Abstract
Importance
Chronic central serous chorioretinopathy (cCSC) is a chorioretinal disease with unknown disease etiology. The glucocorticoid receptor and the mineralocorticoid receptor, 2 glucocorticoid-binding receptors, might be involved in the pathogenesis of cCSC.
Objective
To assess the association of functional variants and haplotypes in the glucocorticoid receptor (NR3C1) and mineralocorticoid receptor (NR3C2) genes with cCSC.
Design, Setting, and Participants
In this case-control genetic association study, 336 patients with cCSC and 1314 unaffected controls, collected at 3 university medical centers from September 1, 2009, to May 1, 2016, underwent KASP genotyping for selected variants in NR3C1 (rs56149945, rs41423247, and rs6198) and NR3C2 (rs2070951 and rs5522).
Main Outcomes and Measures
Genetic associations of 3 NR3C1 variants and 2 NR3C2 variants with cCSC.
Results
Among the 336 patients (274 men and 62 women; mean [SD] age, 52 [10] years), after correction for multiple testing, rs2070951 in the NR3C2 gene was significantly associated with cCSC (odds ratio, 1.29; 95% CI, 1.08-1.53; P = .004). Moreover, the GA haplotype of single-nucleotide polymorphisms rs2070951 and rs5522 in NR3C2 conferred risk for cCSC (odds ratio, 1.39; 95% CI, 1.15-1.68; P = .004), whereas the CA haplotype decreased risk for cCSC (odds ratio, 0.72; 95% CI, 0.60-0.87; P < .001). Three known variants in NR3C1 that alter the activity of the glucocorticoid receptor (rs56149945, rs41423247, and rs6198) were not associated with cCSC.
Conclusions and Relevance
In this study, the variant rs2070951 and the GA haplotype in NR3C2 were associated with an increased risk for cCSC. Results of this genetic study support a possible role for the mineralocorticoid receptor in the pathogenesis of cCSC. Since these haplotypes have previously been associated with perceived stress, this study provides a clue to bridging clinical risk factors for cCSC to underlying genetic associations.
This case-control, genetic association study assesses the association of functional variants and haplotypes in the glucocorticoid receptor (NR3C1) and mineralocorticoid receptor (NR3C2) genes with chronic central serous chorioretinopathy.
Key Points
Question
Are functional variants and haplotypes in the glucocorticoid receptor (NR3C1) and mineralocorticoid receptor (NR3C2) genes associated with chronic central serous chorioretinopathy (cCSC)?
Findings
In this case-control, genetic association study, rs2070951 in the NR3C2 gene was significantly associated with cCSC. The GA haplotype of single-nucleotide polymorphisms rs2070951 and rs5522 in NR3C2 conferred risk for cCSC, whereas the CA haplotype decreased the risk for cCSC.
Meaning
Results of this genetic study support a possible role for the mineralocorticoid receptor in the pathogenesis of cCSC.
Introduction
In chronic central serous chorioretinopathy (cCSC), it has been suggested that dysfunction of the retinal pigment epithelium due to congestion, thickening, and hyperpermeability of the underlying choroid leads to subretinal fluid accumulation with an associated detachment of the neuroretina. The exact etiology of the disease is currently unknown, but clinical associations point toward an involvement of steroid signaling. Endogenous hypercortisolism (Cushing syndrome), exogenous glucocorticoid exposure, and possibly stress and type A personality are associated with cCSC. It has been hypothesized that the glucocorticoid receptor (GR) and the mineralocorticoid receptor (MR), 2 glucocorticoid-binding receptors, may also be involved in the pathogenesis of cCSC.
The involvement of the MR in the pathogenesis of cCSC has been suggested based on the results of studies in rats, in which choroidal findings similar to those seen in cCSC occurred after intravitreal injection of either corticosterone or aldosterone. Involvement of the MR was further supported by ophthalmologic findings in patients with primary hyperaldosteronism (Conn syndrome). Moreover, studies evaluating the administration of MR antagonists for patients with cCSC have shown possible beneficial effects. However, clinical results were variable and not permanent, and no prospective randomized placebo-controlled clinical trials have been published to this date to study the role of MR antagonists in the treatment of cCSC.
The GR is the most widely expressed cortisol receptor in the body; it regulates metabolism and the cardiovascular system and plays a role in immune suppression and stress response. As stress and both exogenous and endogenous hypercortisolism may be involved in the etiology of cCSC, the GR may also be a player in the pathogenesis of cCSC.
