Abstract
The present study aimed to investigate the protective effects of rhein on cerebral ischemic/reperfusion (I/R) injury in rats. The present study focused on the effect of rhein on oxidative stress and apoptotic factors, which are considered to serve an important role in the onset of I/R injury. Sprague-Dawley rats were subjected to middle cerebral artery occlusion. Neurological functional scores (NFSs) were evaluated according to the Zea Longa's score criteria and the area of brain infarct was determined by triphenyltetrazolium chloride staining. The morphology of the nerve cells in the cortex was observed following hematoxylin and eosin staining. In addition, levels of oxidative stress were assessed by measuring the levels of superoxide dismutase (SOD), glutathione-peroxidase (GSH-Px), catalase (CAT) and malondialdehyde (MDA). Levels of B-cell lymphoma-2 (Bcl-2), apoptosis regulator Bax (BAX), caspase-9, caspase-3 and cleaved caspase-3 expression were analyzed using western blot analysis. Levels of caspase-9 and caspase-3 mRNA expression were obtained using reverse transcription-quantitative polymerase chain reaction. The results revealed that treatment with 50 or 100 mg/kg rhein significantly improved the NFS and markedly attenuated the area of infarction. Rhein also significantly reduced the content of MDA and significantly increased SOD, GSH-Px and CAT activity. Western blot analysis indicated that rhein significantly decreased the expression of BAX and enhanced the expression of Bcl-2. Compared with the I/R group, levels of caspase-9, caspase-3 and cleaved caspase-3 protein expression were significantly decreased in the rhein treatment groups. Additionally, rhein treatment significantly reduced levels of caspase-9 and caspase-3 mRNA expression. These results suggest that rhein exhibits protective effects during cerebral I/R injury and its underlying mechanism of action may involve the inhibition of oxidative stress and apoptosis.
Keywords: rhein, cerebral ischemic reperfusion, oxidative stress, apoptosis, caspase-9, caspase-3, B-cell lymphoma-2, apoptosis regulator Bax
Introduction
Ischemic stroke is a leading cause of mortality and disability worldwide (1). Survivors typically suffer from permanent brain damage, resulting in a reduced quality of life and increased social burden (2). Cerebral blood flow reperfusion is the primary treatment option for stroke (3). However, cerebral ischemia/reperfusion (I/R) induces a series of pathophysiological processes, which may cause further damage, including I/R injury (4).
Oxidative stress and neuronal apoptosis are important factors in the pathological process of I/R injury that occurs in cerebral ischemic stroke followed by reperfusion (5). Oxidative stress refers to a comparative surplus of reactive oxygen species (ROS) caused by an imbalance between oxidants and antioxidants (6). The brain tissue is sensitive to oxidative stress as it contains low levels of endogenous antioxidant enzymes, including superoxide dismutase (SOD), glutathione-peroxidase (GSH-Px), and catalase (CAT), which act as cellular defenses against ROS (7). Malondialdehyde (MDA) is primarily induced by ROS and is commonly used as a biomarker of oxidative stress (8). Experimental and clinical studies have demonstrated that the expression of SODs, CATs and GSH-Px are significantly reduced (7,9-12) and MDA is significantly elevated in animal models of stroke and in patients that have experienced an ischemic stroke (12).
Oxidative stress is implicated in the initiation of apoptosis and it has been suggested that the balance between the anti-apoptotic protein B-cell lymphoma-2 (Bcl-2) and the pro-apoptotic apoptosis regulator Bax (BAX) protein regulates apoptosis (13). BAX mediates the activation of caspase-9, which is upregulated following ischemia in human brain tissue (14). In animal models of ischemic stroke, caspase-9 leads to the activation of caspase-3, which is considered to be a key mediator of apoptosis. Wagner et al (15) revealed that cleaved caspase-3 was predominantly associated with cellular responses to stroke and this has been supported by the results of other studies (16-18). Therefore, modulation of Bcl-2/BAX and the suppression of caspase-9, caspase-3 and cleaved caspase-3 expression may serve a protective role in the treatment of cerebral I/R injury.
Rhein is a naturally occurring anthraquinone compound isolated from Radix et Rhizoma Rhei and is involved in a number of biological activities, including the suppression of ROS, downregulation of intracellular calcium concentration, promotion of microcirculatory function, inhibition of neutrophil migration and the promotion of anti-inflammatory effects (19-22). The blood-brain barrier is disrupted during cerebral I/R injury and previous studies have revealed that segments of rhein are able to pass through the disrupted blood-brain barrier (23), inhibit cerebral infarction and improve the neurological functional score (NFS) (24). Therefore, rhein may have potential beneficial effects in anti-cerebral I/R injury. However, to the best of our knowledge, the signaling mechanisms that underlie the protective effects of rhein against I/R remain unknown.
There have been few previous studies investigating the effect of rhein on cerebral I/R injury. Therefore, the present study aimed to evaluate the effects of rhein on ischemic injury, neurological outcomes, oxidative stress and apoptosis biomarkers in rats following ischemic stroke induced by middle cerebral artery occlusion (MCAO).
Materials and methods
Reagents
Rhein (molecular formula, C15H8O6; molecular weight, 284.225) was purchased from the National Institute for Food and Drug Control (Beijing, China). Nimodipine tablets were provided by Bayer AG (Leverkusen, Germany) and 2,3,5-triphenyltetrazolium chloride (TTC) was obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). A Total Extraction Sample kit and a BCA protein assay kit were purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). Antibodies against caspase-9 (cat. no. ab2013), caspase-3 (cat. no. ab44976) and GAPDH (cat. no. ab9485) were obtained from Abcam (Cambridge, UK). Cleaved caspase-3 (cat. no. 9661), Bcl-2 (cat. no. 2876), BAX (cat. no. 2772) and β-actin (cat. no. 4970) antibodies were procured from Cell Signaling Technology, Inc. (Danvers, MA, USA). Horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (cat. no. ZB-2301) was obtained from OriGene Technologies, Inc. (Beijing, China). A Total RNA kit was obtained from Tiangen Biotech Co., Ltd. (Beijing, China). A RevertAid First Strand cDNA Synthesis kit was obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) was purchased fromTakara Bio, Inc. (Otsu, Japan). The total superoxide dismutase (T-SOD) assay kit (cat. no. A001-1-1), glutathione peroxidase (GSH-PX) assay kit (cat. no. A005), catalase (CAT) assay kit (cat. no. A007-1-1) and malondialde-hyde (MDA) assay kit (cat. no. A003-1) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
High-performance liquid chromatography to determine rhein content
The content of rhein was determined using an L-2000 Liquid Chromatography system (Hitachi, Ltd., Tokyo, Japan) equipped with an L-2200 auto-injection device. Chromatographic analysis was preformed with a reverse-phase YMC-Pack Pro C18 column (5 μm; 4.6×250 mm). The isocratic mobile phase comprised 0.5% aqueous acetic acid solution and methanol (40:60, v/v) at a flow rate of 1.0 ml/min and detection was performed at 435 nm using an L-2455 UV-VIS detector (Hitachi, Ltd.). Total analysis time was 15 min and analysis was performed at a temperature of 40°C. The injection volume of rhein was 10 μl. The results revealed that the exact purity of rhein was 98%, which was consistent with the result provided by the manufacturer.
