Abstract
Transforming growth factor β (TGF-β) signaling either promotes or inhibits tumor formation and/or progression of many cancer types including squamous cell carcinoma (SCC). Canonical TGF-β signaling is mediated by a number of downstream proteins including Smad family proteins. Alterations in either TGF-β or Smad signaling can impact cancer. For instance, defects in TGF-β type I and type II receptors (TGF-βRI and TGF-βRII) and in Smad2/3/4 could promote tumor development. Conversely, increased TGF-β1 and activated TGF-βRI and Smad3 have all been shown to have tumor-promoting effects in experimental systems of human and mouse SCCs. Among TGF-β/Smad signaling, only TGF-βRII or Smad4 deletion in mouse epithelium causes spontaneous SCC in the mouse model, highlighting the critical roles of TGF-βRII and Smad4 in tumor suppression. Herein, we review the dual roles of the TGF-β/Smad signaling pathway and related mechanisms in SCC, highlighting the potential benefits and challenges of TGF-β/Smad-targeted therapies.
Keywords: TGF-β signaling, Smad proteins, SCC, tumor suppression, tumor promotion, therapeutic targets
Introduction
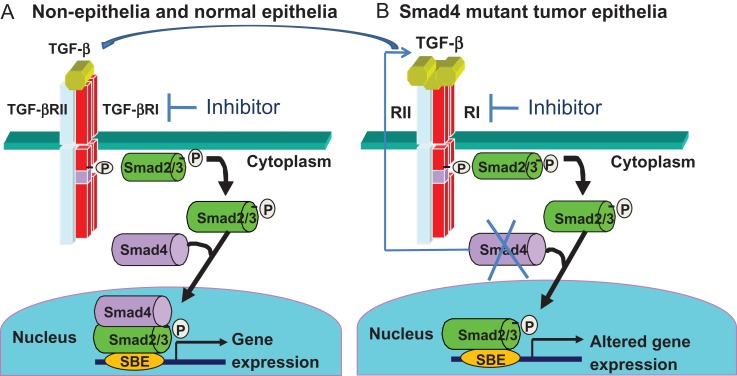
In mammals, transforming growth factor β (TGF-β) signaling has been extensively studied and is known to impact diverse cellular processes including differentiation, proliferation, migration, extracellular matrix remodeling, and apoptosis, all of which could be involved in various biological events including embryogenesis, immunity regulation, fibrosis, wound healing and tumor progression [1]. Typically, TGF-β ligands bind to TGF-β type II receptor (TGF-βRII), which can phosphorylate TGF-β type I receptor (TGF-βRI). The binding of TGF-βRII and TGF-βRI propagates signaling by phosphorylating cytoplasm mediators, Smad2 and Smad3, which then complex with Smad4 and translocate into the nucleus. In the nucleus, the phosphorylated Smad2/3–Smad4 complex binds to specific DNA sequence known as Smad binding elements (SBE), subsequently regulating transcription of TGF-β target genes [2] (Fig. 1A).
Figure 1.
Normal and altered Smad4 signaling (A) Canonical TGF-β signaling in normal cells or stromal cells. (B) Smad4 loss in tumor epithelia causes compensatory TGF-β overproduction that signals through Smad2/3 in tumor cells and paracrine TGF-β signaling in stromal cells through Smad2/3/4. TGF-β signaling can be blocked by a TGF-βRI inhibitor.
The functions of the TGF-β signaling pathway in cancer suppression and progression have also been extensively studied. Studies regarding the suppressive role of TGF-β signaling in cancers suggest that it could inhibit tumor formation mainly through inhibition of proliferation and by inducing growth arrest and apoptosis [3,4]. However, TGF-β also acts as a potent inducer of angiogenesis, inflammation, epithelial–mesenchymal transition (EMT) and immune suppression thereby promoting tumor progression and metastasis. Furthermore, depletions or mutations in genes encoding TGF-β receptors and Smads can cause spontaneous tumor development in mouse models and correlate to poor survival in human cancer [5,6]. However, novel molecular targets of TGF-β signaling that mediate tumor suppression and promotion effects, especially the ones that may serve as druggable targets, still remain to be identified.
Squamous cell carcinomas (SCC), as one of the most common cancers, mainly derives from stratified squamous epithelial cells in the upper digestive track and skin, causing more than 1 million deaths each year worldwide [7]. This review will focus on the paradoxical roles of TGF-β/Smad signaling in SCC in an effort to highlight the consequences of the signaling and how a better understanding of these outcomes may be utilized to design more targeted therapeutic approaches for patients with SCC.
TGF-β1 and Its Roles in SCC
TGF-β1 expression in SCC
TGF-β1 is a potent inhibitor of epithelial proliferation. Hence, it was unexpected when Akhurst et al. identified that TGF-β1 was overexpressed in chemical carcinogen-induced mouse skin SCCs [8]. Further studies from her laboratory have shown that TGF-β1 exhibits biphasic actions in murine skin SCC: suppressing the benign tumor growth but enhancing malignant conversion [9]. By creating a transgenic mouse model in which TGF-β1 can be inducibly expressed at discrete stages of skin carcinogenesis, we have further defined that the tumor suppression and promotion effects are stage-specific: inducing TGF-β1 overexpression prior to tumor formation suppresses benign papilloma formation [10], whereas inducing TGF-β1 overexpression after benign tumor formation promotes malignant transformation and metastasis [11]. We have also found that in human head and neck SCCs (HNSCCs) and skin SCCs, TGF-β1 is also overexpressed in ~78% and 52.9% of specimens, respectively [12,13]. Similarly, other laboratories have also identified elevated expression of TGF-β1 in esophageal SCC (ESCC) (Table 1). However, Logullo et al. argued that increased TGF-β1 expression exhibited no significant correlation with clinicopathological parameters in HNSCC [14]. Given the stage-specific effects of TGF-β1 found in experimental models described above, attempts of using TGF-β1 as a prognostic marker would need careful considerations for the stages of cancer samples being examined.
Table 1.
