Abstract
Background:
Our aim was to follow up patients prescribed lurasidone over 1 year to determine factors predicting treatment persistence.
Methods:
We used noninterventional, observational, prospective follow up of patients consecutively prescribed lurasidone in a large inner-city NHS mental health trust. We also performed retrospective analysis of outcomes from patient case notes.
Results:
Data were available for 69 patients consecutively prescribed lurasidone, of whom three (4%) were lost to follow up. Out of the 66 patients not lost to follow-up, 21 (32%) remained on lurasidone at 1 year. The main reasons for discontinuation were perceived ineffectiveness (49% of discontinuers) and adverse effects (36% of discontinuers), whilst a further seven refused all treatment. Median treatment time on lurasidone was 154 days (95% confidence interval (CI), 33–275). Patients who were not treatment-resistant had a substantially reduced risk of discontinuation, relative risk (RR) 0.18 [95% CI 0.08, 0.41, p < 0.001]. Medium doses (>37–74 mg) of lurasidone reduced the risk of discontinuation by 75% [RR 0.25 (95% CI 0.11, 0.58, p = 0.001)]; high doses (>74–148 mg) reduced the risk of discontinuation by 86% [RR 0.14 (95% CI 0.06, 0.35, p < 0.001)]. Risk of discontinuation was approximately doubled when the reason for prescribing lurasidone was poor tolerability of prior treatment [RR 2.01 (95% CI 1.05, 3.85, p = 0.035)].
Conclusion:
The likelihood of treatment continuation with lurasidone can be vastly improved by targeting individuals most likely to benefit and by using optimal doses.
Keywords: antipsychotics, discontinuation, effectiveness, lurasidone, naturalistic, relapse
Introduction
Lurasidone is a second-generation antipsychotic (SGA), recently licensed for the treatment of schizophrenia in adults in the UK.1 Lurasidone is also approved for treating schizophrenia in adolescents2 and depressive episodes in bipolar 1 in the United States.3 It acts as a potent antagonist at D2, 5HT2a, and 5HT7 receptors.4 Lurasidone has a comparatively benign metabolic side effect profile, possibly related to a low affinity for H1 and 5HT2c receptors,4 and has been recommended as an alternative when hoping to avoid metabolic adverse events or weight gain.5 Efficacy has been shown in a number of short-term randomized controlled trials (RCTs) and a double-blind extension study.6 However, in a comparative network meta-analysis of RCTs, lurasidone was ranked as having a higher rate of drug discontinuation than any other SGA currently available in the UK.7 Higher rates of all-cause discontinuation have been shown in direct comparison with risperidone.8 So far, there has been limited exploration of how lurasidone fares in clinical practice. One study has reported lower rates of discontinuation with lurasidone compared with other SGAs when measuring health insurance administrative claims,9 but was unable to determine predictors of treatment continuation.
Although clinical trials can show the efficacy of antipsychotic treatment under specific controlled conditions, such conditions are unlikely to be replicated in practice. This can lead to difficulties in precisely predicting real-life efficacy and in identifying patient subgroups that benefit from treatment in practice. To our knowledge, the predictors of treatment continuation with lurasidone have not previously been determined elsewhere.
Procedure
Lurasidone was approved for use in our large inner-city NHS mental health trust in July 2014. Patients were not required to give informed consent to the study; the recording of patient details and monitoring of outcomes for this study was approved by the trust’s Drug and Therapeutics Committee, the locally designated approval committee for all noninterventional prescribing outcome surveys, and the analysis used anonymized clinical data. Information was sent to all prescribers, informing them that lurasidone could be prescribed for suitable patients on submission of an initiation form. The form included details about the patient (age, diagnoses), current medication, inpatient/outpatient status, reasons for prescribing and the starting dose of lurasidone. Treatment initiation was an independent prescribing decision without restrictions and all patients received care as normal. Our standard method had previously been designated (by our local ethical committee) as not requiring ethical committee approval, since treatment was not affected by our method and because data were anonymized. Those viewing patient-specific data as part of the data collection process were clinicians who could have seen these data as part of their normal working practice.
