Abstract
Posterior condylar canal dural arteriovenous fistula (PCC dAVF) is a rare entity with only three cases having been reported so far in the English literature. We describe the clinical presentation, imaging, and endovascular management of an elderly man with left PCC dAVF presenting with subarachnoid haemorrhage (SAH). Endovascular management of such cases requires thorough understanding of the vascular anatomy around the craniovertebral junction (CVJ) and variable bridging vein draining patterns. The fistula in our case was fed by the posterior meningeal branch of the left vertebral artery and was draining through a dilated and tortuous medullary bridging vein into the antero-lateral pontomedullary venous system. Transarterial glue embolisation was performed with complete exclusion of the fistula and venous pouches. The patient developed intractable hiccough and left-sided facial pain on the second post-procedural day, and MRI showed focal diffusion restriction in the left dorso-lateral medulla. He recovered completely after a short course of steroids.
Keywords: Posterior condylar canal, dural arteriovenous fistula (dAVF), medulla bridging vein, subarachnoid haemorrhage (SAH), glue embolisation, transarterial
Introduction
Posterior condylar canal dural arteriovenous fistula (PCC dAVF) draining via the medullary bridging vein (BV) is an extremely rare type of intracranial dural AVF, and only three cases have been reported so far to the best of our knowledge. Clinical presentations depend on the pattern of venous drainage/reflux causing either a benign feature like pulsatile tinnitus or an aggressive feature such as subarachnoid haemorrhage (SAH) or spinal venous congestion symptoms (quadriparesis) or a combination thereof. 1 Here we report a case of a PCC BV-draining dAVF presenting with SAH, treated successfully with transarterial glue embolisation. We also review the literature on this rare entity and describe the challenges associated with its management.
Case report
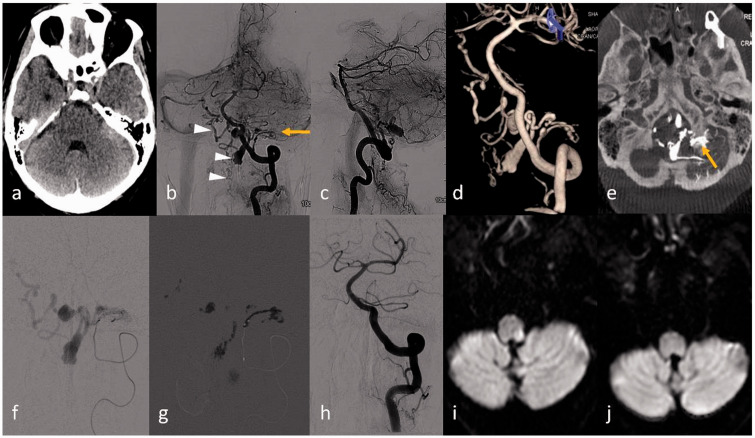
A 72-year-old man presented with sudden-onset severe headache, vomiting and neck rigidity to our emergency department. Non-contrast computed tomography (NCCT) of the head (Figure 1(a)) revealed SAH in the left cerebello-pontine angle cistern and bilateral lateral ventricles. Intra-arterial diagnostic cerebral angiography (Figure 1(b) and (c)) showed arterio-venous shunting with multiple dilated tortuous venous channels in the left side of the craniovertebral junction (CVJ) region. Rotational three-dimensional (3D) angiographic images (Figure 1(d)) clearly depicted a left PCC dAVF fed by a single feeding artery via the posterior meningeal branch of the left V3 vertebral artery. Multiplanar reconstruction (MPR) reformatted images of the rotational 3D data helped to exactly localise the site of the fistula to the left PCC (Figure 1(e)). The venous drainage was through the ipsilateral lateral medullary BV emptying into the anterior medullary-anterior pontomesencephalic (AM-APM) longitudinal venous axis and finally into the contralateral superior petrosal sinus via the petrosal vein. Mild venous reflux was also noted into the upper cervical anterior spinal venous plexus. Multiple venous pouches with focal segmental stenosis were noted along the course of the draining veins. Note was made of a left anterior inferior cerebellar artery (AICA)-posterior inferior cerebellar artery (PICA) complex and hypoplastic right vertebral artery terminating as PICA.
Figure 1.
