Key Points
Question
Can temporal macular atrophy found in sickle cell disease affect visual function?
Findings
This case series of 3 young patients with homozygous sickle cell disease found macular atrophy associated with central and paracentral scotomas, impaired color vision, and loss of contrast sensitivity in the setting of preserved visual acuity.
Meaning
These findings suggest that patients with sickle cell disease and abnormal findings on optical coherence tomographic imaging consistent with temporal macular atrophy can exhibit central and paracentral scotomas despite relatively preserved visual acuity.
Abstract
Importance
Temporal macular involvement in sickle cell disease can now easily be detected by optical coherence tomography (OCT). However, while recent studies have demonstrated its high prevalence, little is known about its potential consequences on visual function.
Objective
To assess the visual function of patients with sickle cell disease with no visual symptoms despite temporal macular atrophy.
Design, Setting, and Participants
This retrospective case series included data collection and explorations made in a single referral center for sickle cell disease in 2016. Three patients with sickle cell disease exhibiting preserved visual acuity but showing temporal macular retinal atrophy were included.
Exposures
Patients underwent the following explorations: best-corrected distance and near visual acuity evaluation; dilated fundus examination; OCT with 12 × 6-mm thickness map; horizontal, vertical, and en face sections; OCT angiography of the 6 × 6-mm perifoveal retina; 30° and 12° central visual fields; Lanthony 15-hue color vision test; automated static contrast sensitivity test; and global electroretinography.
Main Outcomes and Measures
The OCT thickness maps were checked for areas of retinal thinning, appearing as blue patches. When present, these areas were compared with the areas of superficial and deep capillary flow loss on OCT angiography and with the scotomas on visual fields. Contrast sensitivity and color vision loss were quantified.
Results
All 3 patients included had homozygous sickle cell disease. They presented with a 20/20 distance visual acuity, and Parinaud 1,5 near visual acuity in both eyes. They were all followed up for a severe cerebral vasculopathy related to sickle cell disease. The areas of atrophy involved the inner retinal layers and were associated with an absence of signal in the deep capillary plexuses in OCT angiography. These patches of retinal thinning were also matching with scotomas in the automated visual fields. Color vision ability and contrast sensitivity were impaired in all patients. Global electroretinography findings were normal.
Conclusions and Relevance
Temporal macular atrophy in sickle cell disease may have direct consequences on visual function, including in children, even when visual acuity is preserved. Optical coherence tomographic imaging may be warranted when evaluating patients with sickle cell disease, even if asymptomatic with 20/20 visual acuity.
This case series assesses the visual function of 3 patients with sickle cell disease and temporal macular atrophy but with no visual symptoms.
Introduction
Macular involvement has been described in patients with sickle cell disease (SCD) since the early 1970s. Initially, macular asymptomatic capillary changes were reported using fluorescein angiography in about 32% of adult patients with homozygous (HbSS) SCD and 36% of those with double heterozygous (HbSC) SCD. In a functional study using 30° automated static perimetry in 1987, Lee et al showed in adults that macular areas of arteriolar occlusions were actually associated with paracentral scotomas. More recently, owing to the development of optical coherence tomography (OCT), asymptomatic areas of thinning of the inner retinal layers, predominating along the median temporal raphe, were found in about 48% of adults with HbSS SCD and 35% of those with HbSC SCD. By using OCT angiography (OCT-A), it was then shown that these areas of retinal thinning were associated with signs of reduced vascular flow in the superficial and deep capillary plexuses. Such atrophy is usually considered asymptomatic; to our knowledge, only 1 series has assessed the visual function of these patients with more than visual acuity evaluation, using scanning laser ophthalmoscope microperimetry and showing correlations between retinal thinning and decreased retinal sensitivity. We report 3 cases of HbSS SCD—2 children and 1 young adult—seen in the context of systematic screening with normal visual acuity, no clinical sign of SCD proliferative retinopathy, and in whom OCT showed bilateral areas of macular and perimacular thinning. Complete evaluation of their visual function was therefore performed.
Report of Cases
The 3 patients were diagnosed as having HbSS SCD and followed up in the ophthalmology department for an annual screening examination. Institutional review board approval from an ethics committee was waived as this is a retrospective medical record review. Consent to use data from medical records was obtained from patients.