There are several genetic variants in the genes encoding the MR and GR that are known to alter MR and GR protein activity. The MR is encoded by the NR3C2 gene (OMIM 600983), consisting of 10 exons, with 2 alternative 5′-UTR exons 1α and 1β allowing tissue-specific promoter activation. The GR is encoded by the NR3C1 gene (OMIM 138040), consisting of 10 exons, of which 1 to 9α are translated into the functional GRα receptor. In this study, we assessed whether genetic variants in NR3C2 (rs2070951 and rs5522) and NR3C1 (rs56149945, rs41423247, and rs6198) are associated with cCSC.
Methods
We included 336 patients with cCSC in this study. Phenotyping was performed from September 1, 2009, to May 1, 2016, by an experienced retina specialist (C.J.F.B.) and was based on results of a complete ophthalmologic examination, including fundoscopy, optical coherence tomography, fluorescein angiography, and indocyanine green angiography. The patients showed the most typical clinical characteristics of cCSC (serous subretinal fluid affecting the fovea and detected on optical coherence tomography, a disease period of >3 months, ≥1 area of “hot spot” leakage [the point where fluid is presumed to leak into the serous fluid pocket] or diffuse leakage in combination with irregular retinal pigment epithelial window defects detected on fluorescein angiography, and a corresponding hyperfluorescence detected on indocyanine green angiography), described as phenotypic subgroup 1 in a previous article on genetic associations in cCSC. Patients with high myopia, evidence of choroidal neovascularization, polypoidal choroidal vasculopathy, and other atypical findings were excluded. For this study, neither previous nor current steroid use was an exclusion criterion. The patient cohort consisted of 234 patients from the Radboud University Medical Center (Nijmegen, the Netherlands), 72 patients from the Leiden University Medical Center (Leiden, the Netherlands), and 30 patients from the University Hospital of Cologne (Cologne, Germany). A total of 1314 unaffected individuals (ie, controls) enrolled in the European Genetic Database (http://www.eugenda.org) were included in the study; they had no signs of either cCSC or age-related macular degeneration when evaluated by multimodal imaging. The study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review boards and the ethics committees of the Radboud University Medical Center, the Leiden University Medical Center, and the University Hospital of Cologne. Written informed consent was provided by all participants.
Genotyping of selected variants was performed using KASP assays (LGC Genomics) according to manufacturer’s instructions. Specific primers with 5-carboxyfluorescein and 5′-hexachloro-fluorescein labels were designed per variant (NR3C1: rs6198, rs5614994, and rs41423247; NR3C2: rs5522 and rs2070951) (eTable 1 in the Supplement), and the polymerase chain reaction (PCR) conditions per primer pair were provided by LGC Genomics. Data were read with the 7900HT Fast Real-Time PCR system (Applied Biosystems by Life Technologies) and analyzed with SDS version 2.4 (Applied Biosystems).
Statistical Analysis
In IBM SPSS Statistics version 22 (SPSS Inc), the 2-sided Pearson χ2 test was used to compare both the genotype and allele frequencies between cases and controls. Bonferroni correction for multiple testing was performed for 5 variants, and P < .01 was considered statistically significant. Logistic regression was performed for the associated rs2070951 variant with the Firth bias-corrected likelihood ratio test implemented in EPACTS version 3.2.6 (Efficient and Parallelizable Association Container Toolbox; http://genome.sph.umich.edu/wiki/EPACTS), correcting for sex.
Using a haplotype analysis, we assessed the combined effect of the 2 variants in NR3C2. Haplotype analysis was performed with R version 3.0.2 (R Core Team; https://www.R-project.org), using the haplo.stats package (version 1.6.8). The 2 most frequent haplotypes were separately used as a reference in the haplo.cc command to determine odds ratios (ORs) for both haplotypes. A logistic regression analysis (haplo.glm) including sex and haplotypes was performed using the most common haplotype as a reference. Only haplotypes with a frequency higher than 5% are shown. P < .05 was considered statistically significant. Power analysis was performed with CaTS version 0.0.2 (University of Michigan), using a multiplicative model in a joint analysis. The power per variant was calculated based on the minor allele frequency in controls, a disease prevalence of 0.0001, and a variable genotype relative risk (1-2.6), and a graph was created with Graphpad Prism version 5.03 (Graphpad Software).
