Abstract
Aim
To test the psychometric properties and clinical usability of a new Pressure Ulcer Risk Assessment Instrument including inter‐rater and test–retest reliability, convergent validity and data completeness.
Background
Methodological and practical limitations associated with traditional Pressure Ulcer Risk Assessment Instruments, prompted a programme to work to develop a new instrument, as part of the National Institute for Health Research funded, Pressure UlceR Programme Of reSEarch (RP‐PG‐0407‐10056).
Design
Observational field test.
Method
For this clinical evaluation 230 patients were purposefully sampled across four broad levels of pressure ulcer risk with representation from four secondary care and four community NHS Trusts in England. Blinded and simultaneous paired (ward/community nurse and expert nurse) PURPOSE‐T assessments were undertaken. Follow‐up retest was undertaken by the expert nurse. Field notes of PURPOSE‐T use were collected. Data were collected October 2012–January 2013.
Results
The clinical evaluation demonstrated “very good” (kappa) inter‐rater and test–retest agreement for PURPOSE‐T assessment decision overall. The percentage agreement for “problem/no problem” was over 75% for the main risk factors. Convergent validity demonstrated moderate to high associations with other measures of similar constructs.
Conclusion
The PURPOSE‐T evaluation facilitated the initial validation and clinical usability of the instrument and demonstrated that PURPOSE‐T is suitable of use in clinical practice. Further study is needed to evaluate the impact of using the instrument on care processes and outcomes.
Keywords: nursing, pressure ulcer, reliability, risk assessment, tissue viability, usability, validity
Why is this research or review needed?
There are methodological and practical limitations associated with the development and use of traditional Pressure Ulcer Risk Assessment Instruments.
Recent work has been undertaken to develop a new Risk Assessment Instrument, incorporating: (1) a systematic review; (2) a consensus study; (3) conceptual framework development; (4) Pre‐test study.
This phase 5 clinical evaluation paper, reports a subsequent fundamental step in the development of the Pressure Ulcer Risk Primary Or Secondary Evaluation Tool (PURPOSE‐T) to confirm its suitability for use in clinical practice.
What are the key findings?
The clinical evaluation of PURPOSE‐T demonstrated the reliability, convergent validity and clinical usability of the instrument when used by expert and ward/community nurses in secondary care and community settings.
The findings emphasize the importance of including skin status in the assessment process facilitate both primary and secondary prevention.
The clinical evaluation aided refinement of the instrument and confirmed its suitability for use in clinical practice.
How should the findings be used to influence policy/practice/research/education?
The clinical evaluation, was an important phase in the instruments development and the methods used should be considered by others developing health‐related instruments.
PURPOSE‐T translates pressure ulcer risk factor evidence and expert opinion into a usable instrument that can facilitate the identification and management of pressure ulcer risk in practice.
PURPOSE‐T should be considered for clinical use in adult hospital and community populations.
1. INTRODUCTION
Pressure Ulcers (PUs) are “localized injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure, or pressure in combination with shear” (NPUAP/EPUAP/PPPIA, 2014). Skin sites susceptible to PUs are those exposed to pressure (e.g. buttocks and heels) in patients with very limited mobility where offloading is difficult. PUs are categorized numerically according to the tissue layers involved; category 1 indicates non‐blanchable erythema and category 4 indicates full thickness tissue Loss (NPUAP/EPUAP/PPPIA, 2014).
PUs remain a considerable patient safety issue worldwide with prevalence in acute care settings being 11.9–15.8% and incidence being 2.8–9.0% (Briggs et al., 2013; Pieper, 2012; Smith, Nixon, Brown, Wilson, & Coleman, 2016). PUs cause undue burden on patients quality of life (Gorecki et al., 2009; Gorecki, Nixon, Madill, Firth, & Brown, 2012) and have a significant financial impact to healthcare organizations (Bennett, Dealey, & Posnett, 2004; Berlowitz et al., 2011; Dealey, Posnett, & Walker, 2012; Schuurman et al., 2009; Severens, Habraken, Duivenvoorden, & Frederiks, 2002). National and international guidelines agree that structured risk assessment is the cornerstone to PU prevention (Beeckman et al., 2013; NICE, 2014, NPUAP/EPUAP/PPPIA, 2014).
2. BACKGROUND
In clinical practice structured risk assessment is underpinned by routine use of PU Risk Assessment Instruments (PU‐RAIs). These assist nurses to identify those at risk with the aim of appropriately targeting preventative interventions. Since the 1960s over 40 PU‐RAIs have been developed (Nixon & McGough, 2001), though their methodological development and conceptual and practical foundation are often limited. This was demonstrated by analysis of 14 PU‐RAIs included in a recent NICE systematic review (NICE, 2014) which identified the following (Coleman, 2014):
Lack of conceptual framework—only two PU‐RAIs (Braden & Bergstrom, 1987; Suriadi, Sanada, Sugama, Thigpen, & Subuh, 2008) were underpinned by a conceptual framework.
Inadequate development methods—only thee PU‐RAIs were developed from limited statistical modelling methods (Page, Barker, & Kamar, 2011; Perneger et al., 2002; Suriadi et al., 2008) with the remainder developed on the basis of clinical opinion and/or out‐dated literature reviews or adaptations of original instruments.
Limited evidence of target population involvement during development (SAC, 2002)—involvement of both clinical nurses and patient/carers is important as while nurses primarily use the instruments, assessment should involve the patient/carer and where possible lead to shared decision‐making about care (Coleman, 2014).
Inconsistent risk factor inclusion—for example only five PU‐RAIs include skin status (Andersen, Jensen, Kvorning, & Bach, 1982; Cubbin & Jackson, 1991; Kwong et al., 2005; Pritchard, 1986; Waterlow, 1985) yet a systematic review identified this as a key predictor of PU development (Coleman et al., 2013).
These issues undermine the content validity of PU‐RAIs which is a fundamental property and raises concern about their ability to identify risk adequately (Coleman, 2014; Gould, Goldstone, Gammon, Kelly, & Maidwell, 2002; Kottner & Balzer, 2010; Nixon & McGough, 2001). There are also practical limitations associated with their use (Coleman, 2014; Coleman, Nelson, et al., 2014; Nixon et al., 2015):
PU‐RAIs are undertaken on all patients, including full assessment of those who are obviously not at risk, which diverts time away from other important care activities.
Failure to distinguish between those with and without an existing PU which is important as those with a PU require intensified secondary prevention/treatment.
Use of condensed numerical scores as a basis for care interventions which do not facilitate consideration of individual risk profiles in care‐planning.
To address these conceptual, methodological and practical limitations we developed the Pressure Ulcer Risk Primary Or Secondary Evaluation Tool, PURPOSE‐T as part of a NIHR funded PU Programme Of Research (PURPOSE: RP‐PG‐0407‐10056). PURPOSE‐T development drew on principles of the MRC complex intervention framework (MRC, 2000, 2008) and incorporated adapted “gold standard” instrument development methods (FDA DHHS, 2009, Mokkink et al., 2012, SAC, 2002; Steyerberg, 2010), in a structured five phase approach:
Systematic review (Coleman et al., 2013)
Consensus study (Coleman, Nelson, et al., 2014)
Conceptual framework development (Coleman, Nixon, et al., 2014)
Design and pre‐testing (Coleman et al., 2016)
Clinical evaluation (Nixon et al., 2015)
The first four phases of this work were concerned with providing evidence of content validity which was indicated along with usability and acceptability in the phase iv) design and pre‐test (Coleman et al., 2016). This led to the development of the preliminary PURPOSE‐T for clinical evaluation.
2.1. Aims
The aim of the study was to test the psychometric properties and clinical usability of PURPOSE‐T including inter‐rater and test–retest reliability, convergent validity and data completeness.
2.2. Design
PURPOSE‐T clinical evaluation comprised a field test of hospital and community patients using observational descriptive methods (Nixon et al., 2015). The psychometric properties assessed included reliability defined as “the extent to which scores for patients who have not changed are the same for repeated measurement under several conditions” (Mokkink et al., 2012). In this study, we considered this over time (test–retest) when assessed by the same nurse and on the same occasion by different nurses (inter‐rater). Convergent validity was assessed for expected correlations between the items and overall assessment decision of PURPOSE‐T and other PU‐RAIs to demonstrate construct validity, that is “evidence that relationships among items, domains and concepts conform to a priori hypotheses concerning logical relationships that should exist with measures of related concepts or scores produced in similar or diverse patient groups” (FDA DHHS, 2009). In addition, the extent to which scale items were completed and used to allocate risk and the experience of using PURPOSE‐T in clinical practice were captured.
3. PARTICIPANTS
3.1. Nurses
Expert nurses were trained how to use PURPOSE‐T via presentation, the use of vignette case studies, user manual provision and researcher (SC) support. The expert nurses used the same material to cascade PURPOSE‐T training to participating ward/community nurses (Nixon et al., 2015).
3.2. Patients
Patients were purposively sampled ensuring a similar number of hospital and community patients and representation of patients across four broad risk levels (Nixon et al., 2015). These comprised those without mobility restriction (i.e. low risk), those with some mobility/activity limitations (i.e. at risk), those who were bedfast/chairfast (i.e. high risk) and those with an existing PU category 1 or above.
