Abstract
Objective
The objective of this study is to test if patients’ health-related quality of life (HRQoL) declines after prostate biopsy to detect Pca, and after subsequent treatment decision-making in case Pca is confirmed, and to test whether personality state and traits are associated with these potential changes in HRQoL.
Methods
Patients who were scheduled for prostate biopsy to detect Pca (N = 377) filled out a baseline questionnaire about HRQoL (EORTC QLQ-C30 and PR25), “big five” personality traits (BFI-10), optimism (LOT-r), and self-efficacy (Decision Self-efficacy Scale) (t0). Patients with confirmed Pca (N = 126) filled out a follow-up questionnaire on HRQoL within 2 weeks after treatment was chosen but had not yet started (t1).
Results
HRQoL declined between t0 and t1, reflected in impaired role and cognitive functioning, and elevated fatigue, constipation, and prostate-specific symptoms. Sexual activity and functioning improved. Baseline HRQoL scores were unrelated to the selection of a particular treatment, but for patients who chose a curative treatment, post-decision HRQoL showed a greater decline compared to patients who chose active surveillance. Optimism was associated with HRQoL at baseline; decisional self-efficacy was positively associated with HRQoL at follow-up. No associations between HRQoL and the “big five” personality traits were found.
Conclusion
Patients who have undergone prostate biopsy and treatment decision-making for Pca experience a decline in HRQoL. Choosing treatment with a curative intent was associated with greater decline in HRQoL. Interventions aimed at optimism and decision self-efficacy could be helpful to reduce HRQoL impairment around the time of prostate biopsy and treatment decision-making.
Keywords: Cancer, Oncology, Prostate cancer, Diagnosis, HRQoL, Decision-making, Optimism, Decisional self-efficacy
Background
An aging population and increased use of prostate cancer (Pca) screening contribute to a growth in Pca detection in The Netherlands and other Western countries [1–3]. When Pca is suspected, patients undergo prostate biopsy [4]. In The Netherlands only, at least 25,000 Dutch men undergo this procedure every year, resulting in approximately 10,000 Pca diagnoses (Netherlands Cancer Registry, 2015) [5]. The largest proportion of Pca diagnoses consist of localized cancer (stage I or II), for which surgery, radiotherapy (either brachy or external beam), and active surveillance (AS) are seen as equally acceptable treatments [4, 6]. However, adverse effects from treatment can impair patients’ health-related quality of life (HRQoL) [7–10]. Common side effects from treatments with curative intent (surgery, radiotherapy) include sexual, urinary, and bowel-related complaints [9, 11], while AS can increase anxiety symptoms due to postponing treatment [12, 13]. Therefore, impact on HRQoL is an important factor when considering treatment options [14–16].
Changes in HRQoL after Pca treatment are well described and generally consist of a major decline in HRQoL in the first 1–2 years after treatment [9, 17–19]. Besides the consequences of treatment, changes in HRQoL are related to psychological factors. Optimism and self-efficacy are associated with better HRQoL outcomes, while anxiety, depression, and personality traits (e.g., neuroticism, distress) are associated with worse HRQoL outcomes [20–23]. However, most of these studies measured HRQoL from diagnosis onwards, lacking a pre-diagnosis baseline to also capture the psychological burden from prostate biopsy, receiving a Pca diagnosis, and treatment selection. Studies that did take a pre-diagnosis baseline focused on aspecific (older) patient population and did not measure immediately before and after diagnosis [24, 25].
To increase our understanding about the impact of Pca on HRQoL, including receiving a Pca diagnosis and choosing treatment, this study measured HRQoL pre-biopsy and post-treatment decision-making. Our hypothesis was that a significant decline in HRQoL would already appear prior to treatment onset from the psychological burden of diagnosis and treatment decision-making. Moreover, we expected changes in HRQoL would be associated with psychological factors (personality traits, optimism, and self-efficacy).
