Summary
Background
In 2013, a herpes zoster vaccination programme was introduced in England for adults aged 70 years with a phased catch-up programme for those aged 71–79 years. We aimed to evaluate the effect of the first 3 years of the vaccination programme on incidence of herpes zoster and postherpetic neuralgia in this population.
Methods
In this population-based study, we extracted data from the Royal College of General Practitioners sentinel primary care network on consultations with patients aged 60–89 years for herpes zoster and postherpetic neuralgia occurring between Oct 1, 2005, and Sept 30, 2016, obtaining data from 164 practices. We identified individual data on herpes zoster vaccinations administered and consultations for herpes zoster and postherpetic neuralgia, and aggregated these data to estimate vaccine coverage and incidence of herpes zoster and postherpetic neuralgia consultations. We defined age cohorts to identify participants targeted in each year of the programme, and as part of the routine or catch-up programme. We modelled incidence according to age, region, gender, time period, and vaccine eligibility using multivariable Poisson regression with an offset for person-years.
Findings
Our analysis included 3·36 million person-years of data, corresponding to an average of 310 001 patients aged 60–89 years who were registered at an RCGP practice each year. By Aug 31, 2016, uptake of the vaccine varied between 58% for the recently targeted cohorts and 72% for the first routine cohort. Across the first 3 years of vaccination for the three routine cohorts, incidence of herpes zoster fell by 35% (incidence rate ratio 0·65 [95% 0·60–0·72]) and of postherpetic neuralgia fell by 50% (0·50 [0·38–0·67]). The equivalent reduction for the four catch-up cohorts was 33% for herpes zoster (incidence rate ratio 0·67 [0·61–0·74]) and 38% for postherpetic neuralgia (0·62 [0·50–0·79]). These reductions are consistent with a vaccine effectiveness of about 62% against herpes zoster and 70–88% against postherpetic neuralgia.
Interpretation
The herpes zoster vaccination programme in England has had a population impact equivalent to about 17 000 fewer episodes of herpes zoster and 3300 fewer episodes of postherpetic neuralgia among 5·5 million eligible individuals in the first 3 years of the programme. Communication of the public health impact of this programme will be important to reverse the recent trend of declining vaccine coverage.
Funding
Public Health England.
Introduction
Herpes zoster (shingles) is a painful, sometimes debilitating condition, characterised by a unilateral dermatomal vesicular rash resulting from reactivation of latent varicella zoster virus. The incidence of shingles increases with age, rising from 3·5 per 1000 person-years among 50–54 year olds to 7·1 per 1000 person-years among 75–79 year olds between 2001 and 2006 in the UK.1 Older individuals are more likely to develop complications, including postherpetic neuralgia, with pain persisting for more than 3 months after rash onset.2
Since 2006, a live attenuated herpes zoster vaccine, Zostavax (Zoster Vaccine Live; Merck & Co, Kenilworth, NJ, USA), has been indicated for use in immunocompetent adults aged 50 years and older. The vaccine contains the same antigen (Oka strain of varicella zoster virus) as the childhood varicella vaccine but at a higher dose. Pre-licensure clinical trials showed the efficacy of a single dose of vaccine for healthy adults aged 70 years and older against herpes zoster to be 38% and against postherpetic neuralgia to be 67% over a mean 3·13 years of follow-up.3 Since then, longer-term follow-up studies have suggested that the efficacy of a single dose remains up to 30% for up to 8 years.4
In 2010, the UK's Joint Committee on Vaccination and Immunisation recommended routine herpes zoster vaccination for 70 year olds with a catch-up programme for 71–79 year olds; the vaccination programme aimed to target 5·5 million people in England during its first 3 years.5, 6 The choice of target age groups was based on cost-effectiveness analysis incorporating the age-specific incidence of herpes zoster7 and postherpetic neuralgia, the decline in vaccine efficacy with age, and the estimated duration of vaccine-induced protection. In the first year of the programme (2013–14), a single dose of vaccine was offered to adults aged 70 years (routine cohort) and 79 years (catch-up cohort) on Sept 1, 2013. In year two (2014–15), in addition to the routine cohort (70 years on Sept 1, 2014), there were two catch-up cohorts (78 and 79 years on Sept 1, 2014). In the third year (2015–16), in addition to the routine cohort (70 years on Sept 1, 2015), vaccine was offered to one catch-up cohort (78 years on Sept 1, 2015); individuals who were not vaccinated in their scheduled cohort remained eligible until their 80th birthday. In England, the programme was delivered in general practice alongside the seasonal influenza vaccination programme. The timing of implementation and phased catch-up took account of availability of vaccine and the impact on general-practice workload.
Research in context.
