Abstract
Background
Psoriasis is a chronic inflammatory skin disease, affecting patients of a wide age range, including elderly patients. Elderly patients can respond differently to drug treatments and can be more vulnerable to adverse reactions. There are limited data on biologic therapies for psoriasis in elderly subjects. Secukinumab, a fully human monoclonal antibody that selectively neutralizes IL-17A, has proven significant efficacy in the treatment of moderate to severe psoriasis.
Aims
A post-hoc analysis of three phase III trials (ERASURE, FIXTURE and CLEAR) was performed to evaluate the efficacy and safety of secukinumab in elderly subjects.
Methods
Studies were multicentre, randomized, parallel-group, double-blind, 52-week phase III trials in subjects with moderate to severe plaque psoriasis. For efficacy analyses, 67 elderly subjects (≥ 65 years) treated with secukinumab 300 mg were compared with 841 younger subjects (18–64 years). Psoriasis Area and Severity Index (PASI), Dermatological Life Quality Index (DLQI) and safety were analysed.
Results
Elderly subjects had higher baseline frequencies of cardiovascular and metabolic disorders. Secukinumab efficacy in elderly subjects was comparable to that in younger subjects throughout 52 weeks of treatment. PASI 75 response was reached by 81.8% of elderly subjects and 79.4% of younger subjects at Week 52. Similar rates of DLQI 0/1 response were observed. The total rate of adverse events was similar between elderly and younger subjects.
Conclusions
Secukinumab at the recommended dose (300 mg) is effective and acceptably safe in subjects aged ≥ 65 years with moderate to severe psoriasis, with quality-of-life benefits, despite an increased prevalence of cardiovascular and metabolic comorbidities in this population.
Electronic supplementary material
The online version of this article (10.1007/s40266-018-0520-z) contains supplementary material, which is available to authorized users.
Key Points
| Elderly patients can respond differently to drug treatments and can be more vulnerable to side effects. |
| Little is known about efficacy and safety of biologic therapies for psoriasis in elderly subjects. |
| Secukinumab, a fully human monoclonal antibody that selectively neutralizes IL-17A, has previously shown significant efficacy in the treatment of psoriasis. |
| This analysis of clinical trial patients by age shows that secukinumab at the recommended dose (300 mg) is effective and well tolerated in people aged ≥ 65 years, including quality-of-life benefits. |
Introduction
Psoriasis vulgaris is a chronic immune-mediated inflammatory disease with a complex genetic background. Psoriasis is characterized by erythematous scaly plaques, and has a broad clinical spectrum, with a massive impact on quality of life. About 2–3% of the population in industrialized countries is affected by psoriasis, which can present as the mild form, affecting only elbows and knees, or as moderate to severe disease, involving large areas of the skin [1]. Psoriasis is established as a systemic inflammatory disease with an increased risk of various comorbidities. Associated comorbidities include cardiovascular diseases (CVD), obesity, diabetes mellitus, metabolic syndrome, depression or psoriatic arthritis (PsA) [2–4].
Due to the chronic nature of psoriasis and to the ageing of the general population, elderly patients are a patient group of emerging clinical relevance. Many elderly subjects with psoriasis are inadequately treated and experience adverse consequences, both physically and psychologically [5]. Elderly individuals with psoriasis are often excluded from clinical trials and studies, leading to limited availability of data about the clinical features and toxicities in this group.
Elderly subjects also show several characteristics that distinguish them in terms of pharmacokinetics and pharmacodynamics. Elderly subjects exhibit altered distribution volumes (e.g., decreased muscle and increased fat content of the body), reduced liver metabolism and reduced renal function. Furthermore, the immune system shows age-dependent changes, the so-called immunosenescence. Due to their increased number of comorbidities, elderly subjects often receive several medications for various indications at the same time (polymedication), which makes them vulnerable to adverse drug interactions and can potentially produce a higher rate of adverse events. It is therefore important that treatments are chosen and monitored carefully in this at-risk population [6].
Secukinumab, a fully human monoclonal antibody that selectively neutralizes IL-17A, has significant efficacy in the treatment of moderate to severe psoriasis and PsA, demonstrating a rapid onset of action and sustained responses with a favourable safety profile [7–10]. In Europe, secukinumab was the first biologic treatment to be approved for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy, without the precondition of failure to respond, contraindication, or intolerance to other systemic therapies [11]. There are limited data available on the efficacy and safety of biologic treatments in elderly subjects with psoriasis. To evaluate general differences in baseline characteristics between elderly and younger patients as well as the safety and efficacy of secukinumab in elderly subjects, we performed a post-hoc analysis of three phase III trials. A total of 67 elderly subjects (≥ 65 years) affected by chronic plaque psoriasis who were treated with secukinumab 300 mg were identified and compared with 841 younger subjects (18–64 years) treated with secukinumab 300 mg.
Methods
Study Design and Subjects
The following three studies were included into this pooled post-hoc analysis: ERASURE (ClinicalTrials.gov number NCT01365455), FIXTURE (NCT01358578) and CLEAR (NCT02074982). Throughout the analysis, subjects were grouped by their age at the time of randomization: elderly subjects (65 years of age or older) and younger subjects (18–64 years of age). The cut-off was chosen as a standard used in the literature and mean age at baseline of the two groups was evaluated and compared for validation.