Methods
Participants and recruitment
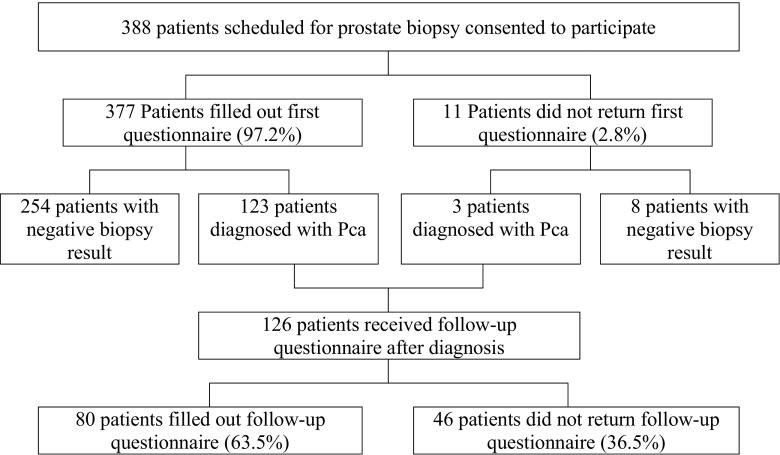
Between January 2013 and May 2014, ten Dutch hospitals participated in this study and recruited 388 patients who were scheduled for a first prostate biopsy due to suspected Pca (M age = 66.5, SD = 6.6; Fig. 1). A host hoc power analysis revealed that this sample size was sufficient to achieve a power of 0.80 for detecting differences with an effect size from Cohen’s d = 0.2 (with alpha 0.05). During consultation, patients were informed that the goal of the study was to investigate quality of care in prostate examination and quality of life of patients undergoing this procedure. Together with an information letter, patients received the first questionnaire (t0) on paper and a pre-stamped envelope to return the questionnaire. Follow-up questionnaires were sent to patients whose biopsy result confirmed Pca. These patients received this second questionnaire and a pre-stamped envelope at their home address within 2 weeks after treatment decision-making (t1). Diagnosis and the moment of treatment decision-making were monitored for all included patients from their (electronic) medical record. After review of the study protocol, the medical ethics review board of the initiating hospital waived the need for formal ethical approval (reference 2012.103) and all participating hospitals approved conducting the study. All patients signed an informed consent.
Fig. 1.
Patient flow
Questionnaires
Demographics and clinical data
Participants were asked to indicate their age, education, marital status, last known prostate-specific antigen (PSA) level, and choice of treatment. PSA levels were asked at both t0 and t1 to control for the possibility that treatment had already taken place before completing the t1 questionnaire.
Health-related quality of life
HRQoL was measured with the Dutch version of the EORTC QLQ-C30 questionnaire, which assesses functional HRQoL aspects (physical, role, cognitive, emotional, and social functioning and global health) and symptoms common for cancer patients (fatigue, nausea, pain, dyspnea, sleep disturbance, appetite loss, constipation, diarrhea, and financial impact) [26]. The prostate cancer-specific EORTC QLQ-PR25 module was added to assess prostate cancer-specific (urinary, bowel, and hormonal) symptoms and (sexual) functioning [27]. Scale reliability was low for the bowel and hormonal symptoms, and sexual activity subscale (alpha’s 0.50–0.60), and adequate (alpha ≥ 0.70) for all other subscales. Similar scale reliability scores have been found earlier [27].
Psychological factors
As possible moderating variables, three measures for individual differences measures were included. First, the Big Five Inventory-10 (BFI-10) was included to measure extraversion, agreeableness, conscientiousness, neuroticism, and openness, also known as the “big five” personality traits [28]. The BFI-10 was included in t0. With only two items per trait, low reliability scores were found (α < 0.50), which is common for this scale [29]. A subsequent confirmatory factor analysis confirmed five underlying factors, with each set of two items per trait yielding highest factor loadings.
Secondly, dispositional optimism, a generalized expectation that good things will happen, was assessed with the Life Orientation Test-Revised (LOT-R) [30]. Some minor textual adjustments were made to an existing and previously validated Dutch version of the LOT-R [31]. Scale reliability was sufficient (α = 0.67).
Thirdly, the Decision Self-Efficacy Scale was used as a subjective measure of the perceived ability to make a healthcare decision [32]. Rather than focusing on one specific decision, the goal of this scale was to measure feelings of self-confidence in a healthcare setting. The scale was included at t0 to measure a person’s baseline decisional self-efficacy before the distress from diagnosis. In the absence of an existing and validated Dutch version of this scale, a forward-backward translation was made by two researchers and the result was evaluated and consented on by two other researchers who were not involved to the translation. Scale reliability was good (α = 0.85).
Statistical analysis
Descriptive statistics are presented as means and standard deviations (SD) for continuous variables and as frequencies and percentages for categorical variables. Mean HRQoL scores at t0 were compared to the scores obtained at t1 using paired-samples t tests. The association between personality traits and HRQoL scores was assessed using bivariate correlation analyses (Pearson’s). Linear regression modeling was carried out with global health as dependent variable and personality characteristics as independent variables, controlling for age, education, PSA levels, and diagnosis (dummy variable; for t0 only). All analyses were performed using SPSS version 22.0 (Statistical Package for Social Sciences, Chicago, IL, USA). p values < 0.05 were considered statistically significant.