Animals
A total of 144 male specific pathogen free Sprague-Dawley rats (weighing 260-300 g, aged 8-10 weeks old) were obtained from the Laboratory Animal Center of Ningxia Medical University (Yinchuan, China). All animal handling procedures were approved by the Animal Research Ethics Committee, School of Ningxia Medical University (Yinchuan, China). Animals were maintained in a 12 h light/dark cycle at room temperature (23±2°C) in 60% humidity. The animals had ad libitum access to food and water. Rats were randomly divided into 6 groups (each, n=24) as follows: Sham, I/R, I/R + 12 mg/kg/day nimodipine (25,26), and I/R + 25, 50 or 100 mg/kg/day rhein (27). Nimodipine was used as a control, as it acts as a neuroprotectant. The sham and I/R groups received 0.5% sodium carboxymethyl cellulose orally for 3 days following cerebral ischemic/reperfusion induced by MCAO, and the other groups received nimodipine (12 mg/kg/day) and rhein (25, 50 or 100 mg/kg/day) orally for 3 days following cerebral ischemic/reperfusion induced by MCAO.
MCAO model
As previously stated, MCAO induces ischemic injury (28). Rats were anesthetized with 7% chloral hydrate (350 mg/kg) via intraperitoneal injection. The right common carotid artery (CCA), the external carotid artery and the internal carotid artery (ICA) were exposed. A nylon wire (1.8±0.5 cm) was advanced from the CCA into the ICA until it blocked the original MCA. Reperfusion was performed by withdrawing the nylon wire following 2 h ischemia. The sham-operated rats were treated by the same surgical procedure however the nylon wire was not introduced.
Determination of the NFS
NFSs were obtained from randomly selected mice from each group (n=8) 72 h post reperfu-sion and they were evaluated according to the Zea Longa's score criteria. The deficit criteria used have been previously described (29). The scoring details were as follows: 0, No noticeable neurological deficit; 1, failed to extend opposite forepaw; 2, circled to the contralateral side; 3, tumbled to its side while walking due to hemiplegia; 4, loss of consciousness or mortality.
Determination of the area of cerebral infarction
Rats were anesthetized by intraperitoneal injection of 7% chloral hydrate (350 mg/kg). Rats were decapitated and then the whole brain was removed. Subsequently, coronary brain slices (2-mm sections) were stained using 2% TTC chloride for 30 min at 37°C and then fixed in 10% formalin solution at 25°C for 1 h (n=6). The infarcted area was stained white, whereas normal brain tissue was red. Stained cerebral slices were photographed using a Nikon D7100 camera (Nikon Corporation, Tokyo, Japan). Cerebral infarct areas were determined using microscope image-analysis software (Image Pro plus, version 6.0; Media Cybernetics, Rockville, MD, USA). The ratio of the infarcted area to the total brain area was calculated using the following equation: Percentage of infarct volume=infarct volume/(infarct volume + normal volume) ×100.
Hematoxylin and eosin staining
Prior to this procedure, rats were anesthetized by intraperitoneal injection of 7% chloral hydrate (350 mg/kg) and perfused transcardially with 100 ml of 0.9% sodium chloride, followed by 200 ml 4% paraformaldehyde. Rats were decapitated and then the whole brain was removed. Rat brains were fixed in 4% formaldehyde at 25°C for 2 h. Brains were soaked in distilled water for 4 h, dehydrated in increasing concentrations of alcohol, hyalinized by dimethylbenzene, embedded in paraffin and sectioned to a thickness of 4-μm. Sections were adhered to glass slides prepared with poly-L-Lysine and stored at 4°C. Following continual dewaxing and routine washing, paraffin-embedded sections were stained with hematoxylin for 5 min, followed by color separation using 1% hydrochloric acid alcohol for 20 sec. Sections were then incubated with 1% ammonia for 30 sec, stained with eosin for 5 min and subsequently dehydrated using alcohol, hyalinized using dimethylbenzene and sealed with neutral gum. All the aforementioned steps were performed at 25°C. A total of five non-overlapping views of the cortex in each section were randomly selected and observed under a light microscope for cell counting at a magnification of ×400. The degenerated cell index (number of degenerated cells/total cells) indicated the degree of damage.
Determination of oxidative stress indicators
Levels of SOD, GSH-Px, CAT and MDA in the ischemic brain were measured 72 h following I/R. The right cortical samples (n=6 per group) were weighed. The SOD, GSH-Px and CAT activities and MDA level were obtained using the T-SOD, GSH-Px, CAT and MDA assay kits according to the manufacturer's protocol (Nanjing Jiancheng Bioengineering Institute, Co., Ltd., Nanjing, China). SOD, GSH-Px and CAT activities were expressed as units/mg protein. The amount of lipid peroxide was obtained as the product of MDA. MDA concentrations were expressed as nmol/mg protein.
Western blot analysis
Frozen right brains were homogenized in cold whole cell lysis buffer (cat. no. KGP2100; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China), sonicated twice on ice for 5 sec each time and centrifuged at 12,000 × g for 5 min at 4°C to remove cell debris. Total protein concentration in the supernatant was determined using the BCA protein assay kit. A total of 80 μg protein per lane was separated using 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% non-fat dried milk for 1 h at 25°C and subsequently incubated with primary antibodies against Bcl-2 (1:500), Bax (1:500), caspase-9 (1:1,000), caspase-3 (1:1,000), cleaved caspase-3 (1:1,000), β-actin (1:1,000) and GAPDH (1:2,000) overnight at 4°C. Membranes were washed with PBST and subsequently incubated with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary antibodies (1:1,000) for 2 h at room temperature. Following washing, membranes were developed using a Chemidoc™ XRS Imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The signal intensities of the bands of interest were quantified and normalized to β-actin or GAPDH using the Image-Pro Plus software version 6.0 (Media Cybernetics, Inc.).