Results
Among the 336 patients, 274 were men and 62 were women; the mean (SD) age was 52 (10) years. The demographic characteristics of the patients and controls enrolled in this study are summarized in Table 1. All described variants were in Hardy-Weinberg equilibrium, both for controls and patients with cCSC. No statistically significant associations between cCSC and variants in the NR3C1 gene (rs56149945, rs41423247, and rs6198) were found (Table 2). After correction for multiple testing, a significant association between cCSC and the rs2070951 variant in the NR3C2 gene was observed (OR, 1.29; 95% CI, 1.08-1.53; P = .004). No association between the variant rs5522 in NR3C2 and cCSC was found (Table 2).
Table 1. Demographic Characteristics .
| Cohort | No. | Age, Mean (SD), y | Men, No. (%) |
|---|---|---|---|
| Patients with cCSC | 336 | 52 (10) | 274 (81.5) |
| Nijmegen, the Netherlands | 234 | 52 (9) | 188 (80.3) |
| Cologne, Germany | 30 | 50 (9) | 24 (80.0) |
| Leiden, the Netherlands | 72 | 52 (10) | 62 (86.1) |
| Controls | 1314 | 70 (7) | 549 (41.8) |
Abbreviation: cCSC, chronic central serous chorioretinopathy.
Table 2. Association Analysis of Variants in NR3C1 and NR3C2 Genes in Patients With cCSC.
| Variant | Gene | Location | Major/Minor Allele | Minor Allele Frequency | P Valuea | OR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| Controls | Patients | Genotype | Allelic | |||||
| rs56149945 | NR3C1 | Exon 2 | A/G | 0.0415 | 0.0357 | .37 | .50 | 0.86 (0.55-1.34) |
| rs41423247 | NR3C1 | Intron 2 | C/G | 0.360 | 0.390 | .16 | .15 | 1.14 (0.95-1.35) |
| rs6198 | NR3C1 | Exon 9 UTR | A/G | 0.169 | 0.158 | .33 | .48 | 0.92 (0.73-1.16) |
| rs2070951 | NR3C2 | c.-2 | C/G | 0.464 | 0.527 | .008 | .004 | 1.29 (1.08-1.53) |
| rs5522 | NR3C2 | Exon 2 | A/G | 0.129 | 0.137 | .84 | .57 | 1.08 (0.83-1.38) |
Abbreviations: cCSC, chronic central serous chorioretinopathy; OR, odds ratio; UTR, untranslated region.
Bonferroni correction for multiple testing was performed for 5 variants, and P < .01 was deemed statistically significant.
Haplotype analysis of the NR3C2 single-nucleotide polymorphisms rs2070951 and rs5522 showed a significantly decreased risk for cCSC for the CA haplotype (OR, 0.72; 95% CI, 0.60-0.87; P < .001) and an increased risk for the GA haplotype (OR, 1.39; 95% CI, 1.15-1.68; P = .004) (Table 3). To account for potential confounding effects of sex between cases and controls, we corrected for this factor in a logistic regression model. When including this variable in the model, the association of rs2070951 was independent of sex (OR, 1.28; 95% CI, 1.16-1.41; P = .009). Similarly, when correcting for sex in the haplotype analysis, setting the most common haplotype (GA) as the reference, we found that the association of the CA haplotype remained significantly associated with cCSC (OR, 0.73; 95% CI, 0.66-0.81; P = .002).
Table 3. Haplotype Analysis of NR3C2 Gene in Patients With cCSC .
| Haplotype | rs2070951 | rs5522 | P Value | Frequency | Direction of Effect | OR (95% CI) |
Direction of Effect | OR (95% CI) |
|
|---|---|---|---|---|---|---|---|---|---|
| Controls | Patients | ||||||||
| H1 | C | A | <.001 | 0.408 | 0.336 | Base | NA | Protective | 0.72 (0.60-0.87) |
| H2 | C | G | .54 | 0.127 | 0.137 | NS | 1.32 (1.00-1.75) |
NS | 0.95 (0.73-1.24) |
| H3 | G | A | .004 | 0.463 | 0.527 | Risk | 1.39 (1.15-1.68) |
Base | NA |
Abbreviations: cCSC, chronic central serous chorioretinopathy; NA, not annotated; NS, not significant; OR, odds ratio.
