Abstract
Purpose
Mild-to-moderate bone pain is a commonly reported adverse event (AE) associated with pegfilgrastim. We evaluated the effect of prophylactic naproxen or loratadine vs no prophylactic treatment on pegfilgrastim-associated bone pain.
Methods
In this open-label study (NCT01712009), women ≥ 18 years of age with newly diagnosed stage I–III breast cancer and an ECOG performance status ≤ 2 who were planning ≥ 4 cycles of adjuvant or neoadjuvant chemotherapy with pegfilgrastim support starting in cycle 1 were randomized 1:1:1 to receive naproxen, loratadine, or no treatment to prevent pegfilgrastim-associated bone pain. The primary endpoint was all-grade bone pain in cycle 1 from AE reporting. Secondary endpoints included bone pain in cycles 2–4 and across all cycles from AE reporting and patient-reported bone pain by cycle and across all cycles.
Results
Six hundred patients were enrolled. Most patients (83.0%) were white, and mean (SD) age was 54.2 (11.1) years. The percentage of patients with all-grade bone pain in cycle 1 from AE reporting in the naproxen, loratadine, and no prophylaxis groups was 40.3, 42.5, and 46.6%, respectively; differences between the treatment groups were not statistically significant. Maximum, mean, and area under the curve for patient-reported bone pain were consistently lower in the naproxen and loratadine groups than in the no prophylaxis group; some of these differences were significant. Loratadine was associated with fewer treatment-related AEs and discontinuations than naproxen.
Conclusions
Given its tolerability, its ease of administration, and its potential benefit, treatment with loratadine should be considered to help prevent bone pain in patients receiving chemotherapy and pegfilgrastim.
Clinical trial registration
Electronic supplementary material
The online version of this article (10.1007/s00520-017-3959-2) contains supplementary material, which is available to authorized users.
Keywords: Bone pain, Breast cancer, Granulocyte colony-stimulating factor, Loratadine, Naproxen, Pegfilgrastim
Background
Febrile neutropenia, the combination of low neutrophil count and fever, is a life-threatening consequence of myelosuppressive chemotherapy that may necessitate hospitalization and administration of intravenous antibiotics [1–3]. Primary prophylaxis with pegfilgrastim reduces the risk of febrile neutropenia in patients with nonmyeloid malignancies receiving chemotherapy [4–7].
Mild-to-moderate bone pain is a commonly reported adverse event (AE) associated with pegfilgrastim [8–11], though the reported incidence of bone pain varies considerably among studies [5, 10–15]. Differences in the incidence of bone pain could be due to differences among patient populations, chemotherapy regimens (e.g., inclusion of taxanes), stage of malignancy and bone involvement, previous therapies, concurrent medication (e.g., use of opioids), and diligence regarding collection of data on AEs.
Bone pain is of considerable concern to patients, because the pain may be severe. Patients may refuse pegfilgrastim, and physicians may stop using pegfilgrastim to avoid bone pain; this may increase the chance of infection, hospitalization, and mortality [3, 16]. Utilization of less intensive regimens as a strategy to reduce the risk of febrile neutropenia may negatively impact treatment outcomes, particularly in curative settings such as early-stage breast cancer [17–19].
Several studies have suggested that naproxen (a nonsteroidal anti-inflammatory drug) and loratadine (an antihistamine) may each be effective in preventing pegfilgrastim-associated bone pain [20–25]. In this randomized, open-label, multicenter study (NOLAN: Naproxen Or Loratadine And Neulasta; NCT01712009), we evaluated the effect of prophylactic naproxen, prophylactic loratadine, or no prophylactic treatment on bone pain in patients with breast cancer receiving chemotherapy and pegfilgrastim.
Methods
Patients
Eligible patients were women ≥ 18 years of age with newly diagnosed stage I–III breast cancer not previously treated with chemotherapy and an Eastern Cooperative Oncology Group performance status ≤ 2, who were planning to receive ≥ 4 cycles of adjuvant or neoadjuvant chemotherapy with pegfilgrastim support starting in cycle 1 and continuing throughout each of the first 4 chemotherapy cycles. Patients were excluded if they were planning to receive weekly chemotherapy, had ongoing chronic pain or other painful conditions requiring treatment (including immediate postoperative treatment), were chronically using oral steroidal and/or nonsteroidal anti-inflammatory drugs or oral antihistamines, had received prior chemotherapy for cancer within 5 years of the current breast cancer diagnosis, or had previously received granulocyte colony-stimulating factor (G-CSF; filgrastim, pegfilgrastim, or other). Patients were also excluded if they had a history of clinically significant gastrointestinal (GI) bleeding, a history of GI ulcers, or active GI bleeding within 6 months prior to randomization. A full listing of inclusion and exclusion criteria is presented in the Supplemental material. This study was conducted in accordance with United States Food and Drug Administration and International Conference on Harmonization Good Clinical Practice regulations/guidelines. All patients provided written informed consent.
Study design
Each eligible patient received adjuvant or neoadjuvant chemotherapy, with pegfilgrastim prophylaxis beginning in the first cycle and continuing through each of the first four chemotherapy cycles. Patients could receive chemotherapy regimens with > 4 cycles; however, data were collected only for the first 4 cycles. Pegfilgrastim (6 mg; Neulasta®, Amgen Inc., Thousand Oaks, CA) was administered as a subcutaneous injection between 24 and 72 h after chemotherapy. Choice of chemotherapy regimen (agent, dose, and schedule) was at the discretion of the treating physician. The investigational medicinal products assessed in the study were naproxen and loratadine; pegfilgrastim and chemotherapy were considered background therapy.
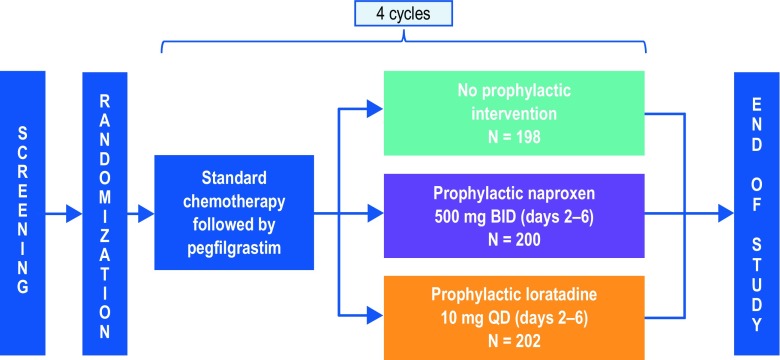
After screening, eligible patients were stratified by age group (< 65 vs ≥ 65 years) and planned chemotherapy type (taxane vs nontaxane). Patients were then randomized in a 1:1:1 ratio within each stratum to receive prophylactic naproxen, prophylactic loratadine, or no prophylactic treatment. Prophylactic naproxen or loratadine was administered orally for 5 days in each of the first 4 chemotherapy cycles, beginning on the day patients received pegfilgrastim. Naproxen (500 mg) was administered twice a day, in the morning and in the evening. Loratadine (10 mg) was administered once a day in the morning. A study schema is shown in Fig. 1.
Fig. 1.
