This case report investigates the feasibility of using microscope-integrated, swept-source optical coherence tomography in the operating room for 2 young children with retinal vascular disease.
Key Points
Question
Is it possible to perform optical coherence tomography angiography in the operating room?
Findings
In this case report of 2 young children with retinal vascular disease who underwent microscope-integrated, intraoperative optical coherence tomography angiography during examination under anesthesia, the microscope-integrated optical coherence tomography angiograms showed more detailed retinal vasculature than was visible in fluorescein angiographic images, especially in areas of previous laser treatment.
Meaning
Intraoperative optical coherence tomography angiography allowed detailed evaluation of retinal vasculature without injection of fluorescein dye in 2 young children who could not cooperate with in-office examinations, suggesting the feasibility of this procedure for similar situations.
Abstract
Importance
Intraoperative optical coherence tomography (OCT) has gained traction as an important adjunct for clinical decision making during vitreoretinal surgery, and OCT angiography (OCTA) has provided novel insights in clinical evaluation of retinal diseases. To date, these two technologies have not been applied in combination to evaluate retinal vascular disease in the operating suite.
Objective
To conduct microscope-integrated, swept-source OCTA (MIOCTA) in children with retinal vascular disease.
Design, Setting, and Participants
In this case report analysis, OCT imaging in pediatric patients, MIOCTA images were obtained during examination under anesthesia from a young boy with a history of idiopathic vitreous hemorrhage and a female infant with familial exudative vitreoretinopathy.
Main Outcomes and Measures
Side-by-side comparison of research MIOCT angiograms and clinically indicated fluorescein angiograms.
Results
In 2 young children with retinal vascular disease, the MIOCTA images showed more detailed vascular patterns than were visible on the fluorescein angiograms although within a more posterior field of view. The MIOCTA system allowed visualization of small pathological retinal vessels in the retinal periphery that were obscured in the fluorescein angiograms by fluorescein staining from underlying, preexisting laser scars.
Conclusions and Relevance
This is the first report to date of the use of MIOCTA in the operating room for young children with retinal vascular disease. Further optimization of this system may allow noninvasive detailed evaluation of retinal vasculature during surgical procedures and in patients who could not cooperate with in-office examinations.
Introduction
Intraoperative optical coherence tomography (OCT) technology has markedly advanced in the past few years. Intraoperative imaging was first performed with handheld and microscope-mounted spectral domain OCT systems during surgical pauses. More recently, both commercial and research spectral-domain, microscope-integrated OCT (MIOCT) systems have been reported. One group showed a custom swept-source, microscope-integrated OCT (SS-MIOCT) system based on a faster 100-kHz laser. The faster scan rate of this laser made possible live volumetric intraoperative imaging and, as reported herein, enabled OCT angiography (OCTA). Optical coherence tomography angiography uses changes in complex OCT signals induced by vascular flow for contrast instead of exogenous fluorescent tracers and has provided substantial insight into retinal vascular abnormalities. Previous OCTA implementations were limited to tabletop clinical OCT systems, which require cooperative and upright patients for imaging. Here, we report on OCTA integrated into our SS-MIOCT system and describe, to our knowledge, its first use in the operating room during examination under anesthesia (EUA) of 2 young children with retinal vascular disease.
Methods
The research system used a 100-kHz swept-source laser (Axsun Technologies) with a center wavelength of 1050 nm. Intraoperative MIOCT angiography (MIOCTA) was performed with the MIOCT scanner and a binocular indirect ophthalmomicroscopic lens (BIOM; Oculus) without microscope illumination. The lens allowed for MIOCTA images to be taken at up to a 30° field of view. The size and location of this field of view is controlled in real time by the operator through manual tracking. Microscope-integrated, swept-source OCTA images were acquired with 300 A-scans per B-scan, 6 repeated B-scans per lateral location, and 150 lateral locations sampled. Total image acquisition time for each MIOCTA scan was 4 seconds. The repeated B-scans were registered with phase correlation to compensate for inter–B-scan motion. Speckle variance was used to compute the angiograms. The inner retinal layers were segmented to create en face projections of the retinal vasculature. Fluorescein angiograms obtained with portable corneal-contact retinal photography (Retcam; Clarity Medical Systems, Inc) using a 130° lens (D1300; Clarity Medical Systems, Inc) were part of the planned clinical examination. All human subject research was performed under a research protocol approved by the Duke University Health System Institutional Review Board and adhered to the Health Insurance Portability and Accountability Act and all tenets of the Declaration of Helsinki. The patients’ families provided written informed consent for the research imaging study.