All three studies were multicentre, randomized, parallel-group, double-blind, 52-week phase III trials assessing the efficacy and safety of secukinumab in different dosing and treatment regimens for the treatment of moderate to severe plaque psoriasis. The ERASURE study compared efficacy and safety of secukinumab at doses of 150 and 300 mg, including a placebo arm, and enrolled 738 subjects. The FIXTURE study compared the efficacy and safety of secukinumab 150, 300 mg and etanercept 50 mg, including a placebo arm, and enrolled 1306 subjects. The CLEAR study compared secukinumab 300 mg with ustekinumab 45/90 mg (per label) and enrolled 676 subjects. Eligibility criteria were similar throughout all three studies. Briefly, subjects were 18 years of age or older with moderate to severe plaque psoriasis that had been diagnosed at least 6 months before randomization and was poorly controlled with topical treatments, phototherapy, previous systemic therapy, or any combination of these. In addition, subjects had a score of 12 or higher on the Psoriasis Area and Severity Index (PASI; a composite evaluation instrument for psoriasis severity, on a scale of 0–72, with higher scores indicating more severe disease), a score of 3 or 4 on the modified Investigator’s Global Assessment (IGA mod 2011 0/1 or IGA; a static, 5-point instrument for rating the clinician’s impression of the overall severity of psoriasis, on a scale from 0 [clear skin] to 4 [severe disease]) [12] and involvement of 10% or more of the body surface area (BSA). Subjects with other forms of plaque psoriasis or drug-induced psoriasis were excluded. In the current analysis population of all three trials, secukinumab 300 mg was applied subcutaneously once weekly at weeks 0, 1, 2, 3 and 4 followed by injections every 4 weeks until week 48. The study design, methods, and results of all three studies were previously described [10, 13, 14]. The studies received ethical approval from the appropriate institutional review boards and were conducted in accordance with the principles of the Declaration of Helsinki.
Data from two further phase III trials (SCULPTURE [15] and STATURE [16]) were not included in the analysis, as these studies did not include non-secukinumab comparator groups and differed considerably in design, and hence did not lend themselves for pooling with the studies included in this analysis.
For the comparison of efficacy and safety, only patients having received secukinumab 300 mg were included in the analysis. For the comparison of baseline characteristics, two analyses were performed. The first analysis included only patients who have received secukinumab 300 mg (analysis set). In addition, a second analysis was performed that included all patients from the three pooled studies, irrespective of their treatment arm (secukinumab 300 mg, secukinumab 150 mg, etanercept, ustekinumab, placebo). This full baseline analysis allowed a maximum possible sample size, making statements about differences between elderly and younger psoriasis subjects more generalizable.
Subjects’ medical history of cardiometabolic comorbidities (only available for ERASURE and FIXTURE, not for CLEAR, which is the reason for the lower number of analysed patients [N] for this parameter), smoking history and psoriasis history were assessed at screening visit in a separate electronic case report form (eCRF) page. All laboratory tests were assessed from blood samples drawn at baseline. The presence of metabolic syndrome was defined as fulfilment of the 2009 consensus definition criteria [17].
Efficacy and Safety Analyses
For the analysis of efficacy and safety parameters, the following groups of subjects were included into the analysis: ERASURE study: secukinumab 300-mg treatment arm; FIXTURE study: secukinumab 300-mg treatment arm; CLEAR study: secukinumab 300-mg treatment arm. The 150-mg arms from ERASURE and FIXTURE were not included in the analysis, as 300 mg is the recommended dose per worldwide labels. There are no efficacy or safety data shown for etanercept or ustekinumab, as the group sizes would be too small for a meaningful comparison between age groups.
Disease activity was assessed by the use of PASI and IGA mod 2011. Efficacy data are presented as percentage of subjects with a score of 0 or 1 on IGA mod 2011 (including a reduction from baseline of ≥ 2) and as percentage of subjects achieving PASI 75, 90 or 100 response. PASI 75, 90 or 100 response means a 75% or more, 90% or more, or 100% improvement from baseline PASI. Health-related quality of life (HRQoL) was assessed by the use of the Dermatology Life Quality Index (DLQI), a validated instrument for dermatologic conditions (scores range from 0–30 points, with higher scores indicating a greater effect of the disease on patient’s QoL [18]. HRQoL data are shown per time point as mean DLQI and the percentage of subjects achieving a DLQI score of 0/1 (corresponding to minimal to no impact of disease on QoL).
Safety was evaluated by monitoring adverse events, including the severity of the event and the relationship of the event to the study drug, and by obtaining clinical laboratory measurements, assessing vital signs, and performing physical examinations at each study visit. There are no safety data shown for etanercept or ustekinumab, as the group sizes would be too small for a meaningful comparison between age groups.
For the entire study period, common adverse events were defined as adverse events that occurred in ≥ 5.0% of either elderly or younger subjects; for the initial 12 weeks of treatment the cut-off was ≥ 2%.