All patients consecutively prescribed lurasidone between August 2014 and August 2015 were included in the study. Each patient prescribed lurasidone was added to a register and the data provided in the initiation form was later confirmed using electronic patient medical notes and pharmacy electronic records. Further information was also collected at baseline, including patient demographics and clinical data, such as the duration of current illness and previous antipsychotic trials (number and type). Prior use of clozapine was confirmed. We also scrutinized notes to find out whether clozapine had previously been considered, but ultimately, not prescribed. Prior clozapine use or consideration of use was deemed to constitute evidence of treatment resistance. Aripiprazole is an already established treatment option with a low risk for metabolic side effects. Lurasidone might be considered as an alternative SGA in patients for whom aripiprazole has previously been ineffective or poorly tolerated. To examine the outcomes of patients who had previously discontinued aripiprazole, the reason for discontinuing aripiprazole was collected retrospectively. The highest dose of lurasidone reached by 1 year (in continuers) or prior to discontinuation (in discontinuers) was also collected (termed simply as ‘dose’ for the purpose of this study). Doses were categorised into low (⩽37 mg), medium (>37–74 mg) and high (>74 mg) dose groups, on the basis that a dosing range would be a more practical predictor to include for prescribing purposes.
Outcomes were collected retrospectively at least 1 year after the initiation date. All patient records were scrutinized for documented adverse effects (including when they first occurred in relation to the initiation date and the dose). When patients discontinued lurasidone, records were searched for the reasons for stopping treatment.
Statistical analysis
Baseline demographics and clinical data were summarized using descriptive statistics. Frequencies and percentages were calculated for categorical data. The main outcome of interest was time to discontinuation of lurasidone and this was determined as the date lurasidone was started until lurasidone was stopped or 1 year of treatment, whichever came soonest, and estimated using a Kaplan–Meier survival curve. Thus, the primary outcome was a measure of treatment continuation, a widely used outcome measure in observational studies,10 which has previously been shown to strongly correlate to clinical improvement as measured by the Clinical Global Impression Scale.11,12 Patients were categorized as censored if they remained on treatment at 1 year, including those who remained on lurasidone when lost to follow-up.
To determine significant variables on survival, log-rank tests were performed. If variables had a significant association with survival (p < 0.2) they were considered for inclusion in the multivariate analysis. Cox proportional hazard regression was performed to establish the most significant factors influencing discontinuation. The final model was selected based on the significance of the variable (p < 0.05) and the Akaike’s Information Criterion (AIC).13 Schoenfield residuals14 were plotted against time to assess for the proportional hazard assumption and Cox–Snell residual plots15 were used to assess goodness of fit. All tests were performed using STATA version 13 (StataCorp LP, College Station, Texas, USA).
Results
Patient characteristics
Full demographic and clinical data were available for the first 69 patients prescribed lurasidone, presented in Table 1. There were significant relationships between discontinuation and diagnosis, evidence of treatment resistance, and the number of previous antipsychotics trialled (Table 1).
Table 1.
Baseline characteristics.
| Cohort characteristics | Totala |
Continued (censored) |
Discontinued |
χ2 (d.f.) | p |
|---|---|---|---|---|---|
|
n = 69 |
n = 24 |
n = 45 |
|||
| n (%) | n (%) | n (%) | |||
| Gender | |||||
| Male | 35 (51) | 13 (37) | 22 (63) | 0.041 (1) | 0.839 |
| Female | 34 (49) | 11 (32) | 23 (68) | ||
| Age (years) | |||||
| 16–25 | 21 (30) | 5 (24) | 16 (76) | 0.169 (2) | 0.919 |
| 26–49 | 32 (46) | 12 (38) | 20 (63) | ||
| ⩾50 | 16 (23) | 7 (44) | 9 (56) | ||
| Ethnicity | |||||
| White | 27 (39) | 11 (41) | 16 (59) | 0.443 (2) | 0.801 |
| Black | 26 (38) | 8 (31) | 18 (69) | ||
| Other | 16 (23) | 5 (31) | 11 (69) | ||
| Diagnosis | |||||
| Schizophrenia | 34 (49) | 11 (32) | 23 (68) | 5.492 (3) | 0.139 |
| Schizoaffective disorder | 12 (17) | 2 (17) | 10 (83) | ||
| Bipolar affective disorder | 18 (26) | 7 (39) | 11 (61) | ||
| Other | 5 (7) | 4 (80) | 1 (20) | ||
| Duration of illness (years) | |||||
| 0–4 | 35 (51) | 12 (34) | 23 (66) | 3.373 (2) | 0.185 |
| 5–9 | 15 (22) | 4 (27) | 11 (73) | ||
| ≥10 | 19 (28) | 8 (42) | 11 (58) | ||
| Care setting at initiation | |||||
| Inpatient | 47 (68) | 15 (32) | 32 (68) | 0.091 (1) | 0.763 |
| Outpatient | 22 (32) | 9 (41) | 13 (59) | ||
| Previous clozapine trial | |||||
| Yes | 23 (33) | 7 (30) | 16 (70) | 1.359 (1) | 0.244 |
| No | 46 (67) | 17 (37) | 29 (63) | ||
| Treatment resistance | |||||
| Yes | 33 (48) | 8 (24) | 25 (76) | 4.594 (1) | 0.033 |
| No | 36 (52) | 16 (44) | 20 (56) | ||
| No. of previous antipsychotics | |||||
| 0–2 | 25 (36) | 13 (52) | 12 (48) | 7.527 (1) | 0.006 |
| >2 | 44 (64) | 11 (25) | 33 (75) | ||
| Started for clozapine augmentation | |||||
| Yes | 7 (10) | 4 (57) | 3 (43) | 0.484 (1) | 0.487 |
| No | 62 (90) | 20 (32) | 42 (68) |
Distributions within the total group are shown as column percentages.