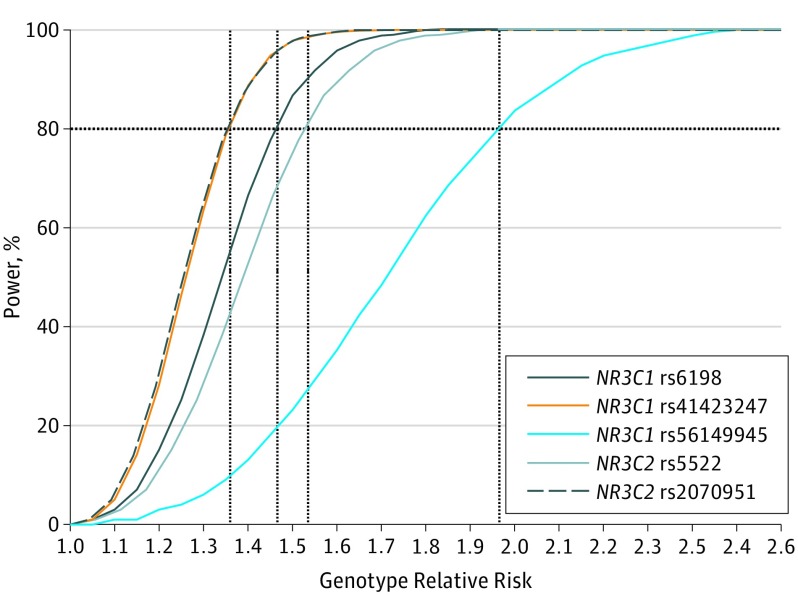
A multiplicative model was used to calculate the power of the study because this model produces a genetic relative risk score that is an estimation of the OR of an allelic model. For each variant, the power of detecting an association was calculated for the current cohort size. For all variants, genotype relative risks less than 2 could be detected with 80% power using this cohort. The detection limits of the genetic relative risk at 80% power were 1.35, 1.36, 1.46, 1.52, and 1.95 for rs2070951, rs41423247, rs6198, rs5522, and rs56149945, respectively (Figure).
Figure. Power Calculation for Each Variant in a Cohort of 336 Patients With Chronic Central Serous Chorioretinopathy and 1314 Unaffected Controls.
For each variant, the power to detect a certain genotype relative risk was assessed using a multiplicative model, with a minor allele frequency in controls and a disease frequency of 0.0001 as input. The 80% power detection limits per variant were 1.35 (rs2070951), 1.36 (rs41423247), 1.46 (rs6198), 1.52 (rs5522), and 1.95 (rs56149945).
Discussion
In this study, we analyzed a possible association with cCSC for 3 known functional variants in NR3C1 and 2 known functional variants in NR3C2. The rs2070951 variant in NR3C2 was significantly associated with cCSC, whereas the rs5522 variant was not associated with cCSC. The NR3C2 CA and GA haplotypes were both significantly associated with cCSC, with a protective and a risk-conferring effect, respectively. Odds ratios of the associated NR3C2 variant and the haplotypes were similar to those of previously described associations for the CFH (OMIM 134370) and ARMS2 (OMIM 611313) genes, and lower compared with the previously described associations in the C4A (OMIM 120810), C4B (OMIM 120820), and CDH5 (OMIM 601120) genes. The 3 variants in the NR3C1 gene (encoding the GR) were not associated with cCSC, which may suggest that MR functionality is more relevant than GR functionality in the etiology of cCSC. However, a larger cohort size is required to exclude the involvement of the 4 variants that were not associated with cCSC in this study.
An abnormal response to the administration of corticosteroids in a subset of patients with cCSC is the strongest risk factor for the disease, with described ORs up to 37. However, the precise mechanism of action of steroids in the pathogenesis of cCSC is unknown. One study showed that both mineralocorticoids and glucocorticoids can activate the MR on choroidal endothelial cells in a rat model. In this animal model, MR activation resulted in vessel dilation via the up-regulation of the endothelial vasodilatory calcium-dependent potassium channel KCa2.3, producing choroidal thickening that is also commonly observed in patients with cCSC. The MR is also present on retinal pigment epithelial cells, and clearance of subretinal fluid through the retinal pigment epithelium toward the choriocapillaris may be influenced by differences in MR haplotypes. In addition, on Müller glial cells, the MR regulates water homeostasis in the eye; dysregulation of this mechanism may contribute to the intraretinal edema observed in a subset of patients with cCSC. However, direct GR overactivation without MR involvement seems to be sufficient to induce cCSC in some patients because synthetic glucocorticoids with strong selectivity for GR over MR have also been described as a risk factor for the disease.