Study schema. Following screening and randomization, patients received chemotherapy followed 24–72 h later by pegfilgrastim. Patients initiated treatment with naproxen or loratadine on the same day they received pegfilgrastim. Data on bone pain AEs were collected on day 1 of cycles 2, 3, and 4 and at the safety follow-up visit. Patients completed bone pain surveys and bone pain medication logs once per day for 5 days, beginning the day they received pegfilgrastim in cycles 1–4. BID twice a day, QD once a day
Data on bone pain were captured by asking patients about any AEs they had experienced since their last clinic visit. On day 1 of cycles 2, 3, and 4 and at the safety follow-up visit that occurred 30–37 days after the last dose of study treatment in cycle 4, healthcare providers asked patients if they had experienced any AEs since their last visit. If patients reported bone pain, the healthcare provider asked the patients to rate the severity of the pain at its worst. Bone pain reported to healthcare providers outside of these scheduled visits was also captured. AEs were coded using the Medical Dictionary for Regulatory Activities, version 18.0. Severity of AEs was graded using Common Terminology Criteria for Adverse Events, version 3.0.
Data on bone pain were also captured using patient surveys. In each of the first 4 chemotherapy cycles, patients were asked to complete a brief bone pain survey once per day for 5 days, beginning the day they received pegfilgrastim. The bone pain survey captured information on the severity of pain (on a scale of 0–10) and the location(s) of pain. The bone pain survey filled out in each cycle by patients in the naproxen arm is shown in Supplemental Fig. 1; similar surveys were filled out by patients in the loratadine and no prophylaxis arms. Patients placed the completed bone pain surveys in sealed envelopes and returned them to the study site at their next clinic visit. The bone pain surveys were retained in their sealed envelopes until patients had completed the study and all AEs reported by the patients had been recorded. Healthcare providers did not supplement AE reports with information gathered from patient surveys.
Patients were asked to record detailed information on any medications taken to alleviate bone pain in a bone pain medication log. This log included fields for the name of the medication taken, the dose of each tablet, and the number of tablets taken per day. Logs were reviewed with healthcare providers at the next clinic visit.
Throughout the study, investigators could prescribe any medications or treatments deemed necessary to provide adequate supportive care, except investigational agents or prophylactic medications for pain relief other than those to which patients were randomized as part of the study. Premedication related to the administration of chemotherapy and use of antiemetics were allowed per usual clinical practice.
Endpoints
The primary endpoint of the study was bone pain (all grades combined) in cycle 1, captured as part of AE reporting. The secondary endpoints were bone pain (all grades combined) by cycle in cycles 2–4 and across all cycles (cycles 1–4) from AE reporting, severe (grade 3 or 4) bone pain by cycle and across all cycles from AE reporting, patient-reported bone pain by cycle and across all cycles, maximum patient-reported bone pain by cycle and across all cycles, and area under the curve (AUC) for patient-reported bone pain (a measure of total pain) by cycle and across all cycles. The safety endpoints were incidence and severity of AEs.
Statistical analyses
This was an estimation study, and it was not powered to demonstrate a statistically significant difference between treatment groups for any endpoints. Differences between treatment groups were not tested formally, and P values are considered nominal. The clinical hypothesis was that an absolute reduction of 10% in all-grade bone pain in the treatment groups compared to the control group could suggest a clinical benefit. The sample size of 600 patients was selected based on data from randomized controlled studies [20, 21].
The following standard summary statistics were calculated: frequency, percentage, and 95% confidence intervals (CIs) for categorical variables; and number, mean, median, minimum, maximum, 95% CI, and standard deviation (SD) for continuous variables. Analysis of the primary and secondary endpoints was based on the full analysis set, which included all patients who received prophylaxis with pegfilgrastim. Analysis was repeated within subgroups based on the stratification factors used in randomization (age < 65 vs ≥ 65 years, taxane vs nontaxane chemotherapy). Since the study did not test any formal statistical hypotheses, no controls for multiple comparisons were employed.
Analysis of AEs was based on the safety analysis set, which included all patients in the full analysis set, according to the prophylactic medication actually received rather than according to randomization. Patient incidence of AEs was summarized for all AEs, treatment-emergent AEs, serious AEs, treatment-related AEs, and AEs leading to withdrawal of naproxen or loratadine.
Missing patient-reported bone pain survey data were imputed using multiple imputation [26]; imputation methodology was applied using SAS® version 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient enrollment and disposition
The study was conducted at 83 centers in the USA between November 1, 2012 (first patient enrolled), and March 18, 2015 (last patient completed the study). Overall, 600 patients were enrolled and randomized: 200 to the naproxen group, 202 to the loratadine group, and 198 to the no prophylaxis group. A total of 391 patients (97.3%) received at least 1 dose of naproxen or loratadine: 193 received naproxen and 198 received loratadine. A total of 587 patients (97.8%) received at least 1 dose of pegfilgrastim (constituting the full analysis set). A CONSORT diagram is shown in Supplemental Fig. 2.
Baseline demographics, disease characteristics, and chemotherapy
All 587 patients in the full analysis set were women. Of these, 83.0% (n = 487) were white, 14.1% (n = 83) were black, and 1.2% (n = 7) were Asian. Mean (SD) age was 54.2 (11.1) years; 80.9% (n = 475) were < 65 years and 19.1% (n = 112) were ≥ 65 years of age. Patient demographics and disease characteristics were largely balanced between the treatment groups. Baseline demographics, disease characteristics, and stratification factors used in randomization are shown in Table 1. Chemotherapy regimens received by patients are shown in Supplemental Table 1. The use of prednisone or dexamethasone, two drugs commonly used in antiemetic regimens, was reported in > 90% of patients (no prophylaxis, 98.4% [n = 188]; naproxen, 94.9% [n = 186]; loratadine, 92.5% [n = 185]).
Table 1.