Results
Three hundred and 88 patients gave informed consent of which 377 patients completed the first questionnaire (t0, response rate 97.2%). All patients whose biopsy confirmed Pca (n = 126 patients, 32%) received the follow-up questionnaire (t1, response rate 63%) (Fig. 1). There were no statistically significant differences in demographics between patients with cancer and patients without cancer at t0, between responders at t0 and t1, or between responders and non-responders at t1. Patient demographics are presented in Table 1.
Table 1.
Demographics
| t0—no cancer (N = 254) | t0—Pca (N = 123) | t1—Pca (N = 80) | |
|---|---|---|---|
| Age at inclusion | |||
| ≤ 65 years | 106 (44%) | 40 (33%) | 24 (30%) |
| 66–75 years | 115 (48%) | 73 (60%) | 50 (63%) |
| ≥ 76 years | 20 (8%) | 9 (7%) | 5 (6%) |
| Education | |||
| Low | 109 (43%) | 48 (39%) | 31 (39%) |
| Medium | 60 (24%) | 37 (30%) | 25 (31%) |
| High | 78 (31%) | 36 (29%) | 23 (29%) |
| Other/not specified | 4 (2%) | 2 (2%) | 1 (1%) |
| Current occupation | |||
| Employed | 70 (28%) | 28 (23%) | 15 (19%) |
| Not employed | 183 (72%) | 93 (77%) | 64 (81%) |
| Partnership | |||
| Partner | 224 (89%) | 115 (94%) | 74 (95%) |
| No partner | 28 (11%) | 7 (6%) | 5 (6%) |
| Children | |||
| Yes | 228 (91%) | 118 (96%) | 77 (96%) |
| No | 24 (9%) | 5 (4%) | 3 (4%) |
| Prostate-specific antigen (PSA) | |||
| ≤ 5 ng/ml | 42 (17%) | 19 (16%) | 19 (25%) |
| 5.01–10 ng/ml | 125 (49%) | 59 (48%) | 37 (49%) |
| ≥ 10.01 ng/ml | 85 (34%) | 44 (36%) | 20 (26%) |
| Selected treatment | |||
| Active surveillance | 26 (34%) | ||
| Radical prostatectomy | 22 (29%) | ||
| Radiotherapy | 28 (37%) | ||
Numbers do not always add up to the same total due to item non-response. Differences between groups did not reach statistical significance
Health-related quality of life
At the pre-biopsy baseline (t0), HRQoL did not differ between patients whose biopsy result confirmed Pca and patients with a negative biopsy result (Table 2). After receiving diagnosis and treatment decision-making (t1), patients reported worse role and cognitive functioning and more symptoms (fatigue, constipation, urinary, bowel, and hormonal). Sexual activity and functioning improved after treatment were chosen (all with p < 0.05; Table 2).
Table 2.
HRQoL scores
| No Pca | Pca | |||
|---|---|---|---|---|
| t0 (N = 254) | t0 (N = 123) | t1 (N = 80) | ||
| HRQoL core | Mean (SD) | Mean (SD) | Mean (SD) | Mean difference (t1−t0)1 |
| Global health | 83.5 (14.8) | 83.7 (15.4) | 80.7 (16.1) | − 3.0 |
| Physical functioning | 94.2 (10.4) | 94.3 (10.1) | 92.8 (12.7) | −1.5 |
| Role functioning | 94.7 (14.9) | 96.0 (12.9) | 86.1 (24.2) | − 9.9*** |
| Emotional functioning | 85.3 (16.0) | 85.0 (16.8) | 83.4 (19.9) | − 1.6 |
| Cognitive functioning | 91.2 (15.0) | 92.3 (12.5) | 88.9 (16.9) | − 3.4* |
| Social functioning | 95.0 (13.9) | 96.2 (10.1) | 93.9 (14.3) | − 2.3 |
| Fatigue | 11.4 (17.3) | 10.7 (15.5) | 17.0 (22.3) | 6.3** |
| Nausea/vomiting | 1.0 (4.7) | 1.1 (5.2) | 2.4 (11.9) | 1.3 |
| Pain | 6.8 (15.8) | 5.8 (12.6) | 9.4 (19.8) | 3.6 |
| Dyspnoea | 7.7 (16.9) | 6.5 (15.3) | 6.8 (17.3) | 0.3 |
| Insomnia | 14.4 (23.8) | 13.8 (21.5) | 15.0 (25.6) | 1.2 |
| Appetite loss | 1.9 (8.2) | 2.0 (7.9) | 4.7 (16.8) | 2.7 |
| Constipation | 1.7 (8.0) | 4.2 (12.7) | 7.7 (20.0) | 3.5* |
| Diarrhea | 4.0 (13.4) | 3.4 (11.0) | 6.8 (18.9) | 3.4 |
| Financial difficulties | 2.6 (12.3) | 0.8 (5.3) | 2.6 (12.9) | 1.8 |
| Prostate specific | ||||