In total, eight patients were not receiving regular antipsychotics at lurasidone initiation (4 of whom were treatment naïve), 17 received olanzapine, 11 aripiprazole, 10 quetiapine, 9 other oral atypicals, 5 oral conventional drugs, and 2 depot antipsychotics. Eight (12%) patients were receiving treatment with clozapine, of whom seven started lurasidone to augment clozapine, and one switched to lurasidone due to adverse effects on clozapine. Overall, the antipsychotic prescribed before lurasidone was not associated with discontinuation.
As a secondary, post hoc analysis, we also dichotomized outcome by prior treatment as either olanzapine or non-olanzapine. The median time on treatment for those switched from olanzapine (n = 17) was 89 days [95% confidence interval (CI), 0–187]. Compared with those switched from other antipsychotics, there was no statistically significant difference in time on treatment (χ2 = 0.21, d.f. = 1, p = 0.645).
The reasons for switching to lurasidone are given in Table 2. Prior poor tolerability of the previous antipsychotic was significantly (p = 0.181) associated with discontinuation (Table 2). In total, 47 (68%) patients had received aripiprazole at any point in their treatment history prior to starting lurasidone, of whom 33 (70%) discontinued aripiprazole due to perceived inefficacy, 11 (23%) due to adverse effects, and for 3 patients the reasons were not recorded. Overall, the recorded reason for discontinuing a previous aripiprazole trial was not significantly associated with lurasidone discontinuation (χ2 = 1.03, d.f. = 1, p = 0.310).
Table 2.
Reasons for switching to lurasidone.
| Reason for switching | Total |
Continued (censored) |
Discontinued |
χ2 (d.f.) | p |
|---|---|---|---|---|---|
|
n = 69 |
n = 24 |
n = 45 |
|||
| n (%) | n (%) | n (%) | |||
| Prior poor effectiveness | 34 (49) | 13 (38) | 21 (62) | 0.18 (1) | 0.671 |
| Prior poor tolerability | 25 (36) | 6 (24) | 19 (76) | 1.79 (1) | 0.181 |
| Other, including patient choice | 10 (14) | 5 (50) | 5 (50) | 1.21 (1) | 0.270 |
Discontinuation
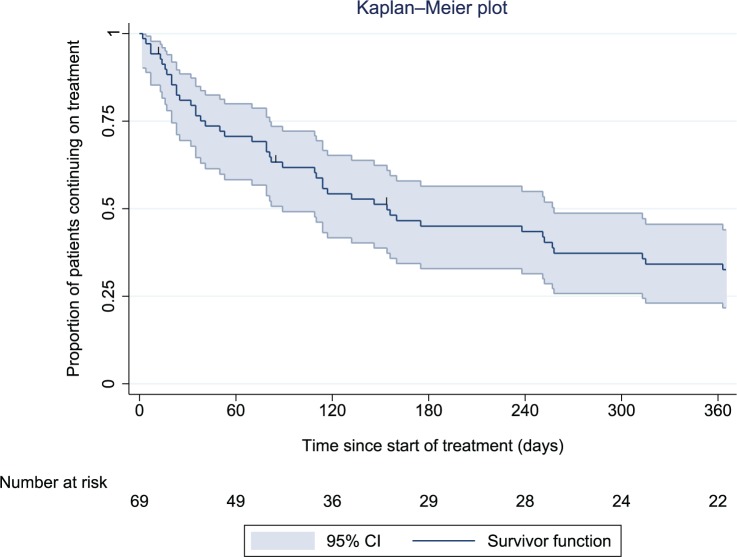
The main outcome was continuation with lurasidone at 1 year. In total, 45 patients (65%) were known to have discontinued lurasidone at 1 year, and 3 (4%) were lost to follow up (all 3 left the trust). The remaining 21 patients (30%) were confirmed as completing 1 year of continuous treatment [21 of 66 (32%) of those not lost to follow-up]. Median survival time on lurasidone for the whole cohort was 154 days (95% CI, 33–275) (Figure 1).