Case 1
A preteen boy was included in a transfusion program for 3 years because of SCD cerebral vasculopathy. Transcranial Doppler had detected an abnormal velocity in the intrapetrous segment of the left internal carotid artery (ICA). The computed tomography angiography confirmed a 36% stenosis of the left ICA. Moreover, a 61% stenosis of the contralateral ICA was found, affecting the ophthalmic artery ostium, undetected by transcranial Doppler for technical reasons. Brain magnetic resonance imaging demonstrated a silent infarct in the left hemisphere on fluid-attenuated inversion recovery–weighted images and a 33% decreased cerebral blood flow in the same hemisphere on arterial spin labeling sequences.
Case 2
A boy in his early teens was included in a transfusion program 7 years prior because of a history of pathological transcranial Doppler in the left middle cerebral artery. Brain magnetic resonance imaging angiography performed a year prior showed a severe narrowing of the left ICA in its terminal part, extended to the middle cerebral artery, and associated with left moyamoya. Fluid-attenuated inversion recovery sequences demonstrated ischemic lesions in the left anterior and middle cerebral arteries territories. Arterial spin labeling sequences showed a decreased cerebral blood flow in the ischemic lesions, but were found normal in all other left and right cortical regions.
Case 3
A man in his mid-20s had severe chronic hemolytic anemia, with a baseline hemoglobin level around 5.5 g/dL (to convert to grams per liter, multiply by 10), and history of several acute chest syndromes, having led to a transfusion program in his teens. Brain magnetic resonance imaging angiography performed the following year showed an absence of signal in the precommunicant segment of the right anterior cerebral artery, suggesting a complete stenosis. Two silent infarcts were found in the right middle cerebral artery territory and 1 in the left anterior watershed territory. Contrast perfusion imaging demonstrated a 50% decreased cerebral blood flow in the left anterior watershed territory.
Results
All patients exhibited 20/20 distance visual acuity and Parinaud 1,5 near visual acuity in both eyes and did not have any visual symptoms, except for case 2, who, when asked about it retrospectively, had noticed a spot in his left eye for several years. Fundus examination only showed 1 black sunburst spot in the left eye of case 1 and in both eyes for case 2. None of the 3 patients showed any sign of proliferative sickle retinopathy (PSR). Figure 1, Figure 2, and Figure 3 show the findings of multimodal imaging and visual function testing for cases 1, 2, and 3, respectively. In all cases, OCT showed bilateral areas of focal macular and perimacular retinal atrophy, appearing as blue areas on the 12 mm × 9-mm thickness maps computed from the OCT volume (OCT RTVue XR100 Avanti Edition; Optovue). This retinal thinning was predominant in the inner layers, as seen on the horizontal, vertical, and en face OCT sections. These areas of atrophy matched with a decreased signal in the deep capillar vasculature, as seen on the 6 × 6 mm central OCT-A. In all cases, alterations of the macular function were noticed on the 30° and 12° automated central visual fields (scotomas matched the areas of retinal thinning), the Lanthony 15-hue color vision test, and an automated static contrast sensitivity test (Metrovision). Global electroretinography (MonPackONE; Metrovision) performed following the revised International Society for Clinical Electrophysiology of Vision recommendations and using DTL electrodes showed normal responses for both the photopic and scotopic systems in all 3 cases compared with our normative data.
