prolonged in the higher stress group than in the lower stress group. We also found a negative correlation between stress and sleep efficiency and a positive correlation between stress and sleep latency (Fig. 1). Thus, we concluded that higher stress can cause less sleep efficiency and delayed sleep latency.
Fig. 1.
Correlation of stress with sleep efficiency, sleep latency, and stage 2 (%). A significant correlation (p < 0.05) was measured using a Pearson’s correlation coefficient test (correlation coefficient: R2 = 0.05, 0.06, and 0.14, respectively). Sleep efficiency (%) [(Total sleep time / total recording time (time until the light is turned on after the test room light is turned off)] × 100.
Moreover, individuals with higher stress showed a longer WASO result (43.66 ± 41.32 min) than those individuals with lower stress (26 ± 26.13 min), indicating that high level of stress may increase the sense of wake. As a result, total sleep quality could be reduced, and the recovery function of sleep could be diminished [14].
For food intake, the mean calories consumed were 1662.99 ± 459.85 kcal in the higher stress group and 1735 ± 394.39 kcal in the lower stress group, which was not a significant difference. There were also no significant differences in consumed carbohydrates, fats, and protein between the groups. Our study results did not corroborate a previous study which suggested the amount of food intake either increase or decrease as the level of stress become higher [15]. Endogenous (appetite-regulating hormones) and exogenous (socio-economic status, access to food, environment, food preference) factors may have influenced the results of the study [16].
The proportion of the caloric intake in two groups met the appropriate levels: carbohydrates 55–65%, fats 15–30%, and protein 7–20%. In particular, the consumption of carbohydrates is consistent with a trend towards lower consumption of carbohydrates in Koreans, 77% in 1980 to 65% in 2015 [17].
The results from the FFQ showed less intake of vegetables among the higher stress group (124.1 ± 104.7 g/day) than in the lower stress group (239.5 ± 164.0 g/day) (p = 0.005). A study on stress and antioxidant vitamins had revealed that vitamin C is an antioxidant nutrient that has a coenzyme role for adrenal hormones and neurotransmitters. The brain and adrenal gland are sensitive to nutritional intake, which means they are vulnerable to vitamin deficiency and vitamin deficiency and could underlie cognitive disorders and ineffective responses towards stress [18,19]. Preliminary studies have asserted that there is an association between stress and antioxidant vitamins and our study supports this association: there was a difference in vegetable consumption between the groups although there was no difference in vitamin intake per se.
The higher stress group consumed 2–3 times more grains (939.1 ± 581.5 g/day vs 492.5 ± 589.6 g/day) and meat and eggs (217.4 ± 253.0 g/day vs 92.6 ± 78.5 g/day; p = 0.023) than the lower stress group. Pork intake was particularly greater in the higher stress group than in the lower stress group. Studies on nutrition and behavior found that stress is associated with high fat and sugar intake, and stressful situations are associated with more harmful eating behavior such as consumption of sweet and fatty foods [20,21].
The higher stress group drank more soju than the lower stress group (87.8 ± 167.5 g/day vs 19.7 ± 59.9 g/day; p = 0.004) (Table 5). According to findings from previous studies, individual perceive that alcohol could ease pain or stress; as a result, individuals who believe that to be true tend to consume greater quantities of alcohol than those individuals who do not hold that perception [22,23]. Substance abuse tobacco and alcohol to release stress can have huge negative impact on general health, following imbalance of nutrition and eating behavior caused by irregular diet and excessive drinking alcohol.
Table 5.
Intake of alcohol, non-alcoholic beverages, snacks, and sweet foods per day as recorded by the food frequency questionnaire
| Variables | Low Stress† (n = 57) | High Stress† (n = 32) | p-value* | |
|---|---|---|---|---|
| Drink and Liquor (g/day) | 49.6 ± 77.4 | 131.4 ± 227.5 | 0.008 | |
| Beer | 20.2 ± 28.6 | 32.7 ± 56.6 | 0.190 | |
| Soju | 19.7 ± 59.9 | 87.8 ± 167.5 | 0.004 | |
| Rawwine | 9.7 ± 26.0 | 10.8 ± 26.1 | 0.463 | |
| Beverages (g/day) | 225.5 ± 286.3 | 561.1 ± 894.1 | 0.005 | |
| Coke | 31.1 ± 121.0 | 15.4 ± 28.6 | 0.154 | |
| Chocolate powder and other formula flavors | 3.0 ± 10.8 | 12.4 ± 54.0 | 0.361 | |
| Chocolate milk | 11.5 ± 40.9 | 15.2 ± 40.4 | 0.676 | |
| Coffee milk | 20.1 ± 62.3 | 12.8 ± 34.0 | 0.149 | |
| Canned coffee | 86.6 ± 138.1 | 27.9 ± 83.2 | 0.297 | |
| Coffee mix | 6.1 ± 6.8 | 20.5 ± 35.6 | 0.002 | |
| Brewed coffee | 34.7 ± 221.3 | 440.8 ± 887.2 | 0.000 | |
| Tea‡ | 13.0 ± 22.2 | 1.2 ± 2.8 | 0.045 | |
| Energy drinks | 17.8 ± 32.6 | 13.9 ± 49.2 | 0.442 | |
| Snack (g/day) | 62.3 ± 112.6 | 119.9 ± 111.3 | 0.557 | |
| Sweets (g/day) | 30.6 ± 131.2 | 38.8 ± 84.7 | 0.612 |
p-values were calculated using an independent t-test, variables were adjusted for age.
BEPSI-K, Korean version of the Brief Encounter Psychosocial Instrument; Low stress ≤ 1.6, High Stress > 1.6.
Tea: black tea, green tea, canned black tea, and canned green tea.