Expression of TGF-β/Smad signaling components in human SCC
| Skin SCC | Oral SCC | Esophageal SCC | References | |||||
|---|---|---|---|---|---|---|---|---|
| N/T | % | N/T | % | N/T | % | |||
| TGF-β1 | Up | 18/34 | 52.9% | 29/79 | 36.7% | 110/258 | 42.6% | [13,14,25,55] |
| 29/80 | 36.3% | |||||||
| TGF-βRI | Up | 49/61 | 80.3% | 0/68 | 0% | [29,30] | ||
| Down | 43/80 | 53.8% | [25] | |||||
| TGF-βRII | Down | 19/34 | 55.9% | 36/68 | 52.9% | 23/80 | 28.8% | [13,25,30,53] |
| 71/108 | 65.7% | |||||||
| Smad2 | Up | 19/48 | 39.6% | [48] | ||||
| Down | 58/83 | 69.9% | 7/80 | 8.8% | [40,47] | |||
| Smad3 | Up | 19/48 | 39.6% | [48] | ||||
| Down | 4/83 | 4.8% | 2/80 | 2.5% | [40,47] | |||
| Smad4 | Down | 58/83 | 69.9% | 66/108 | 61.1% | 175/258 | 67.8% | [40,53,55] |
N/T: number of positive cases in total cases; Up: overexpression in mRNA or protein level; Down: decreased or loss in mRNA, protein or genetic level.
Autocrine and paracrine effects of TGF-β1 and the tumor suppressive roles/mechanisms in SCC
Studies described above have revealed that the functions of TGF-β1 largely depend on tumor stage; it predominantly acts as a tumor suppressor during the early stage of tumorigenesis, while exerts a promotive role at the late stages of tumor development. During early tumorigenesis, components of TGF-β signaling pathway such as TGF-βRII, Smad2 and Smad4 have not yet become depleted or mutated; thus, endogenous TGF-β1 overexpression exerts a growth inhibitory effect. In epithelial cells, after the secretion and activation of endogenous TGF-β1 ligands, the ligands suppressed tumor development by inducing cell cycle inhibitory genes including p15InK4b and p21Waf1/Cip1. Furthermore, the TGF-β1 ligands downregulate c-Myc expression, and this downregulation is involved in proliferation inhibition [15,16]. Similarly, the increased TGF-β1 induced by inhibition of ANRIL (CDKN2B-AS1), a 3.8-kb long noncoding RNA, augments p15InK4b expression, thus inhibiting cellular proliferation in ESCC cell lines [17]. Finally, in a tongue SCC study, endogenous TGF-β1 slightly upregulated Smad4-induced p21 expression and delayed matrix metalloproteinase-2 (MMP-2) expression to promote apoptosis and inhibit proliferation of tumor cells [18]. However, we have shown that TGF-β1-induced inflammation in mouse oral mucosa overrides TGF-β1-mediated growth arrest [12]. Therefore, growth inhibition could not solely explain tumor suppressive effects of TGF-β1 in SCCs. Glick et al. have identified a critical role for TGF-β1 in DNA damage repair [19], which could be critical to prevent cancer formation at early stages.
Autocrine and paracrine effects of TGF-β1 and the tumor-promoting roles/mechanisms in SCC
We have shown that the non-malignant tissue adjacent to human HNSCC or skin SCC exhibits TGF-β1 overexpression [12,13]. To understand the role of TGF-β1 in this early stage of SCC carcinogenesis, we have generated transgenic mice in which TGF-β1 is overexpressed in keratinocytes of the skin or oral cavity [12,20]. TGF-β1 overexpression elicited profound inflammation in the skin and oral cavity, including the increased secretions of inflammatory cytokines including IL-1, IL-6, and IL-8 [20]. We have found that dramatic epithelial hyperplasia and increased expression of IL-1β, tumor necrosis factor α, and NF-κB are all present in TGF-β1-transgenic stroma and epithelium [12]. Our data also suggest that through paracrine signaling to endothelial cells, TGF-β1 transgene induction resulted in angiogenesis through upregulated expression of ALK1/pSmad1/5/8 [12]. Increased inflammation and angiogenesis in turn increase keratinocyte proliferation [20]. These mice never develop spontaneous SCC, suggesting that angiogenesis, profound inflammation and associated epithelial proliferation are insufficient for SCC initiation. However, these TGF-β1 effects play an important role in SCC progression [11]. We have also found that TGF-β1-induced EMT plays a critical role in early onset SCC metastasis [11]. Additionally, endogenous TGF-β1 secreted by stromal cells can also facilitate tumor progression in SCC. For instance, TGF-β1 secreted by cancer-associated fibroblasts (CAFs) increased matrix stiffness through activation of Yap1 and MMPs, subsequently facilitating invasion in OSCC [21,22].
In sum, the studies of molecular mechanisms described above illustrate that TGF-β1 plays dual roles in facilitating tumor inhibition and promoting tumor progression.
TGF-βRI and Its Roles in SCC
Although TGF-βRI mutation has been detected in ~19% of HNSCC patients with metastasis [23], its mutation or loss is quite rare in all cases of human HNSCC overall [24] (Table 1). However, in a study of human ESCC, ~53.8% of patients exhibited reduced expression of TGF-βRI, and this reduced expression correlated to depth of invasion, metastasis, and pathological stage [25] (Table 1), illustrating that TGF-βRI could play a suppressive role in SCC.
To understand the suppressive function of TGF-βRI in tumors, Yasuyuki et al. have generated a mouse model with conditional knockout of TGF-βRI in neurons by neurofilament (NF-H) Cre and found that ~35% TGF-βRI-knockout mice developed SCC in the periorbital or perianal regions 6 months after birth [26]. However, these spontaneous SCCs were negative for the neuronal marker (neuron specific enolase) and did not harbor TGF-βRI deletion, suggesting the SCCs were not derived from TGF-βRI null neural cells [26]. Instead, it is likely the SCCs were derived from TGF-βRI wild type skin epithelial cells as the result of crosstalk between TGF-βRI knockout neurons and epithelial stem cells [26]. In their study, 33% of SCCs from TGF-βRI-knockout mice exhibited IL-13Rα2 and its expression might be involved in the tumorigenesis of SCC, probably enhancing paracrine effects of TGF-β in escaping from immunosurveillance [26]. Another study also showed that TGF-βRI depletion in head and neck epithelia alone is insufficient to initiate spontaneous HNSCC development but accelerates carcinogen 7,12-dimethybenz(a)anthracene (DMBA) initiated SCC in mice [27]. Interestingly, Goudie et al. have found that TGF-βRI mutation causes multiple self-healing squamous epithelioma, an autosomal dominant skin cancer characterized by spontaneous regression [28]. Together, these data indicate that abrogating tumor suppressive effects of TGF-βRI can contribute to the development of both malignant SCC as well as benign squamous tumors.