Baseline demographics, disease characteristics, and stratification factors used in randomization (full analysis set)
| No prophylaxis (N = 191) | Naproxen 500 mg BID (N = 196) | Loratadine 10 mg QD (N = 200) | Total (N = 587) | |
|---|---|---|---|---|
| Race, n (%) | ||||
| White | 148 (77.5) | 166 (84.7) | 173 (86.5) | 487 (83.0) |
| Black (or African American) | 33 (17.3) | 27 (13.8) | 23 (11.5) | 83 (14.1) |
| Asian | 3 (1.6) | 3 (1.5) | 1 (0.5) | 7 (1.2) |
| Othera | 7 (3.7) | 0 (0.0) | 3 (1.5) | 10 (1.7) |
| Age, years | ||||
| Mean (SD) | 54.8 (10.7) | 53.2 (11.7) | 54.6 (10.9) | 54.2 (11.1) |
| Min, max | 29, 82 | 24, 82 | 18, 81 | 18, 82 |
| Age groupb, n (%) | ||||
| Stratified to < 65 was < 65 years | 156 (81.7) | 158 (80.6) | 162 (81.0)c | 476 (81.1)c |
| Stratified to ≥ 65 was ≥ 65 years | 35 (18.3) | 38 (19.4) | 38 (19.0) | 111 (18.9) |
| Calculated BSAd, m2 | ||||
| Mean (SD) | 1.899 (0.235) | 1.875 (0.240) | 1.881 (0.217) | 1.885 (0.231) |
| Min, max | 1.38, 2.62 | 1.36, 2.77 | 1.33, 2.64 | 1.33, 2.77 |
| Disease stage, n (%) | ||||
| I | 58 (30.4) | 41 (20.9) | 51 (25.5) | 150 (25.6) |
| II | 94 (49.2) | 96 (49.0) | 104 (52.0) | 294 (50.1) |
| III | 39 (20.4) | 59 (30.1) | 45 (22.5) | 143 (24.4) |
| Histology, n (%) | ||||
| Ductal carcinoma | 158 (82.7) | 167 (85.2) | 169 (84.5) | 494 (84.2) |
| Lobular carcinoma | 18 (9.4) | 18 (9.2) | 19 (9.5) | 55 (9.4) |
| Othere | 15 (7.9) | 11 (5.6) | 12 (6.0) | 38 (6.5) |
| Nodal status, n (%) | ||||
| 0 | 87 (45.5) | 85 (43.4) | 93 (46.5) | 265 (45.1) |
| 1–3 | 81 (42.4) | 82 (41.8) | 89 (44.5) | 252 (42.9) |
| ≥ 4 | 19 (9.9) | 28 (14.3) | 14 (7.0) | 61 (10.4) |
| Missing | 4 (2.1) | 1 (0.5) | 4 (2.0) | 9 (1.5) |
| Chemotherapy regimenb, n (%) | ||||
| Stratified to taxane received taxane | 88 (46.1) | 94 (48.0) | 92 (46.0) | 274 (46.7) |
| Stratified to taxane received nontaxane | 19 (9.9) | 17 (8.7) | 21 (10.5) | 57 (9.7) |
| Stratified to nontaxane received nontaxane | 83 (43.5) | 84 (42.9) | 85 (42.5) | 252 (42.9) |
| Stratified to nontaxane received taxane | 1 (0.5) | 1 (0.5) | 2 (1.0) | 4 (0.7) |
The full analysis set includes all patients who received primary prophylaxis with pegfilgrastim
BID twice a day, BSA body surface area, QD once a day, SD standard deviation
aOther includes American Indian or Alaska Native, multiple races, native Hawaiian or other Pacific Islander, and other
bStratification factor used in randomization
cOne patient stratified to < 65 was actually ≥ 65 years of age
dCalculated BSA = square root of (height in cm × weight in kg)/3600
eOther includes inflammatory breast cancer, colloid carcinoma, metaplastic carcinoma, and other
Bone pain from AE reporting
All-grade bone pain from AE reporting
In cycle 1, all-grade bone pain was reported by 46.6% (n = 89) of patients in the no prophylaxis group, 40.3% (n = 79) in the naproxen group, and 42.5% (n = 85) in the loratadine group. The differences (95% CIs) between the percentages of patients who reported all-grade bone pain in the naproxen group and the no prophylaxis group and between the loratadine group and the no prophylaxis group in cycle 1 were − 6.3% (− 16.7, 4.1%) and − 4.1% (− 14.5, 6.3%), respectively. None of the differences between the treatment groups in cycle 1 were nominally significant at the 5% level. Differences between the treatment groups in cycles 2–4 were not meaningful; all-grade bone pain was most frequently reported in cycle 1. All-grade bone pain from AE reporting is shown in Table 2.
Table 2.
All-grade bone pain from AE reporting (full analysis set)
| All-grade bone pain | No prophylaxis (N = 191) | Naproxen 500 mg BID (N = 196) | Loratadine 10 mg QD (N = 200) | Difference (naproxen minus no prophylaxis) | Difference (loratadine minus no prophylaxis) | Difference (loratadine minus naproxen) |
|---|---|---|---|---|---|---|
| Cycle 1, n (%)a | 191 (100.0) | 196 (100.0) | 200 (100.0) | |||
| Patients who reported bone pain | 89 | 79 | 85 | |||
| Percentage | 46.6 | 40.3 | 42.5 | − 6.3 | − 4.1 | 2.2 |
| 95% CI for percentageb | (39.4, 53.9) | (33.4, 47.5) | (35.6, 49.7) | (− 16.7, 4.1) | (− 14.5, 6.3) | (− 8.0, 12.4) |
| Cycle 2, n (%)a | 178 (93.2) | 180 (91.8) | 193 (96.5) | |||
| Patients who reported bone pain | 61 | 62 | 67 | |||
| Percentage | 34.3 | 34.4 | 34.7 | 0.2 | 0.4 | 0.3 |
| 95% CI for percentageb | (27.3, 41.7) | (27.5, 41.9) | (28.0, 41.9) | (− 10.2, 10.6) | (− 9.8, 10.7) | (− 9.9, 10.5) |
| Cycle 3, n (%)a | 165 (86.4) | 176 (89.8) | 188 (94.0) | |||
| Patients who reported bone pain | 56 | 60 | 68 | |||
| Percentage | 33.9 | 34.1 | 36.2 | 0.2 | 2.2 | 2.1 |
| 95% CI for percentageb | (26.8, 41.7) | (27.1, 41.6) | (29.3, 43.5) | (− 10.5, 10.8) | (− 8.3, 12.8) | (− 8.3, 12.4) |
| Cycle 4, n (%)a | 162 (84.8) | 169 (86.2) | 179 (89.5) | |||
| Patients who reported bone pain | 66 | 68 | 68 | |||
| Percentage | 40.7 | 40.2 | 38.0 | − 0.5 | − 2.8 | − 2.2 |
| 95% CI for percentageb | (33.1, 48.7) | (32.8, 48.0) | (30.9, 45.5) | (− 11.7, 10.7) | (− 13.7, 8.2) | (− 13.1, 8.6) |
| Across all cycles, n (%)a | 191 (100.0) | 196 (100.0) | 200 (100.0) | |||
| Patients who reported bone pain | 121 | 116 | 122 | |||
| Percentage | 63.4 | 59.2 | 61.0 | − 4.2 | − 2.4 | 1.8 |
| 95% CI for percentagec | (56.1, 70.2) | (52.0, 66.1) | (53.9, 67.8) | (− 14.4, 6.0) | (− 12.5, 7.8) | (− 8.3, 12.0) |
The full analysis set includes all patients who received primary prophylaxis with pegfilgrastim
AE adverse event, BID twice a day, CI confidence interval, N number of patients in the analysis set, QD once a day
aFor individual cycles, n and percentage are based on the number of patients who entered the cycle. For across all cycles, n and percentage are based on the number of patients who started chemotherapy
bCIs for percentages are calculated using binomial distribution. CIs for the difference between percentages are calculated using Fleiss’s method with continuity correction
Across all cycles, all-grade bone pain was reported by 63.4% (n = 121) of patients in the no prophylaxis group, 59.2% (n = 116) in the naproxen group, and 61.0% (n = 122) in the loratadine group. The differences (95% CIs) between the percentages of patients who reported all-grade bone pain in the naproxen group and the no prophylaxis group and between the loratadine group and the no prophylaxis group across all cycles were − 4.2% (− 14.4, 6.0%) and − 2.4% (− 12.5, 7.8%), respectively (Table 2).
Analysis of bone pain from AE reporting in subgroups based on stratification factors at randomization (age < 65 vs ≥ 65 years, taxane vs nontaxane chemotherapy) did not show any meaningful differences between the treatment groups in the number of patients who reported bone pain.