(a) Non-contrast computed tomography (NCCT) of the head showing focal subarachnoid haemorrhage (SAH) in the left cerebello-pontine angle. (b), (c) Left vertebral artery angiogram antero-posterior and lateral views revealed dural arteriovenous fistula (AVF) (arrow) in the left lateral aspect of the foramen magnum fed by the posterior meningeal branch of the left V3 vertebral artery and venous drainage through the medulla bridging vein with reflux into the contralateral anterior medullary-anterior pontomesencephalic (AM-APM) venous system (arrowhead) and anterior spinal vein (arrowhead). (d) Three-dimensional (3D) image depicts better anatomy of the dural AVF; (e) multiplanar reconstruction (MPR) reformatted from the 3D source image shows the exact site of the fistula at the left posterior condylar canal (arrow) with venous pouch at the dorsal aspect of the foramen magnum. (f) Microcatheter injection through the feeding artery and (g) glue cast in the fistula and also into the draining venous pouches are shown. (h) Control angiogram through the left vertebral artery revealed complete obliteration of the AVF. Diffusion-weighted imaging (DWI) revealed focal restricted diffusion along the left dorso-lateral medulla (i), resolved in the subsequent DWI image (j) performed after 10 days.
Treatment
Transarterial embolisation of the fistula via the left posterior meningeal artery was planned. Through a co-axial system with a 6F guiding catheter (Envoy; Cordis Neurovascular), an initial attempt of using a flow-directed microcatheter (Marathon; Covidien, Mansfield, MA, USA) to navigate to the fistula was not successful due to acute angulation of the origin of the feeding artery. Thereafter, a braided microcatheter (Eschelon 10; Covidien) commonly used for coiling was subsequently tried. With the assistance of a balloon catheter (Hyperglide balloon 4 × 15 mm; Covidien) placed just distal to the origin of the feeder in the vertebral artery, the microcatheter could be successfully navigated into the feeding artery until closer to the fistula (Figure 1(f) – microcatheter injection). Dilute glue (N-butyl-2-cyanoacrylate; NBCA) at a concentration of 25% was slowly injected into the fistulous site, allowing adequate penetration up to the venous pouch (Figure 1(g) – glue cast), also taking due care not to allow any significant reflux into the parent artery. Control angiogram showed total exclusion of the fistula and venous pouch (Figure 1(h)). No significant periprocedural complication was observed. On the second post-procedural day the patient developed intractable hiccoughs and left-sided facial pain. Magnetic resonance imaging (MRI) revealed thrombosis of the draining vein and the venous pouches with a small focal area of T2-weighted (T2W)/fluid-attenuated inversion recovery (FLAIR) hyperintensity and diffusion restriction in the left dorso-lateral medulla (Figure 1(i)) adjacent to one of the venous pouches. This abnormal signal intensity was suspected to be due to a focal ischaemic infarction due to device manipulation and the balloon catheter parked at the V4 vertebral artery during the procedure. However, with the delayed onset of symptoms (second post-procedural day) and spontaneous recovery with short-course steroids, the possibility of venous sac thrombosis inducing reactive inflammation and or oedema was also considered. A repeat MRI performed 10 days after the procedure revealed near complete resolution of the abnormal signal intensity in the left dorso-lateral medulla (Figure 1(j) – diffusion-weighted imaging (DWI)). Follow-up digital subtraction angiography performed after six months revealed no residual dAVF or abnormal veins (images not shown).
Discussion
Dural AVFs in and around the CVJ are rare and pose great diagnostic and management challenges due to the highly complex vascular anatomy of the region. 2 Among them also, PCC dAVF draining via a medulla BV are extremely rare. Mitsuhashi et al. first used the term medulla bridging vein-draining dAVFs for dAVFs around the CVJ. 3 The BVs draining the vicinity of the medulla develop embryologically through annexation of the ventral myelencephalic vein, hypoglossal vein, and first cervical intersegmental vein. They may also drain into veins in the hypoglossal canal, marginal sinus, jugular bulb and suboccipital cavernous sinus. Developmentally, these BVs around the medulla are considered homologs of the lateral emissary bridging veins of the spine. 3 These medulla BVs connect the APM-anterior medullary longitudinal venous system (AMV), which collects venous drainage from the brainstem and cerebellum to either the marginal sinus, suboccipital sinus or to the jugular bulb respectively depending on the location. 4 In dAVF of the CVJ, these BVs may cause venous reflux retrogradely into the APM-AMV and subsequently drain into multiple different venous systems, i.e. into the galenic group via the vein of Galen, the petrosal group into the superior petrosal sinuses, and the tentorial group into the torcular herophili or spinal through the anterior spinal venous plexus. 3 On the other hand, these dAVFs can also drain anterogradely directly into the extradural venous systems mentioned above (marginal sinus, jugular bulb, etc). 5 Depending on the location and pattern of venous drainage, presenting clinical features may differ, including SAH, myelopathy, brainstem dysfunction, radiculopathy, and cranial nerve palsy. 2
In our case, the lateral medulla BV which drained the PCC dAVF was seen refluxing into the AM-APM longitudinal venous system with multiple venous pouches and stenosis, which subsequently emptied into the superior petrosal sinus via the petrosal vein. Mild reflux was also noted inferiorly into the anterior spinal venous plexus in the delayed phase of the angiogram. BV-draining dAVF can be associated with high incidence of aggressive presentation, like SAH or paraparesis due to spinal venous congestion. SAH is the common presentation when there is venous reflux into the tortuous and ectatic long leptomeningeal veins of the AM-APM longitudinal venous system, which are prone to rupture from high systemic arterial pressure of the fistula, while quadriparesis or symptoms of spinal venous congestion are likely if there is inferior venous reflux into the anterior spinal venous plexus of the cervical spine. 3 The case reported by Mondel et al. 1 had a similar presentation and venous drainage pattern as ours; however, the arterial feeding pattern was different: Our case demonstrated a single arterial feeder from the posterior meningeal branch of the left vertebral artery as against multiple feeding arteries from ascending pharyngeal and bilateral vertebral arteries in theirs. Tinnitus was the main clinical presentation in the cases reported by Kiyosue et al. 6 and Maus et al., 7 in which the posterior condylar vein which drained the AVF empties directly into the suboccipital sinus and sigmoid sinus without BV vein reflux, thus explaining the presentation.