On the other hand, TGF-βRI can also be overexpressed beyond a physiological level. For example, 80.3% of patients with skin SCC exhibited overexpressed TGF-βRI [29] (Table 1), and continuous expression of TGF-βRI correlated with high pSmad2/3 in skin SCC compared to the surrounding epidermis with the strongest TGF-βRI expression in tumors on sun-exposed skin [29]. However, to date, there is not an experimental model to assess the role of TGF-βRI overexpression in SCC.
TGF-βRII and Its Roles in SCC
The decreased expression and inhibitory roles of TGF-βRII in human SCC have been identified (Table 1). We have found that decreased or lost expression of TGF-βRII occurs in 35.3% of human OSCC on the protein level and in more than 70% of human HNSCC by mRNA levels [30,31]. The fact that TGF-βRII reduction occurs only in HNSCCs but not in adjacent mucosa suggests that loss of TGF-βRII is a relatively late-stage event of SCC carcinogenesis. In late-stage OSCC, the E221V/N238I mutation of TGF-βRII enhanced TGF-β signaling and delayed the internalization of TGF-βRII, subsequently leading to more invasive phenotypic changes [32]. To define the role of TGF-βRII in SCC, several mouse models with keratinocyte-specific TGF-βRII deletion have been established. In oral keratinocytes with TGF-βRII deletion, we have found no spontaneous tumor formation in mice, which is consistent with TGF-βRII loss in human HNSCCs but not in early lesions [31]. These data suggest that TGF-βRII loss in oral keratinocyte is not an initiation event. Indeed, when we introduced a Kras or Hras mutation in this model as an SCC initiation event, these mice developed HNSCC [31]. This model represents the first genetically engineered mouse model with full penetrance of HNSCC. Of note, when TGF-βRII is deleted in mouse airway epithelial cells, it causes increased size and number of Kras-initiated lung SCC [33]. Contrary to our findings, Guasch et al. have shown that K14-Cre/TGF-βRII–/– mice could develop spontaneous anal and genital SCC derived from the transition zone between mucosal epithelium of large intestine and stratified squamous epithelium of anal skin [34,35], suggesting that such transition zones are uniquely susceptible to tumorigenesis in contrast to the more refractory oral and skin epithelium. These data illustrate that TGF-βRII loss can be a tumor-initiating event but exhibits tissue specificity. All these studies support the notion that TGF-βRII loss promotes SCC progression in vivo, and can also act as tumor initiator with tissue and temporal specificity.
Mechanistically, we showed that TGF-βRII depletion could increase the expression of endogenous TGF-β1 secreted by both the epithelia and stroma, though the increase was more significant in the stroma [31]. Consequently, the resultant overexpression of TGF-β1 may increase angiogenesis and inflammation, subsequently promoting tumor progression in HNSCC [31]. Similarly, a mouse model with TGF-βRII knockdown in airway epithelia also exhibited increased TGF-β1 ligand expression, enhancing lung tumor development through increased proliferation and local inflammation but without increasing angiogenesis [33]. From these studies, it is clear that TGF-βRII deletion in keratinocytes causes inflammation during tumor progression. To further examine its role in inflammation, Cohen et al. have developed an oral-specific TGF-βRII-mutant model, and have shown that mutant TP53 might serve as an upstream repressor of TGF-βRII expression and TGF-βRII depletion in tumor epithelial cells results in activated NF-κB1/RelA (p50/p65)[36]. Conversely, we have shown that TGF-β1 overexpression activates NF-κB [20], and increased NF-κB activation in TGF-βRII depletion tumor cells could be via a mechanism independent of TGF-β signaling. Additionally, TGF-βRII deletion increases tumor cell migration and invasion in human bronchial epithelial cell line [33]. Further supporting the suppressive role of TGF-βRII in SCC, and confirmed by both in vitro and in vivo studies, TGF-βRII ablation in epidermal keratinocytes, in coordination with oncogenic mutations in Hras, promotes hyperproliferation and maintains low apoptosis, thereby leading to destabilized homeostasis and tumorigenesis in stratified epithelium [35]. In addition, TGF-βRII deficiency also enhances cell migration and invasion, mainly through integrin-FAK-Src signaling [35]. Furthermore, tumor-initiating stem cells or cancer stem cells (CSCs) from the anal canal and rectum transition zone with TGF-βRII loss enhance tumor cell invasion and metastasis in SCC through de-repression of ELMO1, a RAC-activating guanine exchange factor specifically located in CSCs of anorectal SCC [34]. Taken together, these data demonstrate that angiogenesis, inflammation, proliferation, apoptosis and tumor cell migration and/or invasion can be involved in TGF-βRII-deficient SCC initiation or progression.
Smad2 and Its Roles in SCC
Smad2 is located on chromosome 18q21, near the Smad4 site in the human genome [37]. Smad2 point mutations are infrequent in human primary HNSCC and HNSCC cell lines; only one study reported a Smad2 mutant HNSCC cell line [38,39]. However, we have shown that ~67% of poorly differentiated skin SCCs exhibit loss of heterozygosity (LOH) at the Smad2 locus [40]. Similarly, in another study, Smad2 LOH was detected in 63% of HNSCC cell lines [41]. Further, 94% and 70% of poorly differentiated human skin SCCs had a Smad2 reduction in mRNA and protein levels, respectively [40] (Table 1). These studies suggest that Smad2 LOH is a common event in pre-transcriptional, transcriptional, and posttranscriptional levels during the SCC progression. With regard to the correlation between decreased or loss of Smad2 and clinical tumor behavior, Smad2 protein loss was most common in poorly differentiated human HNSCC [39]. Intriguingly, in a study related to posttranscriptional regulation of Smad2, epigenetically decreased disabled homolog 2 (DAB2) in SCC cell lines inhibits Smad2 phosphorylation and its activation, thereby promoting tumor progression [42]. Conversely, re-expression of DAB2 in SCC cell lines with DAB2-downregulation results in renewed growth prohibitive responses to TGF-β [42]. Taken together, these two studies suggest that DAB2 loss could act as a switch to transition TGF-β pathway signaling from tumor suppressive to promoting, and the critical protein for this transition may be Smad2.