Both of the variants in NR3C2 that were tested in this study influence the transactivational capacity of the MR after exposure to both cortisol and dexamethasone, and they have been shown to affect salivary cortisol levels, especially during the morning cortisol awakening peak. The rs2070951 G variant, which is associated with cCSC in this study, leads to lower expression of MR and reduced transactivation. One study showed that male carriers of the rs2070951 GG genotype in NR3C2 had higher systolic blood pressure. This finding is particularly relevant in the context of cCSC because hypertension is a known risk factor for the disease. The effect of this genetic variant on systolic blood pressure was observed only in male patients, which is interesting because cCSC is more common in men than in women. In our data set, the association for rs2070951 was also observed only in male patients with cCSC when the data were stratified for sex (males: OR, 1.28; 95% CI, 1.04-1.58; P = .02 vs females: OR, 1.21; 95% CI, 0.84-1.75; P = .31 [eTable 2 in the Supplement]). However, this is likely owing to the limited number of female patients with cCSC included in the analysis, and based on the current data we therefore cannot definitively conclude that sex differences exist in this genetic association.
Haplotypes in NR3C2 have previously been associated with differences in perceived chronic stress, another postulated risk factor for cCSC. We found that the haplotype of the single-nucleotide polymorphism rs2070951 and rs5522, GA, which has been previously associated with increased susceptibility to stress, conferred risk for developing cCSC in our cohort. The haplotype that was associated with an optimistic attitude (and a tendency to recover from or adjust easily to misfortune or change), CA, was protective against the development of cCSC. This finding could indicate that, for patients with cCSC who have the GA haplotype, both the MR-mediated pathways and chronic stress are of significant importance, whereas other not-yet-identified factors could play a bigger role in patients with cCSC who have the CA haplotype. In addition, there is a likelihood that patients with the different haplotypes carry additional unknown genetic variants that might also contribute to an increased or decreased risk of cCSC.
Clinical studies that tested the potential of MR antagonists in the treatment of cCSC have yielded mixed results. Our findings may partly explain this variable response to MR antagonists because carriers of different MR haplotypes may respond differently to MR antagonists. The results of our study may lead to the stratification of patients with cCSC into subgroups based on MR haplotype. Treatment of these stratified patient subgroups with MR antagonists could result in group-specific effects. For patients with the CA haplotype, other (thus far unknown) factors could contribute to the development of cCSC to a greater extent. The results of this study may therefore indicate that a more personalized treatment approach to cCSC may be useful. Further studies on the response to treatment of patients with cCSC who have different MR genotypes are needed to test this hypothesis.
Limitations
Our study contains some limitations. The sample size of our cohort is insufficient to detect genetic variants with low ORs. A larger cohort is needed to completely rule out the association of the 4 remaining variants with cCSC.
Conclusions
In this study, rs2070951 in the NR3C2 gene, encoding the MR receptor, was significantly associated with cCSC. In addition, haplotypes of NR3C2 that have previously been associated with perceived stress were also associated with cCSC, which may be the first clue to bridging clinical risk factors for cCSC to underlying genetic associations. The functional effects of this variant and the associated haplotype in the MR gene may contribute to the disease mechanisms of cCSC.