Eligible patients included those who were: ≥18 years, an inpatient in an acute setting or nursing patient in a community setting, able to provide written informed consent/verbal witnessed consent/consultee agreement and expected to be available for PURPOSE‐T retest. Patients were excluded if they were from obstetric, paediatric, day case surgery or psychiatric settings, deemed by the attending healthcare professional to be too unwell to be approached or complete the study assessment schedule.
3.3. Sample size
The sample size was calculated using PASS software is based on a measure of reliability, specifically the kappa coefficient, κ, defined as the proportion of agreement after chance agreement is removed from consideration (Cohen, 1960). Assuming a null hypothesis of κ = 0.6,5% significance level and 15% withdrawal/non‐compliance in paired assessments and 25% of patients are assessed as “not at risk” and 75% of patients are assessed as “at risk,” 230 patients were required to be recruited to the study to detect a statistically significant value of κ ≥ 0.8 (alternative hypothesis) with at least 90% power. For the evaluation of screening instruments, no examples of formal sample size estimation methods were identified in the literature (Nixon et al., 2015). Therefore, literature relating to the psychometric evaluation of rating scales was considered. The “rule of thumb” recommendation of 5–10 patients for every item in a questionnaire was used to estimate the sample size of 115–230 patients (Blazeby, Sprangers, Cull, Groenvold, & Bottomley, 2002; Nunnally & Bernstein, 1994). Therefore, the proposed sample size of 230 to assess inter‐rater reliability of the instrument, with >95% expert nurse data compliance (based on previous research experience), was expected to provide a sufficient number of patients to assess the validity of the risk assessment instrument.
3.4. Data collection
Hospital inpatients and community nursing patients were invited to participate. Ward/community‐based nurses identified suitable patients from their area of practice. Attending clinical staff or a member of the tissue viability team provided the patient with a verbal explanation of the study and an information leaflet before they were invited to provide informed, written consent. Assessment of eligibility and informed consent/consultee agreement was undertaken by a member of the tissue viability team. Participants were registered centrally using the CTRU automated 24‐hour telephone registration system (Nixon et al., 2015).
Participant baseline assessment was undertaken by an expert nurse (tissue viability nurse consultant/specialist/clinical research nurse) and incorporated the collection of demographic data including type of NHS facility, type of admission/referral (e.g. elective/acute), ward specialty (hospital patients), date of birth, gender, ethnicity and clinical assessment comprising subscales of established PU‐RAIs, the Braden scale (Bergstrom, Braden, Laguzza, & Holman, 1987) and Waterlow score (Waterlow, 1988). Braden and Waterlow were selected as they have undergone the most scrutiny in the literature, reflecting their widespread use in practice (Gould et al., 2002; NICE, 2014; Pancorbo‐Hidalgo et al., 2006). Waterlow incorporates mobility, moisture, perfusion, nutrition, gender, age, sensory perception, orthopaedic surgery/below waist fracture, skin condition and medication, whereas the Braden incorporates mobility, activity, friction and shear, moisture, sensory perception and nutrition. Both are ordinal scoring systems where scores for each risk factor are added together to give the patients overall score. This overall score is then compared with a standard reference value to allocate a risk category (e.g. high risk, moderate risk, at risk).
In addition, a paired PURPOSE‐T assessment was undertaken by the expert nurse and a ward/community nurse, incorporating detailed skin assessment and when applicable, PU classification (1–4 and unstageable categories) (NPUAP/EPUAP/PPPIA, 2014). These were conducted simultaneously by both assessors, but recorded separately with blinding maintained (Nixon et al., 2015).
The expert nurse also undertook a second visit and completed PURPOSE‐T (blind to their initial assessment) and recorded significant clinical changes to the patient's condition since the baseline assessment (Nixon et al., 2015).The length of the test–retest interval was planned to be short enough to ensure that clinical change in PU risk was unlikely to occur but sufficiently long to be confident that the expert nurse did not recall responses from the first assessment. Nurses were asked to plan their retest visit 1–3 days and 1‐7 days after baseline for hospital and community patients, respectively, taking into account anticipated recovery/deterioration/stability of each patient's condition and, for hospital patients, length of stay. Expert nurses also kept field notes of their experience of using PURPOSE‐T and comments from ward/community nurses (Nixon et al., 2015).
3.5. The instrument
The preliminary PURPOSE‐T incorporates instructions to support nurse decision‐making facilitated by the use of colour to weight risk factor items. This was based on the overall strength of epidemiological evidence and/or wider scientific evidence, its clinical resonance and its role in the PU causal pathway (Coleman et al., 2013; Coleman, Nelson, et al., 2014; Coleman, Nixon, et al., 2014). In PURPOSE‐T blue indicates “no problem;” yellow indicates a potential impact on PU risk; orange indicates risk and; pink indicates the patient has a PU or scar from a previous PU. This colour code is integrated throughout the three‐step assessment process:
Step 1—screening assessment comprises 4 mobility items (one blue, three yellow) and four skin status items (one blue, two yellow, one pink). This allows those who are clearly not at risk (with only blue items) to be quickly screened out and those with potential risk or actual PUs to proceed to the full assessment.
Step 2—full assessment comprises items for analysis of independent movement (five items which include parameters relating to the extent and frequency of movement; one yellow, four orange); sensory perception (two items; one blue, one orange), detailed skin assessment (13 skin sites each with three items relating to; normal skin (blue), vulnerable skin (orange) or PU (pink) and there is an option to add further skin sites assessed as vulnerable or with a PU in addition to the pre‐specified list); previous PU history (three items; one blue, one yellow and a potential additional pink, yellow or blue); perfusion (three items; one blue two orange), nutrition (five items; one blue, four orange), moisture (three items; one blue, two yellow) and diabetes (two items; one blue, one yellow).
Step 3—requires consideration of step 2 responses to inform 1 of 3 assessment decisions comprising “no PU not currently at risk” for those with only yellow or blue items ticked; “no PU but at risk” for those with any orange (but no pink items) ticked or if yellow/blue boxes are ticked and the nurse assesses the patient to be at risk based on their overall risk profile; and “PU category 1 or above or scarring from previous PU” for those with any pink items ticked.
The final version of PURPOSE‐T is available at http://medhealth.leeds.ac.uk/accesspurposet.
3.6. Ethical considerations
Patients at risk of PUs are often elderly, frail and considered vulnerable. NHS ethical approval for the study was sought through the Health Research Authority and the Integrated Research Application System and Research Ethics Committee.
3.7. Data analysis
3.7.1. Study risk definitions
For the purposes of describing the study population and to assess the convergent validity of PURPOSE‐T with other PU‐RAIs, “at risk” was defined as decision pathways “No PU but at risk” and “PU category 1 or above or scarring from previous PU,” whereas “not at risk” was defined as decision pathway “no PU not currently at risk”. The cut‐points used to identify patients at risk for the two other PU‐RAIs were ≤18 for Braden (Bergstrom, Braden, Kemp, Champagne, & Ruby, 1998) and ≥10 for Waterlow (Waterlow, 1988).
3.7.2. Analysis
For each assessment data completeness was summarized for each element of PURPOSE‐T including the percentage of missing item‐level data and risk categories allocated. We produced the simple kappa statistic with 95% confidence interval (CI) and a weighted kappa statistic which incorporates the distribution of disagreements. We calculated weighted Kappa using (Cicchetti & Allison, 1971) weights for comparisons of outcomes with more than two levels. Prevalence‐adjusted bias‐adjusted kappa (PABAK) statistics were also produced to take into account the prevalence of risk status and bias between observers (Byrt, Bishop, & Carlin, 1993). The final kappa statistic calculated to assess the inter‐rater and test–retest reliability for overall risk status was the maximum value of kappa (κmax) statistic. Cross‐tabulations of overall risk status by rater/retest were also produced. We examined the extent of agreement for individual PURPOSE‐T items using cross‐tabulations by rater/retest. In addition, we produced kappa (with 95% CI) and weighted kappa statistics to assess inter‐rater reliability of the PURPOSE‐T decision pathway and produced corresponding cross‐tabulations by rater/retest. We used published benchmarks to interpret estimates of the kappa coefficient; Poor κ < 0.20, Fair 0.21 ≤ κ ≤ 0.40, Moderate 0.41 ≤ κ ≤ 0.60, good 0.61 ≤ κ ≤ 0.80, very good 0.81 ≤ κ ≤ 1.00 (Bland, 2008; Landis & Koch, 1977).
Cross‐tabulations of overall risk status for PURPOSE‐T, Braden and Waterlow were produced to explore convergent validity. Cross‐tabulations of corresponding items between PURPOSE‐T, Braden and/or Waterlow were produced and correlation coefficients were calculated to assess convergent validity. The Spearman rank correlation coefficient was used when each of the items being compared had more than two levels and the phi correlation coefficient was calculated when dichotomous variables were compared. For exploratory purposes, the following hypotheses were used as guides to the magnitude of correlations: high correlation r > 0.7; moderate correlation 0.3 ≤ r ≤ 0.7; low correlation r < 0.3 (Burnand, Kernan, & Feinstein, 1990; Cohen, 1960). Moderate to high correlations (r ≥ 0.3) were predicted for comparison of PURPOSE T with relevant Braden and Waterlow items. SAS 9.2 was used for the analysis.