Severe (grade 3 or 4) bone pain from AE reporting
No grade 4 bone pain was reported in this study. In cycle 1, severe (grade 3) bone pain was reported by 4.7% (n = 9) of patients in the no prophylaxis group, 3.1% (n = 6) in the naproxen group, and 4.5% (n = 9) in the loratadine group. Across all cycles, severe bone pain was reported by 5.8% (n = 11) of patients in the no prophylaxis group, 4.1% (n = 8) in the naproxen group, and 4.5% (n = 9) in the loratadine group. Severe bone pain from AE reporting is shown in Supplemental Table 2. None of the differences between the treatment groups were nominally significant at the 5% level, and none of the analyses of severe bone pain in patient subgroups showed any meaningful differences between the treatment groups.
Bone pain from patient surveys
Patient-reported maximum bone pain
Missing values for patient-reported bone pain scores were imputed. The amount of missing bone pain survey data across all cycles was 8.4% in the no prophylaxis arm, 7.2% in the naproxen arm, 3.4% in the loratadine arm, and 6.3% total.
Estimated differences in maximum patient-reported bone pain indicated a nominally significant treatment benefit for naproxen in cycle 4 (P = 0.032) and for loratadine in cycle 1 (P = 0.006), cycle 4 (P = 0.032), and across all cycles (P = 0.041) (Table 3).
Table 3.
Patient-reported maximum bone pain (scale 0–10) by cycle and across all cycles (full analysis set)
| Maximum patient-reported bone paina | No prophylaxis (N = 191) | Naproxen 500 mg BID (N = 196) | Loratadine 10 mg QD (N = 200) | Difference (naproxen minus no prophylaxis) | Difference (loratadine minus no prophylaxis) | Difference (loratadine minus naproxen) |
|---|---|---|---|---|---|---|
| Cycle 1 | ||||||
| n b | 191 | 196 | 200 | |||
| Mean (SE) | 3.9 (0.2) | 3.3 (0.2) | 3.0 (0.2) | − 0.6 (0.3) | − 0.9 (0.3) | − 0.2 (0.3) |
| 95% CIc | (3.4, 4.4) | (2.8, 3.7) | (2.6, 3.4) | (− 1.3, 0.0) | (− 1.5, − 0.3) | (− 0.9, 0.4) |
| P valuec | 0.059 | 0.006 | 0.427 | |||
| Cycle 2 | ||||||
| n b | 178 | 180 | 193 | |||
| Mean (SE) | 3.0 (0.2) | 2.4 (0.2) | 2.6 (0.2) | − 0.6 (0.3) | − 0.4 (0.3) | 0.1 (0.3) |
| 95% CIc | (2.5, 3.4) | (2.0, 2.8) | (2.2, 2.9) | (− 1.2, 0.0) | (− 1.0, 0.2) | (− 0.5, 0.7) |
| P valuec | 0.071 | 0.148 | 0.673 | |||
| Cycle 3 | ||||||
| nb | 165 | 176 | 188 | |||
| Mean (SE) | 2.7 (0.2) | 2.2 (0.2) | 2.5 (0.2) | − 0.5 (0.3) | − 0.2 (0.3) | 0.3 (0.3) |
| 95% CIc | (2.3, 3.2) | (1.8, 2.6) | (2.1, 2.9) | (− 1.1, 0.1) | (− 0.8, 0.4) | (− 0.3, 0.9) |
| P valuec | 0.092 | 0.487 | 0.287 | |||
| Cycle 4 | ||||||
| n b | 162 | 169 | 179 | |||
| Mean (SE) | 2.8 (0.2) | 2.1 (0.2) | 2.1 (0.2) | − 0.7 (0.3) | − 0.6 (0.3) | 0.0 (0.3) |
| 95% CIc | (2.3, 3.3) | (1.7, 2.5) | (1.8, 2.5) | (− 1.3, − 0.1) | (− 1.2, − 0.1) | (− 0.5, 0.6) |
| P valuec | 0.032 | 0.032 | 0.889 | |||
| Across all cycles | ||||||
| n b | 191 | 196 | 200 | |||
| Mean (SE) | 4.7 (0.2) | 4.2 (0.2) | 4.1 (0.2) | − 0.5 (0.3) | − 0.7 (0.3) | − 0.2 (0.3) |
| 95% CIc | (4.3, 5.2) | (3.8, 4.7) | (3.6, 4.5) | (− 1.1, 0.2) | (− 1.3, − 0.0) | (− 0.8, 0.5) |
| P valuec | 0.147 | 0.041 | 0.569 | |||
Note: All missing values of patient-reported bone pain within any cycle were imputed. P values < 0.05 are shown in bold. The full analysis set includes all patients who received primary prophylaxis with pegfilgrastim
BID twice a day, CI confidence interval, N number of patients in the analysis set, QD once a day, SE standard error
aMaximum patient-reported bone pain is the maximum value of each patient’s bone pain values across survey days 1–5 within each cycle. Across all cycles, the maximum is the maximum value of each patient’s reported bone pain values across all survey days 1–5 across all cycles
bFor individual cycles, n is based on the number of patients who entered the cycle. For across all cycles, n is based on the number of patients who started chemotherapy
cThe 95% CI and P value are calculated using an analysis of variance (ANOVA) model, with differences calculated using pairwise least-squares mean differences
Among patients < 65 years of age, a treatment benefit for naproxen was observed in cycle 1 (P = 0.015) and cycle 4 (P = 0.032). A treatment benefit for loratadine was observed in cycle 1 (P = 0.002), cycle 4 (P = 0.020), and across all cycles (P = 0.015) (Supplemental Table 3). Among patients ≥ 65 years of age, no treatment benefit for either naproxen or loratadine was observed (Supplemental Table 4).
Table 4.