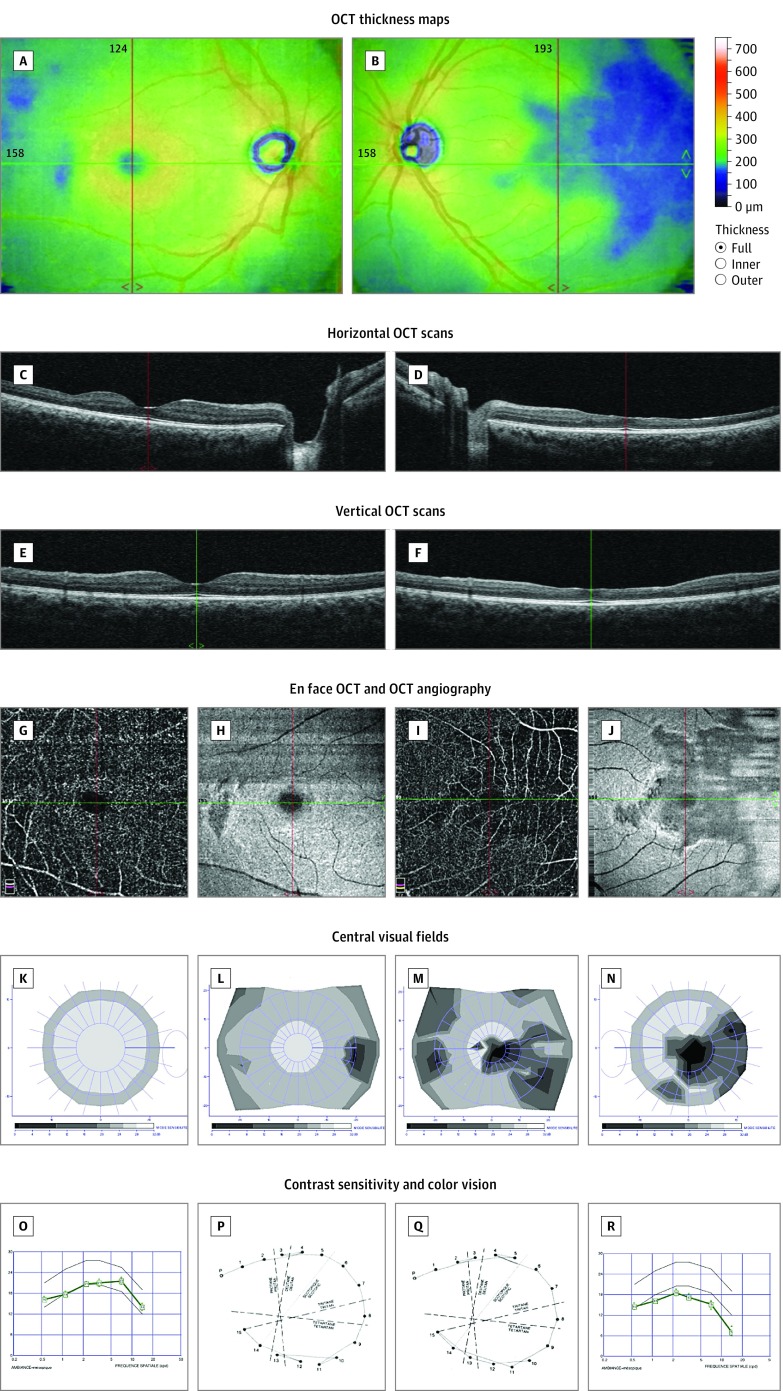
Figure 1. Multimodal Imaging and Detailed Macular Function Evaluation in Case 1.
Optical coherence tomography (OCT) thickness maps showing areas of retinal thinning in both eyes (A and B). Horizontal (C and D), vertical (E and F), and en face (H and J) OCT scans showing thinning of the inner retinal layers, with loss of the normal foveal pit in the left eye. OCT angiography showing no signal in the deep capillary plexuses in the areas of thinning (G and I). Twelve-degree and 30° central visual fields showing scotomas matching the retinal defects (K-N). Contrast sensitivity (O and R) and color vision (P and Q) were significantly affected in the left eye. Composite figures are shown to illustrate a clear parallel between the anatomical lesions and the impaired visual function. A, C, E, G, H, K, L, O, and P are of the right eye; B, D, F, I, J, M, N, Q, and R are of the left eye.
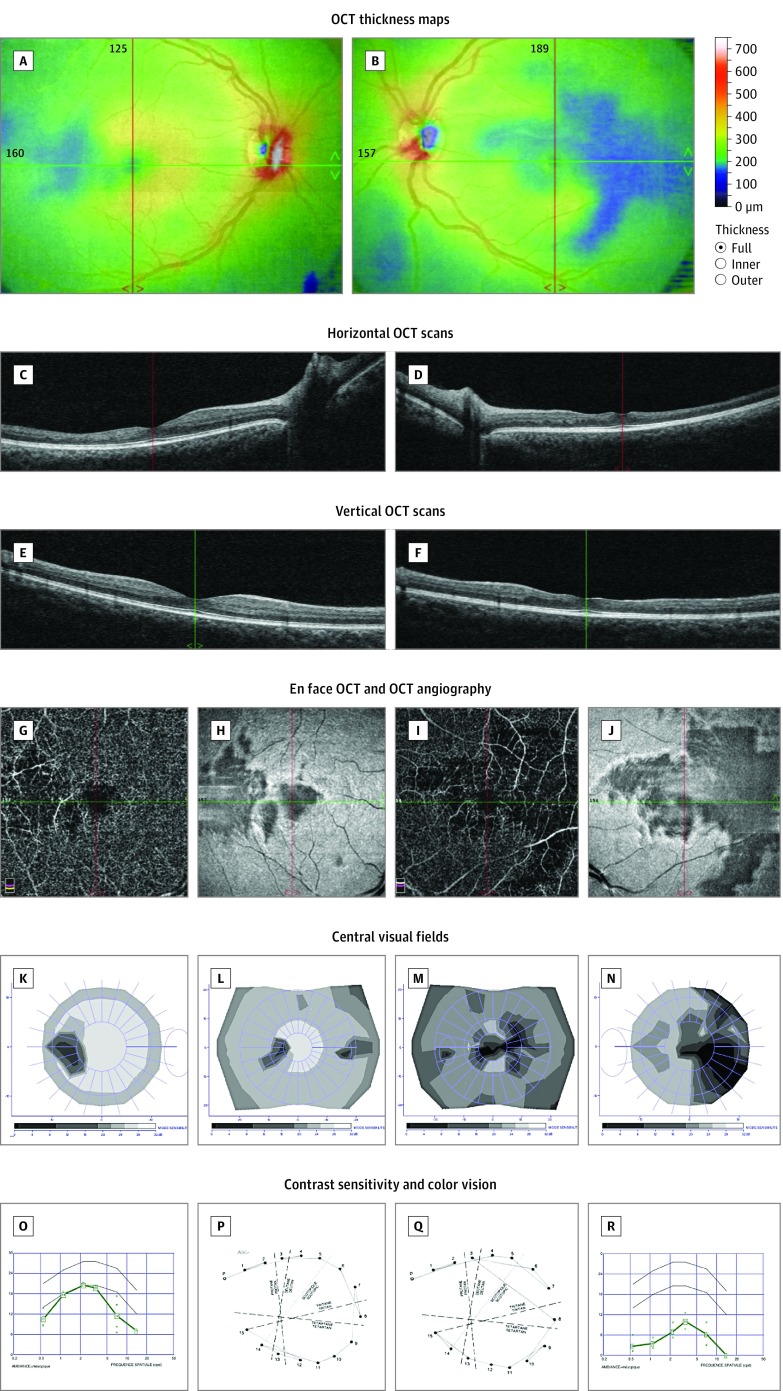
Figure 2. Multimodal Imaging and Detailed Macular Function Evaluation in Case 2.
Optical coherence tomography (OCT) thickness maps showing areas of retinal thinning in both eyes (A and B). Horizontal (C and D), vertical (E and F), and en face (H and J) OCT scans showing thinning of the inner retinal layers, with loss of the normal foveal pit in the left eye. OCT angiography showing no signal in the deep capillary plexuses in the areas of thinning (G and I). Twelve-degree and 30° central visual fields