3.8. Validity and reliability
To maintain blinding between assessors (expert and ward/community nurses) and assessments (baseline and follow‐up), special adhesive data collection forms were used that were sealed on completion, only being opened by CTRU for analysis (Nixon et al., 2015).
To ensure the study population was representative of the clinical population assessed in the course of usual care, approval was sought and gained for witnessed consent (for patients who were capable of giving consent but physically unable to complete the consent form) and consultee agreement (for patients who lacked capacity).
4. RESULTS
In total, 230 of 394 patients screened were registered to the study between 3 October 2012–25 January 2013 (Figure 1), from four acute hospital (108 (47.0%) patients) and four community NHS trusts (122 (53.0%) patients) in England, with numbers of patients registered at each centre ranging from 14 to 54. The 230 patients recruited were assessed in part or full using PURPOSE‐T at baseline, providing a total of 230 paired assessments undertaken by 11 expert nurses and 73 ward/community nurses. Table 1 shows the characteristics of participants, indicating a mainly Caucasian population with good representation from each of the four broad levels of risk. Of the 230 patients registered, 217 (94.3%) had retest assessments completed by the expert nurse (Figure 1).
Figure 1.
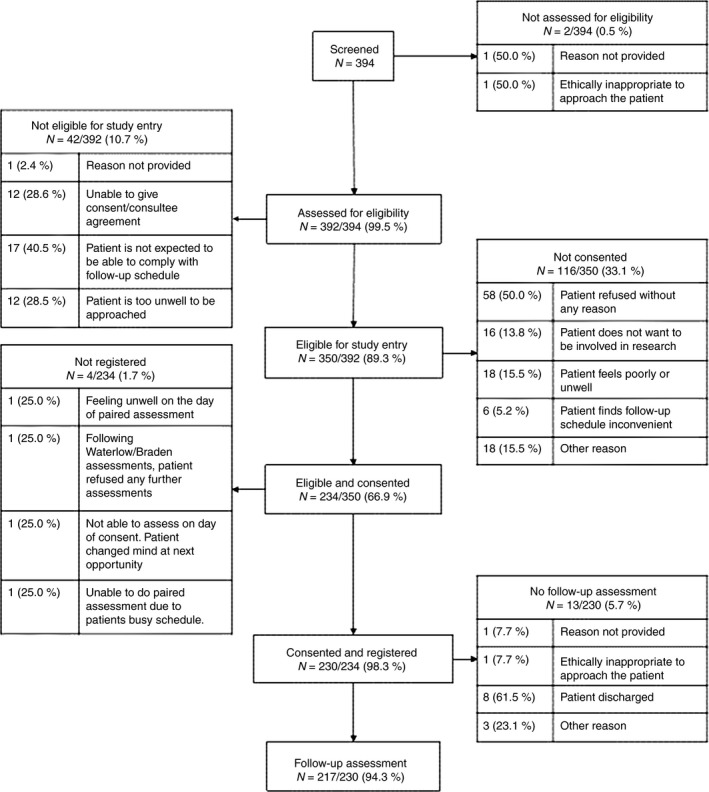
Flow of participants
Table 1.
Baseline characteristics
| Variable | PU at baselinea (N = 60) | No PU at baselinea (N = 169) | Missing PU statusa (N = 1b) | Totalc |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 73.8 (15.9) | 72.1 (18.3) | – | 72.6 (17.6) |
| Median (range) | 76 (29, 98) | 78 (19,102) | 78 (N/A) | 77 (19, 102) |
| Sex, N (%) | ||||
| Male | 27 (27.3%) | 72 (72.7%) | 0 (0.0%) | 99 (43.0%) |
| Female | 33 (25.2%) | 97 (74.0%) | 1 (0.8%) | 131 (57.0%) |
| Ethnicity N (%) | ||||
| Caucasian | 58 (25.9%) | 165 (73.7%) | 1 (0.4%) | 224 (97.4%) |
| Other | 2 (33.3%) | 4 (66.7%) | 0 (0.0%) | 6 (2.6%) |
| Setting, N (%) | ||||
| Community | 37 (30.3%) | 84 (68.9%) | 1 (0.1%) | 122 (53.0%) |
| Secondary care hospital | 23 (21.3%) | 85 (78.7%) | 0 (0.0%) | 108 (47.0%) |
| Mobility status, PURPOSE‐T step 1, N (%) | ||||
| Walks independently with or without walking aids | 10 (12.7%) | 69 (87.3%) | 0 (0.0%) | 79 (34.3%) |
| Needs help of another person to walk | 6 (22.2%) | 21 (77.8%) | 0 (0.0%) | 27 (11.7%) |
| Spends all/majority of time in bed/chair | 16 (28.1%) | 40 (70.2%) | 1 (1.8%) | 57 (24.8%) |
| Remains in same position for long periods | 28 (42.4%) | 38 (57.6%) | 0 (0.0%) | 66 (28.7%) |
| Not completed | 0 (0.0%) | 1 (100%) | 0 (0.0%) | 1 (0.4%) |
| Braden score, N (%) | ||||
| At risk (≤18) | 35 (41.2%) | 50 (58.8%) | 0 (0.0%) | 85 (37.0%) |
| Not at risk (>18) | 25 (17.2%) | 119 (82.1%) | 1 (0.7%) | 145 (63.0%) |
| Waterlow total score | ||||
| At risk (≥10) | 60 (31.1%) | 132 (68.4%) | 1 (0.5%) | 193 (83.9%) |
| Not at risk (<10) | 0 (0.0%) | 37 (100%) | 0 (0.0%) | 37 (16.1%) |
| PURPOSE‐T risk categorization | ||||
| Secondary prevention/treatment pathway | 60 (83.3%) | 12 (16.7%) | 0 (0.0%) | 72 (31.3%) |
| Primary prevention pathway | 0 (0.0%) | 111 (100%) | 0 (0.0%) | 111 (48.3%) |
| Not currently at risk pathway | 0 (0.0%) | 46 (97.9%) | 1 (2.1%) | 47 (20.4%) |
Percentages in the PU status columns correspond to the proportion of patients within that characteristic who do (or do not) have a PU at baseline (e.g. 27.3% (27 out of 99) of the male population were observed to have a PU at baseline).
There was one community patient for whom their PU status at baseline could not be determined as there were no skin assessments recorded by the tissue viability team member.
Percentages in the total column correspond to the proportion of patients from the overall population with that characteristic (e.g. 43.0% of overall population were male; 57.0% female).
Based on the baseline PURPOSE‐T assessment conducted by expert nurses, there were 60 (26.1%) patients who presented with a category 1 or above PU (Table 1). There were a total of 96 PUs across the 60 patients including 21 (21.9%) category 1, 56 (58.3%) category 2, 6 (6.3%) category 3, 6 (6.3%) category 4 and 7 (7.3%) unstageable ulcers. PURPOSE‐T identified 183(79.6%) patients as “at risk,” Waterlow identified 193 (83.9%) patients as “at risk” and Braden identified 85 (37.0%) patients as “at risk.” Of the 145 patients identified as “not at risk” on Braden 25 (17.2%) had an existing PU (Table 1).
4.1. Data completeness and usability
Compliance with the completion guidelines for steps 1–3 were quantified together with a review of the field notes from the expert nurses (Table 2). At least 94.9% data completeness for each construct was observed with the exception of previous PU details (54.7% and 66.7%) and the decision pathway allocation by the ward/community nurses for patients who should not have progressed to step 2 (85.7%) (Table 2).
Table 2.