eTable 1. KASP Probe Sequences and PCR Programs
eTable 2. Associations for Genetic Variant rs2070951
References
- 1.Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Exp Ophthalmol. 2013;41(2):201-214. [DOI] [PubMed] [Google Scholar]
- 2.Daruich A, Matet A, Dirani A, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82-118. [DOI] [PubMed] [Google Scholar]
- 3.Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye (Lond). 2010;24(12):1743-1756. [DOI] [PubMed] [Google Scholar]
- 4.Yannuzzi LA. Central serous chorioretinopathy: a personal perspective. Am J Ophthalmol. 2010;149(3):361-363. [DOI] [PubMed] [Google Scholar]
- 5.Warrow DJ, Hoang QV, Freund KB. Pachychoroid pigment epitheliopathy. Retina. 2013;33(8):1659-1672. [DOI] [PubMed] [Google Scholar]
- 6.van Dijk EH, Dijkman G, Biermasz NR, van Haalen FM, Pereira AM, Boon CJ. Chronic central serous chorioretinopathy as a presenting symptom of Cushing syndrome. Eur J Ophthalmol. 2016;26(5):442-448. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho-Recchia CA, Yannuzzi LA, Negrão S, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109(10):1834-1837. [DOI] [PubMed] [Google Scholar]
- 8.Jonas JB, Kamppeter BA. Intravitreal triamcinolone acetonide and central serous chorioretinopathy. Br J Ophthalmol. 2005;89(3):386-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haimovici R, Koh S, Gagnon DR, Lehrfeld T, Wellik S; Central Serous Chorioretinopathy Case-Control Study Group . Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111(2):244-249. [DOI] [PubMed] [Google Scholar]
- 10.Bouzas EA, Scott MH, Mastorakos G, Chrousos GP, Kaiser-Kupfer MI. Central serous chorioretinopathy in endogenous hypercortisolism. Arch Ophthalmol. 1993;111(9):1229-1233. [DOI] [PubMed] [Google Scholar]
- 11.Yannuzzi LA. Type-A behavior and central serous chorioretinopathy. Retina. 1987;7(2):111-131. [DOI] [PubMed] [Google Scholar]
- 12.Zhao M, Célérier I, Bousquet E, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122(7):2672-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dijk EH, Nijhoff MF, de Jong EK, Meijer OC, de Vries AP, Boon CJ. Central serous chorioretinopathy in primary hyperaldosteronism. Graefes Arch Clin Exp Ophthalmol. 2016;254(10):2033-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bousquet E, Beydoun T, Zhao M, Hassan L, Offret O, Behar-Cohen F. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2013;33(10):2096-2102. [DOI] [PubMed] [Google Scholar]
- 15.Bousquet E, Beydoun T, Rothschild PR, et al. Spironolactone for nonresolving central serous chorioretinopathy: a randomized controlled crossover study. Retina. 2015;35(12):2505-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breukink MB, den Hollander AI, Keunen JE, Boon CJ, Hoyng CB. The use of eplerenone in therapy-resistant chronic central serous chorioretinopathy. Acta Ophthalmol. 2014;92(6):e488-e490. [DOI] [PubMed] [Google Scholar]
- 17.Cakir B, Fischer F, Ehlken C, et al. Clinical experience with eplerenone to treat chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254(11):2151-2157. [DOI] [PubMed] [Google Scholar]
- 18.Keenan CR, Lew MJ, Stewart AG. Biased signalling from the glucocorticoid receptor: renewed opportunity for tailoring glucocorticoid activity. Biochem Pharmacol. 2016;112:6-12. [DOI] [PubMed] [Google Scholar]
- 19.Muhtz C, Zyriax BC, Bondy B, Windler E, Otte C. Association of a common mineralocorticoid receptor gene polymorphism with salivary cortisol in healthy adults. Psychoneuroendocrinology. 2011;36(2):298-301. [DOI] [PubMed] [Google Scholar]
- 20.van Leeuwen N, Caprio M, Blaya C, et al. The functional c.-2G>C variant of the mineralocorticoid receptor modulates blood pressure, renin, and aldosterone levels. Hypertension. 2010;56(5):995-1002. [DOI] [PubMed] [Google Scholar]
- 21.van Leeuwen N, Kumsta R, Entringer S, et al. Functional mineralocorticoid receptor (MR) gene variation influences the cortisol awakening response after dexamethasone. Psychoneuroendocrinology. 2010;35(3):339-349. [DOI] [PubMed] [Google Scholar]
- 22.Derijk RH, Schaaf MJ, Turner G, et al. A human glucocorticoid receptor gene variant that increases the stability of the glucocorticoid receptor beta-isoform mRNA is associated with rheumatoid arthritis. J Rheumatol. 2001;28(11):2383-2388. [PubMed] [Google Scholar]
- 23.van Rossum EF, Roks PH, de Jong FH, et al. Characterization of a promoter polymorphism in the glucocorticoid receptor gene and its relationship to three other polymorphisms. Clin Endocrinol (Oxf). 2004;61(5):573-581. [DOI] [PubMed] [Google Scholar]