| Urinary symptoms | 15.9 (13.4) | 13.3 (11.8) | 17.6 (15.6) | 4.3* |
| Bowel symptoms | 3.0 (6.3) | 2.7 (5.8) | 5.6 (10.5) | 2.9** |
| Hormonal symptoms | 3.5 (5.8) | 3.8 (5.8) | 7.0 (9.4) | 3.2*** |
| Sexual activity | 63.1 (21.6) | 61.5 (22.2) | 65.4 (21.3) | 3.9** |
| Sexual functioning | 22.9 (20.3) | 23.4 (19.6) | 34.5 (24.0) | 11.1* |
All scales are 0–100; for functioning subscales, full functioning is represented by a score of 100; for symptoms, absence of symptoms is represented by a score of 0. All comparisons at t0 between patients with and without cancer were non-significant
1Paired comparison t1 vs t0 (N = 70)
*p < 0.05
**p < 0.01
***p < 0.001
Treatment choice
In case Pca was detected, symptoms and functioning reported prior to biopsy (t0) were not associated with selection of a particular treatment. At the time point after treatment decision-making (t1), men who chose a curative treatment reported reduced functioning and more symptoms compared to men who selected AS (Table 3). No associations were found between treatment choice and personality characteristics (data not shown).
Table 3.
HRQoL changes grouped per treatment decision
| AS N = 23 |
Curative treatment (RP or RT) N = 38 |
|||
|---|---|---|---|---|
| t0 Mean (SD) |
t1 Mean (SD) |
t0 Mean (SD) |
t1 Mean (SD) |
|
| HRQoL core | ||||
| Global health | 86.4 (15.8) | 87.9 (10.5) | 81.4 (14.4) | 75.0 (18.8) |
| Physical functioning | 93.9 (10.1) | 94.2 (10.7) | 93.3 (13.2) | 92.3 (15.3) |
| Role functioning | 97.0 (9.8) | 97.0 (9.8) | 95.5 (16.0) | 79.7 (29.3)** |
| Emotional functioning | 89.8 (14.5) | 92.0 (13.0) | 85.1 (17.7) | 77.6 (23.7)* |
| Cognitive functioning | 90.5 (13.5) | 92.1 (10.2) | 91.2 (12.1) | 85.5 (20.9)* |
| Social functioning | 93.1 (11.0) | 99.2 (3.6)* | 96.8 (8.6) | 90.5 (18.7)* |
| Fatigue | 10.1 (12.8) | 8.6 (12.8) | 10.5 (16.1) | 21.6 (26.8)** |
| Nausea/vomiting | 3.0 (8.4) | 2.3 (5.9) | 0 (0.0) | 16.5 (2.7) |
| Pain | 5.3 (14.9) | 3.0 (8.4) | 7.0 (14.3) | 15.4 (25.8) |
| Dyspnoea | 7.6 (14.9) | 6.1 (16.7) | 7.9 (19.7) | 8.8 (20.0) |
| Insomnia | 9.1 (15.2) | 6.1 (16.7) | 16.7 (24.2) | 22.8 (31.1) |
| Appetite loss | 4.5 (11.7) | 1.5 (7.1) | 2.6 (9.1) | 8.8 (22.8) |
| Constipation | 1.5 (7.1) | 1.5 (7.1) | 4.4 (13.8) | 13.2 (26.3)* |
| Diarrhea | 3.0 (9.8) | 3.0 (9.8) | 4.4 (11.4) | 11.4 (24.8) |
| Financial difficulties | 0.0 (0.0) | 1.5 (7.1) | 0.9 (5.4) | 3.5 (17.0) |
| Prostate specific | ||||
| Urinary symptoms | 19.3 (12.9) | 14.1 (10.4) | 10.3 (8.7) | 19.0 (18.4)** |
| Bowel symptoms | 2.2 (6.3) | 2.2 (4.5) | 3.1 (5.2) | 8.6 (13.4)* |
| Hormonal symptoms | 3.9 (4.9) | 5.6 (6.7) | 3.0 (4.9) | 6.4 (10.0)* |
| Sexual activity | 60.9 (27.3) | 63.0 (18.1) | 58.6 (20.3) | 68.0 (20.9)* |
| Sexual functioning | 25.0 (17.9) | 23.8 (19.6) | 22.2 (16.4) | 29.6 (18.4) |
All scales are 0–100; for functioning subscales, full functioning is represented by a score of 100; for symptoms, absence of symptoms is represented by a score of 0
AS active surveillance, RP radical prostatectomy, RT radiotherapy
*p < 0.05
**p < 0.01
Psychological variables
Prior to biopsy (t0), optimism was a significant predictor for global health (B = .31, p < .001). After receiving diagnosis and treatment decision-making (t1), a positive association was found between global health and decisional self-efficacy (B = 0.29, p = .04). Of the big five traits, extraversion (B = 0.14, p = .03) and neuroticism (B = − 0.17, p = .01) were significant predictors for global health at t0; no relations were found at t1.