Data completeness and completion
| Construct | Data completeness | Completion of PURPOSE‐T according to guidance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of items requiring completion | Expert nurse baseline assessment | Ward/Community nurse assessment | Expert nurse follow‐up assessment | Denominator (i.e. number of items expected to have been completed) | Completion of PURPOSE‐T according to guidance | Expert nurse baseline assessment | Ward/Community nurse assessment | Expert nurse follow‐up assessment | |
| Step 1 Screening | |||||||||
| Mobility | 1 of 4 | 99.6% (229/230) | 99.6% (229/230) | 100.0% (217/217) | All patients, as all were required complete step 1 mobility | Completed | 229 (99.6%) | 229 (99.6%) | 217 (100%) |
| Not completed | 1 (0.4%) | 1 (0.4%) | 0 (0.0%) | ||||||
| Total | 230 (100.0%) | 230 (100.0%) | 217 (100.0%) | ||||||
| Skin status | 1 of 4 (if required) | 98.7% (78/79) | 96.6% (84/87) | 100.0% (63/63) | All patients for whom only the blue box was ticked for step 1 mobility | Appropriate completion (no mobility limitation) | 78 (33.9%) | 84 (36.5%) | 63 (29.0%) |
| Inappropriate non‐completion (no mobility limitation) | 1 (0.4%) | 3 (1.3%) | 0 (0.0%) | ||||||
| Appropriate non‐completion (mobility limitation) | 114 (49.6%) | 83 (36.5%) | 117 (53.9%) | ||||||
| Inappropriate completion (mobility limitation) | 36 (15.7%) | 59 (25.7%) | 37 (17.1%) | ||||||
| Completed but no mobility assessment | 0 (0.0%) | 1 (0.4%) | 0 (0.0%) | ||||||
| Not completed and no mobility assessment | 1 (0.4%) | 0 (0.0%) | 0 (0.0%) | ||||||
| Total | 230 (100.0%) | 230 (100.0%) | 217 (100.0%) | ||||||
| Decision pathway allocated | 1 | 95.3% (41/43) | 85.7% (36/42) | 100.0% (38/38) | All patients for whom only the blue box was ticked for both step 1 mobility and step 1 skin status | ||||
| Progression to step 2 | 195 | 197 | 182 | Appropriate progression to step 2 | 185 (80.4%) | 185 (80.4%) | 179 (82.5%) | ||
| Inappropriate progression to step 2 | 9 (3.9%) | 12 (5.2%) | 3 (1.4%) | ||||||
| Inappropriate non‐progression to step 2 | 0 (0.0%) | 1 (0.4%) | 0 (0.0%) | ||||||
| Appropriate non‐progression to step 2 | 35 (15.2%) | 32 (13.9%) | 35 (16.1%) | ||||||
| Step 2 completed but step 1 not completed | 1 (0.4%) | 0 (0.0%) | 0 (0.0%) | ||||||
| Total | 230 (100.0%) | 230 (100.0%) | 217 (100.0%) | ||||||
| Step 2 Full Assessment | |||||||||
| Step 1 Mobility | 1 of 4 | 99.5% (194/195) | 99.5% (196/197) | 100.0% (182/182) | All patients who progressed to step 2, as all were required to complete step 1 mobility | ||||
| Step 1 skin status | 1 of 4 (if required) | 97.7% (43/44) | 96.3% (52/54) | 100.0% (28/28) | All patients for whom only the blue box was ticked in step 1 mobility who progressed to step 2 | ||||
| Analysis of independent movement | 1 of 5 | 99.0% (193/195) | 99.0% (195/197) | 98.9% (180/182) | All patients who progressed to step 2 | ||||
| Sensory perception and response | 1 of 2 | 96.9% (189/195) | 94.9% (187/197) | 98.4% (179/182) | |||||
| Current detailed skin assessment | 13 | 95.5% (2421/2535) | 95.3% (2440/2561) | 97.5% (2307/2366) | 13 (number of main skin sites) x number of patients who progressed to step 2 | ||||
| Previous PU history | 1 of 2 | 99.0% (193/195) | 95.9% (189/197) | 98.4% (179/182) | All patients who progressed to step 2 | ||||
| Previous PU details | At least 1 | 66.7% (40/60) | 54.7% (29/53) | 57.4% (35/61) | All patients reported to have a PU history | ||||
| Perfusion | At least 1 | 97.9% (191/195) | 97.5% (192/197) | 97.3% (177/182) | All patients who progressed to step 2 | ||||
| Nutrition | At least 1 | 99.0% (193/195) | 99.5% (196/197) | 97.8% (178/182) | |||||
| Moisture | 1 of 3 | 99.5% (194/195) | 97.0% (191/197) | 96.7% (176/182) | |||||
| Diabetes | 1 of 2 | 99.0% (193/195) | 95.9% (189/197) | 96.7% (176/182) | |||||
| Decision pathway allocated | 1 of 3 | 100.0% (194/195) | 99.0% (195/197) | 100.0% (182/182) | |||||
| Assessment Decision | |||||||||
| Pathway allocated at step 1 or step 3 | Appropriate pathway | 226 (98.3%) | 219 (95.2%) | 215 (99.1%) | |||||
| Inappropriate pathway | 4 (1.7%) | 10 (4.3%) | 2 (0.9%) | ||||||
| No pathway selected | 0 (0.0%) | 1 (0.4%) | 0 (0.0%) | ||||||
| Total | 230 (100.0%) | 230 (100.0%) | 217 (100.0%) | ||||||
Appropriate completion of Step 1 (i.e. in line with the recommended assessment flow) by the expert nurses was similar for baseline (83.5%:192/230) and follow‐up (82.9%:180/217), respectively, compared with 72.6% (167/230) of ward/community nurses assessments (Table 2). Progression/non‐progression to step 2 was completed in line with the recommended assessment flow for 95.7% (220/230) of patients assessed by an expert nurse at baseline and 98.6% (214/217) assessed at follow‐up. For ward/community nurses this was 94.3% (217/230) (Table 2). A step1/step 3 decision pathway was allocated to all patients by the expert nurses at baseline and follow‐up and just one patient (0.4%) was not allocated a decision pathway by the ward/community nurses (Table 2).
Patients were allocated (Tables 2 and 3) to the correct decision pathway (i.e. in line with PURPOSE‐T decision rules) by expert nurses at baseline (98.3%:226/230) and follow‐up (99.1%: 215/217) and by ward/community nurses (95.2%:219/230). Expert and ward/community nurses allocated most of patients to the “not at risk” decision pathway when they completed only yellow and blue boxes with 95.6% (43/45), 95.9% (47/49) and 97.7% (42/43) for expert nurses at baseline, ward/community nurses and expert nurses at follow‐up respectively (Table 3).
Table 3.
PURPOSE T decision pathway by colour of boxes ticked
| PURPOSE‐T decision pathway | Colour of boxes ticked N (%) | Total N (%) | ||
|---|---|---|---|---|
| At least one pink box ticked | No pink boxes and at least one orange box ticked | Only blue and yellow boxes ticked | ||
| Expert nurse baseline | ||||
| PU Category 1 or above or scarring | 72 (31.3) | 0 (0.0) | 0 (0.0) | 72 (31.3) |
| No PU, but at risk | 0 (0.0) | 109 (47.4) | 2 (0.9) | 111 (48.3) |
| No PU, not currently at risk | 0 (0.0) | 4 (1.7) | 43 (18.7) | 47 (20.4) |
| Total | 72 (31.3) | 113 (49.1) | 45 (19.6) | 230 (100.0) |
| Ward/Community nurse | ||||
| PU Category 1 or above or scarring | 63 (27.4) | 0 (0.0) | 0 (0.0) | 63 (27.4) |
| No PU, but at risk | 5 (2.2) | 107 (46.5) | 2 (0.9) | 114 (49.6) |
| No PU, not currently at risk | 0 (0.0) | 5 (2.2) | 47 (20.4) | 52 (22.6) |
| Missing | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1 (0.4) |
| Total | 68 (29.6) | 113 (49.1) | 49 (21.3) | 230 (100.0) |
| Expert nurse follow‐up | ||||
| PU Category 1 or above or scarring | 68 (31.3) | 0 (0.0) | 0 (0.0) | 68 (31.3) |
| No PU, but at risk | 2 (0.9) | 104 (47.9) | 1 (0.5) | 107 (49.3) |
| No PU, not currently at risk | 0 (0.0) | 0 (0.0) | 42 (19.4) | 42 (19.4) |
| Total | 70 (32.3) | 104 (47.9) | 43 (19.8) | 217 (100.0) |
4.2. Inter‐rater reliability
At baseline, there were 230 paired assessments by the expert nurse and the ward/community nurse for evaluating inter‐rater reliability. The patient population included patients for whom the assessment was completed by both raters regardless of compliance with the recommended assessment flow (Table 2).
There was agreement in the three‐way decision pathway between expert and ward/community nurses for 81.7% (187/229) of paired assessments (Table 4). Under the assumption that the expert nurse and ward/community nurse would complete PURPOSE‐T in a similar manner, the corresponding simple kappa statistic of 0.71 (95% CI 0.63 to 79) and weighted kappa statistic of 0.76 (95% CI 0.69 to 0.83) indicate good inter‐rater reliability. When classified dichotomously as “at risk”/”not at risk” there was agreement between the expert nurse and ward/community nurse for 93.4% (214/229) paired assessments (Table 4). The corresponding simple kappa statistic of 0.81 (95% CI 0.71 to 0.90), PABAK of 0.87 and κmax of 0.94 indicate very good inter‐rater reliability.
Table 4.