Figure 1.
Kaplan–Meier plot showing the proportion of patients (with 95% confidence interval) prescribed lurasidone over time since treatment initiation.
CI, confidence interval.
The most common reasons for discontinuation were perceived inefficacy in 22 patients (49%) and adverse effects in 16 (36%), whilst 7 (16%) refused all treatment with oral medications. After discontinuing lurasidone, 43 of 45 patients switched to an alternative antipsychotic. Overall, 15 (33%) of discontinuing patients were switched to olanzapine, 9 (20%) clozapine, 6 (13%) aripiprazole, 5 (11%) quetiapine, 4 (9%) other oral atypicals, 3 (7%) depot antipsychotics, and 2 (4%) oral conventional drugs. Two (4%) patients were not switched to an antipsychotic; one commenced a non-antipsychotic mood stabilizer, and one ceased all treatment.
Amongst patients all defined as treatment-resistant, 20 of 33 (60%) patients stopped because of inefficacy. Of the 26 treatment-resistant patients not using lurasidone for clozapine augmentation, 24 (85%) discontinued treatment by 1 year.
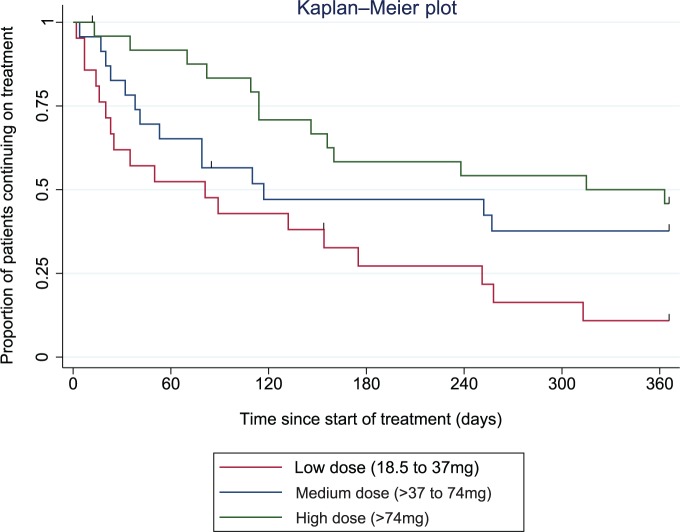
Dose
The mean starting dose of lurasidone was 31 mg [standard deviation (SD) 13.0] and there was no statistical difference between those who continued and discontinued treatment (p = 0.808). Patients were categorized into low (⩽37 mg), medium (>37–74 mg), and high (>74 mg) dose groups, as presented in Table 3, according to the highest dose of lurasidone reached before outcome. The dose of lurasidone was significantly associated with treatment discontinuation (Table 3, Figure 2). Of those discontinuing lurasidone due to perceived inefficacy (n = 22), 6 (27%) patients received low doses of lurasidone, 6 (27%) medium doses, and 10 (45%) high doses.
Table 3.
Dose of lurasidone prescribed (n = 69).
| Dose range of lurasidone | Total |
Continued (censored) |
Discontinued |
χ2 (d.f.) | p |
|---|---|---|---|---|---|
|
n = 69 |
n = 24 |
n = 45 |
|||
| n (%) | n (%) | n (%) | |||
| Low dose (18.5–37 mg) | 21 (30) | 3 (14) | 18 (86) | 8.718 (2) | 0.013 |
| Medium dose (>37–74 mg) | 23 (33) | 9 (39) | 14 (61) | ||
| High dose (>74–148 mg) | 25 (36) | 12 (48) | 13 (52) |
Figure 2.