Evidence before this study
We searched PubMed with the terms “herpes zoster (MeSH)” in combination with “vaccine impact”, “vaccine efficacy”, or “vaccine effectiveness” without age, language, or time restrictions to identify papers up to June 30, 2017. We included papers if they contained primary data on the live attenuated herpes zoster vaccine either collected from clinical trials to estimate vaccine efficacy against zoster or postherpetic neuralgia or from post-marketing studies to estimate vaccine impact and effectiveness in both immunocompetent and immunocompromised groups. We excluded immunogenicity studies and modelling papers predicting the epidemiological and economic impact of a herpes zoster vaccination programme. After reviewing 463 abstracts, we identified 11 relevant studies. These included clinical trials that showed the initial efficacy against herpes zoster and postherpetic neuralgia in immunocompetent individuals aged 70 years and older to be 55·4% and 66·8%, respectively, declining to 22·4% and 49·7%, respectively, after 5–11 years of follow up. Post-marketing surveillance data were available only from the USA, where a national recommendation has been in place since 2006 and where vaccine coverage was reported at 27·9% in 2014. Four large retrospective cohort studies in the USA have assessed vaccine effectiveness among immunocompetent individuals and estimated effectiveness of a single dose against herpes zoster to be 55% (95% CI 52–58), 51% (41–59), and 51% (50–53) and effectiveness against postherpetic neuralgia to be 59% (21–79). More recently estimates of effectiveness against herpes zoster and postherpetic neuralgia in the USA at 3 years of follow-up were 35% (33–37) and 55% (46–62), respectively.
Added value of this study
To our knowledge, this is the first population-based study assessing the impact of the herpes zoster vaccination programme in England since its introduction in 2013 using a sentinel primary care dataset. In the first 3 years of the programme, vaccine coverage for the routine cohort (aged 70 years) declined in the participating practices from 63% to 61% to 58%. Our findings show a clear effect on primary care consultations for herpes zoster and postherpetic neuralgia in the first 3 years of the programme, with an estimated 35% (95% CI 28–40) and 33% (26–39) decline in herpes zoster incidence amongst the routine and catch-up cohorts across the first 3 years of the programme, equivalent to 17 000 fewer zoster consultations among the 5·5 million individuals eligible for vaccination. We estimate a 50% (33–62) decline in postherpetic neuralgia incidence amongst the three routine cohorts across the first 3 years of the vaccination programme, with a 38% (21–50) decline in the catch-up cohorts. These results are consistent with short-term vaccine effectiveness of 62% against herpes zoster and 70–88% against postherpetic neuralgia in the year of vaccination, which is higher than the efficacy shown in the pre-licensure clinical trials and post-marketing surveillance studies in the USA.
Implications of all the available evidence
Our findings indicate that the real world impact of the herpes zoster vaccination programme is somewhat higher than estimated in clinical studies, consistent with the vaccine resulting in fewer patients with severe forms of disease presenting to health-care services. These results suggest the initial cost-effectiveness of the UK programme might have been underestimated. The shown impact of the programme should be communicated to health professionals and the public to improve coverage and maximise public health benefits in all countries where national recommendations exist.
In this paper, we present the first evaluation of the effect of the herpes zoster vaccination programme in England, 3 years after its introduction, using data on primary care consultations for herpes zoster and postherpetic neuralgia.
Methods
Data source
The Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) is a sentinel primary care network, representing over 1% of the English population, and is geographically representative of the general population.8
In March, 2017, we obtained data for the period Oct 1, 2005, to Sept 30, 2016, for patients aged 60–89 years from the 164 practices in the RCGP RSC network. This lag allowed for full capture of data after any delay in updating primary care records. We obtained denominator data for patients registered each month and stratified by age on Sept 1, 2013; year and month of vaccination; gender; and general practitioner (GP) practice. We included patients in the denominators if they were registered at the start and end of each month. Owing to the nature of the data obtained, we were able to generate person-years but could not precisely count included individuals. We also obtained aggregated numerator data to estimate vaccine coverage and incidence of primary care consultations for herpes zoster and postherpetic neuralgia (pain persisting beyond 90 days from rash onset) based on Read codes (a coded thesaurus of clinical terms used for recording patient findings and procedures; see appendix) using the same strata as for the denominator data.
We did not include consultations that included other codes suggestive of herpes zoster or antiviral prescriptions, unless they also coded a herpes zoster diagnosis.9 We also omitted any herpes zoster consultations that occurred within 6 months of a previous consultation.
This study meets the UK Health Research Authorities definition of usual practice in public health, so research ethics committee approval was not required. It was an observational study of usual care, using pseudonymised data extracted by the RCGP RSC after approval by the RCGP.
Statistical analysis
We defined age cohorts by participants' age on Sept 1, 2013, to identify participants targeted in the first (aged 70 or 79 years), second (aged 69, 77, or 78 years), and third (aged 68 or 76 years) years of the programme (table 1). We calculated cumulative vaccine uptake by summing the vaccine uptake within each month from Sept 1, 2013, to Aug 31, 2016, for all cohorts. We calculated average cumulative uptake by taking the mean of the cumulative uptake values in each month from October to September of the relevant year and cohorts.
Table 1.
Eligibility for herpes zoster vaccination in England by date of birth
| Age on Sept 1, 2013 (years) | First became eligible | |
|---|---|---|
| Routine cohorts | ||
| Sept 2, 1942–Sept 1, 1943 | 70 | 2013–14 |
| Sept 2, 1943–Sept 1, 1944 | 69 | 2014–15 |
| Sept 2, 1944–Sept 1, 1945 | 68 | 2015–16 |
| Catch-up cohorts | ||
| Sept 2, 1933–Sept 1, 1934 | 79 | 2013–14 |
| Sept 2, 1934–Sept 1, 1936 | 77, 78 | 2014–15 |
| Sept 2, 1936–Sept 1, 1937 | 76 | 2015–16 |
Individuals remain eligible until their 80th birthday.