dAVF may be treated by endovascular, microsurgical or radiosurgical approaches. Microsurgical treatment of DAVF at this location is technically difficult. Radiosurgery is less preferred due to a long waiting period for adequate results, and radiation at this location may be associated with complications of cranial nerve injury. Endovascular management via transarterial, transvenous or a combined approach may be adopted depending on the angioarchitecture and venous drainage pattern. The transvenous approach is best suited when an easily accessible draining vein of the fistula is available, as was in the cases described by Kiyosue et al. 6 and Maus et al. 7 It was considered difficult and risky in our case as the draining veins involved are tortuous, stenotic and ectatic leptomeningeal veins. Complications in this approach may include intracranial haemorrhage due to wire perforation, cranial nerve injury, or incomplete occlusion of the fistula. Hence, a transarterial approach was adopted in our case with distal catheterisation of the fistula and embolisation with dilute glue to achieve maximum penetration into the fistula and venous pouches. Nevertheless, a transarterial route may also be associated with difficult navigation into the small and tortuous arterial feeders as in our case, vasospasm, arterial injury, embolic material reflux into the parent artery and its complications, inadequate penetration of embolic agent into the fistula and embolisation into normal pial arteries, etc. A thorough knowledge of the angioarchitecture, venous anatomy and variable BV draining patterns at the CVJ are the key for successfully treating such rare cases.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Mondel PK, Saraf R, Limaye US. Acute subarachnoid hemorrhage in posterior condylar canal dural arteriovenous fistula: Imaging features with endovascular management. J Neurointerv Surg 2015; 7: e26. [DOI] [PubMed] [Google Scholar]
- 2.Zhao J, Xu F, Ren J, et al. Dural arteriovenous fistulas at the craniocervical junction: A systematic review. J Neurointerv Surg 2016; 8: 648–653. [DOI] [PubMed] [Google Scholar]
- 3.Mitsuhashi Y, Aurboonyawat T, Pereira VM, et al. Dural arteriovenous fistulas draining into the petrosal vein or bridging vein of the medulla: Possible homologs of spinal dural arteriovenous fistulas. Clinical article. J Neurosurg 2009; 111: 889–899. [DOI] [PubMed] [Google Scholar]
- 4.Kiyosue H, Tanoue S, Sagara Y, et al. The anterior medullary-anterior pontomesencephalic venous system and its bridging veins communicating to the dural sinuses: Normal anatomy and drainage routes from dural arteriovenous fistulas. Neuroradiology 2008; 50: 1013–1023. [DOI] [PubMed] [Google Scholar]
- 5.Spittau B, Millán DS, El-Sherifi S, et al. Dural arteriovenous fistulas of the hypoglossal canal: Systematic review on imaging anatomy, clinical findings, and endovascular management. J Neurosurg 2015; 122: 883–903. [DOI] [PubMed] [Google Scholar]
- 6.Kiyosue H, Okahara M, Sagara Y, et al. Dural arteriovenous fistula involving the posterior condylar canal. Am J Neuroradiol 2007; 28: 1599–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maus V, Söderman M, Rodesch G, et al. Endovascular treatment of posterior condylar canal dural arteriovenous fistula. J Neurointerv Surg 2017; 9: e7. [DOI] [PubMed] [Google Scholar]