Kaplan–Meier plot showing the proportion of patients prescribed lurasidone over time since treatment initiation, by the dose of lurasidone.
Adverse effects
The adverse effects leading to lurasidone discontinuation are summarized in Table 4. The most common adverse effects were acute movement disorders, reported on average 111 days after initiation at a mean dose of 34 mg (SD 21.6); akathisia/agitation 65 days after initiation at 44mg (SD 18.5); and insomnia 49 days after initiation at 31mg (SD 21.4). Of those patients discontinuing lurasidone due to adverse effects (n = 16), 9 (56%) received low doses, 6 (38%) medium doses, and 1 (6%) received high-dose treatment.
Table 4.
Adverse effects leading to lurasidone discontinuation (n = 16).
| Adverse effect | n a |
|---|---|
| Acute movement disorderb | 6 |
| Akathisia/agitation | 4 |
| Insomnia | 3 |
| Nausea | 2 |
| Irritability | 2 |
| Unclear or unspecified | 1 |
| Abdominal pain | 1 |
| Sedation | 1 |
| Anxiety | 1 |
| Reduced appetite | 1 |
| Allergy | 1 |
Some patients reported more than one adverse effect.
Parkinsonism, n = 4; dystonia, n = 1; oculogyric crisis, n = 1.
Predictors of discontinuation
The variables included in the Cox regression model included: patient diagnosis, duration of illness, whether or not there was evidence of treatment resistance, the number of prior antipsychotics, the reason for initiation, and the dose of lurasidone. Three variables were significantly associated with treatment discontinuation (Table 5). The results of the model estimated that not being treatment-resistant at initiation was associated with an 82% decrease in the hazard of discontinuing treatment compared with being treatment-resistant (p < 0.001). Prior poor tolerability of the antipsychotic prescribed immediately before lurasidone was significantly associated with an estimated 101% increase in the risk of discontinuation (p = 0.035). Medium and high doses of lurasidone were associated with a 75% and 86% decrease in the hazard of discontinuing treatment compared with low doses (p = 0.001, p < 0.001), respectively.
Table 5.
Cox model regression results.
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| No evidence of treatment resistance | 0.531 | 0.294, 0.960 | 0.036 | 0.184 | 0.083, 0.407 | <0.001 |
| Prior poor tolerabilitya | 1.492 | 0.825, 2.700 | 0.185 | 2.009 | 1.049, 3.845 | 0.035 |
| Dose of lurasidone at outcomeb | 0.017 | <0.001 | ||||
| Medium dosec | 0.532 | 0.264, 1.075 | 0.079 | 0.254 | 0.112, 0.575 | 0.001 |
| High dosec | 0.370 | 0.181, 0.755 | 0.005 | 0.142 | 0.058, 0.347 | <0.001 |
Versus other reasons for switching.
p value for whole variable.
Versus low doses.
HR, hazard ratio; CI, confidence interval.
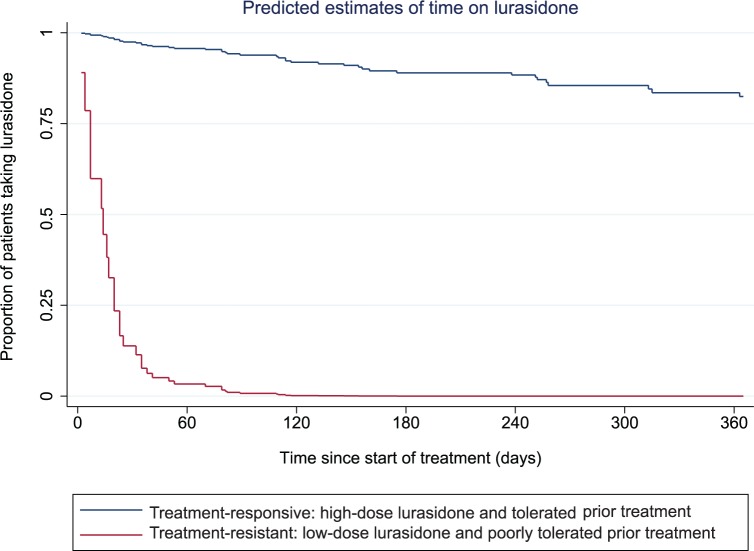
No other variable was statistically associated with discontinuation. The predicted estimates of time on lurasidone treatment at best and worse conditions are presented in Figure 3 using the multivariate model of predictors of treatment continuation and discontinuation.