Patients reporting treatment-related AEs, serious treatment-related AEs, and treatment-related AEs leading to discontinuation of naproxen or loratadine (safety analysis set)
| Naproxen 500 mg BID (N = 193) n (%) |
Loratadine 10 mg QD (N = 198) n (%) |
|
|---|---|---|
| Patients reporting treatment-related AEsa | 30 (15.5) | 7 (3.5) |
| Worst grade of ≥ 2 | 11 (5.7) | 3 (1.5) |
| Worst grade of ≥ 3 | 2 (1.0) | 0 (0.0) |
| Worst grade of ≥ 4 | 0 (0.0) | 0 (0.0) |
| Serious AEs | 1 (0.5) | 0 (0.0) |
| Life-threatening AEs | 0 (0.0) | 0 (0.0) |
| Fatal AEs | 0 (0.0) | 0 (0.0) |
| Patients reporting treatment-related AEs by system organ class and preferred term (≥ 1% of patients in any group), n (%) | ||
| Gastrointestinal disorders | 21 (10.9) | 1 (0.5) |
| Nausea | 5 (2.6) | 1 (0.5) |
| Abdominal pain | 3 (1.6) | 0 (0.0) |
| Constipation | 3 (1.6) | 1 (0.5) |
| Dyspepsia | 3 (1.6) | 0 (0.0) |
| Diarrhea | 2 (1.0) | 1 (0.5) |
| Gastroesophageal reflux disease | 2 (1.0) | 0 (0.0) |
| Vomiting | 2 (1.0) | 0 (0.0) |
| General disorders and administration site conditions | 5 (2.6) | 5 (2.5) |
| Fatigue | 3 (1.6) | 4 (2.0) |
| Nervous system disorders | 3 (1.6) | 2 (1.0) |
| Headache | 3 (1.6) | 1 (0.5) |
| Skin and subcutaneous tissue disorders | 3 (1.6) | 1 (0.5) |
| Blood and lymphatic system disorders | 2 (1.0) | 0 (0.0) |
| Musculoskeletal and connective tissue disorders | 2 (1.0) | 1 (0.5) |
| Patients reporting treatment-related AEs leading to discontinuation of naproxen or loratadine | 9 (4.7) | 0 (0.0) |
| Patients reporting AEs leading to discontinuation of naproxen or loratadine by system organ class and preferred term (≥ 1% of patients in any treatment group) | ||
| Gastrointestinal disorders | 7 (3.6) | 0 (0.0) |
| Abdominal pain | 3 (1.6) | 0 (0.0) |
The safety analysis set includes all patients who received primary prophylaxis with pegfilgrastim; allocation to treatment groups is based on prophylactic medication actually received
AE adverse event, BID twice a day, QD once a day
aTreatment-related AEs are AEs associated with naproxen and loratadine (not chemotherapy or pegfilgrastim)
Among patients in the taxane-based chemotherapy stratum, no treatment benefit for naproxen was observed, but a treatment benefit for loratadine was observed in cycle 1 (P = 0.040) (Supplemental Table 5). Among patients in the nontaxane-based chemotherapy stratum, no treatment benefit for naproxen was observed, but a treatment benefit for loratadine was observed in cycle 1 (P = 0.039), cycle 2 (P = 0.019), cycle 4 (P = 0.021), and across all cycles (P = 0.035) (Supplemental Table 6).
Patient-reported mean bone pain
Estimated differences in mean patient-reported bone pain indicated a nominally significant treatment benefit for naproxen in cycle 4 (P = 0.029) and for loratadine in cycle 1 (P = 0.016) and across all cycles (P = 0.044) (Supplemental Table 7).
Among patients < 65 years of age, a treatment benefit for naproxen was observed in cycle 1 (P = 0.028), cycle 4 (P = 0.020), and across all cycles (P = 0.029). A treatment benefit for loratadine was observed in cycle 1 (P = 0.006) and across all cycles (P = 0.016) (Supplemental Table 8). Among patients ≥ 65 years of age, no treatment benefit for either naproxen or loratadine was observed (Supplemental Table 9).
Among patients in the taxane-based chemotherapy stratum, no treatment benefit for either naproxen or loratadine was observed (Supplemental Table 10). Among patients in the nontaxane-based chemotherapy stratum, no treatment benefit for naproxen was observed, but a treatment benefit for loratadine was observed in cycle 1 (P = 0.024), cycle 2 (P = 0.007), cycle 4 (P = 0.023), and across all cycles (P = 0.014) (Supplemental Table 11).
AUC for patient-reported bone pain
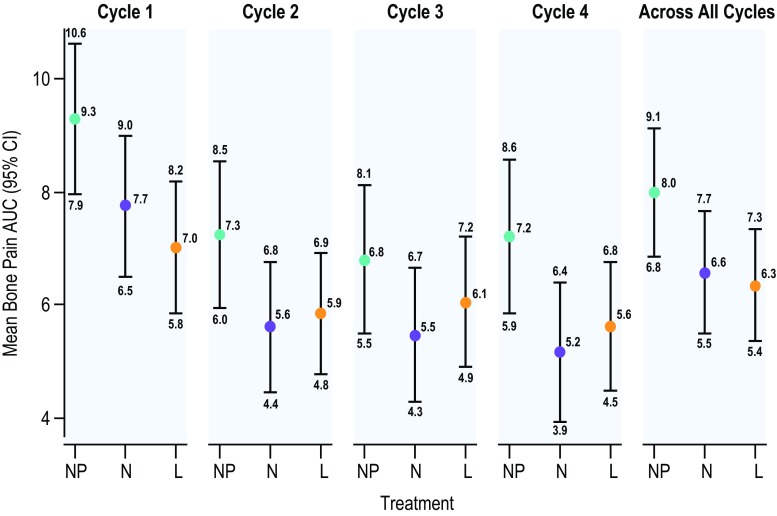
Estimated differences in AUC for patient-reported bone pain over the first 5 days in each cycle (beginning the day patients received pegfilgrastim) indicated a nominally significant treatment benefit for naproxen in cycle 4 (P = 0.027) and for loratadine in cycle 1 (P = 0.012) and across all cycles (P = 0.033) (Fig. 2 and Supplemental Table 12). AUC for patient-reported bone pain was highest for all treatment groups in cycle 1.
Fig. 2.
AUC for patient-reported bone pain (scale 0–10) by cycle and across all cycles. Patient-reported bone pain AUC was calculated using the trapezoidal rule with bone pain scores from day 1 through 5 of each cycle. Numerical values for the means and the 95% CIs are noted. Mean AUC across cycles was the average of AUCs across the cycles. AUC area under the curve, CI confidence interval, L loratadine, N naproxen, NP no prophylaxis
Among patients < 65 years of age, a treatment benefit for naproxen was observed in cycle 1 (P = 0.028), cycle 4 (P = 0.020), and across all cycles (P = 0.028). A treatment benefit for loratadine was also observed in cycle 1 (P = 0.005), cycle 4 (P = 0.049), and across all cycles (P = 0.010) (Supplemental Table 13). Among patients ≥ 65 years of age, no treatment benefit for either naproxen or loratadine was observed (Supplemental Table 14).
Among patients in the taxane-based chemotherapy stratum, no treatment benefit for either naproxen or loratadine was observed (Supplemental Table 15). Among patients in the nontaxane-based chemotherapy stratum, no treatment benefit for naproxen was observed, but a treatment benefit for loratadine was observed in cycle 1 (P = 0.016), cycle 2 (P = 0.004), cycle 4 (P = 0.016), and across all cycles (P = 0.009) (Supplemental Table 16).
Additional bone pain medications from medication logs
Patients recorded information on medications taken reactively to alleviate bone pain in bone pain medication logs. In cycle 1, 63.9% of patients in the no prophylaxis group, 33.2% in the naproxen group, and 42.5% in the loratadine group took additional bone pain medications. Across all cycles, 73.8% of patients in the no prophylaxis group, 44.9% in the naproxen group, and 57.5% in the loratadine group took additional bone pain medications. The percentage of patients who took additional bone pain medications declined from cycle 1 to cycle 2 to cycles 3 and 4. Data on patients who took additional bone pain medications are shown in Supplemental Fig. 3 and Supplemental Table 17. The additional analgesics taken by the most patients were paracetamol, ibuprofen, and vicodin; the additional NSAIDs taken by the most patients were ibuprofen and naproxen sodium; the additional antihistamine taken by the most patients was loratadine. Additional analgesics, antihistamines, and NSAIDs from medication logs are listed in Supplemental Table 18.