A literature search of the PubMed database on October 24, 2016, for microscope-integrated or intraoperative and optical coherence tomography angiography did not identify publications that address this new technique.
Report of Cases
Case 1
A 2-year-old, otherwise healthy boy presented to the pediatric retina service for evaluation of new-onset esotropia and was found to have unilateral vitreous hemorrhage of the right eye. He underwent vitrectomy to remove old vitreous hemorrhage and was found to have a presumed vascular stump superonasal to the optic disc, with focal adherent vitreous and blood but no clear vascular anomaly. He returned 4 months after surgery for a second EUA, fluorescein angiography (FA), and research MIOCTA.
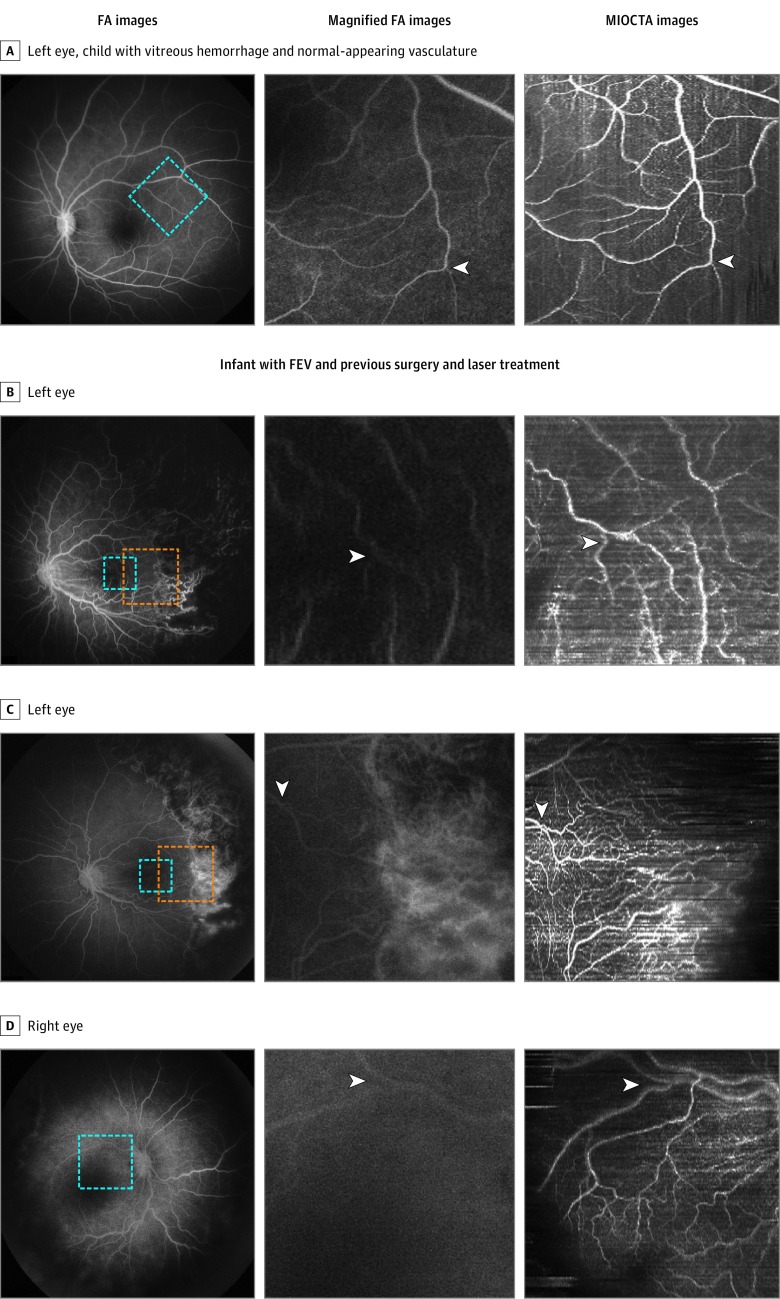
Fluorescein angiography and MIOCTA were performed to evaluate macular (Figure, A) and peripheral vasculature. Fluorescein angiography of the left eye showed a normal macular and peripapillary vascular pattern, which was confirmed by en face MIOCTA (Figure, A and data not shown). MIOCTA was not able to reach the more peripheral focal hemorrhagic site in the right eye during the time allocated for research imaging. Fluorescein angiography demonstrated normal peripheral vasculature beyond the focal hemorrhagic site. In this case, MIOCTA demonstrated the ability to document vascular imaging.