Reverse transcription-quantitative-polymerase chain reaction analysis (RT-qPCR)
RT-qPCR was used to assess the mRNA expression levels of caspase-9 and caspase-3 72 h following reperfusion. Total RNA was extracted using a total RNA kit according to the manufacturer's protocol. Labeled cDNA was prepared using the RevertAid First Strand cDNA Synthesis kit from the total RNA samples. For PCR amplification, a CFX96 Real-Time system was used. cDNA was added to each reaction to a final reaction volume of 25 μl containing 1X SYBR Premix Ex Taq II. The thermocycling conditions used were as follows: Initial denaturation at 95°C for 30 sec., followed by 40 cycles comprising of denaturation at 95°C for 5 sec, annealing at 60°C for 30 sec, elongation at 72°C for 30 sec and a final extension step at 72°C for 5 min. β-actin was used as the reference gene for the normalization of different transcript values and normalized mRNA levels were expressed as 2−ΔΔCq (ΔΔCq=Cqtarget-Cqβ-actin) (30). Only one produced PCR product was subjected to melting curve analysis. Sequences of the primers used are listed in Table I.
Table I.
The primers for reverse transcription-quantitative polymerase chain reaction.
| Gene | Sequence (5′-3′) |
|---|---|
| Caspase-3 | F: AAAGGATGACTGGGAGTGG |
| R: ATGACGACCTGGAACATCG | |
| Caspase-9 | F: TATGGCACAGATGGATGCTC |
| R: CTTTCTGCTCACCACCACAG | |
| β-actin | F: CCCATCTATGAGGGTTACGC |
| R: TTTAATGTCACGCACGATTTC |
F, forward; R, reverse.
Statistical analysis
Statistical analysis was conducted using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). The results were presented as the mean ± standard deviation. Differences among >2 groups were assessed using one-way analysis of variance followed by the least significant difference post hoc test. Differences between two groups were analyzed using an unpaired t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Rhein ameliorates NFSs
NFSs were obtained using the Zea Longa's criteria 72 h following cerebral reperfusion. Severe dysfunction on each test was indicated by a high NFS score of 2-3. The NFSs of the I/R group were significantly higher than those of the sham group (P<0.01; Table II). However, the NFSs were significantly decreased in the groups treated with 50 and 100 mg/kg rhein (P<0.05 and P<0.01, respectively) and nimodipine (P<0.05) compared with the I/R group, thereby indicating that rhein treatment improves neurological function impaired by cerebral I/R.
Table II.
The protective effects of rhein on neurological functional score in rats 72 h post reperfusion.
| Group | Neurological functional score |
|---|---|
| Sham | 0.0±0.0 |
| I/R | 2.2±0.8a |
| Nimodipine | 1.0±0.7b |
| Rhein (25 mg/kg) | 2.0±0.9 |
| Rhein (50 mg/kg) | 1.6±0.5b |
| Rhein (100 mg/kg) | 1.1±0.9c |
Data are presented as the mean ± standard deviation. n=8/group.
P<0.01 vs. the sham group;
P<0.05 and
P<0.01 vs. the I/R group. I/R, ischemic/reperfusion.
Rhein reduces the area of cerebral infarction
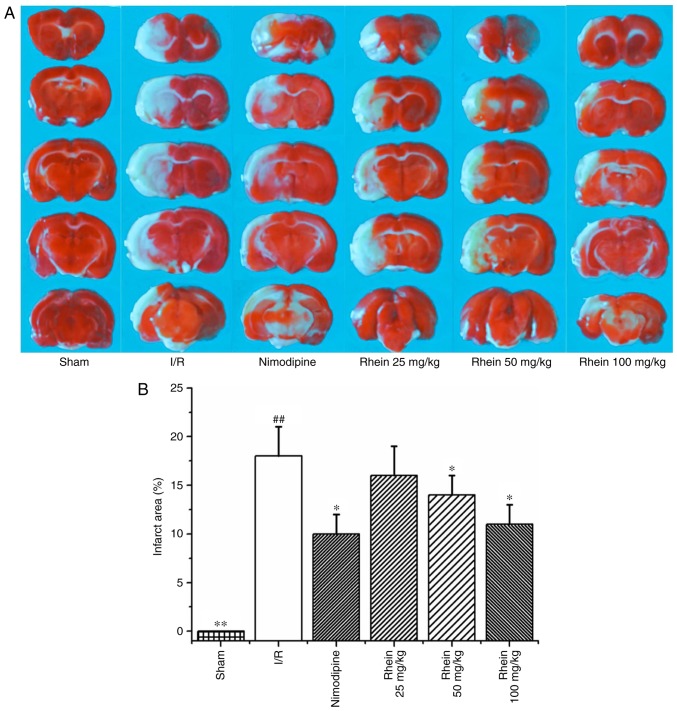
Infarcted cerebral volume was measured 72 h following cerebral reperfusion by TTC staining. The infarcted area (white) was primarily located at the striatum and parts of the cortex frontoparietal lobe in the right hemisphere (Fig. 1A). The infarcted cerebral area of the I/R group was significantly greater than that of the sham group (P<0.01). By contrast, theinfarcted cerebral area of the nimodipine and 50 and 100 mg/kg rheingroups were significantly smaller than the I/R group (each P<0.05; Fig. 1B), thereby indicating that treatment with rhein reduces cerebral injury induced by cerebral I/R.
Figure 1.
Effect of rhein on the area of cerebral infarction in I/R rats. (A) TTC staining. (B) Quantification of TTC staining results. Values are presented as the mean ± standard deviation. n=6/group. ##P<0.01 vs. the sham group; *P<0.05 and **P<0.01 vs. the I/R group. I/R, ischemic/reperfusion; TTC, 2,3,5-triphenyltetrazolium chloride.