Cross tabulation of expert nurse PURPOSE‐T decision pathway at baseline by ward/community nurse decision pathway and expert nurse decision pathway at follow‐up
| Inter‐rater | ||||
|---|---|---|---|---|
| Expert nurse baseline | Ward/community nurse | |||
| PU Category 1 or above or scarring | No PU but at risk | Not PU, not currently at risk | Total | |
| PU Category 1 or above or scarring | 54 (23.6) | 18 (7.9) | 0 (0.0) | 72 (31.4) |
| No PU, but at risk | 9 (3.9) | 91 (39.7) | 10 (4.4) | 110 (48.0) |
| No PU, not currently at risk | 0 (0.0) | 5 (2.2) | 42 (18.3) | 47 (20.5) |
| Total | 63 (27.5) | 114 (49.8) | 52 (22.7) | 229 (100.0) |
| At risk | Not at risk | Total | |
|---|---|---|---|
| At risk | 172 (75.1) | 10 (4.4) | 182 (79.5) |
| Not at risk | 5 (2.2) | 42 (18.3) | 47 (20.5) |
| Total | 177 (77.3) | 52 (22.7) | 229 (100.0) |
| Test–retest | ||||
|---|---|---|---|---|
| Expert nurse baseline | Expert nurse follow‐up | |||
| PU Category 1 or above or scarring | No PU but at risk | Not PU, not currently at risk | Total | |
| PU Category 1 or above or scarring | 64 (30.0) | 5 (2.3) | 0 (0.0) | 69 (32.4) |
| No PU, but at risk | 3 (1.4) | 95 (44.6) | 5 (2.3) | 103 (48.4) |
| No PU, not currently at risk | 0 (0.0) | 4 (1.9) | 37 (17.4) | 41 (19.2) |
| Total | 67 (31.5) | 104 (48.8) | 42 (19.7) | 213 (100.0) |
| At risk | Not at risk | Total | |
|---|---|---|---|
| At risk | 167 (78.4) | 5 (2.3) | 172 (80.8) |
| Not at risk | 4 (1.9) | 37 (17.4) | 41 (19.2) |
| Total | 171 (80.3) | 42 (19.7) | 213 (100.0) |
Table 5 shows the level of agreement between expert nurses and ward/community nurses for specific risk factor items. In terms of complete agreement, the lowest level was 59.2% (113/191) for the analysis of independent movement and the highest was 94.2% (180/191) for diabetic status. We also looked at the levels agreement in terms of the presence of a problem or not and the levels of agreement; ranged from 72.8% (139/191) for perfusion status to 94.2% for diabetic status.
Table 5.
Levels of agreement between expert nurses and ward/community nurses and between expert nurses at baseline and at follow‐up for specific risk factor items
| Item | Expert nurse vs. ward nurse | Expert nurse baseline vs. follow‐up |
|---|---|---|
| Step 1: Mobility (absolute agreement) | 156/228 (68.4%) | 165/212 (77.8%) |
| Step 1: Mobility (agreement on presence/absence of problem) | 207/228 (90.8%) | 197/212 (92.9%) |
| Skin status [step 1 and 2 combined] (absolute agreement) | 189/230 (82.2%) | 191/213 (89.7%) |
| Skin status [step 1 and 2 combined] (agreement on presence/absence of problem) | 204/230 (88.7%) | 200/213 (93.9%) |
| Analysis of independent movement (absolute agreement) | 113/191 (59.2%) | 114/177 (64.4%) |
| Analysis of independent movement (agreement on presence/absence of problem) | 165/191 (86.4%) | 147/177 (83.1%) |
| PU History | 160/191 (83.7%) | 165/177 (93.2%) |
| Sensory perception | 151/191 (79.1%) | 154/177 (87.0%) |
| Nutrition (problem vs. no problem) | 156/191 (81.7%) | 154/177 (87.0%) |
| Unplanned weight loss | 159/191 (83.2%) | 159/177 (89.8%) |
| Poor nutritional intake | 163/191 (85.3%) | 159/177 (89.8%) |
| Low BMI | 176/191 (92.1%) | 170/177 (96.0%) |
| High BMI | 170/191 (89.0%) | 165/177 (93.2%) |
| Diabetic status | 180/191 (94.2%) | 166/177 (93.8%) |
| Perfusion status (absolute agreement) | 125/191 (65.4%) | 138/177 (78.0%) |
| Perfusion status (agreement on presence/absence of problem) | 139/191 (72.8%) | 154/177 (87.0%) |
| Moisture status (absolute agreement) | 145/191 (75.9%) | 155/177 (87.6%) |
| Moisture status (agreement on presence/absence of problem) | 155/191 (81.2%) | 159/177 (89.8%) |
4.3. Test–retest reliability
There were up to 217 paired assessments by the expert nurse at baseline and at follow‐up for evaluating test–retest reliability, however, four were excluded due to a change in the patients' clinical condition providing an analysis population of 213. The median number of days between the baseline and the retest expert nurse assessment was three (range 1–7). As with the inter‐rater reliability, the patient population included patients for whom the assessment was completed at both time points regardless of compliance with the recommended assessment flow.
There was agreement in the three‐way decision pathway between the baseline and follow‐up assessments for 92.0% (196/213) of paired assessments (Table 4). The corresponding simple kappa statistic of 0.87 (95% CI 0.81‐93) and weighted kappa statistic of 0.89 (95% CI 0.84‐0.94) indicate good test–retest reliability. When classified dichotomously as “at risk”/”not at risk” there was agreement between the baseline and follow‐up assessment for 204 (95.8%) paired assessments (Table 4). The corresponding simple kappa statistic of 0.87(95% CI 0.78‐0.95), PABAK of 0.92 and κmax of 0.99 indicate very good test–retest reliability.
Table 5 shows the level of agreement between expert nurse's baseline and follow‐up assessments for specific risk factor items. In terms of complete agreement, the lowest level was 64.4% (114/177) for the analysis of independent movement and the highest was 96.0% (170/177) for low BMI. We also looked at the levels of agreement in terms of the presence of a problem or not and levels of agreement ranged from 87.0% (154/177) for perfusion status to 96.0% (170/177) for low BMI.
4.4. Convergent validity
The overall risk status on PURPOSE‐T was compared with Waterlow for all 230 patients PU free at baseline. A moderate association was observed between PURPOSE‐T and Waterlow with a phi correlation coefficient of 0.63 (Table 6). A moderate association was also observed between PURPOSE‐T and Braden for 169 PU‐free patients, as assessed by the expert nurse at baseline, with a phi correlation coefficient of 0.40. Individual constructs on PURPOSE‐T were compared with relevant constructs on Braden and Waterlow with moderate to high correlations observed in each case (Tables 7 & 8).
Table 6.
Cross tabulation of PURPOSE‐T with the Waterlow and the Braden scales—overall risk
| PURPOSE‐T overall risk status | Waterlow overall risk status N (%) | Correlation coefficient | ||
|---|---|---|---|---|
| At risk (≥10) | Not at risk (<10) | Total | ||
| At risk | 175 (76.1) | 8 (3.5) | 183 (79.6) | Phi 0.63—Moderate |
| Not at risk | 18 (7.8) | 29 (12.6) | 47 (20.4) | |
| Total | 193 (83.9) | 37 (16.1) | 230 (100.0) | |
| PURPOSE‐T overall risk status | Braden overall risk status N (%) | Correlation coefficient | ||
| At risk (≤18) | Not at risk (>18) | Total | ||
| At risk | 50 (29.6) | 73 (43.2) | 123 (72.8) | Phi 0.40—Moderate |
| Not at risk | 0 (0.0) | 46 (27.2) | 46 (27.2) | |
| Total | 50 (29.6) | 119 (70.4) | 169 (100.0) | |
Table 7.
Cross tabulations of dichotomized PURPOSE‐T constructs with relevant constructs on the Waterlow and the Braden scales
| PURPOSE T mobility Step 1 | Braden Mobility | Correlation coefficient | ||
|---|---|---|---|---|
| No limitation | Slightly/very limited/completely immobile | Total | ||
| No problem | 69 (30.1%) | 10 (4.4%) | 79 (34.5%) | Phi 0.60—Moderate |
| Problem | 37 (16.2%) | 113 (49.3%) | 150 (65.5%) | |
| Total | 106 (46.3%) | 123 (53.7%) | 229 (100.0%) | |
| PURPOSE T mobility Step 1 | Braden Activity | Correlation coefficient | ||
|---|---|---|---|---|
| Walks frequently | Walks occasionally, chairfast or bedfast | Total | ||
| No problem | 56 (24.5%) | 23 (10.0%) | 79 (34.5%) | Phi 0.66—Moderate |
| Problem | 11 (4.8%) | 139 (60.7%) | 150 (65.5%) | |
| Total | 67 (29.3%) | 162 (70.7%) | 229 (100.0%) | |
| PURPOSE T Sensory response and Perception | Braden Sensory | Correlation coefficient | ||
|---|---|---|---|---|
| No impairment | Slightly, very or completely limited | Total | ||
| No problem | 134 (70.9%) | 7 (3.7%) | 141 (74.6%) | Phi 0.74—High |
| Patient unable to feel and/or respond appropriately to discomfort from pressure | 11 (5.8%) | 37 (19.6%) | 48 (25.4%) | |
| Total | 145 (76.7%) | 44 (23.3%) | 189 (100.0%) | |
| PURPOSE T nutrition | Braden Nutrition | Correlation coefficient | ||
|---|---|---|---|---|
| Excellent or adequate | probably inadequate or very poor | Total | ||
| No problem | 93 (48.2%) | 1 (0.5%) | 94 (48.7%) | Phi 0.58—Moderate |
| Problem | 47 (24.4%) | 52 (26.9%) | 99 (51.3%) | |
| Total | 140 (72.5%) | 53 (27.5%) | 193 (100.0%) | |
| PURPOSE T nutrition | Waterlow malnutrition screening tool: Patient eating poorly or lack of appetite | Correlation coefficient | ||
|---|---|---|---|---|
| Yes | No | Total | ||
| Problem | 64 (33.7%) | 35 (18.4%) | 99 (52.1%) | Phi 0.60—Moderate |
| No problem | 6 (3.2%) | 85 (44.7%) | 91 (47.9%) | |
| Total | 70 (36.8%) | 120 (63.2%) | 190 (100.0%) | |
| PURPOSE T nutrition—poor nutritional intake | Braden Nutrition | Correlation coefficient | ||
|---|---|---|---|---|
| Probably inadequate or very poor | Excellent or adequate | Total | ||
| Yes | 50 (25.9%) | 12 (6.2%) | 62 (32.1%) | Phi 0.82—High |
| No | 3 (1.6%) | 128 (66.3%) | 131 (67.9%) | |
| Total | 53 (27.5%) | 140 (72.5%) | 193 (100.0%) | |
| PURPOSE T nutrition—poor nutritional intake | Waterlow malnutrition screening tool: Patient eating poorly or lack of appetite | Correlation coefficient | ||
|---|---|---|---|---|
| Yes | No | Total | ||
| Yes | 57 (30.0%) | 5 (2.6%) | 62 (32.6%) | Phi 0.79—High |
| No | 13 (6.8%) | 115 (60.5%) | 128 (67.4%) | |
| Total | 70 (36.8%) | 120 (63.2%) | 190 (100.0%) | |
| PURPOSE T nutrition—low BMI | Waterlow build or weight for height | Correlation coefficient | ||
|---|---|---|---|---|
| BMI < 20 | BMI ≥ 20 | Total | ||
| Yes | 21 (11.0%) | 0 (0.0%) | 21 (11.0%) | Phi 0.72—High |
| No | 16 (8.4%) | 154 (80.6%) | 170 (89.0%) | |
| Total | 37 (19.4%) | 154 (80.6%) | 191 (100.0%) | |
| PURPOSE T nutrition—High BMI | Waterlow build or weight for height | Correlation coefficient | ||
|---|---|---|---|---|
| BMI ≥ 30 | BMI < 30 | Total | ||
| Yes | 22 (11.5%) | 4 (2.1%) | 26 (13.6%) | Phi 0.74—High |
| No | 9 (4.7%) | 156 (81.7%) | 165 (86.4%) | |
| Total | 31 (16.2%) | 160 (83.8%) | 191 (100.0%) | |
Table 8.