Figure. Fluorescein Angiography (FA) vs Microscope-Integrated Optical Coherence Tomography Angiography (MIOCTA) During Pediatric Examination Under Anesthesia.
Fluorescein angiographic, magnified FA, and corresponding MIOCTA images depict the vasculature of a 2-year-old child with previous vitreous hemorrhage and a 7-month-old infant with familial exudative vitreoretinopathy (FEV) and previous vitreoretinal surgery and retinal laser treatment. A, Magnified FA image (area of blue box on FA) shows the microvasculature of the retina (arrowhead), which can be compared with that visible on the MIOCTA image. B, Magnified FA image (area of blue box on FA in B and C) shows small retinal vessels visible in the temporal macula (arrowhead) in contrast to the MIOCTA image of the same area. C, Magnified FA image (area of orange box on FA in B and C) shows small retinal vessels (arrowhead) visible at the margin of laser treatment scars; the MIOCTA image of the same area shows retinal vessels that are visible over laser treatment scars. D, Enlarged images (area of blue box on FA) show small retinal vessels (arrowhead) that are more visible on the MIOCTA image than on the magnified FA image.
Case 2
A 7-month-old infant girl (born between 31 and 32 weeks’ gestation owing to oligohydramnios with a birth weight of 1134 g and requiring a short stay in the neonatal intensive care unit) was referred to the pediatric retina service for evaluation. She had a history of peripheral laser treatment in both eyes for what was described as stage 3 retinopathy of prematurity. During the initial EUA, she was found to have 360° laser scars in both eyes and a temporal neovascular ridge posterior to laser scars in the right eye. In the left eye, she had a partial vitreous detachment with subhyaloid hemorrhage, macular fold with epiretinal membrane, and neovascularization temporally. She underwent fill-in laser photocoagulation in the right eye and vitrectomy, membrane peel, and endolaser in the left eye. Based on her gestational age and the late onset of progressive neovascularization, she was diagnosed with familial exudative vitreoretinopathy. She received additional laser photocoagulation to nonperfused retina in both eyes during follow-up EUAs. Six months after the initial surgery during another EUA with FA, she underwent research MIOCTA.
MIOCTA showed a vascular pattern similar to that seen on FA as well as fine perifoveal vasculature that was not evident on the FA (Figure, B and D). In the left eye, MIOCTA of the retinal periphery demonstrated disorganized peripheral microvasculature adjacent to preexisting laser scars that were difficult to discern on FA (Figure, C). Although a limitation of OCTA is the absence of fluorescein leakage as a marker of abnormal retinal vessels, these fine vascular abnormalities seen on MIOCTA were not masked by leakage or staining from preexisting laser scars that can obsure them on FA. In the right eye, the peripapillary capillary network was evident (Figure, D). The peripheral vascular capillary network in the temporal periphery was more organized compared with the network in the left eye. Because of the leakage noted on FA, additional laser treatment was applied to temporal nonperfused retina in both eyes.