showing scotomas matching the retinal defects (K-N). Contrast sensitivity (O and R) and color vision (P and Q) were affected in both eyes. Composite figures are shown to illustrate a clear parallel between the anatomical lesions and the impaired visual function. A, C, E, G, H, K, L, O, and P are of the right eye; B, D, F, I, J, M, N, Q, and R are of the left eye.
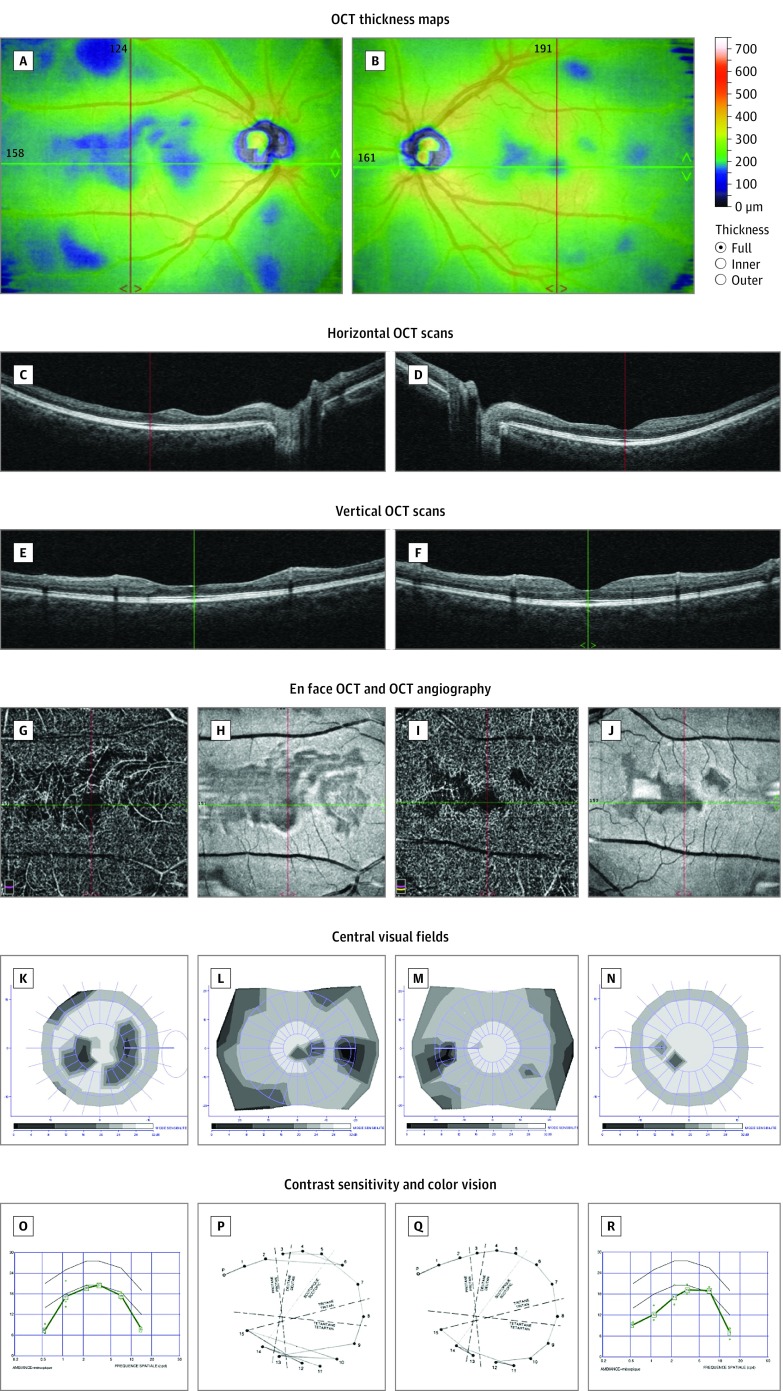
Figure 3. Multimodal Imaging and Detailed Macular Function Evaluation in Case 3.
Optical coherence tomography (OCT) thickness maps showing areas of retinal thinning in both eyes (A and B). Horizontal (C and D), vertical (E and F), and en face (H and J) OCT scans showing thinning of the inner retinal layers, with loss of the normal foveal pit in the right eye. OCT angiography showing no signal in the deep capillary plexuses in the areas of thinning (G and I). Twelve-degree and 30° central visual fields showing scotomas matching the retinal defects (K-N). Contrast sensitivity was affected in both eyes (O and R), and color vision defect predominated in the right eye (P and Q). Composite figures are shown to illustrate a clear parallel between the anatomical lesions and the impaired visual function. A, C, E, G, H, K, L, O, and P are of the right eye; B, D, F, I, J, M, N, Q, and R are of the left eye.
Discussion