Figure 3.
Predicted estimates (using the Cox regression model) of time on lurasidone*.
*Multivariate regression estimates.
Discussion
The main finding in this study of an early cohort prescribed lurasidone is that 65% discontinued treatment by 1 year. The majority of discontinuers stopped treatment owing to perceived inefficacy, which might be explained by the unusually high proportion of treatment-resistant patients. Higher doses of lurasidone were a positive predictive factor for treatment continuation. Treatment resistance and prior poor treatment tolerability were negative predictive factors. Outcomes for patients with a specific combination of moderating factors were predicted to be substantially improved: around 80% of patients without treatment resistance that tolerated their prior treatment and received the higher doses of lurasidone would complete 1 year of treatment. The proportion of patients continuing at 1 year with treatment resistance who poorly tolerated their prior treatment and received the lower doses of lurasidone would be close to zero. Adverse effects were broadly in line with those previously documented in pooled study data.6
Our findings provide direction to improve treatment outcomes with lurasidone, which begins with selecting patients most likely to benefit from treatment. There may be a temptation to prescribe new antipsychotics like lurasidone to treatment-refractory patients (just under half of this early cohort had evidence of treatment resistance) who have not tolerated prior antipsychotics. Outcomes for these patients were poor. Continuation with treatment will likely be improved by using lurasidone in treatment-responsive patients. The rationale for switching to lurasidone after prior poor treatment tolerability should be closely assessed. The main comparative advantage of lurasidone is an improved cardiometabolic side-effect profile, yet most of those switched due to prior poor tolerability (15 of 25) were for noncardiometabolic reasons. Most patients in this study were switched to lurasidone during inpatient admissions, and can reasonably be assumed to be in an acute phase of their illness. Better outcomes have been reported in nonacute-phase outpatients after switching lurasidone from a broad range of antipsychotics, with notable improvements in metabolic parameters.16,17 Prior poor treatment tolerability may be a clinically useful reason for switching to lurasidone, particularly for those with cardiometabolic concerns.
Our findings also have value in optimizing the use of lurasidone. The use of lurasidone in doses higher than 37 mg (the recommended starting dose) strongly predicted treatment continuation. Higher doses did not appear to compromise tolerability; discontinuations due to adverse effects were in fact more common with lower doses (9 of 18) than medium (6 of 14) and high (1 of 13) doses. Our observation is confounded by the fact that doses tend to increase as treatment persists; that is, continuation with treatment could be likely to predict higher doses. However, our findings are in line recent RCT evidence that demonstrated lurasidone early nonresponders benefit from dose escalation as early as 2 weeks after initiation without compromising tolerability.18 In patients switching to lurasidone from other antipsychotics, starting doses of 74 mg/day appear to be well tolerated.16 Patients in our low-dose group (18.5 mg to 37 mg/day) presumably reflect those who were not subjected to dose escalation after being commenced on the recommended starting dose of 37 mg/day. Continuation with treatment is likely to be improved by reviewing patients on lower doses of lurasidone (18.5–37 mg/day) for dose escalation where possible.
Limitations
This was a naturalistic observational study in which prescribing practices were not influenced. There were no controls on treatment differences between continuers and discontinuers. Although a number of potentially influencing factors were taken into account, there are others for which we have no information. Our results represent local outcomes in our particular clinical environment; one in which the use of lurasidone was not restricted. As such, the generalizability of our results in other healthcare systems is difficult to establish. The study sample size was relatively small; a larger sample could have allowed risk factors that are more moderately associated with treatment continuation to be demonstrated. There was no direct measure of clinical effectiveness, although a clear relationship between treatment continuation and clinical improvement has previously been demonstrated.10,11,19
Conclusion
The rates of treatment continuation with lurasidone are likely to be improved by targeting those most likely to benefit from treatment (patients without evidence of treatment-resistance) and by early dose optimization.
Footnotes
Funding: This research was funded by an unrestricted grant from Sunovion. The sponsor had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The sponsor was shown a final draft of the report before submission to allow for the alerting of factual errors regarding their product. No changes were suggested. IJO and SM declare no competing interests.
Conflict of interest statement: This research was funded by an unrestricted grant from Sunovion. DT has received speaker honoraria from Janssen, Servier, Otsuka, Lundbeck and Eli Lilly.