Patient-reported bone pain by survey day and range in cycle 1
The frequency distribution of patient-reported bone pain on each survey day in cycle 1 was calculated. The percentage of patients across groups who reported moderate (score 4–6) or severe bone pain (score 7–10) was 10.6% (n = 62) on survey day 1 (the day of pegfilgrastim administration), 22.8% (n = 134) on day 2, 30.7% (n = 180) on day 3, 27.8% (n = 163) on day 4, and 22.0% (n = 129) on day 5. The percentage of patients across groups who reported no bone pain (score 0) or mild bone pain (score 1–3) was 84.8% (n = 498) on day 1, 71.7% (n = 421) on day 2, 64.2% (n = 377) on day 3, 66.6% (n = 391) on day 4, and 71.7% (n = 421) on day 5. Bone pain (score 1–10) across groups was lowest on day 1 and highest on day 3. These data are presented in Supplemental Fig. 4 and Supplemental Table 19.
Safety
Thirty patients (15.5%) in the naproxen group and 7 patients (3.5%) in the loratadine group reported treatment-related AEs (Table 4). One patient in the naproxen group had two serious treatment-related AEs: GI hemorrhage and peptic ulcer. Treatment-related GI disorders were reported by 21 patients (10.9%) in the naproxen group and 1 patient (0.5%) in the loratadine group. GI disorders were the most common reason for discontinuation of naproxen. There were no reports of treatment-related neutropenia or febrile neutropenia in this study, and there were no treatment-related life-threatening or fatal AEs. Cases of treatment-emergent neutropenia and febrile neutropenia were generally balanced across the treatment groups. Treatment-emergent AEs that occurred in ≥ 5% of patients in any treatment group are shown in Supplemental Table 20.
Discussion
Bone pain is a commonly reported AE associated with pegfilgrastim use [5, 9–15], and some patients experience such severe pain that they opt to discontinue pegfilgrastim [22]. This may increase the incidence of infection [17, 27, 28] and may negatively affect outcomes, as dose delays, dose reductions, and reduced chemotherapy dose intensity may increase the cancer recurrence rate and decrease overall survival [1, 18, 29, 30].
We evaluated the effect of prophylactic naproxen, prophylactic loratadine, or no prophylactic treatment on pegfilgrastim-associated bone pain. The primary objective was to estimate the differences between the treatment groups in the percentage of patients who reported all-grade bone pain in cycle 1 as part of AE reporting. The differences between the treatment groups that we observed were not nominally significant at the 5% level. A reduction of 10% in bone pain in the naproxen or loratadine groups compared to the no prophylaxis group was used as a benchmark for clinically meaningful benefit. However, the differences between the groups that we observed were less than 10%. Differences in the percentage of patients who reported all-grade bone pain in cycles 2–4 and across all cycles and the percentage of patients who reported severe (grade 3 or 4) bone pain by cycle and across all cycles were not meaningful. While these results have implications for the efficacy of naproxen and loratadine, they also have implications for AE reporting as an instrument for data collection. Asking patients once per chemotherapy cycle (i.e., every 2, 3, or 4 weeks) whether they have experienced any AEs since their last visit may not be an adequately sensitive way of collecting information on subjective measures such as pain [31, 32].
Patient-reported outcomes are potentially a much better way of collecting data on pain than AE reporting [33–35], and they are gradually gaining acceptance as instruments for reporting symptoms [36–38]. Patient surveys revealed that maximum bone pain, mean bone pain, and AUC for bone pain were lower in the naproxen and loratadine groups than in the no prophylaxis group in some cycles. Some of these differences were nominally significant at the 5% level. Most importantly, patient-reported bone pain was consistently lower in the naproxen and loratadine groups than in the no prophylaxis group by every measure.
A large amount of information on bone pain severity was collected in this study. Across treatment groups, severe bone pain from AE reporting was more common in cycle 1 than in subsequent cycles. Maximum patient-reported bone pain was highest in cycle 1 and consistently lower in cycles 2, 3, and 4. In cycle 1, the percentage of patients across treatment groups who reported moderate (score 4–6) or severe bone pain (score 7–10) increased from day 1 (the day of pegfilgrastim administration) to day 2, peaked on day 3, and then declined on day 4 and then again on day 5. This information may be of interest to patients and healthcare providers as they prepare for treatment or guide the treatment of others.
Among patient subgroups based on stratification factors at randomization (age < 65 vs ≥ 65 years, taxane vs nontaxane chemotherapy), there was consistent evidence for a treatment benefit for naproxen and loratadine among patients < 65 years of age and those stratified to nontaxane-based chemotherapy. Little treatment benefit was observed among patients ≥ 65 years of age and those stratified to taxane-based chemotherapy. As this was an estimation study, and controls for multiple comparisons were not employed, results of the subgroup analyses should be interpreted with caution.
The number of patients with treatment-related AEs was higher in the naproxen group than in the loratadine group; the difference between the arms in treatment-related AEs was greatest for gastrointestinal disorders. The number of patients with ≥ grade 3 treatment-related AEs and serious treatment-related AEs was higher in the naproxen arm than in the loratadine arm, and more patients discontinued naproxen. Loratadine may therefore be a better choice than naproxen for patients receiving chemotherapy. Naproxen may also be avoided in patients receiving myelosuppressive chemotherapy due to its inhibition of platelet aggregation and its antipyretic effects.
This study lends support to previous studies that have shown a treatment benefit for naproxen and loratadine in the prophylaxis of pegfilgrastim-associated bone pain. Kirshner et al. showed that naproxen reduced the incidence and severity of pegfilgrastim-induced bone pain [22], and showed in pilot studies that loratadine may also reduce bone pain [21, 23]. Two case reports reported similar findings on loratadine and bone pain [24, 39]. Pawloski et al. reported that loratadine was the most common medication used to treat bone pain, but did not assess the effectiveness of this intervention [40]. A study by Moukharskaya et al. reported that loratadine did not significantly reduce pain among patients who reported significant back or leg bone pain following an initial dose of pegfilgrastim [41]. The authors proposed a number of possible explanations for this outcome: only patients who experienced significant pain in cycle 1 entered the treatment phase of the trial, patients were permitted to use analgesics for bone or other pain, and patients filled out the FACT-BP questionnaire only twice over two cycles of chemotherapy [41]. A recent study by Gavioli et al. reported that double histamine blockade (using a combination of famotidine and loratadine) was effective in alleviating bone pain secondary to G-CSF use [42].
The present study was a prospective, randomized, three-arm trial conducted in 600 patients. As such, it is the largest study conducted to date to assess prophylactic agents for the prevention of pegfilgrastim-induced bone pain. Despite these strengths, the study had several limitations. This was an estimation study with no formal hypothesis testing, and P values should therefore be considered nominal. There was no statistical adjustment for multiple comparisons, and the number of patients in some subgroups was small. Treatment with naproxen and loratadine was open-label, and patients in the no prophylaxis group did not receive placebo. Patients were free to take medications reactively for bone pain during the study, and more patients in the no prophylaxis group than in the naproxen or loratadine groups took medications for bone pain; this may have reduced the observed treatment benefit for naproxen and loratadine. Patients in this study received a variety of chemotherapy regimens, and different levels of bone pain may be associated with these regimens. Premedication related to the administration of chemotherapy and use of antiemetics were allowed per usual clinical practice. Use of prednisone and dexamethasone, two drugs commonly used in antiemetic regimens, was balanced across the treatment arms, but both premedication and antiemetics may have reduced the impact of the interventions in this study. All patients in the study were women and had early-stage breast cancer, so these results may not translate to men or other tumor types and stages. Subgroup analyses were based on stratification factors at randomization, and treatment did not always align with stratification.