Statistical Analysis
Statistical analyses were all conducted post hoc. The full analysis set for subjects treated with secukinumab 300 mg (label dose) was used for efficacy parameter analyses (PASI, IGA, DLQI), the safety set was used for the safety analysis. Non-responder imputation was applied for PASI response, percentage of patients with PASI ≤ 1, 2, 3, 5 and percentage of patients with a score of 0 or 1 on IGA. Last observation carried forward (LOCF) imputation was applied for DLQI data. For the comparison of baseline characteristics, a 2 sample t test was used for continuous variables (e.g. age, weight) and a Chi square test for categorical variables (e.g. percentage of smokers). No correction for multiple testing was applied. Because of the post-hoc nature of the analysis and the high imbalance in patient numbers between the two groups, no statistical testing was performed for efficacy data.
Results
Baseline Characteristics of Elderly Psoriasis Subjects in Comparison to Younger Psoriasis Subjects
A broad range of patient characteristics at baseline were analysed in order to differentiate the clinical characteristics of elderly and younger psoriasis subjects. In the analysis set treated with secukinumab 300 mg (elderly [n = 67] and younger subjects [n = 842]), baseline characteristics are presented in Table 1, including psoriasis history, medical history of cardiometabolic comorbidity, and laboratory assessment. There was a significant difference in mean age between elderly and younger groups (69.3 years vs 42.9 years, p < 0.0001), underlining the validity of the ‘65 years’ cut-off for division of the population into elderly and younger subjects. Elderly subjects showed longer disease duration than younger subjects. There were significant differences in the prevalence of several cardiovascular and metabolic comorbidities, including diabetes and hypertension, which showed higher prevalence in the elderly patient population (Table 1). Furthermore, elderly patients showed significantly higher creatinine values, reflecting a decline in kidney function. A slightly higher psoriasis disease severity was seen in younger patients, who were also more likely to smoke than elderly patients (Table 1).
Table 1.
Baseline characteristics of the analysis population comprising patients treated with secukinumab 300 mg for up to 52 weeks in ERASURE, FIXTURE and CLEAR
| Variable | Elderly patients (≥ 65 years) | Younger patients (18 to < 65 years) | p valued |
|---|---|---|---|
| N | 67 | 842 | |
| Demographic characteristics | |||
| Age, mean years | 69.3 | 42.9 | < 0.0001 |
| Male sex, % | 61.2 | 69.0 | 0.0069 |
| Race, % | |||
| Asian | 14.9 | 16.4 | |
| Black | 1.5 | 1.1 | |
| Caucasian | 77.6 | 76.5 | |
| Unknown/other | 6.0 | 6.1 | |
| Weight, mean kg (SD) | 82.3 (17.4) | 86.5 (22.1) | NS |
| BMI, mean kg/m2 (SD) | 29.6 (5.4) | 29.1 (6.6) | NS |
| Waist circumference, mean cm | 101.7 | 99.0 | NS |
| Current smokers, % | 14.9 | 40.3 | < 0.0001 |
| Psoriasis history | |||
| Baseline PASI score, mean (SD) | 20.2 (7.5) | 22.9 (9.4) | 0.0081 |
| % of subjects with PASI ≤ 20 | 62.7 | 49.8 | |
| % of subjects with PASI > 20 to ≤ 30 | 25.4 | 30.4 | |
| % of subjects with PASI > 30 | 11.9 | 19.8 | |
| Baseline DLQI score, mean (SD) | 9.9 (7.0) | 13.8 (7.4) | < 0.0001 |
| Psoriatic arthritis present, % | 20.9 | 19.2 | NS |
| Time since first diagnosis of psoriasis, mean years (SD) | 26.6 (18.9) | 17.0 (11.3) | 0.0001 |
| Previous systemic treatment | |||
| Any, % | 68.7 | 65.2 | NS |
| Biologic agent, % | 19.4 | 17.0 | NS |
| TNF inhibitor, % | 16.4 | 10.8 | NS |
| Previous TNF inhibitor failure, % | 11.9 | 6.1 | NS |
| Medical history of cardiometabolic comorbiditiesa | |||
| Diabetesb, % | 23.1 | 9.4 | 0.0066 |
| Hypertension, % | 71.8 | 20.8 | < 0.0001 |
| Dyslipidaemia, % | 41.0 | 15.9 | 0.0001 |
| Myocardial infarction, % | 7.7 | 1.9 | 0.0186 |
| Stable coronary artery disease, % | 10.3 | 1.7 | 0.0005 |
| Atrial fibrillation, % | 2.6 | 0.9 | NS |
| Peripheral arterial disease, % | 0.0 | 0.2 | NS |
| Stroke, any type, % | 2.6 | 0.6 | NS |
| Metabolic syndrome presentc, % | 35.8 | 31.7 | NS |
| Laboratory assessment (all mean values) | |||
| Creatinine, mg/dL | 86.9 | 77.3 | 0.0022 |
| GOT, U/L | 23.7 | 24.7 | NS |
| GPT, U/L | 22.3 | 28.2 | 0.0006 |
| γGT, U/L | 29.2 | 32.6 | NS |
| Haemoglobin, g/L | 140.8 | 146/2 | 0.0022 |
| LDL cholesterol, mmol/L | 3.0 | 3.2 | NS |
| HDL cholesterol, mmol/L | 1.4 | 1.3 | NS |
| Triglycerides, mmol/L | 1.5 | 1.6 | NS |
| Fasting plasma glucose, mg/dL | 110.8 | 99.2 | 0.0054 |
aPrior and ongoing conditions are included. For all cardiometabolic comorbidities except metabolic syndrome: N = 39 for elderly patients and N = 533 for younger patients (without CLEAR patients). For metabolic syndrome: N = 67 for elderly patients and N = 842 for younger patients
bIncludes type 1 and type 2 diabetes mellitus
cMetabolic syndrome defined as ≥ 3 of five criteria: (1) SBP/DBP ≥ 130/85; (2) fasting plasma glucose ≥ 100 mg/dL; (3) HDL < 40 mg/dL in men/< 50 mg/dL in women; (4) triglycerides ≥ 150 mg/dL; (5) elevated waist circumference as per ethnicity
dp values < 0.05 were regarded as statistically significant
BMI body mass index, DBP diastolic blood pressure, DLQI Dermatology Life Quality Index, GOT glutamic-oxaloacetic transaminase, GPT glutamic-pyruvic transaminase, HDL high-density lipoprotein, LDL low-density lipoprotein, NS not significant, PASI Psoriasis Area and Severity Index, SBP systolic blood pressure, SD standard deviation, γGT gamma glutamyl transferase
Baseline characteristics were additionally assessed in the full set of elderly subjects (n = 189) and younger subjects (n = 2531) randomized to any treatment arm in the three clinical trials (Supplementary Table 1, see electronic supplementary material [ESM]). Similar trends were observed, indicating that the baseline information for the analysis set is reflective of a larger population.