Cross‐tabulations of dichotomized PURPOSE‐T constructs with relevant constructs on the Waterlow and the Braden scales
| PURPOSE T Step 2 Analysis of independent movement | Braden mobility | Correlation coefficient | |||
|---|---|---|---|---|---|
| Completely immobile | Very or slightly limited | No limitation | Total | ||
| Does not Move | 7 (3.6%) | 4 (2.1%) | 0 (0.0%) | 11 (5.7%) | Spearman rank 0.62—Moderate |
| Moves occasionally and slight or major position changes or moves frequently with slight position changes | 1 (0.5%) | 96 (49.7%) | 26 (13.5%) | 123 (63.7%) | |
| Moves frequently and major position changes | 0 (0.0%) | 12 (6.2%) | 47 (24.4%) | 59 (30.6%) | |
| Total | 8 (4.1%) | 112 (58.0%) | 73 (37.8%) | 193 (100.0%) | |
| PURPOSE T Step 2 Analysis of independent movement | Braden Activity | Correlation coefficient | |||
|---|---|---|---|---|---|
| Bedfast | Chairfast or walks occasionally | Walks frequently | Total | ||
| Does not Move | 6 (3.1%) | 5 (2.6%) | 0 (0.0%) | 11 (5.7%) | Spearman rank 0.55—Moderate |
| Moves occasionally and slight or major position changes or moves frequently with slight position changes | 15 (7.8%) | 102 (52.8%) | 6 (3.1%) | 123 (63.7%) | |
| Moves frequently and major position changes | 0 (0.0%) | 31 (16.1%) | 28 (14.5%) | 59 (30.6%) | |
| Total | 21 (10.9%) | 138 (71.5%) | 34 (17.6%) | 193 (100.0%) | |
| PURPOSE T skin status | Waterlow skin status | Correlation coefficient | |||
|---|---|---|---|---|---|
| Healthy | Tissue paper dry oedematous clammy | Discoloured grade 1 or broken spots grade 2–4 | Total | ||
| Normal Skin | 47 (20.6%) | 18 (7.9%) | 0 (0.0%) | 65 (28.5%) | Spearman rank 0.83—High |
| Vulnerable skin | 11 (4.8%) | 79 (34.6%) | 13 (5.7%) | 103 (45.2%) | |
| PU Category | 1 (0.4%) | 0 (0.0%) | 59 (25.9%) | 60 (26.3%) | |
| Total | 59 (25.9%) | 97 (42.5%) | 72 (31.6%) | 228 (100.0%) | |
| PURPOSE T Moisture | Braden Moisture | Correlation coefficient | |||
|---|---|---|---|---|---|
| Rarely or occasionally moist | Very moist | Constantly moist | Total | ||
| No problem/Occasional | 154 (79.4%) | 2 (1.0%) | 0 (0.0%) | 156 (80.4%) | Spearman Rank 0.67—Moderate |
| Frequent (2–4 times a day) | 18 (9.3%) | 12 (6.2%) | 0 (0.0%) | 30 (15.5%) | |
| Constant | 0 (0.0%) | 4 (2.1%) | 4 (2.1%) | 8 (4.1%) | |
| Total | 172 (88.7%) | 18 (9.3%) | 4 (2.1%) | 194 (100.0%) | |
4.5. Summary of expert nurse field notes
The field notes described positive and problem aspects of using PURPOSE‐T in practice (Table 9). More general issues associated with all PU‐RAIs were reported (Nixon et al., 2015):
Lack of knowledge of PU classification
Difficulty assessing:
Mobility when the patient is unable to communicate and after only a short assessment period
Sensory perception
Medical history in community setting
Poor nutritional intake
BMI in community setting
Table 9.
Summary of expert nurse field notes
| Characteristic | Positive aspects of using PURPOSE | Problem aspects of using PURPOSE T |
|---|---|---|
| Layout |
|
|
| Format |
|
|
| Content |
|
|
| Usability |
|
|
5. DISCUSSION
The PURPOSE‐T clinical evaluation involved 230 patients, assessed by both expert and ward/community nurses. Overall the level of data completion for each construct on PURPOSE‐T was high at >90%, except for previous PU history. The inter‐rater and test–retest reliability as determined by the kappa statistic was “very good” for the assessment decision overall. The observed percentage agreement for the assessment of “problem/no problem” for the eight risk factors (mobility, skin, previous PU, sensory perception, perfusion, nutrition, moisture and diabetes) was high for both inter‐rater reliability and test–retest reliability. The lowest levels of absolute agreement were for the analysis of independent movement, a matrix item. This is likely related to the increased number of assessment options available (i.e. does not move/does not move, moves occasionally/slight position changes, move occasionally/major position changes, move frequently/slight position changes and moves frequently/major position changes) when compared with the other items. Indeed when agreement was considered on presence/absence of a problem, agreement was in line with other assessment items. As predicted moderate to high associations were demonstrated for convergent validity, assessed by comparison with the same or similar constructs on other PU‐RAIs (Braden and Waterlow) (Nixon et al., 2015).
An important feature of PURPOSE‐T is the inclusion of skin status which was identified, as a key predictor of PU development (Coleman et al., 2013). This allows us to identify those who are at risk of PU development and require primary prevention and those who have an existing PU and require secondary prevention. Traditional RAIs were designed to identify “at risk” patients, that is before they develop a PU, yet in practice they are used on all patients (those with and without PUs) and do not distinguish between these two groups (Coleman, 2014). Without skin assessment nurses may not identify the presence of an existing PU and fail to initiate escalated interventions, leading to the progression of a severe PU (Pinkney et al., 2014). The results indicate that for Waterlow and PURPOSE‐T which incorporate skin status, all patients with an existing PU were identified as “at risk” (for PURPOSE‐T the red “Secondary prevention/treatment pathway” was allocated), whereas 17.2% of those assessed as “not at risk” by Braden, which does not include skin status actually had an existing PU. It maybe that these patients were recovering and not considered “at risk” of new PU development but they should still be considered a priority in clinical practice. It was beyond the scope of this study to assess whether their “not at risk” status had an impact on the intensity of the interventions received.
The field notes recorded by the expert nurses highlighted positive and problem aspects of using PURPOSE‐T in the clinical environment (Nixon et al., 2015). Negative aspects included difficulties in assessing some of PURPOSE‐T items and concerns about reliability, but these were not evidenced in the formal evaluation of inter‐rater and test–retest reliability. It is of note that where only yellow and blue data items were present both expert and ward/community nurses allocated the majority of patients (>95%) to the “not at risk” category, with clinical decision‐making reflecting systematic review evidence (Coleman et al., 2013) that there is a weaker relationship between these factors and PU development (Nixon et al., 2015).
The issues raised in the field notes and emerging evidence from the PURPOSE pain cohort study (Nixon et al., 2015) indicating pain as a predictor of PU development were considered in a post‐clinical evaluation review of PURPOSE‐T by the expert group and PU Research Service User Network (PURSUN UK http://www.pursun.org.uk/) involved in the earlier consensus study (Coleman, Nelson et al., 2014). The review resulted in final amendments to PURPOSE‐T and the inclusion of pain in the stage 2 skin assessment section. PURPOSE‐T and supporting literature is freely available for academic research and clinical use and can be downloaded following web‐based registration http://medhealth.leeds.ac.uk/accesspurposet). It has since been implemented in community and acute NHS Trusts and early indications are positive, though further evaluation of its impact in practice is needed.