Discussion
This study investigated the HRQoL impacts of undergoing prostate biopsy, receiving Pca diagnosis, and choosing treatment. Prior to prostate biopsy, when Pca is suspected but not yet confirmed, HRQoL was similar between patients who were later confirmed to have Pca and patients without Pca. When a Pca diagnosis was received, and treatment was chosen but had not yet started, patients reported more symptoms and reduced functioning compared to the pre-biopsy baseline. HRQoL at baseline did not predict treatment choice, but patients who chose a curative treatment instead of AS reported more symptoms and reduced functioning compared to patients who chose AS. Overall global health at baseline was related to optimism; after diagnosis and treatment selection, an association with decisional self-efficacy was found.
HRQoL outcomes
Differences in HRQoL between patients who selected curative treatment over AS are not surprising. Men eligible for AS could be expected to be in a more favorable condition compared to men who need (immediate) curative treatment [33]. However, it is remarkable that most HRQoL differences were not present in our sample at baseline but were only reported after diagnosis and treatment selection. Moreover, the highest level of urinary symptoms at t0 was reported by men who later selected AS, while after the treatment decision was made, most symptoms were reported by men who selected a curative treatment. Therefore, changes in HRQoL appear to be influenced by the impact of diagnosis and treatment decision-making, rather than by changes in the patient’s physical condition. Possibly, the Pca diagnosis made men more aware of their symptoms and led them to attribute their overall condition more to their disease. Increased symptom burden and impaired functioning at t1 could also be explained by cognitive dissonance reduction [34]; consequently of a finalized treatment decision, men could be motivated to justify this decision as being the right one. This could have resulted in a revised HRQoL evaluation at t1 to make it consonant with the characteristics that would fit to the selected treatment [35, 36]. If biopsy itself caused a decline in HRQoL, all patients should have reported lower HRQoL at t1, while this was only the case for patients who chose a curative treatment, patients from the AS group even reported (non-significant) improvements [37].
Earlier studies on physical and psychological outcomes in Pca patients highlighted the perceived masculinity threat men could experience [38, 39]. This threat affects how men cope with their condition and the perceived threat could cause a further decline of HRQoL after treatment. Although most of the work on masculinity threats in Pca patients focused on post-treatment outcomes, it is likely that this perceived threat is already present from diagnosis onwards. In our results, reduced role functioning and increased sexual functioning (compensatory behavior) could be indicative for the presence of a masculinity threat [40, 41].
Personality factors
Optimism and decisional self-efficacy were associated with better global health; this is in line with previous research that found optimism and decisional self-efficacy to be associated with less distress and better coping [21, 42]. In the current study, patients scoring higher on optimism report better HRQoL prior to biopsy, when Pca was suspected but not yet confirmed. After diagnosis, and a treatment decision was required, optimism seemed to play less of a role and decisional self-efficacy, the subjective feeling of being able to take the right action, making good decisions, and to ask questions, was positively associated with HRQoL. This adds to previous findings about knowledgeable (and therefore possibly more self-efficated) patients reporting better HRQoL [43].
Instead of focusing on a single trait (e.g., neuroticism), this study investigated a broader spectrum of the big five personality traits. At t0, extraversion and neuroticism were related to global health, while at t1, no relations were present anymore. Hence, we found no evidence of a moderating role of specific traits affecting changes in HRQoL. Another explanation could be that the brief measure we used was not sensitive enough to also detect statistically significant differences in the smaller t1 sample. Future studies should use more extensive measures to investigate this relation in more detail.