Efficacy of Secukinumab in Elderly Subjects Compared with Younger Subjects with Psoriasis
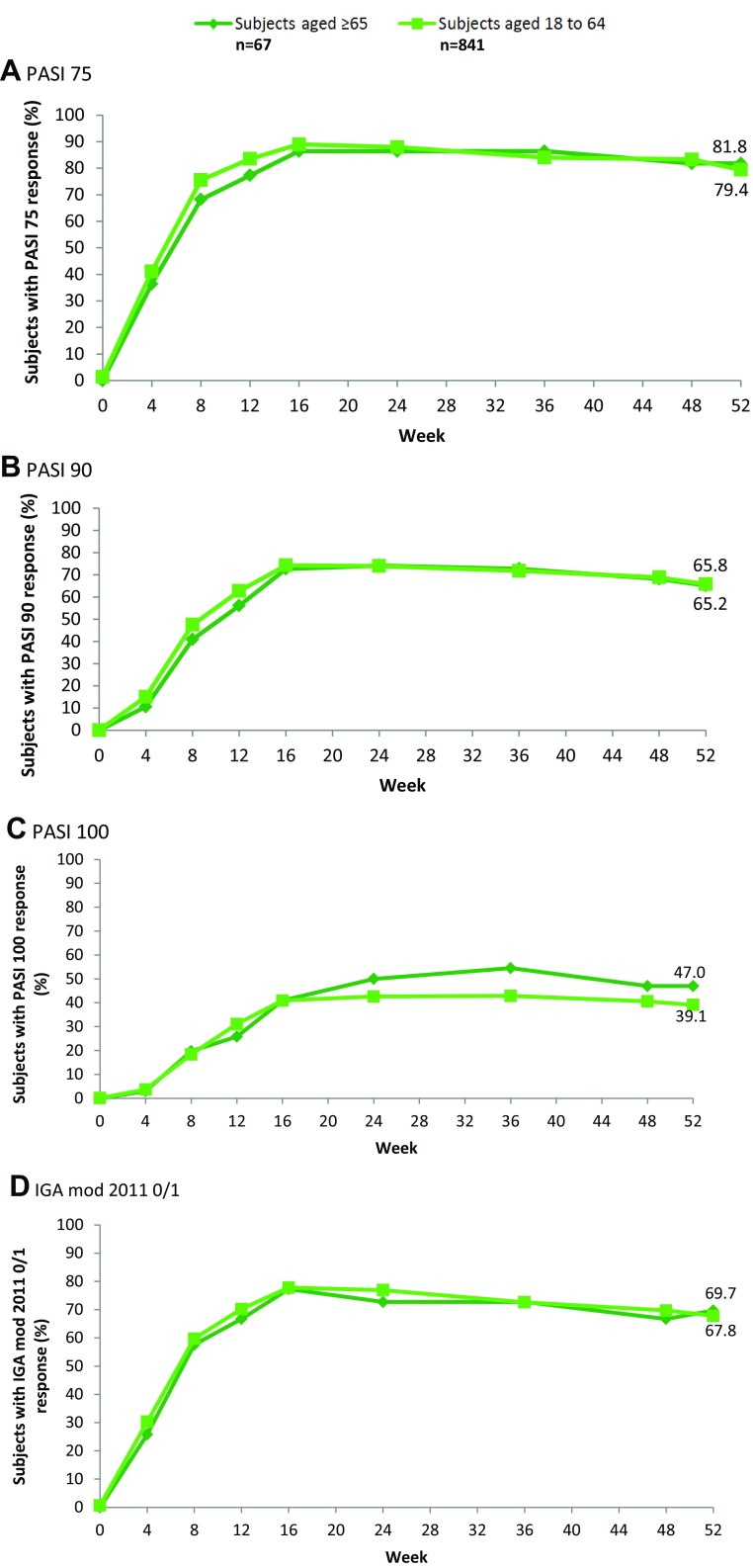
For the efficacy analysis, only those patients in the pooled study population who received secukinumab 300 mg were included. This population comprised 67 elderly patients and 841 younger patients. The percentage of subjects achieving PASI 75, 90 and 100 and IGA responses under secukinumab 300 mg treatment over time is shown in Fig. 1. At Week 16, 86.4%, 72.7% and 40.9% of elderly patients and 89.0, 74.3 and 40.9% of younger patients achieved PASI 75, 90, and 100 responses, respectively. The efficacy of secukinumab in elderly subjects is comparable to its efficacy in younger subjects up to Week 52, at which point 81.8% of elderly subjects and 79.4% of younger subjects were PASI 75 responders. Secukinumab showed a similar time to onset as well as sustained efficacy until Week 52 in both groups (Fig. 1).