To assess the impact of the vaccination programme, we modelled the incidence of herpes zoster primary care consultations according to age (in years), region (London, Midlands, north England, and south England), gender, period of vaccination (through the years 2005–16), and vaccine eligibility. Vaccine eligibility is the factor that measures impact and was determined for each incidence stratum according to age and period and assigned as not eligible, eligible in that year (defined as October–September), eligible in the previous year, and eligible 2 years previously. The reason for starting in October before measuring impact was to allow time for immunity to develop and to take into account low uptake in the September of each year.
We modelled the data using multivariable Poisson regression with an offset for person-years. This model measures impact as the relative incidence in the vaccine eligible cohorts compared with unvaccinated cohorts.
Initially, we constructed a complex model including vaccine eligibility; linear, quadratic, and cubic age; and period effects; alongside gender, region, and month; and interactions between age, region, and period. We then simplified this model by dropping variables if the estimates of impact (incidence rate ratio for the vaccine eligibility factor) did not change by more than 3%, although a linear period effect was retained in all models. To predict the expected incidence and number of events in the absence of vaccination, we also fitted the final model using data from the cohorts who were not eligible for vaccination and used this modelled incidence to extrapolate to the vaccine eligible cohorts. We also used this model to generate figures of observed compared with modelled incidence, and a table of observed and expected numbers of events along with expected incidence and incidence reduction. To check robustness of impact estimates, we fitted a random effects model with GP as the random effect and a model with age and period included as factors rather than as polynomials. We used the same final model for postherpetic neuralgia. We did sample size calculations prior to obtaining data to ensure reductions of 25% could be detected (with 80% power and 5% significance). We calculated vaccine effectiveness consistent with impact and coverage by dividing the impact (percentage reduction) by the average cumulative coverage in the period and age cohort assessed. For example, a vaccine effectiveness of 60% is consistent with a 30% reduction and 50% coverage. Precision of vaccine effectiveness is based on the 95% CIs for impact. We did all analyses in Stata 13.
Role of the funding source
The funding source had no role in the study design; collection, analysis, and interpretation of the data; and writing of the manuscript. The corresponding author had full access to all the data and the final responsibility to submit for publication.
Results
The number of patients aged 60–89 years at Oct 1 in each year who were registered at one of the 164 RCGP practices was 274 383 in 2005, increasing to 335 402 in 2015, with an average of 310 001 per year over the 11 years. These patients contributed a total of 3·36 million person-years and are described in table 2 by vaccine eligibility, year, age, region, and gender according to person-years contributed and herpes zoster and postherpetic neuralgia events. Uptake for each age cohort was calculated on the basis of only those individuals eligible for vaccination each year, which increased from 20 951 in 2013–14 to 51 798 in 2014–15 to 72 710 in 2015–16.
Table 2.
Description of the aggregated RCGP cohort person-years according to vaccine eligibility and year, age, region, and gender
|
Eligible for vaccination |
Not eligible for vaccination |
|||||
|---|---|---|---|---|---|---|
| Total person-years* | Herpes zoster events (rate per 1000 person-years) | Postherpetic neuralgia events (rate per 1000 person-years) | Total person-years* | Herpes zoster events (rate per 1000 person-years) | Postherpetic neuralgia events (rate per 1000 person-years) | |
| Year | ||||||
| 2005–06 | ·· | ·· | ·· | 270 216 | 2096 (7·8) | 297 (1·1) |
| 2006–07 | ·· | ·· | ·· | 279 264 | 2194 (7·9) | 306 (1·1) |
| 2007–08 | ·· | ·· | ·· | 289 991 | 2391 (8·2) | 313 (1·1) |
| 2008–09 | ·· | ·· | ·· | 297 811 | 2394 (8·0) | 330 (1·1) |
| 2009–10 | ·· | ·· | ·· | 305 087 | 2535 (8·3) | 349 (1·1) |
| 2010–11 | ·· | ·· | ·· | 310 829 | 2551 (8·2) | 334 (1·1) |
| 2011–12 | ·· | ·· | ·· | 315 872 | 2620 (8·3) | 338 (1·1) |
| 2012–13 | ·· | ·· | ·· | 317 643 | 2644 (8·3) | 364 (1·1) |
| 2013–14 | 21 192 | 151 (7·1) | 18 (0·8) | 295 483 | 2480 (8·4) | 348 (1·2) |
| 2014–15 | 52 558 | 298 (5·7) | 43 (0·8) | 271 590 | 2309 (8·5) | 353 (1·3) |
| 2015–16 | 73 844 | 467 (6·3) | 67 (0·9) | 256 406 | 2043 (8·0) | 287 (1·1) |
| Age, years | ||||||
| 60–69† | ·· | ·· | ·· | 1 646 415 | 11 487 (7·0) | 1134 (0·7) |
| 70–73 | 80 669 | 463 (5·7) | 50 (0·6) | 411 416 | 3636 (8·8) | 478 (1·2) |
| 74–77† | ·· | ·· | ·· | 423 441 | 3939 (9·3) | 648 (1·5) |
| 78–82 | 66 925 | 453 (6·8) | 78 (1·2) | 358 625 | 3546 (9·9) | 636 (1·8) |
| 83–89† | ·· | ·· | ·· | 370 298 | 3649 (9·9) | 723 (2·0) |
| Region | ||||||
| London | 15 578 | 105 (6·7) | 16 (1·0) | 348 701 | 2323 (6·7) | 383 (1·1) |
| Midlands and east England | 30 649 | 163 (5·3) | 24 (0·8) | 637 363 | 5084 (8·0) | 687 (1·1) |
| North England | 49 349 | 323 (6·5) | 42 (0·9) | 1 091 739 | 9517 (8·7) | 1232 (1·1) |
| South England | 52 019 | 325 (6·2) | 46 (0·9) | 1 132 391 | 9333 (8·2) | 1317 (1·2) |
| Gender | ||||||
| Female | 78 700 | 550 (7·0) | 80 (1·0) | 1 725 459 | 15 872 (9·2) | 2180 (1·3) |
| Male | 68 894 | 366 (5·3) | 48 (0·7) | 1 484 735 | 10 385 (7·0) | 1439 (1·0) |
RCGP=Royal College of General Practitioners.