Histological changes
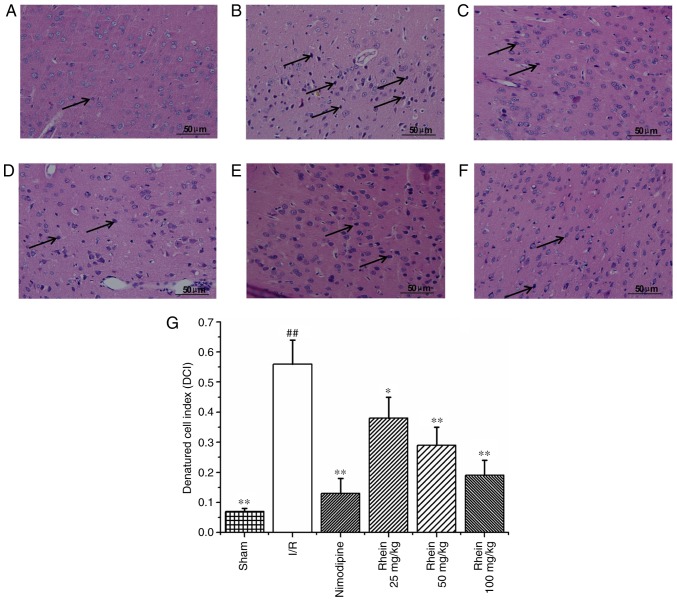
Hematoxylin and eosin staining (Fig. 2) revealed that nerve cell arrangement was well organized, exhibiting a complete structure in the control group (Fig. 2A). However, 72 h following ischemia, in the I/R group the neuron arrangement became irregular with deeply colored and condensed nuclei, increased gaps around the nerves and degenerative changes, including the formation of vacuoles and necrosis in a number of the neurons (Fig. 2B). In addition, the denatured cell index (DCI) values in the I/R group were significantly higher than those of the sham group (P<0.01; Fig. 2G). In the nimodipine and rhein groups, nerve cell damage was milder compared with that of the model group and neurons were well arranged, with clear outlines, nuclear condensation, and shriveled bodies. DCI values in all the rhein groups and the nimodipine group were significantly decreased compared with the I/R group (P<0.05 or P<0.01; Fig. 2G). Deep coloration was observed in only a few cells and there were no significant differences in DCI values between the different rhein groups.
Figure 2.
Effect of rhein on nerve cell morphology and structure in the cortices of I/R rats. Hematoxylin and eosin staining in the (A) sham, (B) I/R, (C) nimodipine, (D) 25, (E) 50 and (F) 100 mg/kg rhein treatment groups. Arrows indicate denatured cells. Magnification, ×400. (G) The denatured cell index for all groups. ##P<0.01 vs. the sham group; *P<0.05 and **P<0.01 vs. the I/R group. I/R, ischemic/reperfusion.
Effect of rhein on oxidative stress indicators induced by I/R
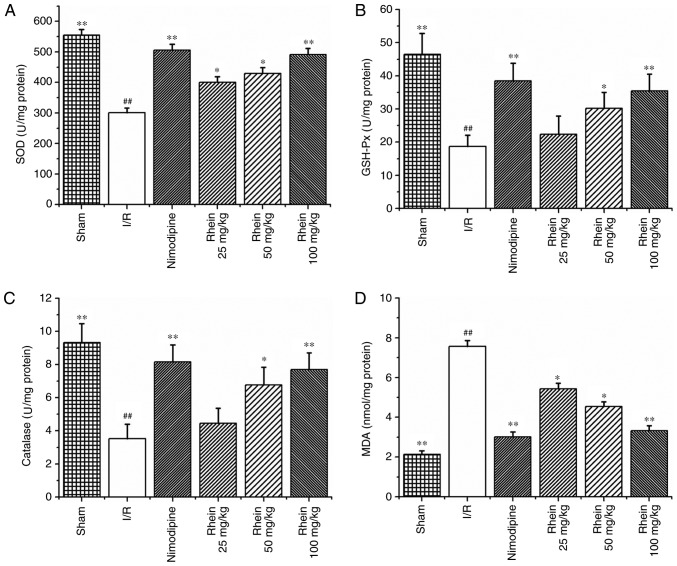
The activities of the SOD, GSH-Px and CAT enzymes were significantly reduced in the I/R group compared with those in the sham group (P<0.01; Fig. 3A–C). However, treatment with nimodipine and 50 and 100 mg/kg rhein, significantly increased the activities of SOD, GSH-Px and CAT compared with the I/R group (P<0.01 or P<0.05). Treatment with 25 mg/kg rhein also significantly increased the activity of SOD compared with the I/R group (P<0.05). MDA was used as a biomarker for lipid peroxidation and oxidative stress. MDA content was significantly higher in the ischemic cortices of the I/R group compared with the sham group (P<0.01; Fig. 3D). However, following treatment with nimodipine and all doses of rhein, MDA content was significantly decreased compared with the I/R group (P<0.01 or P<0.05).
Figure 3.
Rhein attenuates oxidative stress following I/R in rats. Effect of rhein administration on (A) SOD, (B) GSH-Px, (C) catalase and (D) MDA expression. Values are presented as the mean ± standard deviation (n=6). ##P<0.01 vs. the sham group; *P<0.05 and **P<0.01 vs. the I/R group. SOD, superoxide dismutase; GSH-Px, glutathione-peroxidase; MDA, malondialdehyde; I/R, ischemic/reperfusion.
Rhein inhibits BAX expression and enhances Bcl-2 expression
Western blot analysis revealed that the expression of Bcl-2 was significantly reduced (P<0.05; Fig. 4A and C) and that BAX protein expression was significantly enhanced in the I/R group compared with the sham group (P<0.05; Fig. 4A and D). Compared with the I/R group, treatment with nimodipine and 100 mg/kg rhein significantly upregulated Bcl-2 expression (P<0.05; Fig. 4A and C). Furthermore, treatment with nimodipine and 50 and 100 mg/kg rhein significantly downregulated BAX expression (P<0.05; Fig. 4A and D). The Bcl-2/BAX ratio was also significantly increased following treatment with nimodipine and 50 or 100 mg/kg rhein compared with the I/R group (P<0.05 or P<0.01; Fig. 4B).
Figure 4.
Effect of rhein on the expression of Bcl-2 and BAX. (A) Representative western blots of Bcl-2 and BAX expression. β-actin was used as the loading control. Quantification of (B) Bcl-2 and (C) BAX expression. (D) The Bcl-2/BAX ratio. Values are presented as the mean ± standard deviation (n=6). #P<0.05 and ##P<0.01 vs. the sham group; *P<0.05 and **P<0.01 vs. the I/R group. I/R, ischemic/reperfusion; Bcl-2, B-cell lymphoma-2; BAX, apoptosis regulator Bax.