To better understand the role of Smad2 loss in stratified epithelia in vivo, we have created a model with inducible and keratinocyte-specific Smad2-knockout mice driven by a keratin-5 promoter (K5.Smad2–/–) [40,43]. Neither the homozygous (K5.Smad2–/–) nor heterozygous (K5.Smad2+/–) loss mice developed spontaneous skin tumors [40], thus Smad2 loss alone is not sufficient for tumor initiation. However, both K5.Smad2–/– and K5.Smad2+/– mice exhibited accelerated tumor formation and malignant conversion when subject to a two-stage chemical skin carcinogen exposure compared to wild type mice [40,44], indicating that Smad2 loss promotes susceptibility to skin tumorigenesis and promotes malignant progression.
Smad2 loss-associated EMT and angiogenesis are the two main processes contributing to tumor progression in SCC. We have found that Smad2 loss recruits Smad4 binding to the SBE of Snail, subsequently leading to Snail expression and contributing to the loss of E-cadherin [40]. Additionally, skin SCC with Smad2 ablation increased the expression of hepatocyte growth factor (HGF), a potent angiogenic factor and a promoter for tumor epithelial cell migration, resulting in activation of the HGF receptor, c-Met, on the endothelial cells [40,44]. These data suggest that EMT and angiogenesis induced by Smad2 loss contribute to SCC susceptibility and progression.
Smad3 and Its Roles in SCC
Smad3 expression in SCC
In human SCCs, Smad3 missense mutations are at a low frequency in HNSCC [13] and Smad3 loss or reduction in protein level is also uncommon (0%–4.8%) in HNSCC, skin SCC or ESCC [40,45,46,47] (Table 1). Increased Smad3 expression at the mRNA level, however, has been reported in 39.6% of OSCCs [48].
Tumor suppressive roles/mechanisms of Smad3 in SCC
In a mouse model with conditional Smad3 knockdown, Bae et al. found that v-RasHa-transduced Smad3–/– keratinocytes developed SCC, while v-RasHa-transduced Smad3+/+ keratinocytes only exhibited papillomas [49]. Similarly, in another study, Vijgayachandra et al. grafted the primary keratinocytes with v-RasHa-transduced Smad3 loss onto nude mice and found that 50% of the Smad3–/– grafts underwent malignant conversion, while 85.7% of the Smad3+/+ ones exhibited benign papillomas [50]. These data indicate that expression of Smad3 can suppress SCC carcinogenesis. Indeed, Smad3 expression can abrogate tumor progression through inducing senescence and regulating inflammation. For instance, overexpression of Smad3 in v-RasHa-transduced keratinocytes increased senescent cells and S phase cells [50].
Tumor promotion roles/mechanisms of Smad3 in SCC
In contradiction to the above v-RasHa-transduced Smad3–/– spontaneous tumor data [49,50], in our study, neither Smad3+/– nor Smad3–/– mice developed spontaneous skin tumors [46]. Surprisingly, Smad3–/– mice have attenuated inflammation and fewer tumor associated macrophages but increased apoptosis [46]. Furthermore, we have found that more than 90% of Smad3+/+ papilloma cells were positive for NF-κB, while only ~50% or less were identified in Smad3+/– and Smad3–/– mice respectively [46], suggesting that inducing a pro-inflammatory response is one of the potential mechanisms of Smad3 acting as a tumor promotor in SCC. In addition to alterations in NF-κB, Smad3 can function in other capacities to promote tumor progression by antagonizing the tumor suppressive roles of TGF-β1. For instance, Smad3 knockdown blocks the ability of TGF-β1 to induce either MMP-9 or uPA gene expression, consequently inhibiting tumor invasion and metastasis [49]. Additionally, Park et al. have found that death-associated protein kinase-related apoptosis-inducing kinase 1 (DRAK1) inhibits TGF-β1 tumor suppressor activity by binding Smad3 in HNSCC, consequently blocking Smad3–Smad4 complex formation [16]. These studies demonstrate that Smad3 might mediate tumor promotion in SCC.
Smad4 and Its Roles in SCC
Smad4 is localized to 18q21–22 chromosome, near the Smad2 locus. Notably, chromosome 18q LOH is a common event in HNSCC and it occurs in 56% of the primary and secondary HNSCC cell lines [5,51,52]. Furthermore, we have also found that 86% of tumors and 67% of adjacent non-malignant mucosa show more than 50% reduction of Smad4 mRNA expression in HNSCC [5], suggesting that Smad4 reduction is an early event during the HNSCC progression. However, by immunostaining, studies of Smad4 loss at a protein level vary significantly from as low as 12% to as high as 61.12% in HNSCC [53,54] (Table 1). These differences might have been caused by different tumor locations, different methods in measurement or even different races. In accordance, by immunostaining, 51.2% of the patients with ESCC exhibited Smad4 loss and 67.8% had a Smad4 reduction at protein level [55,56]. In both human HNSCC and ESCC, attenuated Smad4 is associated with more advanced tumor characteristics including invasion and poor prognosis [53,55–57]. Taken together, these observations reveal that Smad4 loss or reduction is a common event even in the early stage of SCC and Smad4 mainly plays a suppressive role in SCC progression. In fact, Smad4 ablation alone causes spontaneous SCC in the skin, oral cavity and stomach of mice [5,58–60]. Intriguingly, Smad4/Dpc4 conditional knockout in mouse mammary glands causes SCC development, representing a trans-differentiation of tumor type [61].