Vaccine eligible person-years includes all time from the point that each age cohort first became eligible to receive vaccine.
Age group not eligible for vaccination.
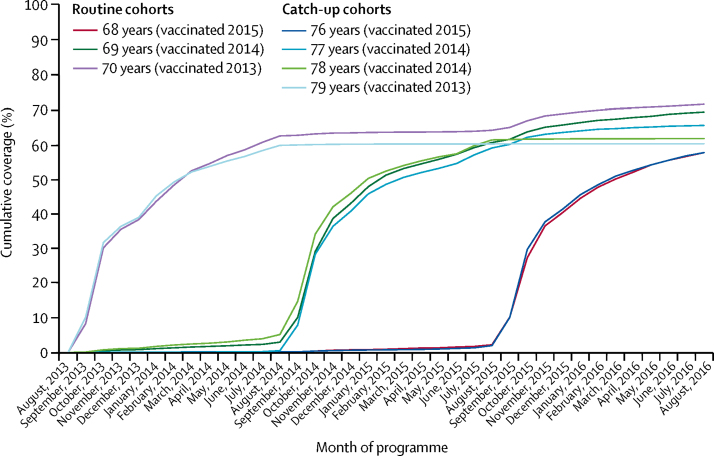
Uptake of the vaccine based on RCGP data shows that in the first year of the programme, coverage for the routine (aged 70 years) and catch-up cohorts (aged 79 years) rapidly increased, reaching approximately 35% by Nov 30, 2013, and then increased at a slower rate, achieving 63% coverage (routine) and 60% coverage (catch-up) by the end of the first year (figure 1). Eligibility of unvaccinated individuals in these cohorts until their 80th birthday is reflected in the higher coverage achieved in the first routine cohort by year 3 of the programme (table 2).
Figure 1.
Cumulative coverage for each age cohort throughout 3-year vaccination programme
Cohorts are grouped by age on Sept 1, 2013.
In the second and third years (2014–16), coverage in the routine cohorts (aged 69 or 68 years on Sept 1, 2013) showed a similar initial increase, but end-of-year coverage was lower (61% for the 2014–15 routine cohort and 58% for the 2015–16 routine cohort; figure 1). There were two catch-up cohorts in 2014–15 and one in 2015–16; end-of-year coverage was 58% in those aged 76 years on Sept 1, 2013; 59% in those aged 77 years; and 61% in those aged 78 years.
Uptake in the participating practices was similar to that reported nationally. Coverage was similar for men and women, except for the catch-up cohorts in the first 2 years of the programme, when coverage was about 5–6% higher in men. Coverage varied by 10% across regions (data not shown).
We included more than 3·35 million person-years of data for individuals aged 60–89, from Oct 1, 2005, to Sept 30, 2016, in the analysis of impact. The unadjusted rates show initial evidence of lower incidence in the vaccine-eligible cohorts than in those ineligible, higher rates in women than in men and in participants outside of London than those in London, and increases with age (table 2). We then modelled the incidence data to estimate the impact. After the fitting process, the final model only included the variable to measure impact (vaccine eligibility), a log-linear time trend (a 0·8% increase per year), and a quadratic age effect (with incidence increasing from 5·9 per 1000 person-years at age 60 years to a peak of 9·9 at age 83 years, then a slight decline to 9·7 by age 89 years—higher than previously reported in the UK1). Region, month, and gender, although associated with herpes zoster incidence, were not confounders (vaccine impact estimates changed by <1%) and we identified no significant interactions (data not shown). The model with factors for age and period gave similar results, as did the random effects model.