The 3 cases described here show that the visual function of asymptomatic patients with SCD with macular atrophy can be affected. Recent years have seen renewed interest for macular involvement in SCD, with the description of areas of inner retinal layers thinning by using spectral-domain OCT and OCT-A. The pathophysiological process leading to the underlying atrophic process remains unknown. Some authors have considered that this retinal atrophy resulted from the same mechanisms as PSR: localized arteriolar occlusions due to sickled erythrocytes, leading to permanent or transient capillary nonperfusion and retinal ischemia and thinning. They suggested that such macular atrophic areas precluded PSR. However, these areas seem to be more frequently encountered in HbSS than in HbSC patients, unlike PSR, which is far less frequent in HbSS than in HbSC patients. In 2012, we hypothesized that the retinal atrophy at the level of the temporal median raphe in SCD could represent the ocular equivalent of a silent borderzone cerebral infarct, both processes occurring in junctional territories of the brain and the eye.
Cerebrovascular complications are a major concern in young patients with HbSS SCD. While the long-term motor and cognitive effects of cerebral strokes have long been known, studies have recently addressed the question of the possible expression of the—even more frequent— “silent” cerebral infarcts in SCD and showed that they were associated with severe cognitive impairment. As a result, the indications of a prevention program with annual screening in children with SCD have been extended.