Discussion
These 2 cases illustrate, to our knowledge, the first use of MIOCTA in young children with retinal vascular disease. In the second case, MIOCTA allowed study of pathological changes in smaller retinal vessels in the retinal periphery that were obscured in FA by leakage or staining. Although a limitation of OCTA is that this imaging method does not reveal sites of vascular leakage, we recognize that FA is a time-dependent procedure and that, once leakage has occurred, it is difficult to obtain imaging of the microvasculature in that area (Figure, C). Our MIOCTA system allowed noninvasive retinal vasculature imaging in supine infants who could not be imaged with the available commercial OCTA systems. A current limitation of this MIOCTA system is that imaging of the peripheral retina is still limited to the midperiphery. A potential application of this system includes monitoring for global or regional loss or gain in perfused vessels during vitreoretinal surgery, such as on removal of fibrovascular proliferation and release of traction during vitreoretinal surgery for pediatric familial exudative vitreoretinopathy or for adults with proliferative diabetic retinopathy.
The intense interest in OCTA for vitreoretinal applications has led to its swift rise in popularity in the clinical setting and important advances in our knowledge and application of OCTA for retinal diseases among adults and children (but not previously among infants). This SS-MIOCT system allowed for the use of MIOCTA and real-time OCT. Further image processing and optimization of image capture are needed to segment layers of deep and superficial capillary network and choroidal vasculature. Improved image capture and processing speed will allow real-time feedback. Enhancement of image resolution and further correlation between FA and MIOCTA images will validate the current system.
Limitations
Our current case report analysis is limited by its small sample size. More patients are being enrolled to analyze additional vascular patterns in children with retinal vascular disease.
Conclusions
The use of MIOCTA to capture images of the macular and midperipheral retinal vascular networks is feasible in supine infants with an SS-MIOCT system. Further optimization and use of this system may provide important insights into neurovascular patterning during human development and disease as well as vascular changes and complications during vitreoretinal surgery.
References
- 1.Dayani PN, Maldonado R, Farsiu S, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009;29(10):1457-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao YK, Ehlers JP, Toth CA, Izatt JA. Intraoperative spectral domain optical coherence tomography for vitreoretinal surgery. Opt Lett. 2010;35(20):3315-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehlers JP, Goshe J, Dupps WJ, et al. Determination of feasibility and utility of microscope-integrated optical coherence tomography during ophthalmic surgery: the DISCOVER Study RESCAN results. JAMA Ophthalmol. 2015;133(10):1124-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falkner-Radler CI, Glittenberg C, Gabriel M, Binder S. Intrasurgical microscope-integrated spectral domain optical coherence tomography-assisted membrane peeling. Retina. 2015;35(10):2100-2106. [DOI] [PubMed] [Google Scholar]
- 5.Carrasco-Zevallos OM, Keller B, Viehland C, et al. Optical coherence tomography for retinal surgery: perioperative analysis to real-time four-dimensional image-guided surgery. Invest Ophthalmol Vis Sci. 2016;57(9):OCT37-OCT50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrasco-Zevallos OM, Keller B, Viehland C, et al. Live volumetric (4D) visualization and guidance of in vivo human ophthalmic surgery with intraoperative optical coherence tomography. Sci Rep. 2016;6:31689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao SS, Jia Y, Zhang M, et al. Optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT27-OCT36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia Y, Bailey ST, Hwang TS, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci U S A. 2015;112(18):E2395-E2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim DY, Fingler J, Zawadzki RJ, et al. Optical imaging of the chorioretinal vasculature in the living human eye. Proc Natl Acad Sci U S A. 2013;110(35):14354-14359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendargo HC, Estrada R, Chiu SJ, Tomasi C, Farsiu S, Izatt JA. Automated non-rigid registration and mosaicing for robust imaging of distinct retinal capillary beds using speckle variance optical coherence tomography. Biomed Opt Express. 2013;4(6):803-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariampillai A, Standish BA, Moriyama EH, et al. Speckle variance detection of microvasculature using swept-source optical coherence tomography. Opt Lett. 2008;33(13):1530-1532. [DOI] [PubMed] [Google Scholar]
- 12.Chiu SJ, Li XT, Nicholas P, Toth CA, Izatt JA, Farsiu S. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express. 2010;18(18):19413-19428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 14.John VJ, McClintic JI, Hess DJ, Berrocal AM. Retinopathy of prematurity versus familial exudative vitreoretinopathy: report on clinical and angiographic findings. Ophthalmic Surg Lasers Imaging Retina. 2016;47(1):14-19. [DOI] [PubMed] [Google Scholar]