Conclusions
This study has implications for both patient quality of life and cancer treatment. Prophylactic use of daily naproxen or loratadine could potentially reduce pegfilgrastim-associated bone pain and help maintain adherence with planned chemotherapy. This may improve treatment outcomes. Healthcare providers should carefully weigh risks and benefits when deciding on a course of treatment for each patient, and they should be cognizant of the potential dangers of polypharmacy. Nevertheless, given its tolerability, its ease of administration, and the consistent reductions in patient-reported bone pain observed in this study, treatment with 5 days of once daily loratadine in each chemotherapy cycle should be considered for patients receiving chemotherapy and pegfilgrastim.
Electronic supplementary material
(PDF 2717 kb)
Acknowledgments
The authors thank the patients, their families, and the staff at participating sites for their contributions to this study. The authors also acknowledge Holly Watson for her contributions to the conception and design of this study. Medical writing support was provided by Micah Robinson (Amgen Inc.).
Funding information
This study was funded by Amgen Inc.
Compliance with ethical standards
Conflict of interest
Jeffrey J Kirshner has no relationships to disclose. Maxwell C McDonald III reports a relationship with Merck (speakers’ bureau). Flavio Kruter has no relationships to disclose. Andrew S Guinigundo reports relationships with Oncology Hematology Care Inc./US Oncology (employment), Amgen (consultancy, speakers’ bureau, travel expenses), Celgene (speakers’ bureau), Genentech (consultancy, speakers’ bureau), Merck (speakers’ bureau), and Pfizer (speakers’ bureau). Linda Vanni reports relationships with Ascension Providence (employment), Collegium (consultancy), Daiichi Sankyo (consultancy), and The Medicine Shop (consultancy, speakers’ bureau). Cathy L Maxwell reports a relationship with Amgen (consultancy, honoraria, speakers’ bureau, travel expenses). Maureen Reiner, Terry E Upchurch, Jacob Garcia, and Phuong Khanh Morrow report a relationship with Amgen (employment, stock/stock options).
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in this study.
Footnotes
Results of this study were presented in part as a poster at the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL; June 3–7, 2016. Results were also presented in part as a poster at JADPRO Live at APSHO, Washington, DC; November 3–6, 2016.
Electronic supplementary material
The online version of this article (10.1007/s00520-017-3959-2) contains supplementary material, which is available to authorized users.
References
- 1.Pettengell R, Schwenkglenks M, Leonard R, Bosly A, Paridaens R, Constenla M, Szucs TD, Jackisch C, Impact of Neutropenia in Chemotherapy-European Study Group Neutropenia occurrence and predictors of reduced chemotherapy delivery: results from the INC-EU prospective observational European neutropenia study. Support Care Cancer. 2008;16:1299–1309. doi: 10.1007/s00520-008-0430-4. [DOI] [PubMed] [Google Scholar]
- 2.Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C, European Organisation for Research and Treatment of Cancer 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Schilling MB, Parks C, Deeter RG. Costs and outcomes associated with hospitalized cancer patients with neutropenic complications: a retrospective study. Exp Ther Med. 2011;2:859–866. doi: 10.3892/etm.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyman GH, Kuderer NM, Djulbegovic B. Prophylactic granulocyte colony-stimulating factor in patients receiving dose-intensive cancer chemotherapy: a meta-analysis. Am J Med. 2002;112:406–411. doi: 10.1016/S0002-9343(02)01036-7. [DOI] [PubMed] [Google Scholar]
- 5.Vogel CL, Wojtukiewicz MZ, Carroll RR, Tjulandin SA, Barajas-Figueroa LJ, Wiens BL, Neumann TA, Schwartzberg LS. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 2005;23:1178–1184. doi: 10.1200/JCO.2005.09.102. [DOI] [PubMed] [Google Scholar]
- 6.Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25:3158–3167. doi: 10.1200/JCO.2006.08.8823. [DOI] [PubMed] [Google Scholar]
- 7.Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, Kris M, Grous J, Picozzi V, Rausch G, Smith R, Gradishar W, Yahanda A, Vincent M, Stewart M, Glaspy J. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325:164–170. doi: 10.1056/NEJM199107183250305. [DOI] [PubMed] [Google Scholar]
- 8.Neulasta® (pegfilgrastim) prescribing information. Amgen
- 9.Crawford J. Safety and efficacy of pegfilgrastim in patients receiving myelosuppressive chemotherapy. Pharmacotherapy. 2003;23:15S–19S. doi: 10.1592/phco.23.9.15S.32889. [DOI] [PubMed] [Google Scholar]
- 10.Kubista E, Glaspy J, Holmes FA, Green MD, Hackett J, Neumann T, Pegfilgrastim Study Group Bone pain associated with once-per-cycle pegfilgrastim is similar to daily filgrastim in patients with breast cancer. Clin Breast Cancer. 2003;3:391–398. doi: 10.3816/CBC.2003.n.003. [DOI] [PubMed] [Google Scholar]
- 11.Kirshner JJ, Hickok J, Hofman M. Pegfilgrastim-induced bone pain: incidence, risk factors, and management in a community practice. Community Oncol. 2007;4:455–459. doi: 10.1016/S1548-5315(11)70107-3. [DOI] [Google Scholar]
- 12.Pinto L, Liu Z, Doan Q, Bernal M, Dubois R, Lyman G. Comparison of pegfilgrastim with filgrastim on febrile neutropenia, grade IV neutropenia and bone pain: a meta-analysis of randomized controlled trials. Curr Med Res Opin. 2007;23:2283–2295. doi: 10.1185/030079907X219599. [DOI] [PubMed] [Google Scholar]
- 13.Sierra J, Harms R, Mo M, Vogel C (2009) Evaluation of reported bone pain in patients (pts) receiving chemotherapy in pegfilgrastim clinical trials. J Clin Oncol 27(15_suppl): Abstract 9621
- 14.Gregory SA, Schwartzberg LS, Mo M, Sierra J, Vogel C. Evaluation of reported bone pain in cancer patients receiving chemotherapy in pegfilgrastim clinical trials: a retrospective analysis. Commun Oncol. 2010;7:297–308. doi: 10.1016/S1548-5315(11)70402-8. [DOI] [Google Scholar]
- 15.Lambertini M, Del Mastro L, Bellodi A, Pronzato P. The five “Ws” for bone pain due to the administration of granulocyte-colony stimulating factors (G-CSFs) Crit Rev Oncol Hematol. 2014;89:112–128. doi: 10.1016/j.critrevonc.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Dulisse B, Li X, Gayle JA, Barron RL, Ernst FR, Rothman KJ, Legg JC, Kaye JA. A retrospective study of the clinical and economic burden during hospitalizations among cancer patients with febrile neutropenia. J Med Econ. 2013;16:720–735. doi: 10.3111/13696998.2013.782034. [DOI] [PubMed] [Google Scholar]