ORCID iD: Ian J. Osborne  http://orcid.org/0000-0002-5412-1452
http://orcid.org/0000-0002-5412-1452
Contributor Information
Ian J. Osborne, Pharmacy Department, Maudsley Hospital, Denmark Hill, London, SE5 8AZ, UK
Shubhra Mace, Pharmacy Department, Maudsley Hospital, London, UK Institute of Pharmaceutical Science, King’s College, London, UK.
David Taylor, Maudsley Hospital, Pharmacy Department, Denmark Hill, London SE5 8AZ, UK Institute of Pharmaceutical Science, King’s College, London, 5th Floor, Franklin-Wilkins Building, 150 Stamford Street, London SE1 9NH, UK.
References
- 1. Dainippon Sumitomo Pharma Co. Ltd. Dainippon Sumitomo Pharma and Takeda announce the European marketing authorization for Latuda® (lurasidone), http://www.ds-pharma.com/ir/news/pdf/ene20140331_1.pdf (2014, accessed 23 June 2017).
- 2. Dainippon Sumitomo Pharma Co. Ltd. Sunovion’s Latuda® (lurasidone HCl) receives FDA approval to treat adolescents with schizophrenia, http://www.ds-pharma.com/ir/news/pdf/ene20170128.pdf (2017, accessed 23 June 2017).
- 3. Dainippon Sumitomo Pharma Co. Ltd. Sunovion Pharmaceuticals Inc. Announces FDA approval of Latuda® (lurasidone HCl) as monotherapy and adjunctive therapy in adult patients with bipolar depression, http://www.ds-pharma.com/ir/news/pdf/ene20130629.pdf (2013, accessed 23 June 2017).
- 4. Ishibashi T, Horisawa T, Tokuda K, et al. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther 2010; 334: 171–181. [DOI] [PubMed] [Google Scholar]
- 5. Scottish Medicines Consortium. Lurasidone (Latuda®), SMC No. (994/14), http://www.scottishmedicines.org.uk/files/advice/lurasidone__Latuda__FINAL_Sept_2014_amended_15.09.14_for_website.pdf (2014, accessed 23 June 2017).
- 6. European Medicines Agency. Assessment report – Latuda, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002713/WC500164684.pdf (2014, accessed 23 June 2017).
- 7. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382: 951–962. [DOI] [PubMed] [Google Scholar]
- 8. Citrome L, Cucchiaro J, Sarma K, et al. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol 2012; 27: 165–176. [DOI] [PubMed] [Google Scholar]
- 9. Rajagopalan K, Wade S, Meyer N, et al. Real-world adherence assessment of lurasidone and other oral atypical antipsychotics among patients with schizophrenia: an administrative claims analysis. Curr Med Res Opin 2017; 33: 813–820. [DOI] [PubMed] [Google Scholar]
- 10. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353: 1209–1223. [DOI] [PubMed] [Google Scholar]
- 11. Taylor DM, Young C, Patel MX. Prospective 6-month follow-up of patients prescribed risperidone long-acting injection: factors predicting favourable outcome. Int J Neuropsychopharmacol 2006; 9: 685–694. [DOI] [PubMed] [Google Scholar]
- 12. Guy W. ECDEU assessment manual for psychopharmacology. Rev ed. Rockville: National Institute of Mental Health, 1976, pp.157–169. [Google Scholar]
- 13. Akaike H. Maximum likelihood identification of Gaussian autoregressive moving average models. Biometrika 1973; 60: 255–265. [Google Scholar]
- 14. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81: 515–526. [Google Scholar]
- 15. Collett D. Modelling survival data in medical research. Boca Raton, FL: CRC Press, 2015. [Google Scholar]
- 16. McEvoy JP, Citrome L, Hernandez D, et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry 2013; 74: 170–179. [DOI] [PubMed] [Google Scholar]
- 17. Citrome L, Weiden PJ, McEvoy JP, et al. Effectiveness of lurasidone in schizophrenia or schizoaffective patients switched from other antipsychotics: a 6-month, open-label, extension study. CNS Spectr 2014; 19: 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loebel A, Silva R, Goldman R, et al. Lurasidone dose escalation in early nonresponding patients with schizophrenia: a randomized, placebo-controlled study. J Clin Psychiatry 2016; 77: 1672–1680. [DOI] [PubMed] [Google Scholar]
- 19. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006; 163: 611–622. [DOI] [PubMed] [Google Scholar]