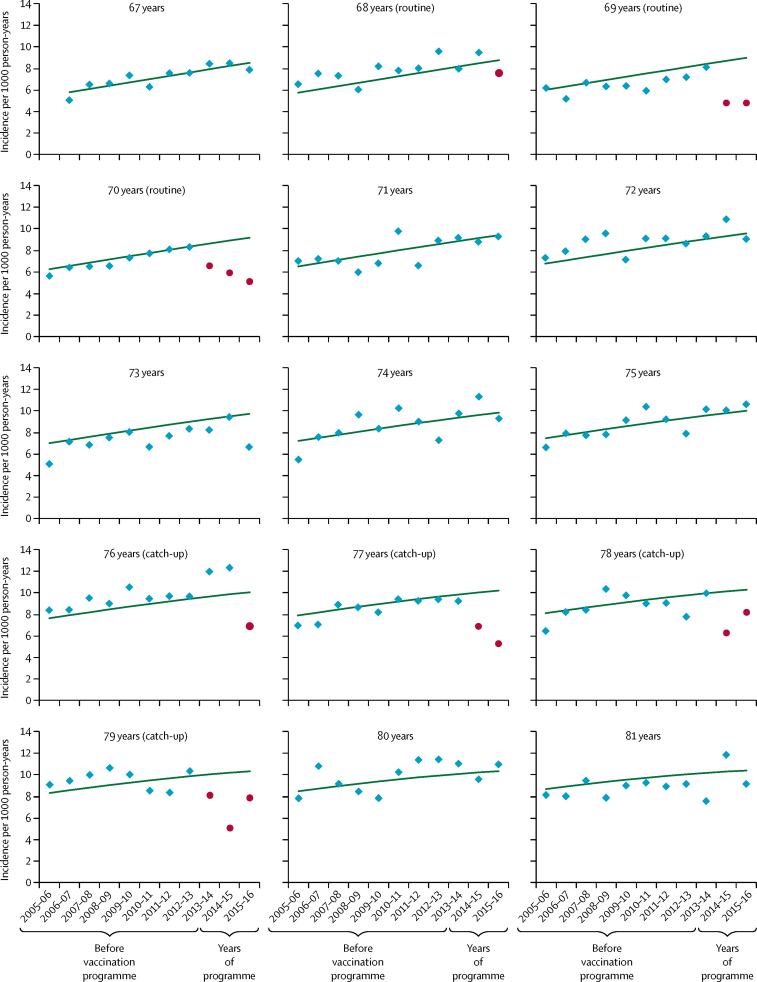
The first year of the programme (2013–14) saw a decline in the observed incidence of herpes zoster for routine and catch-up cohorts, whereas incidence in other cohorts showed a general increasing trend over time as the cohorts aged (figure 2). In the second year (2014–15), the decline in incidence in those eligible for vaccination in the first year improved as coverage improved. A decline in incidence was also observed in the third year (2015–16) for the additional routine cohort targeted in the second year—ie, those aged 69 years in Sept 1, 2013—as well as in the catch-up cohort of those aged 77 years on Sept 1, 2013, but the decline reversed for those aged 78 years on Sept 1, 2013. In the third year, the decline seen in the first routine cohort continued, but was reversed in the first catch-up cohort.
Figure 2.
Observed zoster incidence and model-predicted incidence in the absence of vaccination by year for each age cohort
Cohorts are grouped by age on Sept 1, 2013. Each year range runs from Sept 1 of the first year to Aug 31 of the second. Blue points indicate observed zoster incidence and red points indicate observed incidence in age cohorts eligible for vaccination. Green lines show the model-predicted incidence in the absence of vaccination.
Cumulative uptake, observed and predicted cases, and relative incidence estimates from the fitted models are summarised for herpes zoster (table 3) and postherpetic neuralgia (table 4). Reductions for herpes zoster incidence were 35% (28–40) across the routine cohorts and 33% (26–39) across the catch-up cohorts in the 3 years of the programme (table 3). The vaccine effectiveness required to generate these observed reductions in the first 3 years after vaccination is about 60–65%, although somewhat lower in the second year after introduction for the catch-up cohort.
Table 3.
Impact of routine and catch-up herpes zoster vaccination on GP-diagnosed herpes zoster by time since cohorts were first eligible for vaccination
| Age on Sept 1, 2013, (age when first eligible for vaccination) | Average cumulative uptake* | Expected events† | Observed events | Incidence rate ratio (95% CI)‡ | Expected incidence per 1000 person-years | Incidence reduction per 1000 person-years (95% CI) | Vaccine effectiveness (95% CI)§ | |
|---|---|---|---|---|---|---|---|---|
| Routine cohorts | ||||||||
| First year after vaccine eligibility | 68–70 years (70–71 years) | 46% | 354 | 255 | 0·72 (0·64–0·82) | 8·7 | 2·4 (1·6–5·6) | 62% (39–78) |
| Second year after vaccine eligibility | 69–70 years (70–71 years) | 65% | 241 | 143 | 0·59 (0·50–0·70) | 8·9 | 3·6 (2·7–4·5) | 62% (54–77) |
| Third year after vaccine eligibility | 70 years (70–71 years) | 70% | 117 | 65 | 0·56 (0·44–0·71) | 9·2 | 4·1 (2·7–5·2) | 64% (41–80) |
| All years of the programme | 68–70 years (70–71 years) | 56% | 712 | 463 | 0·65 (0·60–0·72) | 8·8 | 3·1 (2·5–3·5) | 62% (50–71) |
| Catch-up cohorts | ||||||||
| First year after vaccine eligibility | 76–79 years (78–80 years) | 46% | 348 | 243 | 0·70 (0·62–0·79) | 10·0 | 3·0 (2·1–3·8) | 65% (46–83) |
| Second year after vaccine eligibility | 77–79 years (78–80 years) | 62% | 251 | 151 | 0·60 (0·51–0·71) | 10·2 | 4·0 (3·0–5·0) | 64% (47–79) |
| Third year after vaccine eligibility | 79 years (79–80 years) | 60% | 78 | 59 | 0·76 (0·59–0·98) | 10·3 | 2·5 (0·2–4·2) | 40% (3–68) |
| All years of the programme | 76–79 years (78–80 years) | 54% | 677 | 453 | 0·67 (0·61–0·74) | 10·1 | 3·1 (2·6–3·9) | 62% (48–72) |
Impact is shown by the incidence rate ratio (observed/expected events) and incidence reduction. GP=general practitioner.