- 24.Huizenga NA, Koper JW, De Lange P, et al. A polymorphism in the glucocorticoid receptor gene may be associated with and increased sensitivity to glucocorticoids in vivo. J Clin Endocrinol Metab. 1998;83(1):144-151. [DOI] [PubMed] [Google Scholar]
- 25.van Rossum EF, Koper JW, van den Beld AW, et al. Identification of the BclI polymorphism in the glucocorticoid receptor gene: association with sensitivity to glucocorticoids in vivo and body mass index. Clin Endocrinol (Oxf). 2003;59(5):585-592. [DOI] [PubMed] [Google Scholar]
- 26.van Leeuwen N, Bellingrath S, de Kloet ER, et al. Human mineralocorticoid receptor (MR) gene haplotypes modulate MR expression and transactivation: implication for the stress response. Psychoneuroendocrinology. 2011;36(5):699-709. [DOI] [PubMed] [Google Scholar]
- 27.Zennaro MC, Keightley MC, Kotelevtsev Y, Conway GS, Soubrier F, Fuller PJ. Human mineralocorticoid receptor genomic structure and identification of expressed isoforms. J Biol Chem. 1995;270(36):21016-21020. [DOI] [PubMed] [Google Scholar]
- 28.de Jong EK, Breukink MB, Schellevis RL, et al. Chronic central serous chorioretinopathy is associated with genetic variants implicated in age-related macular degeneration. Ophthalmology. 2015;122(3):562-570. [DOI] [PubMed] [Google Scholar]
- 29.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 30.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27-38. doi: 10.1093/biomet/80.1.27 [DOI] [Google Scholar]
- 31.Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet. 2006;38(2):209-213. [DOI] [PubMed] [Google Scholar]
- 32.Horita N, Kaneko T. Genetic model selection for a case-control study and a meta-analysis. Meta Gene. 2015;5:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miki A, Kondo N, Yanagisawa S, Bessho H, Honda S, Negi A. Common variants in the complement factor H gene confer genetic susceptibility to central serous chorioretinopathy. Ophthalmology. 2014;121(5):1067-1072. [DOI] [PubMed] [Google Scholar]
- 34.Schubert C, Pryds A, Zeng S, et al. Cadherin 5 is regulated by corticosteroids and associated with central serous chorioretinopathy. Hum Mutat. 2014;35(7):859-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breukink MB, Schellevis RL, Boon CJ, et al. Genomic copy number variations of the complement component C4B gene are associated with chronic central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2015;56(9):5608-5613. [DOI] [PubMed] [Google Scholar]
- 36.Golestaneh N, Picaud S, Mirshahi M. The mineralocorticoid receptor in rodent retina: ontogeny and molecular identity. Mol Vis. 2002;8:221-225. [PubMed] [Google Scholar]
- 37.Zhao M, Valamanesh F, Celerier I, et al. The neuroretina is a novel mineralocorticoid target: aldosterone up-regulates ion and water channels in Müller glial cells. FASEB J. 2010;24(9):3405-3415. [DOI] [PubMed] [Google Scholar]
- 38.DeRijk RH, Wüst S, Meijer OC, et al. A common polymorphism in the mineralocorticoid receptor modulates stress responsiveness. J Clin Endocrinol Metab. 2006;91(12):5083-5089. [DOI] [PubMed] [Google Scholar]
- 39.Tittl MK, Spaide RF, Wong D, et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. 1999;128(1):63-68. [DOI] [PubMed] [Google Scholar]
- 40.Eom Y, Oh J, Kim SW, Huh K. Systemic factors associated with central serous chorioretinopathy in Koreans. Korean J Ophthalmol. 2012;26(4):260-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980-2002. Ophthalmology. 2008;115(1):169-173. [DOI] [PubMed] [Google Scholar]
- 42.Wang M, Munch IC, Hasler PW, Prünte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86(2):126-145. [DOI] [PubMed] [Google Scholar]
- 43.Spahn C, Wiek J, Burger T. Operationalized psychodynamic diagnostics (OPD) in patients with central serous chorioretinopathy [in German]. Psychother Psychosom Med Psychol. 2004;54(2):52-57. [DOI] [PubMed] [Google Scholar]
- 44.Conrad R, Bodeewes I, Schilling G, Geiser F, Imbierowicz K, Liedtke R. Central serous chorioretinopathy and psychological stress [in German]. Ophthalmologe. 2000;97(8):527-531. [DOI] [PubMed] [Google Scholar]
- 45.de Kloet ER, Otte C, Kumsta R, et al. Stress and depression: a crucial role of the mineralocorticoid receptor. J Neuroendocrinol. 2016;28(8). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. KASP Probe Sequences and PCR Programs
eTable 2. Associations for Genetic Variant rs2070951