Study limitations
Some limitations need to be discussed. First, no detailed clinical data about tumor stage was available, and PSA was self-reported by participants. However, patients were only eligible for inclusion if Pca was suspected, following pre-biopsy screening (rectal examination and PSA testing). Therefore, we were still able to sample a homogeneous patient population. And although we had no registration of the number of patients refusing participation, the average Pca detection rate in our sample was similar to what was expected based on literature [5]. Secondly, dropout of men without Pca diagnosis and non-response at t1 led to a limited number of patients per treatment group available for further analyses. Moreover, the comparison between t1 and t0 on group level had sufficient power; however, the subgroup comparisons were lacking power. As we found no statistically significant differences in patient characteristics between responders and non-responders, we estimate the risk for selection bias was low. Our results should therefore be seen as exploratory findings on the development of HRQoL in Pca patients with a pre-diagnosis baseline. Follow-up studies preferably use larger samples.
Future studies
Based on the changes in HRQoL we found in this study, future studies should focus on determining the impact of the individual aspects of undergoing biopsy, receiving Pca diagnosis, and selecting treatment. Compared to the current design, this would require an additional measurement in between receiving diagnosis and making a treatment decision.
Furthermore, the current study did not follow up on patients with a negative biopsy result. To have a complete comparison of HRQoL after prostate biopsy, post-biopsy HRQoL should also be compared between patients with a positive and patients with a negative biopsy result. Recently, a prospective study found similar HRQoL before and after diagnosis between Pca patients on AS and a non-cancer control group, indicating HRQoL of patients on AS is similar to that of patients without cancer [44]. However, it would be interesting to investigate if decisional self-efficacy is still associated with HRQoL outcomes when no treatment decision has to be made.
Clinical implications
This study emphasizes the impact of undergoing prostate biopsy, receiving a Pca diagnosis, and selecting treatment. Clinicians should be aware that optimism and decisional self-efficacy are associated with HRQoL prior to treatment onset. To ensure that optimism does not backfire post-treatment, it is important to ensure accurate risk perceptions in patients about the chances of treatment success and the occurrence of treatment side effects. Interventions to stimulate shared decision-making, like decision aids, could be helpful for achieving this, as well as to contribute to patients’ decisional self-efficacy levels [45].
Conclusion
So far, most studies investigating HRQoL in Pca patients have focused on the impact of treatment, while neglecting the psychological burden caused by diagnosis and the treatment selection process. This study showed that prior to treatment onset, patients reported reduced functioning, more symptoms, and lower overall global health, in particular if a curative treatment was selected. During clinical counseling, managing optimism when Pca is suspected (before and after biopsy) and (decisional) self-efficacy when Pca is confirmed could help to reduce the pre-treatment impact on HRQoL.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Contributor Information
Maarten Cuypers, Email: M.Cuypers@uvt.nl.
Romy E. D. Lamers, Email: R.Lamers@etz.nl
Erik B. Cornel, Email: E.Cornel@zgt.nl
Lonneke V. van de Poll-Franse, Email: L.vandePoll@iknl.nl
Marieke de Vries, Email: Marieke.deVries@ru.nl.
Paul J. M. Kil, Email: P.Kil@etz.nl
References
- 1.Arnold M, Karim-Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: analysis of the European cancer observatory. Eur J Cancer. 2015;51(9):1164–1187. doi: 10.1016/j.ejca.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–EE86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Cremers RGHM, Karim-Kos HE, Houterman S, Verhoeven RHA, Schröder FH, van der Kwast TH, et al. Prostate cancer: trends in incidence, survival and mortality in the Netherlands, 1989–2006. Eur J Cancer. 2010;46(11):2077–2087. doi: 10.1016/j.ejca.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 4.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Lane BR, Zippe CD, Abouassaly R, Schoenfield L, Magi-Galluzzi C, Jones JS. Saturation technique does not decrease cancer detection during follow up after initial prostate biopsy. J Urol. 2008;179(5):1746–1750. doi: 10.1016/j.juro.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 6.Sobin LH, Fleming IDTNM. Classification of malignant tumors. Cancer. 1997;80(9):1803–1804. doi: 10.1002/(SICI)1097-0142(19971101)80:9<1803::AID-CNCR16>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Pardo Y, Guedea F, Aguiló F, Fernández P, Macías V, Mariño A, et al. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment. J Clin Oncol. 2010;28(31):4687–4696. doi: 10.1200/JCO.2009.25.3245. [DOI] [PubMed] [Google Scholar]