Calculated by taking the mean of the cumulative uptake values in each month from October to September of the relevant years and cohorts.
Expected if the vaccine had not been introduced; based on model results for unvaccinated cohorts.
Estimated from the Poisson regression model with a log-linear time trend, quadratic age effect, and the factor for vaccine eligibility.
Effectiveness required to generate the observed reductions in the first 3 years after vaccination.
Table 4.
Impact of routine and catch-up herpes zoster vaccination on GP-diagnosed postherpetic neuralgia by time since cohorts were first eligible for vaccination
| Age on Sept 1, 2013, (age when first eligible for vaccination) | Average cumulative uptake* | Expected events† | Observed events | Incidence rate ratio (95% CI)‡ | Expected incidence per 1000 person-years | Incidence reduction per 1000 person-years (95% CI) | Vaccine effectiveness (95% CI)§ | |
|---|---|---|---|---|---|---|---|---|
| Routine cohorts | ||||||||
| First year after vaccine eligibility | 68–70 years (70–71 years) | 46% | 47·8 | 24 | 0·50 (0·34–0·75) | 1·17 | 0·58 (0·82–0·77) | 100% (54–100) |
| Second year after vaccine eligibility | 69–70 years (70–71 years) | 65% | 43·1 | 21 | 0·62 (0·40–0·95) | 1·26 | 0·48 (0·06–0·76) | 59% (8–92) |
| Third year after vaccine eligibility | 70 years (70–71 years) | 70% | 17·3 | 5 | 0·29 (0·12–0·70) | 1·35 | 0·96 (0·41–1·19) | 100% (43–100) |
| All years of the programme | 68–70 years (70–71 years) | 56% | 99·1 | 50 | 0·50 (0·38–0·67) | 1·23 | 0·61 (0·41–0·76) | 88% (59–100) |
| Catch-up cohorts | ||||||||
| First year after vaccine eligibility | 76–79 years (78–80 years) | 46% | 63·0 | 38 | 0·60 (0·44–0·83) | 1·82 | 0·83 (0·31–1·02) | 86% (37–100) |
| Second year after vaccine eligibility | 77–79 years (78–80) | 62% | 47·1 | 31 | 0·66 (0·46–0·94) | 1·91 | 0·64 (0·11–1·03) | 55% (10–87) |
| Third year after vaccine eligibility | 79 years (79–80 years) | 60% | 15·0 | 9 | 0·60 (0·31–1·16) | 1·99 | 0·80 (−0·32–1·37) | 66% (27–100) |
| All years of the programme | 76–79 years (78–80 years) | 54% | 125·1 | 78 | 0·62 (0·50–0·79) | 1·88 | 0·68 (0·39–0·94) | 70% (39–93) |
Impact is shown by the incidence rate ratio (observed/expected events) and incidence reduction. GP=general practitioner.
Calculated by taking the mean of the cumulative uptake values in each month from October to September of the relevant years and cohorts.
Expected if the vaccine had not been introduced; based on model results for unvaccinated cohorts.
Estimated from the Poisson regression model with a log-linear time trend, quadratic age effect, and the factor for vaccine eligibility.
Effectiveness required to generate the observed reductions in the first 3 years after vaccination.
Reductions were greater for postherpetic neuralgia, with a 50% (33–62) reduction in postherpetic neuralgia incidence in the routine cohorts across the first 3 years and a 38% (21–50) reduction in the catch-up cohorts (table 4). These results would be equivalent to a reduction in postherpetic neuralgia episodes of 0·6–0·7 per 1000 person-years, consistent with vaccine effectiveness of about 88% for the routine cohorts and 70% for the catch-up cohorts (table 4). The incidence rate ratios for postherpetic neuralgia, however, are based on small numbers with wide CIs.