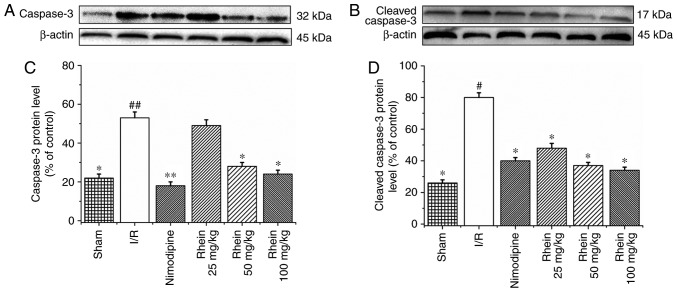
Rhein inhibits the expression of caspase-9, caspase-3 and cleaved caspase-3
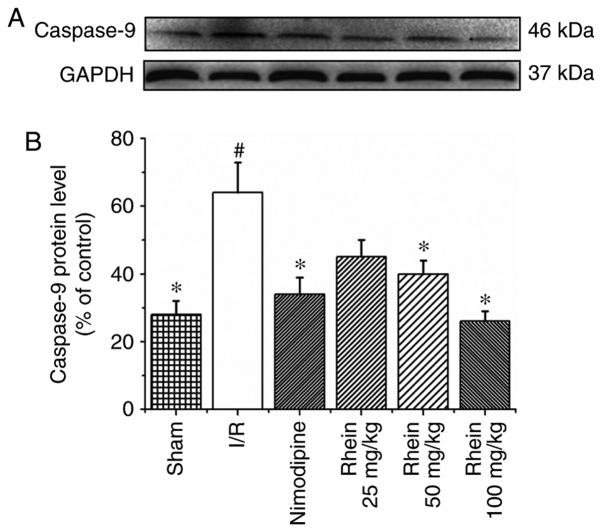
Western blot analysis revealed that the expression of caspase-9 in the I/R group was significantly higher than in the sham group (P<0.05; Fig. 5). Treatment with 50 and 100 mg/kg rhein significantly reduced the expression of caspase-9 compared with the I/R group (P<0.05). In the I/R group, levels of caspase-3 (P<0.01) and cleaved caspase-3 (P<0.05) expression were significantly higher than in the sham group (Fig. 6). The administration of nimodipine and 50 and 100 mg/kg rhein significantly reduced the expression of caspase-3 (P<0.05), whereas administration of nimodipine and all doses of rhein significantly reduced the expression of cleaved caspase-3 (P<0.05).
Figure 5.
Effect of rhein on caspase-9 expression. (A) Representative western blots of caspase-9 expression. GAPDH was used as the loading control. (B) Quantification of caspase-9 expression. Values are presented as the mean ± standard deviation (n=6). #P<0.05 vs. the sham group; *P<0.05 vs. the I/R group. I/R, ischemic/reperfusion.
Figure 6.
Effect of rhein on the protein expression levels of caspase-3 and cleaved caspase-3. Western blot analysis of (A) caspase-3 and (B) cleaved caspase-3 expression. β-actin was used as the loading control. Quantitative data of (C) caspase-3 and (D) cleaved caspase-3 are given as percentages of the value in control rats. Values are presented as the mean ± standard deviation (n=6). #P<0.05 and ##P<0.01 vs. the sham group; *P<0.05 and **P<0.01 vs. the I/R group. I/R, ischemic/reperfusion.
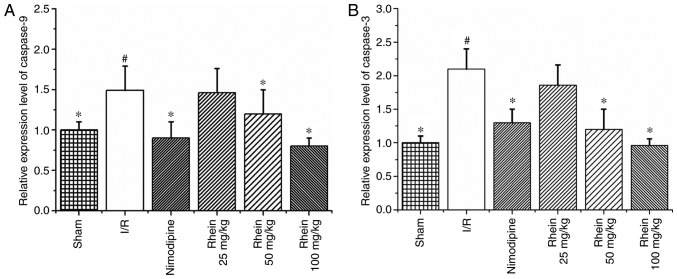
RT-qPCR results revealed that levels of caspases-9 and -3 mRNA expression were significantly increased in the I/R group compared with the sham group (P<0.05; Fig. 7). However, treatment with 50 and 100 mg/kg rhein significantly decreased levels of caspases-9 and -3 mRNA (P<0.05).
Figure 7.
Effect of rhein on the mRNA expression of caspases-9 and -3. (A) Expression of caspase-9 mRNA. (B) The expression of caspase-3 mRNA. Values are presented as the mean ± standard deviation (n=6). #P<0.05 vs. the sham group; *P<0.05 vs. the I/R group. I/R, ischemic/reperfusion.
Discussion
In the present study, the infarcted cerebral area was identified by TTC staining and NFS was evaluated 72 h following reper-fusion. Treatment with rhein markedly decreased the infarcted cerebral area and significantly improved the NFS. These results indicate that rhein may protect against cerebral I/R injury.
Ischemic stroke is the cumulative effect of numerous mechanisms including oxidative stress, excitotoxicity, intracellular calcium overload, inflammation and apoptosis (31). Oxidative stress secondary to ROS formation and neuron apoptosis is crucial in stimulating the progression of ischemic stroke (32). Under normal physiological conditions, low levels of ROS exist within the brain (6). Excessive ROS are removed by endogenous antioxidant enzymes including SODs (33), GSH-Px and CATs (34). SOD converts superoxide anions into hydrogen peroxide and GSH-Px uses GSH to reduce H2O2 to water (35). MDA, which is a poisonous end product of lipid peroxidation, is able to directly reflect the rate and extent of lipid peroxidation and indirectly reflects the capacity of free radical elimination (36). Previous studies have demonstrated that increasing the SOD, CAT and GSH-Px levels and decreasing MDA concentrations significantly protects against oxidative stress during ischemic stroke (7,9-11). The results of the present study were consistent with the aforementioned previous studies. The protective effects of rhein in I/R injury may be associated with its enhancing effect on endogenous antioxidant capability, which mitigates oxidative stress during cerebral I/R. Therefore, the enhancement of the antioxidant system represents a potential target for novel therapies to treat patients with ischemic stroke.
Ischemic stroke results in ROS overproduction in the mitochondria (37) and specific ROS, including H2O2 or superoxide, are crucial mediators of apoptosis (38,39). Apoptosis is induced via extrinsic and intrinsic pathways (40). In the intrinsic pathway, the loss of transmembrane potential leads to mitochondrial respiration failure and enhanced ROS generation, which accelerates cytochrome c release from the mitochondria (41). The release of cytochrome c via this mechanism may be associated with the Bcl-2 family of proteins (40,42). When cytochrome c forms a complex with apoptotic protease-activating factor 1 apoptosomes are formed, which activate caspase-9 and consequently activate caspase-3 (43), DNA-breaking enzymes, including endonucleases (44), and repair enzymes, including poly ADP-ribose polymerase (45), leading to cell death. The extrinsic apoptosis pathway activates caspase-8, which either activates executioner caspases, such as caspase-3, or the intrinsic apoptotic pathway (46,47).