Clinical evaluation allowed construct validity (convergent and discriminant), inter‐rater and test–retest reliability and clinical utility to be evaluated. These are important building blocks in the development of RAIs, yet have often been overlooked in the development of previous RAIs (Coleman, 2014) with only a few reporting the evaluation of reliability during instrument validation (Bergstrom et al., 1987; Lindgren, Unosson, Krantz, & Ek, 2002; Suriadi et al., 2006) and a focus on establishing predictive validity (Coleman, 2014). While this is an important property, its evaluation is hampered by several important factors, including the subjective nature of PU risk factor measurement; the lack of a reference gold standard test (Kottner & Balzer, 2010)); the instigation of preventative interventions in routine practice having an impact on instrument performance (Deeks, 1996; Defloor & Grypdonck, 2004; Gould et al., 2002) and; studies often only assess the predictive validity of one instrument, rather than multiple RAIs in the same study population to allow comparison (Ferrante di Ruffano, Hyde, Mccaffery, Bossuyt, & Deeks, 2012).
A major limitation of this traditional approach to PU‐RAI validation and evaluation, has been a failure to consider them as complex interventions where their delivery contains several interacting components (MRC, 2008) including the assessment itself, the potential outcomes and decisions about care interventions set in the context of complex healthcare environments. Recent complex intervention guidance advocates the use of process evaluations allowing causal mechanisms, contextual factors and the quality of implementation to be considered alongside clinical outcomes (Moore et al., 2015; MRC, 2008; Richards & Hallberg, 2015). Therefore in addition to predictive validity testing (alongside other RAIs), the ongoing evaluation of PURPOSE‐T will involve a realist process evaluation to identify and test theories and underlying assumptions regarding its use in practice to learn how it can be best used in different contexts, to enhance the probability of effectiveness. This will allow us to establish whether there is sufficient confidence that PURPOSE‐T can “reasonably be expected to have a worthwhile effect” (MRC, 2008) over a standard RAI and inform a potential future RCT.
5.1. Limitations
The sample included in this study were overwhelmingly Caucasian. The results, demonstrating the performance of PURPOSE‐T should not be applied to other groups without consideration of the importance of skin assessment as a core (and novel) element of this instrument and the potential differences in skin assessment for people with non‐white skin.
The level of “training” in PURPOSE‐T use, with expert nurses trained by the researcher which was then cascaded to local nurses was designed to replicate the roll‐out of tools to NHS nurses. However, the focussed training of the experts and their direct access to PURPOSE‐T development team may mean that the achieved level of competence might not necessarily be replicated in routine clinical practice.
6. CONCLUSION
PURPOSE‐T evaluation facilitated the initial validation and clinical usability of the instrument. The results indicate very good inter‐rater and retest reliability, high levels of data completion and moderate to high associations for convergent validity, when the same or similar constructs of PURPOSE‐T were compared with other PU‐RAIs.
The expert nurse field notes allowed the positive and negative aspects of using the instrument to be captured. These along with the other results were reviewed and some finals amendments were made to PURPOSE‐T. This culminated in the development of a new evidenced‐based RAI for use in adult populations and is now being used in clinical practice. Further evaluation of its impact on care processes and patient outcomes is planned.
CONFLICT OF INTEREST
No conflict of interest has been declared by the author(s).
AUTHOR CONTRIBUTIONS
All authors have agreed on the final version and meet at least one of the following criteria (recommended by the ICMJE [http://www.icmje.org/recommendations/]):
substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data;
drafting the article or revising it critically for important intellectual content.
ACKNOWLEDGEMENTS
Pressure Ulcer Research Service User Network (PURSUN). Clinical nurses who participated in the clinical evaluation.
Coleman S, Smith IL, McGinnis E, et al. Clinical evaluation of a new pressure ulcer risk assessment instrument, the Pressure Ulcer Risk Primary or Secondary Evaluation Tool (PURPOSE T). J Adv Nurs. 2018;74:407–424. https://doi.org/10.1111/jan.13444
Funding information
This publication presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (RP‐PG‐0407‐10056). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health
REFERENCES
- Andersen, K. E. , Jensen, O. , Kvorning, S. A. , & Bach, E. (1982). Prevention of pressure sores by identifying patients at risk. British Medical Journal Clinical Research Ed., 284, 1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeckman, D. , Matheï, C. , Van Lancker, A. , Van Houdt, S. , Vanwalleghem, G. , Gryson, L. , … Van Den Heede, K. A. (2013). A National Guideline for the prevention of pressure ulcers: Good Clinical Practice (GCP), KCE Reports 193C. Belgium: Belgian Health Care Knowledge Centre (KCE). [Google Scholar]
- Bennett, G. , Dealey, C. , & Posnett, J. (2004). The cost of pressure ulcers in the UK. Age and Ageing, 33, 230–235. [DOI] [PubMed] [Google Scholar]
- Bergstrom, N. , Braden, B. , Kemp, M. , Champagne, M. , & Ruby, E. (1998). predicting pressure ulcer risk: A multisite study of the predictive validity of the Braden scale. Nursing Research September/October, 47, 261–269. [DOI] [PubMed] [Google Scholar]
- Bergstrom, N. , Braden, B. J. , Laguzza, A. , & Holman, V. (1987). The braden scale for predicting pressure sore risk. Nursing Research, 36, 205–210. [PubMed] [Google Scholar]
- Berlowitz, D. , Vandeusen Lukas, C. , Parker, V. , Niederhauser, A. , Silver, J. , Logan, C. , & Ayello, E. (2011). Preventing pressure ulcers in hospitals: A toolkit for improving quality of care. Retrieved from http://www.ahrq.gov/research/ltc/pressureulcertoolkit/putool1.htm
- Bland, J. (2008). measuring health and disease: Cohen's kappa. England: Department of Health Sciences, University of York. [Google Scholar]
- Blazeby, J. , Sprangers, M. , Cull, A. , Groenvold, M. , & Bottomley, A. (2002). Guidelines for developing questionnaire modules, 3rd Edition Revised. http://groups.eortc.be/qol/sites/default/files/archives/guidelines_for_developing_questionnaire-_final.pdf (accessed 9 March 2015). EORTC Quality of Life Group.
- Braden, B. , & Bergstrom, N. (1987). A conceptual schema for the study of the etiology of pressure sores. Rehabilitation Nursing, 12, 8–16. [DOI] [PubMed] [Google Scholar]
- Briggs, M. , Collinson, M. , Wilson, L. , Rivers, C. , Mcginnis, E. , Dealey, C. , … Nixon, J. (2013). The prevalence of pain at pressure areas and pressure ulcers in hospitalised patients. BMC Nursing, 12, 19 http://www.biomedcentral.com/1472-6955/12/19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnand, B. , Kernan, W. N. , & Feinstein, A. R. (1990). Indexes and boundaries for “quantitative significance” in statistical decisions. Journal of Clinical Epidemiology, 43, 1273–1284. [DOI] [PubMed] [Google Scholar]
- Byrt, T. , Bishop, J. , & Carlin, J. B. (1993). Bias, prevalence and kappa. Journal of Clinical Epidemiology, 46, 423–429. [DOI] [PubMed] [Google Scholar]
- Cicchetti, D. , & Allison, T. (1971). A new procedure for assessing reliability of scoring EEG sleep recordings. American Journal of EEG Technology, 11, 101–110. [Google Scholar]
- Cohen, J. (1960). A coefficient of agreement for nominal scales. Educational and Psychological Measurement, 20, 37–46. [Google Scholar]
- Coleman, S. (2014). The development of a pressure ulcer risk assessment framework and minimum data set. England: PhD, University of Leeds. [Google Scholar]
- Coleman, S. , Gorecki, C. , Nelson, E. A. , Closs, S. J. , Defloor, T. , Halfens, R. , … Nixon, J. (2013). Patient risk factors for pressure ulcer development: Systematic review. International Journal of Nursing Studies, 50, 974–1003. [DOI] [PubMed] [Google Scholar]
- Coleman, S. , Nelson, E. A. , Keen, J. , Wilson, L. , Mcginnis, E. , Dealey, C. , … Nixon, J. (2014). Developing a pressure ulcer risk factor minimum data set and risk assessment framework. Journal of Advanced Nursing, 70, 2339–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, S. , Nixon, J. , Keen, J. , Muir, D. , Wilson, L. , Mcginnis, E. , … Nelson, E. A. (2016). Using cognitive pre‐testing methods in the development of a new evidenced‐based pressure ulcer risk assessment instrument. BMC Research Methodology, 16, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, S. , Nixon, J. , Keen, J. , Wilson, L. , Mcginnis, E. , Dealey, C. , … Nelson, E. A. (2014). A new pressure ulcer conceptual framework. Journal of Advanced Nursing, 70, 2222–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubbin, B. , & Jackson, C. (1991). Trial of a pressure area risk calculator for intensive therapy patients. Intensive Care Nursing, 7, 40–44. [DOI] [PubMed] [Google Scholar]