- 17.Crawford J, Dale DC, Kuderer NM, Culakova E, Poniewierski MS, Wolff D, Lyman GH. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Cancer Netw. 2008;6:109–118. doi: 10.6004/jnccn.2008.0012. [DOI] [PubMed] [Google Scholar]
- 18.Lyman GH, Dale DC, Tomita D, Whittaker S, Crawford J. A retrospective evaluation of chemotherapy dose intensity and supportive care for early-stage breast cancer in a curative setting. Breast Cancer Res Treat. 2013;139:863–872. doi: 10.1007/s10549-013-2582-2. [DOI] [PubMed] [Google Scholar]
- 19.Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Cancer Netw. 2009;7:99–108. doi: 10.6004/jnccn.2009.0009. [DOI] [PubMed] [Google Scholar]
- 20.Kirshner JJ, Heckler CE, Dakhil SR, Hopkins JO, Coles C, Morrow GR (2010) Prevention of pegfilgrastim-induced bone pain (PIP): a URCC CCOP randomized, double-blind, placebo-controlled trial of 510 cancer patients. J Clin Oncol 28 (15_suppl):Abstract 9014 [DOI] [PMC free article] [PubMed]
- 21.Kirshner JJ, Heckler CE, Tiffany S, Reichel C, McAuliffe C, Morrow GR (2011) A phase II study of loratidine (L) to prevent pegfilgrastim-induced pain (PIP). J Clin Oncol 29 (15_suppl):Abstract e19704
- 22.Kirshner JJ, Heckler CE, Janelsins MC, Dakhil SR, Hopkins JO, Coles C, Morrow GR. Prevention of pegfilgrastim-induced bone pain: a phase III double-blind placebo-controlled randomized clinical trial of the University of Rochester Cancer Center Clinical Community Oncology Program Research Base. J Clin Oncol. 2012;30:1974–1979. doi: 10.1200/JCO.2011.37.8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirshner JJ, Heckler CE, Reichel C, Morrow GR (2012) A phase II study of loratidine (L) and naproxen (N) to prevent pegfilgrastim-induced pain (PIP). J Clin Oncol 30 (15_suppl):Abstract e19510
- 24.Romeo C, Li Q, Copeland L. Severe pegfilgrastim-induced bone pain completely alleviated with loratadine: a case report. J Oncol Pharm Pract. 2015;21:301–304. doi: 10.1177/1078155214527858. [DOI] [PubMed] [Google Scholar]
- 25.Gewandter J, Heckler C, Kirshner J, Roscoe J, Morrow G. Naproxen reduces chemotherapy and pegfilgrastim-induced sleep disturbance possibly mediated through reduced bone pain. J Pain. 2012;13:S65. doi: 10.1016/j.jpain.2012.01.271. [DOI] [Google Scholar]
- 26.Little RJA, Rubin DB. Statistical analysis with missing data. Hoboken: Wiley; 2002. [Google Scholar]
- 27.Culakova E, Thota R, Poniewierski MS, Kuderer NM, Wogu AF, Dale DC, Crawford J, Lyman GH. Patterns of chemotherapy-associated toxicity and supportive care in US oncology practice: a nationwide prospective cohort study. Cancer Med. 2014;3:434–444. doi: 10.1002/cam4.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langeberg WJ, Siozon CC, Page JH, Morrow PK, Chia VM. Use of pegfilgrastim primary prophylaxis and risk of infection, by chemotherapy cycle and regimen, among patients with breast cancer or non-Hodgkin’s lymphoma. Support Care Cancer. 2014;22:2167–2175. doi: 10.1007/s00520-014-2184-5. [DOI] [PubMed] [Google Scholar]
- 29.Budman DR, Berry DA, Cirrincione CT, Henderson IC, Wood WC, Weiss RB, Ferree CR, Muss HB, Green MR, Norton L, Frei E., 3rd Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst. 1998;90:1205–1211. doi: 10.1093/jnci/90.16.1205. [DOI] [PubMed] [Google Scholar]
- 30.Leonard RC, Miles D, Thomas R, Nussey F, UK Breast Cancer Neutropenia Audit Grou Impact of neutropenia on delivering planned adjuvant chemotherapy: UK audit of primary breast cancer patients. Br J Cancer. 2003;89:2062–2068. doi: 10.1038/sj.bjc.6601279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sivendran S, Galsky MD. Adverse event reporting in oncology clinical trials—lost in translation? Expert Opin Drug Saf. 2016;15:893–896. doi: 10.1080/14740338.2016.1175429. [DOI] [PubMed] [Google Scholar]
- 32.Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362:865–869. doi: 10.1056/NEJMp0911494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basch E, Jia X, Heller G, Barz A, Sit L, Fruscione M, Appawu M, Iasonos A, Atkinson T, Goldfarb S, Culkin A, Kris MG, Schrag D. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101:1624–1632. doi: 10.1093/jnci/djp386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkinson TM, Li Y, Coffey CW, Sit L, Shaw M, Lavene D, Bennett AV, Fruscione M, Rogak L, Hay J, Gönen M, Schrag D, Basch E. Reliability of adverse symptom event reporting by clinicians. Qual Life Res. 2012;21:1159–1164. doi: 10.1007/s11136-011-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Maio M, Basch E, Bryce J, Perrone F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol. 2016;13:319–325. doi: 10.1038/nrclinonc.2015.222. [DOI] [PubMed] [Google Scholar]
- 36.Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167. doi: 10.1136/bmj.f167. [DOI] [PubMed] [Google Scholar]
- 37.Basch E, Rogak LJ, Dueck AC. Methods for implementing and reporting patient-reported outcome (PRO) measures of symptomatic adverse events in cancer clinical trials. Clin Ther. 2016;38:821–830. doi: 10.1016/j.clinthera.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basch E. Patient-reported outcomes—harnessing patients’ voices to improve clinical care. N Engl J Med. 2017;376:105–108. doi: 10.1056/NEJMp1611252. [DOI] [PubMed] [Google Scholar]
- 39.Moore K, Haroz R. When hydromorphone is not working, try loratadine: an emergency department case of loratadine as abortive therapy for severe pegfilgrastim-induced bone pain. J Emerg Med. 2017;52:e29–e31. doi: 10.1016/j.jemermed.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Pawloski PA, Larsen M, Thoresen A, Giordana MD. Pegfilgrastim use and bone pain: a cohort study of community-based cancer patients. J Oncol Pharm Pract. 2016;22:423–429. doi: 10.1177/1078155215585188. [DOI] [PubMed] [Google Scholar]
- 41.Moukharskaya J, Abrams DM, Ashikaga T, Khan F, Schwartz J, Wilson K, Verschraegen C, Openshaw T, Valentine J, Eneman J, Unger P, Ades S. Randomized phase II study of loratadine for the prevention of bone pain caused by pegfilgrastim. Support Care Cancer. 2016;24:3085–3093. doi: 10.1007/s00520-016-3119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gavioli E, Abrams M. Prevention of granulocyte-colony stimulating factor (G-CSF) induced bone pain using double histamine blockade. Support Care Cancer. 2017;25:817–822. doi: 10.1007/s00520-016-3465-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 2717 kb)