Apoptosis is caused by an imbalance between pro-apoptotic and anti-apoptotic signals. Bcl-2 is an anti-apoptotic protein that intercepts the release of cytochrome c in response to various apoptotic signals, thereby inhibiting apoptosis. BAX is a pro-apoptotic protein that accelerates cytochrome c release from the mitochondria, thereby leading to cell death (48-51). It has been demonstrated that the Bcl-2/BAX ratio is decreased in the cerebrum of rats following I/R (52,53). The results of the present study illustrated that the expression of Bcl-2 was significantly inhibited whereas the expression of BAX was significantly increased following I/R, which is consistent with the results of previous studies. The Bcl-2/BAX ratio was significantly reduced in the cerebrum of rats following I/R; however the administration of 100 mg/kg rhein significantly reversed the decrease in Bcl-2 expression. In addition, 50 and 100 mg/kg rhein significantly inhibited the increase in BAX expression. The ratio of Bcl-2/BAX was significantly increased following the administration of 50 and 100 mg/kg rhein. The administration of 100 mg/kg rhein was more effective than 50 mg/kg rhein. The results indicate that rhein may prevent cerebral I/R injury by upregulating Bcl-2 and downregulating BAX expression, thereby increasing the Bcl-2/BAX ratio and inhibiting apoptosis.
Caspases are central regulators of apoptosis and serve keys roles in ischemia-induced cytotoxicity (54). Caspases may be classified into two groups, initiators (caspase-9) and effectors (caspase-3), based on their function (55). Typically, initiator caspases activate downstream effector caspases through a proteolytic cascade, resulting in the cleavage of cellular substrates involved in apoptosis (56). Caspase-3 may be activated by caspase-9 via the mitochondria-dependent cytochrome c/caspase-9 intrinsic pathway (57). In addition, increased BAX expression may induce the activation of caspase-9 within the mitochondria (58). Caspases-9 and -3 are upregulated and activated in ischemic brain tissues (59) and pharmacological or genetic inhibition of caspase-3 reduces neuronal death in the ischemic brain (60). The present study demonstrated that oral rhein administration maintains low caspase-9 levels. Rhein at doses of 50 and 100 mg/kg significantly inhibited caspase-9 expression. Caspase-3 expression in the I/R group was significantly higher than in the sham group; however, administration with 100 mg/kg rhein significantly inhibited its expression. Cleaved caspase-3 is a key executioner of apoptosis. The results of the present study revealed that cleaved caspase-3 expression in the I/R group was significantly higher than that of the sham group, which was consistent with the results obtained by Wang et al (61). However, administration of 50 and 100 mg/kg rhein significantly inhibited the expression of cleaved caspase-3.
In conclusion, the present study demonstrated that treatment with rhein markedly reduces the area of brain infarction and reduces neuronal apoptosis in a rat model of I/R injury. The protective effects of rhein were mediated through the augmentation of endogenous antioxidant defenses and the inhibition of the oxidative stress pathway in the ischemic rat brain. In addition, rhein exhibited anti-apoptotic effects. These results suggest that rhein may be beneficial in the treatment of ischemic stroke. However, further studies are required to confirm these results prior to its use in humans.
Acknowledgments
Not applicable.
Footnotes
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81660700 and 81260679), Ningxia College first-class discipline construction project (Chinese medicine) funded project (grant no. NXYLXK2017A06), the college students innovation training program of Ningxia Hui Autonomous Region (grant nos. NXCX2015183 and NXCX2016162).
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author upon reasonable request.
Authors' contributions
QZ, XW and XC constructed the MCAO model, performed hematoxylin and eosin staining, western blot analysis and reverse transcription-quantitative polymerase chain reaction. AC and GZ determined the NFS, the area of cerebral infarction and oxidative stress indicators. YZ and YH analyzed and interpreted the data. JS revised the manuscript critically for important intellectual content. YZ and QZ designed the study, supervised the research group and gave final approval of the version to be published. The final version of the manuscript has been read and approved by all authors and each author believes that the manuscript represents honest work.
Ethics approval and consent to participate
All procedures were approved by the Animal Research Ethics Committee, School of Ningxia Medical University (Yinchuan, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, et al. Heart disease and stroke statistics-2016 update: A report from the American heart association. Circulation. 2016;133:e338–e360. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Hua F, Tang H, Wang J, Prunty MC, Hua XD, Sayeed L, Stein DG. TAK-242, an antagonist for Toll-like receptor 4, protects against acute cerebral ischemia/reperfusion injury in mice. J Cereb Blood Flow Metab. 2015;35:536–542. doi: 10.1038/jcbfm.2014.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Amki M, Wegener S. Improving cerebral blood flow after arterial recanalization: A novel therapeutic strategy in stroke. Int J Mol Sci. 2017;18:E2669. doi: 10.3390/ijms18122669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Chen L, Dang X, Liu J, Ito Y, Sun W. Neuroprotective effects of total steroid saponins on cerebral ischemia injuries in an animal model of focal ischemia/reperfusion. Planta Med. 2014;80:637–644. doi: 10.1055/s-0034-1368584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melani A, Dettori I, Corti F, Cellai L, Pedata F. Time-course of protection by the selective A2A receptor antagonist SCH58261 after transient focal cerebral ischemia. Neurol Sci. 2015;36:1441–1448. doi: 10.1007/s10072-015-2160-y. [DOI] [PubMed] [Google Scholar]
- 6.Zhang R, Xu M, Wang Y, Xie F, Zhang G, Qin XY. Nrf2-a promising therapeutic target for defensing against oxidative stress in stroke. Mol Neurobiol. 2017;54:6006–6017. doi: 10.1007/s12035-016-0111-0. [DOI] [PubMed] [Google Scholar]