There are a variety of potential mechanisms related to carcinogenesis induced by Smad4 loss in SCC (Fig. 1B). For example, we have shown that Smad4 loss increases cell proliferation and reduces apoptosis in Smad4–/– mucosa and Smad4–/– SCC when compared to Smad+/+ mucosa [11]. These alterations effectively abrogate the early tumor suppression induced by TGF-β via relieving the TGF-β-mediated growth arrest. However, these changes do not explain why Smad4 depletion alone is an initiation event for SCC, as similar changes were found in our study on TGF-βRII ablation which required combination with Kras or Hras mutation for HNSCC tumorigenesis [31]. Intriguingly, we have identified that Smad4 loss downregulates the expression of Brca/Fanc (Breast cancer susceptibility/Fanconi anemia complementation) genes, which are critical for double-stranded DNA repair [5]. Furthermore, decreased expression of Brca/Fanc induced by Smad4 loss is essential for accumulation of DNA damage to initiate SCC formation [5]. In support of this, clinical data indicate that Fanconi anemia patients with Brca/Fanc mutations have markedly increased susceptibility to HNSCC compared to general population [62]. Interestingly, Brca1 depletion leads to development of SCC in the skin, the inner ear canal and the oral epithelium [63,64]. In addition, Smad4 loss could increase the overexpression of TGF-β1 and activate Smad3, subsequently leading to inflammation [5]. Intriguingly, leukocyte infiltration in Smad4–/– tissue was decreased significantly when these mice were bred into the Smad3+/– background [5]. These results suggest that Smad3 contributes to TGF-β1-associated inflammation during abrogation of Smad4 in SCC. A study using human HNSCC cells showed that Smad4 downregulation induces EMT while enhancing cetuximab resistance in HNSCC [65]. Similarly, we have shown that when Smad4 deletion is targeted to K15+ stem cells, SCCs have a high incidence of EMT [66]. Conversely, studies have also shown that Smad4 is required for TGF-β-mediated EMT [40,67]. Therefore, EMT in HNSCCs with low Smad4 may be an indirect effect of Smad4 loss or independent of TGF-β signaling. To this end, Ozawa et al. have found that activated JNK and MAPK pathways contribute to cetuximab resistance in human HNSCC cell lines with Smad4 loss [68]. Importantly, the use of JNK and MAPK inhibitors sensitized the Smad4-loss HNSCC cell lines to cetuximab [68]. Lastly, changes in Smad4 can impact the surrounding stromal microenvironment. For example, Smad4 abrogation in the oral mucosa increases infiltration of macrophages, granulocytes and T lymphocytes in the stroma adjacent to Smad4–/– mucosa and the tumor stroma of Smad4–/– SCC, consequently increasing inflammation [5]. Furthermore, SCCs with Smad4 loss escape CD8+ T cell-mediated immune surveillance by activation and exhaustion of CD8+ T cells with co-expression of programmed cell death-1 (PD-1) and lymphocyte activation gene-3 (LAG-3), and dual inhibition of PD-1 and LAG-3 on CD8+ T cells suppresses tumor growth in SCC with Smad4 loss [69]. In sum, these data demonstrate that Smad4 loss in the epithelium promotes tumor formation through its direct effects in the epithelium and indirect effects in the stroma.
Potential Strategies for Targeting TGF-β/Smad Signaling in Cancer Therapy
Considering the paradoxical roles of TGF-β/Smad signaling pathway in tumor suppression and promotion of SCC, careful considerations are warranted in the development of cancer therapies targeting TGF-β signaling. Many therapeutic agents including neutralizing antibodies, antisense oligonucleotides (ASOs), and receptor kinase inhibitors that block TGF-β/Smad signaling have already been developed for suppressing tumor progression after tumors have lost early TGF-β-mediated tumor suppression. For example, in mouse models, two neutralizing antibodies, 2G7 and 1D11, can bind all three TGF-β isoforms and reduce their biological activity in tumors [70,71]. Another strategy to reduce TGF-β ligand synthesis is achieved by ASOs. ASOs are designed to hybridize to TGF-β isoforms’ complementary sequence in RNA and increase the mRNA degradation [72]. ASOs to TGF-β1 and TGF-β2 have already been investigated as an approach for cancer therapy. Clinically, the suppression of TGF-β2 production by ASOs (AP12009) has been employed in clinical trials for glioblastoma and astrocytoma [73]. Although ASOs inhibit the activities of TGF-β ligands, they are unable to block receptor signaling directly, suggesting that inhibiting receptors may be more effective at tumor suppression. For instance, unlike ASOs, miR-211 and miR-17/20a can bind to TGF-βRII directly, thereby attenuating the phosphorylation of Smad2 or Smad3 and promoting SCC progression [74,75]. In addition, TGF-βRI/ALK5 inhibitors such as Ki26894 and LY364937 block TGF-β signaling in pancreatic, hepatocellular cancers and glioblastoma [72,76]. Finally, in skin SCC, LY2109761, another ALK5 inhibitor, exhibited tumor suppression through reducing carcinoma myofibroblasts and disrupting vascular integrity [77]. Due to the early tumor suppressive actions of TGF-β signaling, TGF-β inhibitor clinical trials are all at the late-stage/metastasis setting. To date, it is unknown if treatment regimens of TGF-β inhibitor are effective in SCC, as no such a clinical trial exist to date in SCCs. For clinical trial designs, any therapeutic strategies developed to exploit the dual roles of TGF-β1, TGF-βRI and Smads should be aware of the temporal transition from tumor suppressor to promotor to optimize treatment efficacy.
Conclusion
The TGF-β/Smad signaling pathway exhibits paradoxical roles by exhibiting both tumor-suppressing and tumor-promoting functions. TGF-β1 and TGF-βRI are identified as tumor suppressors during the early stage of tumorigenesis, while they exert promotive roles in later stages. Smad2, TGF-βRII, and Smad4 mainly act as tumor suppressors in SCC. However, these TGF-β signaling components are also required for tumor-promoting effects of TGF-β signaling. Only TGF-βRII or Smad4 deletion in the epithelium could develop spontaneous SCC in mouse models, indicating that TGF-βRII and Smad4 play a key role in the suppression of SCC. Any therapeutic strategies designed to inhibit the tumor-promoting role of TGF-β, TGF-βRI, TGF-βRII, and Smads should focus on, or be aware of, the mechanism and timing of the switch from tumor suppressor to promoter. Notably, efforts in drug development of TGF-β inhibitors are now gearing towards selectively blocking the tumor-promoting effects of TGF-β/Smad signaling, while avoiding toxicity.
Funding
F.W. is supported by China Scholarship Council (File No. 201606240201). K.W. is supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Awards T32CA17468 and 1F32DE027285-01. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The original work from the Wang lab is supported by NIH grants.
Acknowledgments
Due to space limitations, we apologize for not being able to cite and discuss all relevant work from different laboratories published to date. The authors declare that they have no competing interests with respect to the authorship and publication of this article.