- 8.Smith DP, King MT, Egger S, Berry MP, Stricker PD, Cozzi P et al (2009) Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ 339 [DOI] [PMC free article] [PubMed]
- 9.Punnen S, Cowan JE, Chan JM, Carroll PR, Cooperberg MR. Long-term health-related quality of life after primary treatment for localized prostate cancer: results from the CaPSURE Registry. Eur Urol. 2015;68(4):600–608. doi: 10.1016/j.eururo.2014.08.074. [DOI] [PubMed] [Google Scholar]
- 10.Drummond FJ, Kinnear H, O’Leary E, Donnelly GA, Sharp L. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, your experience) study. J Cancer Surviv. 2015;9(2):361–372. doi: 10.1007/s11764-014-0419-6. [DOI] [PubMed] [Google Scholar]
- 11.Mols F, Korfage IJ, Vingerhoets AJJM, Kil PJM, Coebergh JWW, Essink-Bot M-L, et al. Bowel, urinary, and sexual problems among long-term prostate cancer survivors: a population-based study. Int J Radiat Oncol Biol Phys. 2009;73(1):30–38. doi: 10.1016/j.ijrobp.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011;29(27):3669–3676. doi: 10.1200/JCO.2011.34.9738. [DOI] [PubMed] [Google Scholar]
- 13.Bellardita L, Valdagni R, van den Bergh R, Randsdorp H, Repetto C, Venderbos LDF, et al. How does active surveillance for prostate cancer affect quality of life? A systematic review. Eur Urol. 2015;67(4):637–645. doi: 10.1016/j.eururo.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Hamdy FC, Donovan JL. Patient-reported outcomes following treatment for localized prostate cancer: helping decision making for patients and their physicians. JAMA. 2017;317(11):1121–1123. doi: 10.1001/jama.2017.1703. [DOI] [PubMed] [Google Scholar]
- 15.Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375(15):1425–1437. doi: 10.1056/NEJMoa1606221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen RC, Basak R, Meyer A, et al. Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. JAMA. 2017;317(11):1141–1150. doi: 10.1001/jama.2017.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang GJ, Sadetsky N, Penson DF. Health related quality of life for men treated for localized prostate cancer with long-term follow up. J Urol. 2010;183(6):2206–2212. doi: 10.1016/j.juro.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Namiki S, Arai Y. Health-related quality of life in men with localized prostate cancer. Int J Urol. 2010;17(2):125–138. doi: 10.1111/j.1442-2042.2009.02437.x. [DOI] [PubMed] [Google Scholar]
- 19.Penson DF, Litwin MS, Aaronson NK. Health related quality of life in men with prostate cancer. J Urol. 2003;169(5):1653–1661. doi: 10.1097/01.ju.0000061964.49961.55. [DOI] [PubMed] [Google Scholar]
- 20.Allison PJ, Guichard C, Fung K, Gilain L. Dispositional optimism predicts survival status 1 year after diagnosis in head and neck cancer patients. J Clin Oncol. 2003;21(3):543–548. doi: 10.1200/JCO.2003.10.092. [DOI] [PubMed] [Google Scholar]
- 21.Curtis R, Groarke A, Sullivan F. Stress and self-efficacy predict psychological adjustment at diagnosis of prostate cancer. Sci Rep. 2014;4:5569. doi: 10.1038/srep05569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Victorson DE, Schuette S, Schalet BD, Kundu SD, Helfand BT, Novakovic K et al (2016) Factors affecting quality of life at different intervals after treatment of localized prostate cancer: unique influence of treatment decision making satisfaction, personality and sexual functioning. J Urol 196(5):1422–1428 [DOI] [PubMed]
- 23.Punnen S, Cowan JE, Dunn LB, Shumay DM, Carroll PR, Cooperberg MR. A longitudinal study of anxiety, depression and distress as predictors of sexual and urinary quality of life in men with prostate cancer. BJU Int. 2013;112(2):E67–E75. doi: 10.1111/bju.12209. [DOI] [PubMed] [Google Scholar]