Fig. 1.
Secukinumab efficacy in elderly and younger subjects in pooled studies. PASI and IGA mod 2011 0/1 response rates in the analysis population comprising patients treated with secukinumab 300 mg for up to 52 weeks in ERASURE, FIXTURE and CLEAR (non-responder imputation). PASI Psoriasis Area and Severity Index, DLQI Dermatological Life Quality Index, IGA mod 2011 0/1 modified Investigator’s Global Assessment
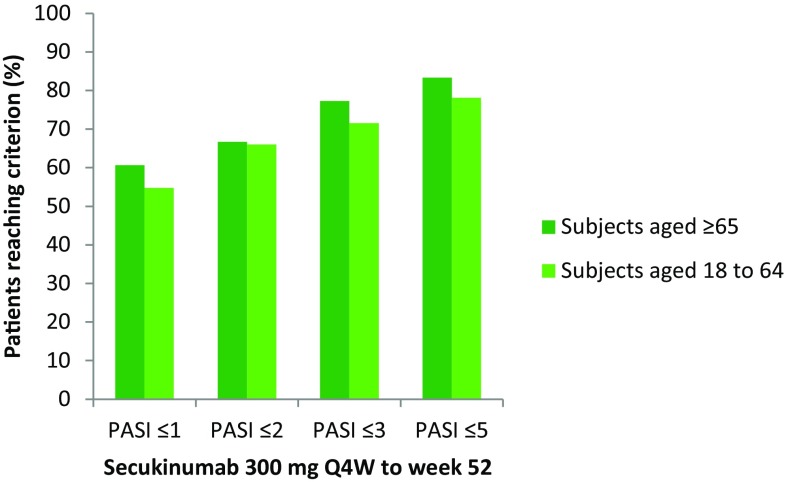
Similar proportions of both treatment groups had an absolute PASI score ≤ 5, ≤ 3, ≤ 2 or ≤ 1 at Week 52 (Fig. 2).
Fig. 2.
Absolute PASI scores at Week 52 in the analysis population comprising patients treated with secukinumab 300 mg for up to 52 weeks in ERASURE, FIXTURE and CLEAR. PASI Psoriasis Area and Severity Index, Q4 W every 4 weeks
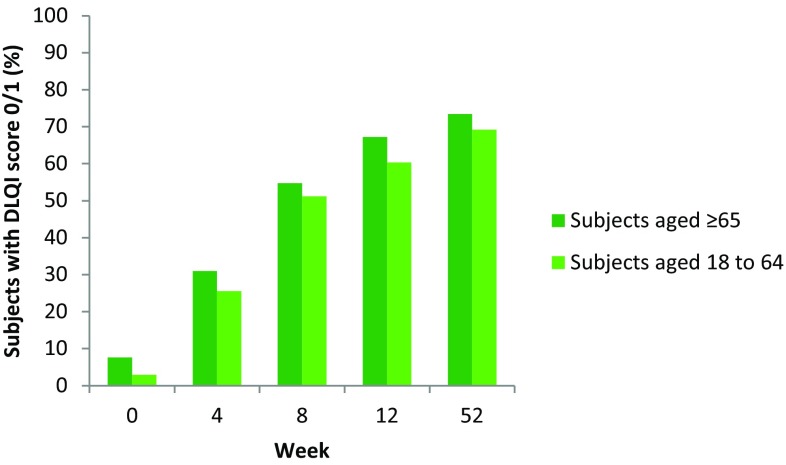
DLQI score decreased from a mean of 9.4 ± 7.0 at baseline to a mean of 2.0 ± 4.1 at Week 52 in elderly subjects and from 13.8 ± 7.4 to 2.2 ± 4.4 in younger subjects (Table 2). The percentage of subjects achieving DLQI 0/1 under secukinumab 300-mg treatment at different time points is shown in Fig. 3. A similar proportion of subjects in each age group attained DLQI 0/1 response at each time point.
Table 2.
Mean absolute DLQI score per visit in elderly and younger subjects—DLQI scores for the analysis population comprising patients treated with secukinumab 300 mg for up to 52 weeks in ERASURE, FIXTURE and CLEAR
| Visit | Mean (SD) DLQI score in patients aged ≥ 65 years (n = 67) | Mean (SD) DLQI score in patients aged 18 to < 65 years (n = 841) |
|---|---|---|
| Baseline | 9.4 (7.0) | 13.8 (7.4) |
| Week 4 | 3.6 (3.8) | 5.5 (5.4) |
| Week 8 | 2.7 (3.7) | 3.0 (4.1) |
| Week 12 | 2.1 (3.5) | 2.4 (3.9) |
| Week 52 | 2.0 (4.1) | 2.2 (4.4) |
DLQI Dermatology Life Quality Index, SD standard deviation
Fig. 3.