For beverages, coffee mix and brewed coffee consumption in the higher stress group (20.5 ± 35.6 g/day; 440.8 ± 887.2 g/day) was significantly greater (p < 0.05) than in the lower group (6.1 ± 6.8 g/day; 34.7 ± 221.3 g/day). This result indicates that consuming coffee with caffeine may act as a factor that reduces sleep quality. The role of caffeine in sleep quality should be addressed in future studies.
Here, we found that stress level and nutrition are associated with objective quality of sleep. Although total sleep time in both the lower and higher stress groups were not significantly different, there were notable discrepancies in sleep quality factors including sleep efficacy, sleep latency, and WASO. Furthermore, nutritional intake differed between groups; the lower stress group tended to have healthier eating behaviors than the higher stress group. Therefore, food and stress management for insomnia should be utilized for improving not only sleep quantity but also sleep quality, and, ultimately, for enhancing overall quality of life.
REFERENCES
- 1.Guilleminault C, Brooks SN. Excessive daytime sleepiness: a challenge for the practising neurologist. Brain. 2001;124:1482–91. doi: 10.1093/brain/124.8.1482. [DOI] [PubMed] [Google Scholar]
- 2.Dijk D-J, Lockley SW. Invited Review: Integration of human sleep-wake regulation and circadian rhythmicity. J Appl Physiol. 2002;92:852–62. doi: 10.1152/japplphysiol.00924.2001. [DOI] [PubMed] [Google Scholar]
- 3.Johnson EW, Kennedy JH. Comprehensive management of Duchenne muscular dystrophy. Arch Phys Med Rehabil. 1971;52:110–4. [PubMed] [Google Scholar]
- 4.Johns MW. A new method for measuring daytime s leepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 5.Morley JE, Levine AS, Rowland NE. Stress induced eating. Life Sci. 1983;32:2169–82. doi: 10.1016/0024-3205(83)90415-0. [DOI] [PubMed] [Google Scholar]
- 6.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–94. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Heatherton TF, Herman CP, Polivy J. Effects of physical threat and ego threat on eating behavior. J Pers Soc Psychol. 1991;60:138–43. doi: 10.1037/0022-3514.60.1.138. [DOI] [PubMed] [Google Scholar]
- 8.Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, Wolf A. Food selection changes under stress. Physiol Behav. 2006;87:789–93. doi: 10.1016/j.physbeh.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 10.Yim JH, Bae JM, Choi SS, Kim SW, Hwang HS, Huh BY. The validity of modified Korean-translated BEPSI (Brief Encounter Psychosocial Instrument) as instrument of stress measurement in outpatient clinic. J Korean Acad Fam Med. 1996;17:42–53. [Google Scholar]
- 11.Yun SH, Shim JS, Kweon SH, Oh KW. Development of a food frequency questionnaire for the Korea National Health and Nutrition Examination Survey: data from the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV) Korean J Nutr. 2013;46:186–96. doi: 10.4163/kjn.2013.46.2.186. [DOI] [Google Scholar]
- 12.Ministry of Health and Welfare, The Korean Nutrition Society. Dietary reference intakes for Koreans. Ministry of Health and Welfare; Sejong, Korea: 2015. 2015. [Google Scholar]
- 13.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Katz ES, Kheirandish-Gozal L. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40–3. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Woo JM, Hyun SY, Lee SH, Kang SG, Lee JS, Kim L, Lee YJ, Yu BH, Kang EH, Ku JI. Productivity time lost by sleep disturbance among workers in Korea. J Korean Neuropsychiatr Assoc. 2011;50:62–8. [Google Scholar]
- 15.Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. J Psychosom Res. 2000;48:195–202. doi: 10.1016/S0022-3999(00)00076-3. [DOI] [PubMed] [Google Scholar]
- 16.McCann BS, Warnick GR, Knopp RH. Changes in plasma lipids and dietary intake accompanying shifts in perceived workload and stress. Psychosom Med. 1990;52:97–108. doi: 10.1097/00006842-199001000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. 2015 Health behavior and chronic disease statistics: Korea national health and nutrition examination survey·Youth health behavior online survey. Ministry of Health & Welfare, Korea Centers for Disease Control and Prevention; Sejong, Korea: 2016. [Google Scholar]
- 18.Meade SM. The effect of social stress and vitamin C on immunity and response to hemorrhagic enteritis virus in Turkeys [Dissertation] Blacksburg, Virginia: Virginia Tech; 2004. English. [Google Scholar]
- 19.Zaidi SM, Banu N. Antioxidant potential of vitamins A, E and C in modulating oxidative stress in rat brain. Clin C him Acta. 2004;340:229–33. doi: 10.1016/j.cccn.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Bergmann NC, Gyntelberg F, Faber J. Chronic stress and the development of the metabolic syndrome: a systematic review of prospective cohort studies. Endocr Connect. 2014 doi: 10.1530/EC-14-0031. EC-14-0031, Epub 2014 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon JH, Kim SH. Depression, stress and how they are related with health behaviors and metabolic syndrome among women over 40 years. J Korean Soc Matern Child Health. 2012;16:263–73. doi: 10.21896/jksmch.2012.16.2.263. [DOI] [Google Scholar]
- 22.Yoon SH, Bae JY, Lee SW, An KE, Kim SE. The effects of job stress on depression, drinking and smoking among Korean men. Health Soc Sci. 2006;19:31–50. [Google Scholar]
- 23.Dawson DA, Grant BF, Ruan WJ. The association between stress and drinking: modifying effects of gender and vulnerability. Alcohol Alcohol. 2005;40:453–60. doi: 10.1093/alcalc/agh176. [DOI] [PubMed] [Google Scholar]