- 7.Žitňanová I, Šiarnik P, Kollár B, Chomová M, Pazderová P, Andrezálová L, JeDoviIová M, Koňariková K, Laubertová L, Krivošíková Z, et al. Oxidative stress markers and their dynamic changes in patients after acute ischemic stroke. Oxid Med Cell Longev. 2016;2016:9761697. doi: 10.1155/2016/9761697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Subcell Biochem. 2008;49:241–268. doi: 10.1007/978-1-4020-8831-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo J, Cheng C, Chen CS, Xing X, Xu G, Feng J, Qin X. Overexpression of Fibulin-5 attenuates ischemia/reperfu-sion injury after middle cerebral artery occlusion in rats. Mol Neurobiol. 2016;53:3154–3167. doi: 10.1007/s12035-015-9222-2. [DOI] [PubMed] [Google Scholar]
- 10.Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis. 2015;30:747–754. doi: 10.1007/s11011-014-9632-2. [DOI] [PubMed] [Google Scholar]
- 11.Ding Y, Chen M, Wang M, Li Y, Wen A. Posttreatment with 11-keto-β-Boswellic acid ameliorates cerebral ischemia reperfusion injury: Nrf2/HO-1 pathway as a potential mechanism. Mol Neurobiol. 2015;52:1430–1439. doi: 10.1007/s12035-014-8929-9. [DOI] [PubMed] [Google Scholar]
- 12.Milanlioglu A, Aslan M, Ozkol H, Çilingir V, Nuri Aydın M, Karadas S. Serum antioxidant enzymes activities and oxidative stress levels in patientswith acute ischemic stroke: Influence on neurological status and outcome. Wien Klin Wochenschr. 2016;128:169–174. doi: 10.1007/s00508-015-0742-6. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Yu S, Zheng W, Feng G, Luo G, Wang L, Zhao Y. Curcumin improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Neurochem Res. 2010;35:374–379. doi: 10.1007/s11064-009-0065-y. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Peng H, Rowat A, Gao F, Zhang Z, Wang P, Zhang W, Wang X, Qu L. The effect of concentration and duration of normobaric oxygen in reducing caspase-3 and -9 expression in a rat-model of focal cerebral ischaemia. Brain Res. 2015;1618:205–211. doi: 10.1016/j.brainres.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 15.Wagner DC, Riegelsberger UM, Michalk S, Härtig W, Kranz A, Boltze J. Cleaved caspase-3 expression after experimental stroke exhibits different phenotypes and is predominantly non-apoptotic. Brain Res. 2011;1381:237–242. doi: 10.1016/j.brainres.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 16.Endres M, Namura S, Shimizu-Sasamata M, Waeber C, Zhang L, Gómez-Isla T, Hyman BT, Moskowitz MA. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J Cereb Blood Flow Metab. 1998;18:238–247. doi: 10.1097/00004647-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, Endres M, Moskowitz MA. Synergistic effects of caspase inhibitors and MK-801 in brain injury after transient focal cerebral ischaemia in mice. Br J Pharmacol. 1998;124:756–762. doi: 10.1038/sj.bjp.0701871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugawara T, Lewén A, Gasche Y, Yu FS, Chan P. Overexpression of SOD1 protects vulnerable motor neurons after spinal cord injury by attenuating mitochondrial cytochrome c release. FASEB J. 2002;16:1997–1999. doi: 10.1096/fj.02-0251fje. [DOI] [PubMed] [Google Scholar]
- 19.Tsang SW, Bian ZX. Anti-fibrotic and Anti-tumorigenic effects of rhein, a natural anthraquinone derivative, in mammalian stellate and carcinoma cells. Phytother Res. 2015;29:407–414. doi: 10.1002/ptr.5266. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Uematsu H, Tsuchida N, Ikeda MA. Essential role of caspase-8 in p53/p73-dependent apoptosis induced by etoposide in head and neck carcinoma cells. Mol Cancer. 2011;10:95. doi: 10.1186/1476-4598-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cong XD, Ding MJ, Dai DZ, Wu Y, Zhang Y, Dai Y. ER stress, p66shc, and p-Akt/Akt mediate adjuvant-induced inflammation, which is blunted by argirein, a supermolecule and rhein in rats. Inflammation. 2012;35:1031–1040. doi: 10.1007/s10753-011-9407-4. [DOI] [PubMed] [Google Scholar]
- 22.Gao Y, Chen X. Rhein exerts pro-and anti-inflammatory actions by targeting IKKβ inhibition in LPS-activated macrophages. Free Radic Biol Med. 2014;72:104–112. doi: 10.1016/j.freeradbiomed.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Fan R, Luo J, Tang T, Xing Z, Xia Z, Peng W, Wang W, Lv H, Huang W, et al. An ultra high performance liquid chromatography with tandem mass spectrometry method for plasma and cerebrospinal fluid pharmacokinetics of rhein in patients with traumatic brain injury after administration of rhubarb decoction. J Sep Sci. 2015;38:1100–1108. doi: 10.1002/jssc.201401197. [DOI] [PubMed] [Google Scholar]
- 24.Lam BY, Lo AC, Sun X, Luo HW, Chung SK, Sucher NJ. Neuroprotective effects of tanshinones in transient focal cerebral ischemia in mice. Phytomedicine. 2003;10:286–291. doi: 10.1078/094471103322004776. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Deng CQ, Chen BY, Zhang SP, Liang Y, Luo XG. Total saponins of Panax Notoginseng modulate the expression of caspases and attenuate apoptosis in rats following focal cerebral ischemia-reperfusion. J Ethnopharmacol. 2009;121:412–418. doi: 10.1016/j.jep.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Q, Cheng X, Wang X, Wang J, Zhu Y, Ma X. Neuroprotective effect and mechanism of Mu-Xiang-You-Fang on cerebral ischemia-reperfusion injury in rats. J Ethnopharmacol. 2016;192:140–147. doi: 10.1016/j.jep.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Lian Y, Xie L, Chen M, Chen L. Effects of an astragalus polysaccharide and rhein combination on apoptosis in rats with chronic renal failure. Evid Based Complement Alternat Med. 2014;2014:271862. doi: 10.1155/2014/271862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genovese T, Mazzon E, Paterniti I, Esposito E, Bramanti P, Cuzzocrea S. Modulation of NADPH oxidase activation in cerebral ischemia/reperfusion injury in rats. Brain Res. 2011;1372:92–102. doi: 10.1016/j.brainres.2010.11.088. [DOI] [PubMed] [Google Scholar]