DLQI response at Week 52 in the analysis population comprising patients treated with secukinumab 300 mg for up to 52 weeks in ERASURE, FIXTURE and CLEAR (last observation carried forward). DLQI Dermatological Life Quality Index
Safety of Secukinumab in Elderly Subjects Compared with Younger Subjects with Psoriasis
Safety data are shown for subjects who received secukinumab 300 mg. Elderly subjects (n = 67) and younger subjects (n = 839) who received at least one dose of study medication were included in the safety analysis (safety set). The total rate of adverse events was 82.1% in elderly subjects and 85.7% in younger subjects (Table 3). The total rate of adverse events ‘possibly related’ (as assessed by the investigator) to study treatment was 34.3% in elderly subjects and 34.2% in younger subjects. The total rate of serious adverse events was numerically higher in elderly subjects compared with younger subjects (14.9 vs 8.2%; Table 3); however, the sample size for elderly patients was small. Serious adverse events possibly related to study treatment occurred in 4.5% of elderly subjects and 1.8% of younger subjects. Over the 52-week duration, 7.5% of elderly subjects and 1.8% of younger subjects had to discontinue study treatment because of adverse events (Table 3). The rate of common adverse events during the entire treatment period (Weeks 0–52) is shown in Table 3. The rate of common adverse events during the initial 12 weeks of treatment is shown in Supplementary Table 2 (ESM).
Table 3.
Summary of adverse events by age group. Treatment-emergent adverse events in the analysis population, comprising patients treated with secukinumab 300 mg for up to 52 weeks in ERASURE, FIXTURE and CLEAR
| Patients aged ≥ 65 years (n = 67) | Patients aged 18 to < 65 years (n = 839a) | |
|---|---|---|
| Mean exposure to study treatment, days (SD) | ||
| Initial treatment period (weeks 0–12) | 92.4 (20.03) | 93.7 (15.99) |
| Entire study period (weeks 0–52) | 336.9 (88.63) | 347.3 (65.98) |
| Summary of adverse events (entire treatment period) | ||
| Any AE, n (%) | 55 (82.1) | 719 (85.7) |
| Death, n | 0 | 0 |
| Non-fatal SAE, n (%) | 10 (14.9) | 69 (8.2) |
| Atrial fibrillation | 1 (1.5) | 0 (0.0) |
| Myocardial infarction | 0 (0.0) | 1 (0.1) |
| Serious infections | 1 (1.5) | 13 (1.5) |
| Hypertension | 7 (10.4) | 36 (4.3) |
| Diarrhoea | 1 (1.5) | 0 (0.0) |
| Discontinuation due to AE, n (%) | 5 (7.5) | 15 (1.8) |
| Infection/infestation, n (%) | 36 (53.7) | 527 (62.8) |
| Cardiac disorders, n (%) | 8 (11.9) | 24 (2.9) |
| Cardiac failure | 0 (0.0) | 1 (0.1) |
| Coronary artery disease | 0 (0.0) | 1 (0.1) |
| Myocardial infarction | 0 (0.0) | 1 (0.1) |
| Angina pectoris | 0 (0.0) | 1 (0.1) |
| Atrial fibrillation | 1 (1.5) | 1 (0.1) |
| Tachycardia | 2 (3.0) | 3 (0.4) |
| AV block first degree | 1 (1.5) | 4 (0.5) |
| Sinus bradycardia | 1 (1.5) | 2 (0.2) |
| Aortic valve incompetence | 1 (1.5) | 0 |
| Otherb | 2 (3.0) | 10 (1.2) |
| Treatment-emergent AEs occurring in ≥ 5% subjects in either group, n (%) (entire study period, weeks 0–52) | ||
| Nasopharyngitis | 13 (19.4) | 219 (26.1) |
| Headache | 4 (6.0) | 115 (13.7) |
| Pruritus | 7 (10.4) | 43 (5.1) |
| Hypertension | 7 (10.4) | 36 (4.3) |
| URTI | 4 (6.0) | 83 (9.9) |
| Diarrhoea | 6 (9.0) | 62 (7.4) |
| Sinusitis | 5 (7.5) | 18 (2.1) |
| Eczema | 5 (7.5) | 30 (3.6) |
| Arthralgia | 4 (6.0) | 60 (7.2) |
| Oropharyngeal pain | 0 | 59 (7.0) |
| Influenza | 2 (3.0) | 56 (6.7) |
| Back pain | 4 (6.0) | 55 (6.6) |
| Cough | 4 (6.0) | 52 (6.2) |
| Bronchitis | 4 (6.0) | 42 (5.0) |
aTwo subjects in the full analysis set for the 18 to < 65 years age group randomized to secukinumab 300 mg did not receive study medication and are not included in the safety set
bIncludes bundle branch block left, palpitations, angina unstable, arteriosclerosis coronary artery, atrial flutter, bradycardia, left ventricular hypertrophy, supraventricular tachycardia, ventricular failure
AE adverse event, AV atrioventricular, SAE serious adverse event, SD standard deviation, URTI upper respiratory tract infection
The total rate of infections was 53.7% in elderly subjects and 62.8% in younger subjects. The most frequent infections were nasopharyngitis, occurring in 19.4% of elderly subjects and 26.1% of younger subjects, and upper respiratory tract infections, occurring in 6.0% of elderly subjects and 9.9% of younger subjects. Blood and lymphatic disorders were numerically more frequent in younger subjects (3.6%) than in elderly subjects (0.0%). There were no cases of leukopenia, neutropenia or lymphopenia among elderly subjects. Drug hypersensitivity occurred in 0.0% of elderly and 0.2% of younger patients.