References
- 1. Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol 2012, 13: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu T, Feng XH. Regulation of TGF-beta signalling by protein phosphatases. Biochem J 2010, 430: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dong M, How T, Kirkbride KC, Gordon KJ, Lee JD, Hempel N, Kelly P, et al. . The type III TGF-beta receptor suppresses breast cancer progression. J Clin Invest 2007, 117: 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malkoski SP, Wang XJ. Two sides of the story? Smad4 loss in pancreatic cancer versus head-and-neck cancer. FEBS Lett 2012, 586: 1984–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bornstein S, White R, Malkoski S, Oka M, Han G, Cleaver T, Reh D, et al. . Smad4 loss in mice causes spontaneous head and neck cancer with increased genomic instability and inflammation. J Clin Invest 2009, 119: 3408–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slattery ML, Herrick JS, Lundgreen A, Wolff RK. Genetic variation in the TGF-beta signaling pathway and colon and rectal cancer risk. Cancer Epidemiol Biomarkers Prev 2011, 20: 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jemal A, Simard EP, Dorell C, Noone AM, Markowitz LE, Kohler B, Eheman C, et al. . Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013, 105: 175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akhurst RJ, Fee F, Balmain A. Localized production of TGF-beta mRNA in tumour promoter-stimulated mouse epidermis. Nature 1988, 331: 363–365. [DOI] [PubMed] [Google Scholar]
- 9. Cui W, Fowlis DJ, Bryson S, Duffie E, Ireland H, Balmain A, Akhurst RJ. TGFbeta1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell 1996, 86: 531–542. [DOI] [PubMed] [Google Scholar]
- 10. Wang XJ, Liefer KM, Tsai S, O’Malley BW, Roop DR. Development of gene-switch transgenic mice that inducibly express transforming growth factor beta1 in the epidermis. Proc Natl Acad Sci U S A 1999, 96: 8483–8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weeks BH, He W, Olson KL, Wang XJ. Inducible expression of transforming growth factor beta1 in papillomas causes rapid metastasis. Cancer Res 2001, 61: 7435–7443. [PubMed] [Google Scholar]
- 12. Lu SL, Reh D, Li AG, Woods J, Corless CL, Kulesz-Martin M, Wang XJ. Overexpression of transforming growth factor beta1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation. Cancer Res 2004, 64: 4405–4410. [DOI] [PubMed] [Google Scholar]
- 13. Han G, Lu SL, Li AG, He W, Corless CL, Kulesz-Martin M, Wang XJ. Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J Clin Invest 2005, 115: 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Logullo AF, Nonogaki S, Miguel RE, Kowalski LP, Nishimoto IN, Pasini FS, Federico MH, et al. . Transforming growth factor beta1 (TGFbeta1) expression in head and neck squamous cell carcinoma patients as related to prognosis. J Oral Pathol Med 2003, 32: 139–145. [DOI] [PubMed] [Google Scholar]
- 15. Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390: 465–471. [DOI] [PubMed] [Google Scholar]
- 16. Park Y, Kim W, Lee JM, Park J, Cho JK, Pang K, Lee J, et al. . Cytoplasmic DRAK1 overexpressed in head and neck cancers inhibits TGF-beta1 tumor suppressor activity by binding to Smad3 to interrupt its complex formation with Smad4. Oncogene 2015, 34: 5037–5045. [DOI] [PubMed] [Google Scholar]
- 17. Chen D, Zhang Z, Mao C, Zhou Y, Yu L, Yin Y, Wu S, et al. . ANRIL inhibits p15(INK4b) through the TGFbeta1 signaling pathway in human esophageal squamous cell carcinoma. Cell Immunol 2014, 289: 91–96. [DOI] [PubMed] [Google Scholar]
- 18. Wang X, Sun W, Zhang C, Ji G, Ge Y, Xu Y, Zhao Y. TGF-beta1 inhibits the growth and metastasis of tongue squamous carcinoma cells through Smad4. Gene 2011, 485: 160–166. [DOI] [PubMed] [Google Scholar]
- 19. Glick AB, Weinberg WC, Wu IH, Quan W, Yuspa SH. Transforming growth factor beta 1 suppresses genomic instability independent of a G1 arrest, p53, and Rb. Cancer Res 1996, 56: 3645–3650. [PubMed] [Google Scholar]
- 20. Li AG, Wang D, Feng XH, Wang XJ. Latent TGFbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. EMBO J 2004, 23: 1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, et al. . Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 2013, 15: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Le Bras GF, Taylor C, Koumangoye RB, Revetta F, Loomans HA, Andl CD. TGFbeta loss activates ADAMTS-1-mediated EGF-dependent invasion in a model of esophageal cell invasion. Exp Cell Res 2015, 330: 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen T, Yan W, Wells RG, Rimm DL, McNiff J, Leffell D, Reiss M. Novel inactivating mutations of transforming growth factor-beta type I receptor gene in head-and-neck cancer metastases. Int J Cancer 2001, 93: 653–661. [DOI] [PubMed] [Google Scholar]
- 24. White RA, Malkoski SP, Wang XJ. TGFbeta signaling in head and neck squamous cell carcinoma. Oncogene 2010, 29: 5437–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fukai Y, Fukuchi M, Masuda N, Osawa H, Kato H, Nakajima T, Kuwano H. Reduced expression of transforming growth factor-beta receptors is an unfavorable prognostic factor in human esophageal squamous cell carcinoma. Int J Cancer 2003, 104: 161–166. [DOI] [PubMed] [Google Scholar]
- 26. Honjo Y, Bian Y, Kawakami K, Molinolo A, Longenecker G, Boppana R, Larsson J, et al. . TGF-beta receptor I conditional knockout mice develop spontaneous squamous cell carcinoma. Cell Cycle 2007, 6: 1360–1366. [DOI] [PubMed] [Google Scholar]
- 27. Bian Y, Terse A, Du J, Hall B, Molinolo A, Zhang P, Chen W, et al. . Progressive tumor formation in mice with conditional deletion of TGF-beta signaling in head and neck epithelia is associated with activation of the PI3K/Akt pathway. Cancer Res 2009, 69: 5918–5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goudie DR, D’Alessandro M, Merriman B, Lee H, Szeverenyi I, Avery S, O’Connor BD, et al. . Multiple self-healing squamous epithelioma is caused by a disease-specific spectrum of mutations in TGFBR1. Nat Genet 2011, 43: 365–369. [DOI] [PubMed] [Google Scholar]