- 29.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Kaushal V, Schlichter LC. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci. 2008;28:2221–2230. doi: 10.1523/JNEUROSCI.5643-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI200422422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 34.Vakili A, Sharifat S, Akhavan MM, Bandegi AR. Effect of lavender oil (Lavandula angustifolia) on cerebral edema and its possible mechanisms in an experimental model of stroke. Brain Res. 2014;1548:56–62. doi: 10.1016/j.brainres.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigo R, Fernández-Gajardo R, Gutiérrez R, Matamala JM, Carrasco R, Miranda-Merchak A, Feuerhake W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol Disord Drug Targets. 2013;12:698–714. doi: 10.2174/1871527311312050015. [DOI] [PubMed] [Google Scholar]
- 36.Schettler V, Methe H, Staschinsky D, Schuff-Werner P, Müller GA, Wieland E. Review: The oxidant/antioxidant balance during regular low density lipoprotein apheresis. Ther Apher. 1999;3:219–226. doi: 10.1111/j.1091-6660.1999.t01-3-.x. [DOI] [PubMed] [Google Scholar]
- 37.Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 38.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carmona-Gutierrez D, Eisenberg T, Büttner S, Meisinger C, Kroemer G, Madeo F. Apoptosis in yeast: Triggers, pathways, subroutines. Cell Death Differ. 2010;17:763–773. doi: 10.1038/cdd.2009.219. [DOI] [PubMed] [Google Scholar]
- 40.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhen YF, Wang GD, Zhu LQ, Tan SP, Zhang FY, Zhou XZ, Wang XD. P53 dependent mitochondrial permeability transition pore opening is required for dexamethasone-induced death of osteoblasts. J Cell Physiol. 2014;229:1475–1483. doi: 10.1002/jcp.24589. [DOI] [PubMed] [Google Scholar]
- 42.Matés JM, Segura JA, Alonso FJ, Márquez J. Intracellular redox status and oxidative stress: Implications for cell proliferation, apoptosis, and carcinogenesis. Arch Toxicol. 2008;82:273–299. doi: 10.1007/s00204-008-0304-z. [DOI] [PubMed] [Google Scholar]
- 43.Sugawara T, Noshita N, Lewén A, Gasche Y, Ferrand-Drake M, Fujimura M, Morita-Fujimura Y, Chan PH. Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation. J Neurosci. 2002;22:209–217. doi: 10.1523/JNEUROSCI.22-01-00209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fahmi T, Branch D, Nima ZA, Jang DS, Savenka AV, Biris AS, Basnakian AG. Mechanism of graphene-induced cytotoxicity: Role of endonucleases. J Appl Toxicol. 2017;37:1325–1332. doi: 10.1002/jat.3462. [DOI] [PubMed] [Google Scholar]
- 45.Zhou X, Patel D, Sen S, Shanmugam V, Sidawy A, Mishra L, Nguyen BN. Poly-ADP-ribose polymerase inhibition enhances ischemic and diabetic wound healing by promoting angiogenesis. J Vasc Surg. 2017;65:1161–1169. doi: 10.1016/j.jvs.2016.03.407. [DOI] [PubMed] [Google Scholar]
- 46.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 47.Rami A, Bechmann I, Stehle JH. Exploiting endogenous anti-apoptotic proteins for novel therapeutic strategies in cerebral ischemia. Prog Neurobiol. 2008;85:273–296. doi: 10.1016/j.pneurobio.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 49.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 50.Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after BAX-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 51.Van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006;16:203–213. doi: 10.1038/sj.cr.7310028. [DOI] [PubMed] [Google Scholar]
- 52.Wang GH, Lan R, Zhen XD, Zhang W, Xiang J, Cai DF. An-Gong-Niu-Huang Wan protects against cerebral ischemia induced apoptosis in rats: Up-regulation of Bcl-2 and down-regulation of BAX and caspase-3. J Ethnopharmacol. 2014;154:156–162. doi: 10.1016/j.jep.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 53.Zhao P, Zhou R, Zhu XY, Hao YJ, Li N, Wang J, Niu Y, Sun T, Li YX, Yu JQ. Matrine attenuates focal cerebral ischemic injury by improving antioxidant activity and inhibiting apoptosis in mice. Int J Mol Med. 2015;36:633–644. doi: 10.3892/ijmm.2015.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SR, Lok J, Rosell A, Kim HY, Murata Y, Atochin D, Huang PL, Wang X, Ayata C, Moskowitz MA, Lo EH. Reduction of hippocampal cell death and proteolytic responses in tissue plasminogen activator knockout mice after transient global cerebral ischemia. Neuroscience. 2007;150:50–57. doi: 10.1016/j.neuroscience.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pérez-Garijo A. When dying is not the end: Apoptotic caspases as drivers of proliferation. Semin Cell Dev Biol. 2017;S1084–9521(17):30500–1. doi: 10.1016/j.semcdb.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 56.Liu J, Chen Z, Zhang Y, Zhang M, Zhu X, Fan Y, Shi S, Zen K, Liu Z. Rhein protects pancreatic β-cells from dynamin-related protein-1-mediated mitochondrial fission and cell apoptosis under hyperglycemia. Diabetes. 2013;62:3927–3935. doi: 10.2337/db13-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu H, Zhou Y, Tang L. Caffeine induces sustained apoptosis of human gastric cancer cells by activating the caspase9/caspase3 signalling pathway. Mol Med Rep. 2017;16:2445–2454. doi: 10.3892/mmr.2017.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Topçu Y, Bayram E, Ozbal S, Yiş U, Tuğyan K, Karaoğlu P, Kumral A, Yılmaz O, Kurul SH. Zonisamide attenuates hyperoxia-induced apoptosis in the developing rat brain. Neurol Sci. 2014;35:1769–1775. doi: 10.1007/s10072-014-1834-1. [DOI] [PubMed] [Google Scholar]
- 59.Rami A, Sims J, Botez G, Winckler J. Spatial resolution of phospholipid scramblase 1 (PLSCR1), caspase-3 activation and DNA-fragmentation in the human hippocampus after cerebral ischemia. Neurochem Int. 2003;43:79–87. doi: 10.1016/S0197-0186(02)00194-8. [DOI] [PubMed] [Google Scholar]
- 60.Le DA, Wu Y, Huang Z, Matsushita K, Plesnila N, Augustinack JC, Hyman BT, Yuan J, Kuida K, Flavell RA, Moskowitz MA. Caspase activation and neuroprotection in caspase-3 deficient mice after in vivo cerebral ischemia and in vitro oxygen glucose deprivation. Proc Natl Acad Sci USA. 2002;99:15188–15193. doi: 10.1073/pnas.232473399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang N, Zhang Y, Wu L, Wang Y, Cao Y, He L, Li X, Zhao J. Puerarin protected the brain from cerebral ischemia injury via astrocyte apoptosis inhibition. Neuropharmacology. 2014;79:282–289. doi: 10.1016/j.neuropharm.2013.12.004. [DOI] [PubMed] [Google Scholar]