Cardiac disorders were more frequent in elderly subjects (11.9%) compared with younger subjects (2.9%; Table 3). Hypertension and atrial fibrillation were more frequent in elderly patients compared with the younger group (Table 3).
Nervous system disorders were more frequent in younger subjects (19.7%) compared with elderly subjects (14.9%).
Discussion
This pooled analysis of three phase III trials evaluated the efficacy and safety of the label dose of secukinumab 300 mg in subjects with moderate to severe plaque psoriasis stratified by age into an elderly and a younger group. Secukinumab showed equivalent efficacy in older subjects, with a safety profile similar to that observed in previous studies, in line with biologic treatments for psoriasis generally, and consistent with an elderly population.
Elderly subjects with psoriasis require distinct treatment considerations in routine clinical practice. Both drug pharmacokinetics and pharmacodynamics may be altered in the elderly [19]. Elderly subjects are more likely to have had longer disease duration than younger subjects. Due to an increased likelihood of comorbid health conditions, elderly subjects may be prescribed concomitant medications that must be considered when selecting psoriasis therapies to avoid adverse events associated with polypharmacy [6].
Other biologic agents have been assessed in elderly subjects with psoriasis, although data remain limited. An observational study of etanercept and adalimumab monotherapy in 89 elderly subjects with plaque-type psoriasis and psoriatic arthritis showed consistent and stable efficacy over 156 weeks [20]. Ustekinumab treatment was also evaluated in 24 elderly subjects with moderate to severe plaque psoriasis [21].
The elderly and younger subjects included in the current analysis showed several distinct differences at baseline. The mean age of both patient groups differed significantly (69.9 years vs 42.8 years, p < 0.0001), which supports the validity of the analysis. Elderly subjects had a significantly higher frequency of cardiovascular and metabolic comorbidities including diabetes, metabolic syndrome, hypertension and coronary artery disease. As may be expected, elderly subjects had significantly longer disease duration than younger subjects. Younger subjects appeared to show greater disease severity at baseline, with higher PASI and DLQI scores recorded, and were more likely to smoke.
Here, we show that secukinumab 300 mg gave rapid and sustained PASI 75, 90 and 100, and IGA responses in all subjects regardless of age. There were no differences in the speed of onset or duration of response between elderly and younger subjects treated with secukinumab.
The elderly subjects had a slightly lower baseline DLQI score than the younger group, but DLQI in both groups decreased considerably over the 52 weeks of secukinumab treatment. The proportion of subjects achieving a DLQI response of 0 or 1, indicating minimal impact of psoriasis on QoL, was similar in both elderly and younger subjects over 52 weeks. These data indicate that in addition to the clinical efficacy shown by the decrease in PASI score, secukinumab improves HRQoL in elderly subjects.
Overall, the tolerability of secukinumab was comparable between elderly and younger subjects. There was no indication of an increased risk of infections in elderly subjects; the overall infection rate was lower among elderly than younger subjects, although this was not a significant difference. Similar rates of different types of infections were observed between the two groups. There was a higher frequency of cardiovascular adverse events in the elderly population. At the same time, the elderly population had a significantly higher baseline rate of several cardiovascular and metabolic comorbidities, which should be considered in this context. There was a greater frequency of treatment discontinuation among elderly subjects. The natural age association with the incidence and prevalence of several comorbid diseases should be taken into consideration when interpreting the safety data of this analysis.
Despite a careful study design, there are a number of limitations to the current analysis, most notably the retrospective, post-hoc nature of the pooled study. The method of imputation was chosen as the most appropriate approach for the analysis of the data, but all imputation methods carry an inherent risk of bias. Furthermore, the number of patients was highly imbalanced between elderly and younger patients, limiting the comparability of the two groups. The number of elderly patients was low, meaning that numerical differences observed could be assigned clinical significance only with caution. Concerning safety data, the relatively small number of elderly patients limits the detection of rare adverse events. Despite these limitations, the data show that the efficacy and tolerability of secukinumab are similar in both elderly and younger populations.
In conclusion, secukinumab at the label dose of 300 mg is effective in elderly and younger populations. Pronounced benefits in HRQoL were also observed in both populations. Upcoming real-world evidence from ongoing non-interventional studies and registries such as PROSPECT [22], SERENA or PsoBest [23] will be of interest to better characterize efficacy and safety of biologic treatments in elderly psoriasis patients. Further prospective studies are required to confirm the findings from this pooled analysis, which supports secukinumab as a treatment option for elderly subjects with psoriasis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Prof Dr Richard Dodel, Geriatrics Clinic, Elisabeth-Krankenhaus Essen and University Duisburg-Essen, Germany, for critical reading of this manuscript. The authors would like to acknowledge the assistance of all investigators, clinical trial staff and participants. The authors thank Evelyn Altemeyer of Novartis Ireland Ltd for providing medical writing support/editorial support, which was funded by Novartis Pharma GmbH, Nuremberg, Germany in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Compliance with Ethical Standards
Funding
The study was funded by Novartis Pharma GmbH, Germany.