- 29. Berger F, Geddert H, Faller G, Werner M, Dimmler A. Pattern of TGFbeta receptor 1 expression differs between kras-mutated keratoacanthomas and squamous cell carcinomas of the skin. Pathol Res Pract 2014, 210: 596–602. [DOI] [PubMed] [Google Scholar]
- 30. Meng W, Xia Q, Wu L, Chen S, He X, Zhang L, Gao Q, et al. . Downregulation of TGF-beta receptor types II and III in oral squamous cell carcinoma and oral carcinoma-associated fibroblasts. BMC Cancer 2011, 11: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu SL, Herrington H, Reh D, Weber S, Bornstein S, Wang D, Li AG, et al. . Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev 2006, 20: 1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park I, Son HK, Che ZM, Kim J. A novel gain-of-function mutation of TGF-beta receptor II promotes cancer progression via delayed receptor internalization in oral squamous cell carcinoma. Cancer Lett 2012, 315: 161–169. [DOI] [PubMed] [Google Scholar]
- 33. Malkoski SP, Haeger SM, Cleaver TG, Rodriguez KJ, Li H, Lu SL, Feser WJ, et al. . Loss of transforming growth factor beta type II receptor increases aggressive tumor behavior and reduces survival in lung adenocarcinoma and squamous cell carcinoma. Clin Cancer Res 2012, 18: 2173–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCauley HA, Chevrier V, Birnbaum D, Guasch G. De-repression of the RAC activator ELMO1 in cancer stem cells drives progression of TGFbeta-deficient squamous cell carcinoma from transition zones. Elife 2017, 6: e22914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, Fuchs E. Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell 2007, 12: 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cohen J, Chen Z, Lu SL, Yang XP, Arun P, Ehsanian R, Brown MS, et al. . Attenuated transforming growth factor beta signaling promotes nuclear factor-kappaB activation in head and neck cancer. Cancer Res 2009, 69: 3415–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eppert K, Scherer SW, Ozcelik H, Pirone R, Hoodless P, Kim H, Tsui LC, et al. . MADR2 maps to 18q21 and encodes a TGFbeta-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell 1996, 86: 543–552. [DOI] [PubMed] [Google Scholar]
- 38. Qiu W, Schonleben F, Li X, Su GH. Disruption of transforming growth factor beta-Smad signaling pathway in head and neck squamous cell carcinoma as evidenced by mutations of SMAD2 and SMAD4. Cancer Lett 2007, 245: 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muro-Cacho CA, Rosario-Ortiz K, Livingston S, Munoz-Antonia T. Defective transforming growth factor beta signaling pathway in head and neck squamous cell carcinoma as evidenced by the lack of expression of activated Smad2. Clin Cancer Res 2001, 7: 1618–1626. [PubMed] [Google Scholar]
- 40. Hoot KE, Lighthall J, Han G, Lu SL, Li A, Ju W, Kulesz-Martin M, et al. . Keratinocyte-specific Smad2 ablation results in increased epithelial-mesenchymal transition during skin cancer formation and progression. J Clin Invest 2008, 118: 2722–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takebayashi S, Ogawa T, Jung KY, Muallem A, Mineta H, Fisher SG, Grenman R, et al. . Identification of new minimally lost regions on 18q in head and neck squamous cell carcinoma. Cancer Res 2000, 60: 3397–3403. [PubMed] [Google Scholar]
- 42. Hannigan A, Smith P, Kalna G, Lo Nigro C, Orange C, O’Brien DI, Shah R, et al. . Epigenetic downregulation of human disabled homolog 2 switches TGF-beta from a tumor suppressor to a tumor promoter. J Clin Invest 2010, 120: 2842–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hoot KE, Oka M, Han G, Bottinger E, Zhang Q, Wang XJ. HGF upregulation contributes to angiogenesis in mice with keratinocyte-specific Smad2 deletion. J Clin Invest 2010, 120: 3606–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tannehill-Gregg SH, Kusewitt DF, Rosol TJ, Weinstein M. The roles of Smad2 and Smad3 in the development of chemically induced skin tumors in mice. Vet Pathol 2004, 41: 278–282. [DOI] [PubMed] [Google Scholar]
- 45. Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, Fakhry C, et al. . Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 2011, 333: 1154–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li AG, Lu SL, Zhang MX, Deng C, Wang XJ. Smad3 knockout mice exhibit a resistance to skin chemical carcinogenesis. Cancer Res 2004, 64: 7836–7845. [DOI] [PubMed] [Google Scholar]
- 47. Fukuchi M, Nakajima M, Miyazaki T, Masuda N, Osawa H, Manda R, Tsukada K, et al. . Lack of activated Smad2 in transforming growth factor-beta signaling is an unfavorable prognostic factor in patients with esophageal squamous cell carcinoma. J Surg Oncol 2006, 94: 51–56. [DOI] [PubMed] [Google Scholar]
- 48. Mangone FR, Walder F, Maistro S, Pasini FS, Lehn CN, Carvalho MB, Brentani MM, et al. . Smad2 and Smad6 as predictors of overall survival in oral squamous cell carcinoma patients. Mol Cancer 2010, 9: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bae DS, Blazanin N, Licata M, Lee J, Glick AB. Tumor suppressor and oncogene actions of TGFbeta1 occur early in skin carcinogenesis and are mediated by Smad3. Mol Carcinog 2009, 48: 441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vijayachandra K, Lee J, Glick AB. Smad3 regulates senescence and malignant conversion in a mouse multistage skin carcinogenesis model. Cancer Res 2003, 63: 3447–3452. [PubMed] [Google Scholar]
- 51. Snijders AM, Schmidt BL, Fridlyand J, Dekker N, Pinkel D, Jordan RC, Albertson DG. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene 2005, 24: 4232–4242. [DOI] [PubMed] [Google Scholar]
- 52. Takebayashi S, Hickson A, Ogawa T, Jung KY, Mineta H, Ueda Y, Grenman R, et al. . Loss of chromosome arm 18q with tumor progression in head and neck squamous cancer. Genes Chromosomes Cancer 2004, 41: 145–154. [DOI] [PubMed] [Google Scholar]