- 24.Reeve BB, Potosky AL, Smith AW, Han PK, Hays RD, Davis WW, et al. Impact of cancer on health-related quality of life of older Americans. J Natl Cancer Inst. 2009;101(12):860–868. doi: 10.1093/jnci/djp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reeve BB, Stover AM, Jensen RE, Chen RC, Taylor KL, Clauser SB, et al. Impact of diagnosis and treatment of clinically localized prostate cancer on health-related quality of life for older Americans. Cancer. 2012;118(22):5679–5687. doi: 10.1002/cncr.27578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 27.van Andel G, Bottomley A, Fosså SD, Efficace F, Coens C, Guerif S, et al. An international field study of the EORTC QLQ-PR25: a questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur J Cancer. 2008;44(16):2418–2424. doi: 10.1016/j.ejca.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 28.Rammstedt B, John OP. Measuring personality in one minute or less: a 10-item short version of the Big Five Inventory in English and German. J Res Pers. 2007;41(1):203–212. doi: 10.1016/j.jrp.2006.02.001. [DOI] [Google Scholar]
- 29.Rammstedt B, Beierlein C. Can’t we make it any shorter? J Individ Differ. 2014;35(4):212–220. doi: 10.1027/1614-0001/a000141. [DOI] [Google Scholar]
- 30.Glaesmer H, Rief W, Martin A, Mewes R, Brähler E, Zenger M, et al. Psychometric properties and population-based norms of the Life Orientation Test Revised (LOT-R) Br J Health Psychol. 2012;17(2):432–445. doi: 10.1111/j.2044-8287.2011.02046.x. [DOI] [PubMed] [Google Scholar]
- 31.Ten Klooster P, Weekers A, Eggelmeijer F, Van Woerkom J, Drossaert C, Taal E, et al. Optimisme en/of pessimisme: factorstructuur van de Nederlandse Life Orientation Test-Revised. Psychologie en Gezondheid. 2010;38(2):89–100. doi: 10.1007/BF03089356. [DOI] [Google Scholar]
- 32.O’Connor AM (2002) User manual—Decision Self-Efficacy Scale Ottawa: Ottawa Hospital Research Institute (OHIR). Available from: https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decision_SelfEfficacy.pdf
- 33.van den Bergh RCN, Ahmed HU, Bangma CH, Cooperberg MR, Villers A, Parker CC. Novel tools to improve patient selection and monitoring on active surveillance for low-risk prostate cancer: a systematic review. Eur Urol. 2014;65(6):1023–1031. doi: 10.1016/j.eururo.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Festinger L. A theory of cognitive dissonance. Stanford: Univer. Press; 1957. [Google Scholar]
- 35.Sprangers MAG, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med. 1999;48(11):1507–1515. doi: 10.1016/S0277-9536(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 36.Gruppen LD, Margolin J, Wisdom K, Grum CM. Outcome bias and cognitive dissonance in evaluating treatment decisions. Acad Med. 1994;69(10):S57–S59. doi: 10.1097/00001888-199410000-00042. [DOI] [PubMed] [Google Scholar]
- 37.Nomura T, Fukuda Y, Sakamoto S, Nasu N, Tasaki Y. Comprehensive evaluation of the health-related quality of life after ultrasound-guided prostate needle biopsy: a prospective study. Andrology (Los Angel) 2016;5(160):2167–0250.1000160. [Google Scholar]
- 38.Mróz LW, Oliffe JL, Davison BJ. Masculinities and patient perspectives of communication about active surveillance for prostate cancer. Health Psychol. 2013;32(1):83–90. doi: 10.1037/a0029934. [DOI] [PubMed] [Google Scholar]
- 39.Hoyt MA, Stanton AL, Irwin MR, Thomas KS. Cancer-related masculine threat, emotional approach coping, and physical functioning following treatment for prostate cancer. Health Psychol. 2013;32(1):66–74. doi: 10.1037/a0030020. [DOI] [PubMed] [Google Scholar]
- 40.Holmes DS. Compensation for ego threat: two experiments. J Pers Soc Psychol. 1971;18(2):234–237. doi: 10.1037/h0030850. [DOI] [PubMed] [Google Scholar]
- 41.Babl JD. Compensatory masculine responding as a function of sex role. J Consult Clin Psychol. 1979;47(2):252–257. doi: 10.1037/0022-006X.47.2.252. [DOI] [PubMed] [Google Scholar]
- 42.Orom H, Nelson CJ, Underwood W, Homish DL, Kapoor DA. Factors associated with emotional distress in newly diagnosed prostate cancer patients. Psycho-Oncology. 2015;24(11):1416–1422. doi: 10.1002/pon.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orom H, Biddle C, Underwood W, Nelson CJ, Homish DL. What is a “good” treatment decision? Decisional control, knowledge, treatment decision making, and quality of life in men with clinically localized prostate cancer. Med Decis Mak. 2016;36(6):714–725. doi: 10.1177/0272989X16635633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pham KN, Cullen J, Hurwitz LM, Wolff EM, Levie KE, Odem-Davis K, et al. Prospective quality of life in men choosing active surveillance compared to those biopsied but not diagnosed with prostate cancer. J Urol. 2016;196(2):392–398. doi: 10.1016/j.juro.2016.02.2972. [DOI] [PubMed] [Google Scholar]
- 45.Durand M-A, Carpenter L, Dolan H, Bravo P, Mann M, Bunn F, et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PLoS One. 2014;9(4):e94670. doi: 10.1371/journal.pone.0094670. [DOI] [PMC free article] [PubMed] [Google Scholar]