Limitations
These are 3 illustrative cases with large and profound areas of retinal atrophy, while in most cases encountered, the atrophic lesions appear less severe on OCT. One can only speculate so far that in all other cases, the impairment of the visual function would be proportional to the severity of the anatomical lesions.
Conclusions
Current recommendations for the ophthalmological follow-up of patients with SCD are exclusively based on the risk for PSR owing to its long-known dramatic possible consequences. Anatomical alterations of the central retina in patients with SCD are currently extensively described and referred to as asymptomatic. They are more frequent than PSR and do not occur in the same patients. Not only are they of considerable interest for the better understanding of the disease pathophysiology, but, as is the case for “silent” borderzone cerebral infarct on cognitive functions, they can also result in visual function impairment. This small case series stresses the need for large studies focusing on the functional consequences and evolution of SCD maculopathy.
References
- 1.Stevens TS, Busse B, Lee CB, Woolf MB, Galinos SO, Goldberg MF. Sickling hemoglobinopathies: macular and perimacular vascular abnormalities. Arch Ophthalmol. 1974;92(6):455-463. [DOI] [PubMed] [Google Scholar]
- 2.Asdourian GK, Nagpal KC, Busse B, et al. Macular and perimacular vascular remodelling sickling haemoglobinopathies. Br J Ophthalmol. 1976;60(6):431-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CM, Charles HC, Smith RT, Peachey NS, Cunha-Vaz JG, Goldberg MF. Quantification of macular ischaemia in sickle cell retinopathy. Br J Ophthalmol. 1987;71(7):540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murthy RK, Grover S, Chalam KV. Temporal macular thinning on spectral-domain optical coherence tomography in proliferative sickle cell retinopathy. Arch Ophthalmol. 2011;129(2):247-249. [DOI] [PubMed] [Google Scholar]
- 5.Mathew R, Bafiq R, Ramu J, et al. Spectral domain optical coherence tomography in patients with sickle cell disease. Br J Ophthalmol. 2015;99(7):967-972. [DOI] [PubMed] [Google Scholar]
- 6.Minvielle W, Caillaux V, Cohen SY, et al. Macular microangiopathy in sickle cell disease using optical coherence tomography angiography. Am J Ophthalmol. 2016;164:137-44.e1. [DOI] [PubMed] [Google Scholar]
- 7.Han IC, Tadarati M, Scott AW. Macular vascular abnormalities identified by optical coherence tomographic angiography in patients with sickle cell disease. JAMA Ophthalmol. 2015;133(11):1337-1340. [DOI] [PubMed] [Google Scholar]
- 8.Chow CC, Genead MA, Anastasakis A, Chau FY, Fishman GA, Lim JI. Structural and functional correlation in sickle cell retinopathy using spectral-domain optical coherence tomography and scanning laser ophthalmoscope microperimetry. Am J Ophthalmol. 2011;152(4):704-711.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCulloch DL, Marmor MF, Brigell MG, et al. ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015;130(1):1-12. [DOI] [PubMed] [Google Scholar]
- 10.Ghasemi Falavarjani K, Scott AW, Wang K, et al. Correlation of multimodal imaging in sickle cell retinopathy [published online August 4, 2016]. Retina. doi: 10.1097/IAE.0000000000001230 [DOI] [PubMed] [Google Scholar]
- 11.Jampol LM, Rabb MF. Vasoocclusive diseases of the posterior pole. Int Ophthalmol Clin. 1981;21(3):201-213. [DOI] [PubMed] [Google Scholar]
- 12.Hoang QV, Chau FY, Shahidi M, Lim JI. Central macular splaying and outer retinal thinning in asymptomatic sickle cell patients by spectral-domain optical coherence tomography. Am J Ophthalmol. 2011;151(6):990-994.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elagouz M, Jyothi S, Gupta B, Sivaprasad S. Sickle cell disease and the eye: old and new concepts. Surv Ophthalmol. 2010;55(4):359-377. [DOI] [PubMed] [Google Scholar]
- 14.Robert MP, Ingster-Moati I, Roche O, et al. Asymptomatic atrophy of the temporal median raphe of the retina associated with cerebral vasculopathy in homozygous sickle cell disease. J AAPOS. 2012;16(4):394-397. [DOI] [PubMed] [Google Scholar]
- 15.Bernaudin F, Verlhac S, Fréard F, et al. Multicenter prospective study of children with sickle cell disease: radiographic and psychometric correlation. J Child Neurol. 2000;15(5):333-343. [DOI] [PubMed] [Google Scholar]