- 53. Wang X, Sun W, Bai J, Ma L, Yu Y, Geng J, Qi J, et al. . Growth inhibition induced by transforming growth factor-beta1 in human oral squamous cell carcinoma. Mol Biol Rep 2009, 36: 861–869. [DOI] [PubMed] [Google Scholar]
- 54. Xie W, Aisner S, Baredes S, Sreepada G, Shah R, Reiss M. Alterations of Smad expression and activation in defining 2 subtypes of human head and neck squamous cell carcinoma. Head Neck 2013, 35: 76–85. [DOI] [PubMed] [Google Scholar]
- 55. Natsugoe S, Xiangming C, Matsumoto M, Okumura H, Nakashima S, Sakita H, Ishigami S, et al. . Smad4 and transforming growth factor beta1 expression in patients with squamous cell carcinoma of the esophagus. Clin Cancer Res 2002, 8: 1838–1842. [PubMed] [Google Scholar]
- 56. Fukuchi M, Masuda N, Miyazaki T, Nakajima M, Osawa H, Kato H, Kuwano H. Decreased Smad4 expression in the transforming growth factor-beta signaling pathway during progression of esophageal squamous cell carcinoma. Cancer 2002, 95: 737–743. [DOI] [PubMed] [Google Scholar]
- 57. Xie W, Bharathy S, Kim D, Haffty BG, Rimm DL, Reiss M. Frequent alterations of Smad signaling in human head and neck squamous cell carcinomas: a tissue microarray analysis. Oncol Res 2003, 14: 61–73. [DOI] [PubMed] [Google Scholar]
- 58. Yang L, Mao C, Teng Y, Li W, Zhang J, Cheng X, Li X, et al. . Targeted disruption of Smad4 in mouse epidermis results in failure of hair follicle cycling and formation of skin tumors. Cancer Res 2005, 65: 8671–8678. [DOI] [PubMed] [Google Scholar]
- 59. Xu X, Brodie SG, Yang X, Im YH, Parks WT, Chen L, Zhou YX, et al. . Haploid loss of the tumor suppressor Smad4/Dpc4 initiates gastric polyposis and cancer in mice. Oncogene 2000, 19: 1868–1874. [DOI] [PubMed] [Google Scholar]
- 60. Qiao W, Li AG, Owens P, Xu X, Wang XJ, Deng CX. Hair follicle defects and squamous cell carcinoma formation in Smad4 conditional knockout mouse skin. Oncogene 2006, 25: 207–217. [DOI] [PubMed] [Google Scholar]
- 61. Li W, Qiao W, Chen L, Xu X, Yang X, Li D, Li C, et al. . Squamous cell carcinoma and mammary abscess formation through squamous metaplasia in Smad4/Dpc4 conditional knockout mice. Development 2003, 130: 6143–6153. [DOI] [PubMed] [Google Scholar]
- 62. Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, Goberdhan A, et al. . High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg 2003, 129: 106–112. [DOI] [PubMed] [Google Scholar]
- 63. Houghtaling S, Timmers C, Noll M, Finegold MJ, Jones SN, Meyn MS, Grompe M. Epithelial cancer in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Genes Dev 2003, 17: 2021–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Berton TR, Matsumoto T, Page A, Conti CJ, Deng CX, Jorcano JL, Johnson DG. Tumor formation in mice with conditional inactivation of Brca1 in epithelial tissues. Oncogene 2003, 22: 5415–5426. [DOI] [PubMed] [Google Scholar]
- 65. Cheng H, Fertig EJ, Ozawa H, Hatakeyama H, Howard JD, Perez J, Considine M, et al. . Decreased SMAD4 expression is associated with induction of epithelial-to-mesenchymal transition and cetuximab resistance in head and neck squamous cell carcinoma. Cancer Biol Ther 2015, 16: 1252–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. White RA, Neiman JM, Reddi A, Han G, Birlea S, Mitra D, Dionne L, et al. . Epithelial stem cell mutations that promote squamous cell carcinoma metastasis. J Clin Invest 2013, 123: 4390–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. David CJ, Huang YH, Chen M, Su J, Zou Y, Bardeesy N, Iacobuzio-Donahue CA, et al. . TGF-beta tumor suppression through a lethal EMT. Cell 2016, 164: 1015–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ozawa H, Ranaweera RS, Izumchenko E, Makarev E, Zhavoronkov A, Fertig EJ, Howard JD, et al. . SMAD4 loss is associated with cetuximab resistance and induction of MAPK/JNK activation in head and neck cancer cells. Clin Cancer Res 2017, 23: 5162–5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mishra AK, Kadoishi T, Wang X, Driver E, Chen Z, Wang XJ, Wang JH. Squamous cell carcinomas escape immune surveillance via inducing chronic activation and exhaustion of CD8+ T cells co-expressing PD-1 and LAG-3 inhibitory receptors. Oncotarget 2016, 7: 81341–81356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fabregat I, Fernando J, Mainez J, Sancho P. TGF-beta signaling in cancer treatment. Curr Pharm Des 2014, 20: 2934–2947. [DOI] [PubMed] [Google Scholar]
- 71. Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, Freeman ML, et al. . Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest 2007, 117: 1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Connolly EC, Freimuth J, Akhurst RJ. Complexities of TGF-beta targeted cancer therapy. Int J Biol Sci 2012, 8: 964–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahapatra AK, Suri A, Balasubramaniam A, et al. . Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol 2011, 13: 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jing C, Ma G, Li X, Wu X, Huang F, Liu K, Liu Z. MicroRNA-17/20a impedes migration and invasion via TGF-beta/ITGB6 pathway in esophageal squamous cell carcinoma. Am J Cancer Res 2016, 6: 1549–1562. [PMC free article] [PubMed] [Google Scholar]
- 75. Chu TH, Yang CC, Liu CJ, Lui MT, Lin SC, Chang KW. miR-211 promotes the progression of head and neck carcinomas by targeting TGFbetaRII. Cancer Lett 2013, 337: 115–124. [DOI] [PubMed] [Google Scholar]
- 76. Herbertz S, Sawyer JS, Stauber AJ, Gueorguieva I, Driscoll KE, Estrem ST, Cleverly AL, et al. . Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther 2015, 9: 4479–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Connolly EC, Saunier EF, Quigley D, Luu MT, De Sapio A, Hann B, Yingling JM, et al. . Outgrowth of drug-resistant carcinomas expressing markers of tumor aggression after long-term TbetaRI/II kinase inhibition with LY2109761. Cancer Res 2011, 71: 2339–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]