Discussion
To our knowledge, this study provides the first evidence of a population effect of the herpes zoster vaccination programme on herpes zoster and postherpetic neuralgia amongst older adults in England. Among the three eligible routine cohorts and four catch-up cohorts during 2013–16, we found a 33–35% decline in the incidence of herpes zoster and a 38–50% decline in postherpetic neuralgia, respectively. This translates to approximately 17 000 fewer herpes zoster episodes and 3300 fewer episodes of postherpetic neuralgia amongst the 5·5 million individuals targeted for vaccination in the first 3 years of the programme. This result is in the context of an increasing incidence of herpes zoster over time, reflecting changes in population demographics and similar to trends observed in other countries.10
In England, coverage was reported as 61·8% in the first year of the programme, 59·0% in the second year, and 54·9% in the third year in the routine cohorts (aged 70 years, 69 years, and 68 years on Sept 1, 2013, respectively) and 59·6% in the first year, 58·1% (57·8% for individuals aged 78 years and 58·5% for individuals aged 77 years on Sept 1, 2013), and 55·5% in the third year for the catch-up cohorts (aged 79 years, 78 or 77 years, and 76 years on Sept 1, 2013, respectively).11, 12, 13 General practices were encouraged to deliver the programme alongside seasonal influenza vaccination between September and January. Cumulative uptake data indicate most vaccines were delivered during the influenza season, although the vaccine is offered opportunistically throughout the year. Uptake in the participating practices showed geographical variation, similar to that observed nationally, with ethnicity and deprivation identified as contributing factors.14 Uptake was lower than for seasonal influenza vaccination in adults aged 65 years and older, at 73·2% for the 2013–14 season, 72·7% for the 2014–15 season, and 71·0% for the 2015–16 season.15
In addition to the routine programme for adults aged 70 years, a phased catch-up programme was implemented for individuals aged between 71 and 79 years. The primary aim of the programme is to provide protection against herpes zoster and its most substantial complication, postherpetic neuralgia, by boosting an individual's pre-existing immunity. Unlike many vaccination pro-grammes in which the herd protection effects contribute to the overall population impact, any impact of the herpes zoster programme occurs through individual direct protection. This, together with the phased implementation adopted in England, provided an opportunity to assess whether a discernible population impact had occurred. By comparing changes in the incidence of herpes zoster and postherpetic neuralgia amongst vaccine eligible and ineligible cohorts, we showed reductions of 30–40% in herpes zoster, and slightly larger reductions in postherpetic neuralgia, in the first 3 years of the programme.
Based on the average RCGP uptake, the estimated incidence reductions are consistent with short-term vaccine effectiveness at 3 years of about 62% against herpes zoster and 70–88% against postherpetic neuralgia. Although these estimates are not based on a formal assessment of vaccine effectiveness and should not be overinterpreted (because potential confounders such as frailty are not assessed), they compare favourably with findings from the pre-licensure clinical trials in which protection against herpes zoster was 55·4% (95% CI 39·9–66·9) and 66·8% (43·3–81·3) against postherpetic neuralgia in adults aged 70 years and older.3, 4 This difference might reflect that patients with herpes zoster in our analysis presented to general practice, and therefore might have been more severe than those detected during the active trial follow-up. This finding would be consistent with the vaccine providing improved protection against more severe forms of disease. If these effectiveness estimates were to be confirmed through a formal evaluation, such a confirmation might suggest that the initial cost-effectiveness of the programme was underestimated. So far, three published US retrospective studies have estimated effectiveness against herpes zoster at 55% (52–58),16 51% (41–59),17 and 51% (50–53)18 and effectiveness against postherpetic neuralgia at 59% (21–79).17 A more recent US study19 estimated vaccine effectiveness against community herpes zoster and postherpetic neuralgia at 3 years in adults aged 70–74 years at 35% (33–37) and 55% (46–62), respectively.19
In clinical trials, long-term follow-up of those individuals aged 70 years and older suggested that the efficacy against herpes zoster declined to 22·4% and against postherpetic neuralgia to 49·7% after 5–11 years.4 The Joint Committee on Vaccination and Immunisation recommendation was based on a cost-effectiveness analysis that considered the age-specific herpes zoster incidence, and fitted the available clinical trial data to a model that predicted a decline in vaccine efficacy over time.6 Our study followed up cohorts into the third year following vaccination. Longer-term population-based follow-up studies are therefore important to determine whether the waning protection assumed in the original cost-effectiveness can be shown in real life.
Because herpes zoster and postherpetic neuralgia are largely clinical diagnoses and patients tend to present to primary care, GP clinical record systems provide a comprehensive data source to monitor trends. The RCGP sentinel surveillance programme was established in 1964 providing longitudinal demographic and clinical information on a population of around 1·2 million registered patients, representative of the general population.8 The benefit of this system over other sentinel primary care systems is that the precise date of birth can be extracted; this is important to accurately identify eligible cohorts, because eligibility is based on age on Sept 1 of the year considered.
Despite the strengths of the RCGP data, assessment of programme impact requires accurate and reliable coding of the outcome (herpes zoster or postherpetic neuralgia consultations) and vaccines administered. Furthermore, we only included consultations in which herpes zoster and postherpetic neuralgia were coded based on diagnosis rather than by prescriptions. Although this approach increases sensitivity, the burden in primary care might be underestimated. Formal vaccine effectiveness assessment was not undertaken as individual level data were not extracted from RCGP. Although most cases of herpes zoster and postherpetic neuralgia are managed in general practice, severe cases that present to hospital might not always be accurately recorded in the primary care record. The herpes zoster vaccination programme is delivered through GP practices in England and is the only live attenuated vaccine routinely offered to that age group. A clinical assessment is required before administration to ensure those for whom the vaccine is contraindicated (particularly those who are immunosuppressed) are not inadvertently vaccinated.2 In the first year of the programme, about 2·9% of the routine cohort and 3·6% of the catch-up cohort were contraindicated to receive the vaccine.2 However, these groups are also at increased risk of herpes zoster and its complications and most likely to benefit from vaccination. The development of an inactivated subunit vaccine, which has shown promising results in pre-licensure studies, might protect high-risk individuals from herpes zoster in the future.20
Despite the encouraging coverage early in the vaccination programme, coverage has declined by 6·9 percentage points since the start of the programme, from 61·8% in 2013–14 to 54·9% in 2015–16.13 Given the demonstrated impact of the programme on herpes zoster and postherpetic neuralgia, the benefits of the programme need to be effectively communicated to health professionals and the public to maximise protection from this potentially debilitating condition in those most at risk.