- Dealey, C. , Posnett, J. , & Walker, A. (2012). The cost of pressure ulcers in the United Kingdom. Journal of Wound Care, 21, 261–266. [DOI] [PubMed] [Google Scholar]
- Deeks, J. J. (1996). Pressure sore prevention: Using and evaluating risk assessment tools. British Journal of Nursing, 5(313–4), 316–320. [DOI] [PubMed] [Google Scholar]
- Defloor, T. , & Grypdonck, M. F. (2004). Validation of pressure ulcer risk assessment scales: A critique. Journal of Advanced Nursing, 48, 613–621. [DOI] [PubMed] [Google Scholar]
- FDA DHHS . (2009). Guidance for industry: Patient‐reported outcome measures: Use in medical product development to support labeling claims. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. [DOI] [PMC free article] [PubMed]
- Ferrante di Ruffano, L. , Hyde, C. J. , Mccaffery, K. J. , Bossuyt, P. M. M. , & Deeks, J. J. (2012). Assessing the value of diagnostic tests: A framework for designing and evaluating trials. BMJ, 344, 1–9. [DOI] [PubMed] [Google Scholar]
- Gorecki, C. , Brown, J. M. , Nelson, E. A. , Briggs, M. , Schoonhoven, L. , Dealey, C. , … European Quality of Life Pressure Ulcer Project, G. (2009). Impact of pressure ulcers on quality of life in older patients: A systematic review. Journal of the American Geriatrics Society, 57, 1175–1183. [DOI] [PubMed] [Google Scholar]
- Gorecki, C. , Nixon, J. , Madill, A. , Firth, J. , & Brown, J. M. (2012). What influences the impact of pressure ulcers on health‐related quality of life? A qualitative patient‐focused exploration of contributory factors. Journal of Tissue Viability, 21, 3–12. [DOI] [PubMed] [Google Scholar]
- Gould, D. , Goldstone, L. , Gammon, J. , Kelly, D. , & Maidwell, A. (2002). Establishing the validity of pressure ulcer risk assessment scales: A novel approach using illustrated patient scenarios. International Journal of Nursing Studies, 39, 215–228. [DOI] [PubMed] [Google Scholar]
- Kottner, J. , & Balzer, K. (2010). Do pressure ulcer risk assessment scales improve clinical practice. Journal of Multidisciplinary Healthcare, 3, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong, E. , Pang, S. , Wong, T. , Ho, J. , Shao‐Ling, X. , & Li‐Jun, T. (2005). Predicting pressure ulcer risk with the modified Braden, Braden and Norton scales in acute care hospitals in Mainland China. Applied Nursing Research, 18, 122–128. [DOI] [PubMed] [Google Scholar]
- Landis, J. R. , & Koch, G. G. (1977). The measurement of observer agreement for categorical data. Biometrics, 33, 159–174. [PubMed] [Google Scholar]
- Lindgren, M. , Unosson, M. , Krantz, A. M. , & Ek, A. C. (2002). A risk assessment scale for the prediction of pressure sore development: Reliability and validity. Journal of Advanced Nursing, 38, 190–199. [DOI] [PubMed] [Google Scholar]
- Mokkink, L. , Terwee, C. , Patrick, D. , Alonso, J. , Stratford, P. , Knol, D. , … De Vet, H. (2012). COSMIN checklist manual. Netherlands: EMGO Institute for Health and Care Research. [Google Scholar]
- Moore, G. F. , Audrey, S. , Barker, M. , Bond, L. , Bonell, C. , Hardeman, W. , … Baird, J. (2015). Process evaluation of complex interventions: Medical research council guidance. BMJ, 350, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MRC . (2000). A framework for development and evaluation of RCTs for complex interventions to improve health.
- MRC . (2008). Developing and evaluating complex interventions: New guidance In Council M. R. (Ed.), Process evaluation of complex interventions (pp. 1–134). London: UK Medical Research Council (MRC) guidance. MRC. [Google Scholar]
- NICE . (2014). Pressure ulcer prevention: The prevention and management of pressure ulcers in primary and secondary care, Clinical Guideline 179, Methods, evidence and recommendations. England: National Clinical Guideline Centre. [Google Scholar]
- Nixon, J. , & McGough, A. (2001). Principles of patient assessment: Screening for pressure ulcers and potential risk In Morison M. (Ed.), The prevention and treatment of pressure ulcers (pp. 17–36). Edinburgh: Mosby. [Google Scholar]
- Nixon, J. , Nelson, E. A. , Rutherford, C. , Coleman, S. , Muir, D. , Keen, J. , … Brown, J. M. (2015). Pressure UlceR Programme Of reSEarch (PURPOSE): Using mixed methods (systematic reviews, prospective cohort, case study, consensus and psychometrics) to identify patient and organisational risk, develop a risk assessment tool and patient‐reported outcome Quality of Life and Health Utility measures. Programme Grants for Applied Research, 3, 71–167. [PubMed] [Google Scholar]
- NPUAP/EPUAP/PPPIA . (2014). National pressure ulcer advisory panel, European pressure ulcer advisory panel and pan pacific pressure injury alliance. Prevention and treatment of pressure ulcers: Clinical practice guideline In Haesler E. (Ed.), Prevention and treatment of pressure ulcers: Clinical practice guideline as already indicated. (pp. 38–59). Obsborne Park Western Australia: Cambridge Media. [Google Scholar]
- Nunnally, J. , & Bernstein, I. (1994). Psychometric Theory. New York, NY: McGraw‐Hill. [Google Scholar]
- Page, K. N. , Barker, A. L. , & Kamar, J. (2011). Development and validation of a pressure ulcer risk assessment tool for acute hospital patients. Wound Repair Regen, 19, 31–37. [DOI] [PubMed] [Google Scholar]
- Pancorbo‐Hidalgo, P. L. , Garcia‐Fernandez, F. P. , Lopez‐Medina, I. M. , & Alvarez‐Nieto, C. (2006). Risk assessment scales for pressure ulcer prevention: a systematic review. Journal of Advanced Nursing, 54, 94–110. [DOI] [PubMed] [Google Scholar]
- Perneger, T. V. , Rae, A. C. , Gaspoz, J. M. , Borst, F. , Vitek, O. , & Heliot, C. (2002). Screening for pressure ulcer risk in an acute care hospital: Development of a brief bedside scale. Journal of Clinical Epidemiology, 55, 498–504. [DOI] [PubMed] [Google Scholar]
- Pieper, B. E. (2012). Pressure Ulcers: Prevalence, Incidence and Implications for the Future. Washinton DC: NPUAP. [Google Scholar]
- Pinkney, L. , Nixon, J. , Wilson, L. , Coleman, S. , Mcginnis, E. , Stubbs, N. , … Keen, J. (2014). Why do patients develop severe pressure ulcers? A retrospective case study. British Medical Journal Open, 4 http://bmjopen.bmj.com/content/4/1/e004303.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard, V. (1986). Pressure sores. Calculating the risk. Nursing Times, 82, 59–61. [PubMed] [Google Scholar]
- Richards, D. A. , & Hallberg, I. R. (2015). Complex interventions in health: An overview of research methods. London: Routledge Taylor and Francis Group. [Google Scholar]
- SAC (2002). Assessing health status and quality‐of‐life instruments: Attributes and review criteria. Quality of Life Research, 11, 193–205. [DOI] [PubMed] [Google Scholar]
- Schuurman, J.‐P. , Schoonhoven, L. , Defloor, T. , Van Engelshoven, I. , Van Ramshorst, B. , & Buskens, E. (2009). Economic evaluation of pressure ulcer care: A cost minimization analysis of preventive strategies. Nursing Economics, 27, 390–400. [PubMed] [Google Scholar]
- Severens, J. L. , Habraken, J. M. , Duivenvoorden, S. , & Frederiks, C. M. A. (2002). The cost of illness of pressure ulcers in The Netherlands. Advances in Skin & Wound Care, 15, 72–77. [DOI] [PubMed] [Google Scholar]
- Smith, I. L. , Nixon, J. , Brown, S. , Wilson, L. , & Coleman, S. (2016). Pressure ulcer and wounds reporting in NHS hospitals in England part 1: Audit of monitoring systems. Journal of Tissue Viability, 25, 3–15. [DOI] [PubMed] [Google Scholar]
- Steyerberg, E. W. (2010). Clinical prediction models: A practical approach to development, validation and updating. New York, NY: Springer. [Google Scholar]
- Suriadi, Sanada, H. , Sugama, J. , Thigpen, B. , Kitagawa, A. , Kinosita, S. , & Murayama, S. (2006). A new instrument for predicting pressure ulcer risk in an intensive care unit. Journal of Tissue Viability, 16, 21–26. [DOI] [PubMed] [Google Scholar]
- Suriadi, Sanada, H. , Sugama, J. , Thigpen, B. , & Subuh, M. (2008). Development of a new risk assessment scale for predicting pressure ulcers in an intensive care unit. Nursing in Critical Care, 13, 34–43. [DOI] [PubMed] [Google Scholar]
- Waterlow, J. (1985). Pressure sores: A risk assessment card. Nursing Times, 81, 49–55. [PubMed] [Google Scholar]
- Waterlow, J. (1988). The Waterlow card for the prevention and management of pressure sores: Towards a pocket policy. Care, Science and Practice, 6, 8–12. [Google Scholar]