Conflict of interest
AK has received honoraria from Novartis, Lilly, Leo Pharma, Almirall, Janssen, UCB, MSD and Pfizer and has received fees for board participation from Novartis, Leo Pharma, Janssen and Lilly. CP is an employee of Novartis Pharma AG. VB is an employee of Novartis Healthcare Pvt. Ltd. MR is an employee of Novartis Pharma GmbH.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s40266-018-0520-z) contains supplementary material, which is available to authorized users.
References
- 1.Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(Suppl 2):ii18–ii23. doi: 10.1136/ard.2004.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33(1):41–55. doi: 10.1016/j.det.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Gan EY, Chong WS, Tey HL. Therapeutic strategies in psoriasis patients with psoriatic arthritis: focus on new agents. BioDrugs Clin Immunother Biopharm. Gene Ther. 2013;27(4):359–373. doi: 10.1007/s40259-013-0025-6. [DOI] [PubMed] [Google Scholar]
- 4.Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146(8):891–895. doi: 10.1001/archdermatol.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phan C, Sigal ML, Esteve E, Reguiai Z, Barthelemy H, Beneton N, et al. Psoriasis in the elderly: epidemiological and clinical aspects, and evaluation of patients with very late onset psoriasis. J Eur Acad Dermatol Venereol. 2016;30(1):78–82. doi: 10.1111/jdv.12850. [DOI] [PubMed] [Google Scholar]
- 6.Balato N, Patruno C, Napolitano M, Patri A, Ayala F, Scarpa R. Managing moderate-to-severe psoriasis in the elderly. Drugs and Aging. 2014;31(4):233–238. doi: 10.1007/s40266-014-0156-6. [DOI] [PubMed] [Google Scholar]
- 7.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2(52):52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 8.Papp KA, Langley RG, Sigurgeirsson B, Abe M, Baker DR, Konno P, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168(2):412–421. doi: 10.1111/bjd.12110. [DOI] [PubMed] [Google Scholar]
- 9.Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Eng J Med. 2015;373(14):1329–1339. doi: 10.1056/NEJMoa1412679. [DOI] [PubMed] [Google Scholar]
- 10.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Eng J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 11.Nast A, Gisondi P, Ormerod AD, Saiag P, Smith C, Spuls PI, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015;29(12):2277–2294. doi: 10.1111/jdv.13354. [DOI] [PubMed] [Google Scholar]
- 12.Langley RG, Feldman SR, Nyirady J, van de Kerkhof P, Papavassilis C. The 5-point Investigator’s Global Assessment (IGA) Scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatol Treat. 2013;26(1):23–31. doi: 10.3109/09546634.2013.865009. [DOI] [PubMed] [Google Scholar]
- 13.Thaci D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409. doi: 10.1016/j.jaad.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Blauvelt A, Reich K, Tsai TF, Tyring S, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76(1):60.e9–69.e9. doi: 10.1016/j.jaad.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Mrowietz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE) J Am Acad Dermatol. 2015;73(1):27.e1–36.e1. doi: 10.1016/j.jaad.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Thaci D, Humeniuk J, Frambach Y, Bissonnette R, Goodman JJ, Shevade S, et al. Secukinumab in psoriasis: randomized, controlled phase 3 trial results assessing the potential to improve treatment response in partial responders (STATURE) Br J Dermatol. 2015;173(3):777–787. doi: 10.1111/bjd.13814. [DOI] [PubMed] [Google Scholar]
- 17.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 18.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 19.Flammiger A, Maibach H. Drug dosage in the elderly: dermatological drugs. Drugs Aging. 2006;23(3):203–215. doi: 10.2165/00002512-200623030-00003. [DOI] [PubMed] [Google Scholar]
- 20.Esposito M, Giunta A, Mazzotta A, Zangrilli A, Babino G, Bavetta M, et al. Efficacy and safety of subcutaneous anti-tumor necrosis factor-alpha agents, etanercept and adalimumab, in elderly patients affected by psoriasis and psoriatic arthritis: an observational long-term study. Dermatology (Basel Switz) 2012;225(4):312–319. doi: 10.1159/000345623. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi M, Umezawa Y, Fukuchi O, Ito T, Saeki H, Nakagawa H. Efficacy and safety of ustekinumab treatment in elderly patients with psoriasis. J Dermatol. 2014;41(11):974–980. doi: 10.1111/1346-8138.12653. [DOI] [PubMed] [Google Scholar]
- 22.Körber A, Thaçi D, Kiedrowski Rv, Bachhuber T, Melzer N, Kasparek T, et al. Assessment of duration of transition from prior treatments to secukinumab treatment in moderate to severe psoriasis: baseline characteristics from the PROSPECT Study. In: American Academy of Dermatology 75th annual meeting. Florida, USA; 2017.
- 23.Trettel A, Spehr C, Korber A, Augustin M. The impact of age on psoriasis health care in Germany. J Eur Acad Dermatol Venereol. 2017;31(5):870–875. doi: 10.1111/jdv.14115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.