Acknowledgments
Acknowledgments
This study was supported by Public Health England. We thank the patients and practices of the Royal College of General Practitioners Research and Surveillance Centre (RCGP RSC) who allowed their pseudonymised clinical medical records data to be used for this study. We also thank colleagues at the RCGP RSC secure data and analytics hub at the University of Surrey.
Contributors
GA, NA, PK, DM, AC, SdL, and MR were all involved in the concept and design of the evaluation of the herpes zoster vaccination programme. AC, DM, and SdL were responsible for providing the RCGP data. GA drafted the paper and all authors were involved in the revision and approval of the final content before submission.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Gauthier A, Breuer J, Carrington D, Martin M, Remy V. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect. 2009;137:38–47. doi: 10.1017/S0950268808000678. [DOI] [PubMed] [Google Scholar]
- 2.Public Health England Immunisation against infectious disease. Shingles (herpes zoster): the green book, chapter 28a. https://www.gov.uk/government/publications/shingles-herpes-zoster-the-green-book-chapter-28a (accessed Dec 1, 2016).
- 3.Oxman MN, Levin MJ, Johnson GR. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 4.Morrison VA, Johnson GR, Schmader KE. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015;60:900–909. doi: 10.1093/cid/ciu918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joint Committee on Vaccination and Immunisation. Statement on varicella and herpes zoster vaccines. March 29, 2010. http://webarchive.nationalarchives.gov.uk/20120907090205/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@ab/documents/digitalasset/dh_133599.pdf (accessed Oct 18, 2016).
- 6.van Hoek AJ, Gay N, Melegaro A, Opstelten W, Edmunds WJ. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine. 2009;27:1454–1467. doi: 10.1016/j.vaccine.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 7.de Lusignan S, Correa A, Pathirannehelage S. RCGP Research and Surveillance Centre Annual Report 2014–2015: disparities in presentations to primary care. Br J Gen Pract. 2017;67:e29–e40. doi: 10.3399/bjgp16X688573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correa A, Hinton W, McGovern A. Royal College of General Practitioners Research and Surveillance Centre (RCGP RSC) sentinel network: a cohort profile. BMJ Open. 2016;6:e011092. doi: 10.1136/bmjopen-2016-011092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lusignan S. Codes, classifications, terminologies and nomenclatures: definition, development and application in practice. Inform Prim Care. 2005;13:65–70. doi: 10.14236/jhi.v13i1.580. [DOI] [PubMed] [Google Scholar]
- 10.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Public Health England Herpes zoster (shingles) immunisation programme 2013 to 2014: evaluation report. Dec 19, 2014. https://www.gov.uk/government/publications/herpes-zoster-shingles-immunisation-programme-2013-to-2014-evaluation-report (accessed Oct 18, 2016).
- 12.Public Health England Herpes zoster (shingles) immunisation programme 2014 to 2015: evaluation report. Dec 4, 2015. https://www.gov.uk/government/publications/herpes-zoster-shingles-immunisation-programme-2014-to-2015-evaluation-report (accessed Oct 18, 2016).
- 13.Public Health England Herpes zoster (shingles) immunisation programme 2015 to 2016: evaluation report. https://www.gov.uk/government/publications/herpes-zoster-shingles-immunisation-programme-2015-to-2016-evaluation-report (accessed Aug 9, 2017).
- 14.Ward C, Byrne L, White JM, Amirthalingam G, Tiley K, Edelstein M. Sociodemographic predictors of variation in coverage of the national shingles vaccination programme in England, 2014/15. Vaccine. 2017;35:2372–2378. doi: 10.1016/j.vaccine.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 15.Public Health England Vaccine uptake guidance and latest coverage data. https://www.gov.uk/government/collections/vaccine-uptake#seasonal-flu-vaccine-uptake:-figures accessed Sept 26, 2017).
- 16.Tseng HF, Smith, Harpaz R. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011;305:160–166. doi: 10.1001/jama.2010.1983. [DOI] [PubMed] [Google Scholar]
- 17.Langan SM, Smeeth L, Margolis DJ. Herpes zoster vaccine effectiveness against herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med. 2013;10:e1001420. doi: 10.1371/journal.pmed.1001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng HF, Harpaz R, Luo Y. Declining effectiveness of herpes zoster vaccine in adults aged >60 years. J Infect Dis. 2016;213:1872–1875. doi: 10.1093/infdis/jiw047. [DOI] [PubMed] [Google Scholar]
- 19.Izurieta HS, Wernecke M, Kelman J. Effectiveness and duration of protection provided by the live-attenuated herpes zoster vaccine in the Medicare population ages 65 years and older. Clin Infect Dis. 2017;64:785–793. doi: 10.1093/cid/ciw854. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham AL, Lal H, Kovac M. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.