Abstract
Helicobacter pylori-associated gastritis leads to the development of gastric cancer. Kyoto global consensus report on H. pylori gastritis recommended H. pylori eradication therapy to prevent gastric cancer. To manage H. pylori infection, it is important to choose the appropriate regimen considering regional differences in resistance to clarithromycin and metronidazole. Quinolones and rifabutin-containing regimens are useful as third- and fourth-line rescue therapies.
Keywords: H. pylori, Gastric cancer, Clarithromycin, Metronidazole, Fluoroquinolone
Introduction
Helicobacter pylori (H. pylori) is one of the main causes of gastric cancer [1, 2]. H. pylori leads to gastric oncogenesis through the injection of the oncoprotein CagA into host cells via a type IV secretion system [1]. Kyoto global consensus report on H. pylori gastritis recommended that all individuals with H. pylori infection should receive eradication therapy to prevent gastric cancer [3, 4]. In particular, H. pylori test-and-treat should be promoted in regions with high incidence of gastric cancer. Since 2013, H. pylori eradication therapy was approved for all cases of H. pylori gastritis by the national health insurance scheme in Japan, before other countries. From the global scale, a higher incidence of gastric cancer is found in Asia than in Europe and North America [5, 6]. In managing effective H. pylori suppression, prevalence of H. pylori infection and incidence of resistance to antimicrobial agents in each region are important factors. In this article, we describe the geographic characteristics of the prevalence of H. pylori infection, gastric cancer, and resistance to antibiotics and discuss the strategy of H. pylori eradication therapy.
Epidemiology of H. pylori infection and gastric cancer incidence
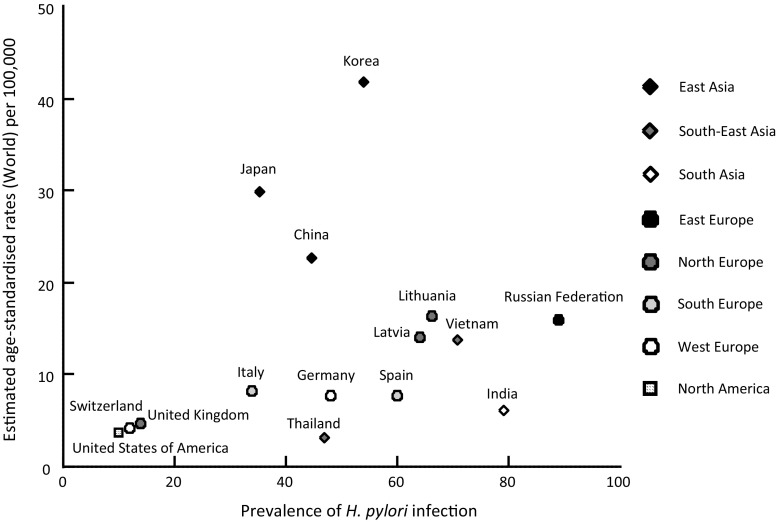
The geographic distribution of the prevalence of H. pylori infection and gastric cancer incidence is shown in Fig. 1. The incidence of gastric cancer is generally in direct proportion to the prevalence of H. pylori infection [6]. However, higher incidences of gastric cancer are found in countries in eastern Asia than in other countries.
Fig. 1.
The relationship between geographic distribution of the prevalence of H. pylori infection and the gastric cancer incidence is described. The gastric cancer incidence is generally in direct proportion to the prevalence of H. pylori infection. However, higher incidences of gastric cancer are found in East Asian countries than in the other countries
Chronic atrophic gastritis, mainly caused by H. pylori infection, is a known precancerous lesion for gastric cancer [7, 8]. A cross-sectional comparative study on chronic gastritis between the United Kingdom and Japan showed gastritis in Japan is histologically more severe, present at an earlier age, and more likely to be corpus predominant or pan-gastritis compared with the United Kingdom, whereas there was no significant difference in the prevalence of H. pylori between these countries [9].
Cytotoxin-associated gene A (CagA) is known as a major pathogenetic factor, which is peculiar to H. pylori [10]. To classify the genetic status of CagA, H. pylori CagA is classified into East Asian CagA and Western CagA [11, 12]. The East Asian CagA protein possesses stronger SHP-2 binding activity than the Western CagA [13]. The grades of inflammation, activity of gastritis, and atrophy are significantly higher in gastritis patients infected with the East Asian CagA-positive strain than in gastritis patients infected with CagA-negative or Western CagA-positive strains [12]. Therefore, the difference of CagA subtypes is the most important factor predicting the risk of gastric cancer.
There has been a progressive and rapid decline in the prevalence of H. pylori infection as well as a fall in the rate of progression of gastric atrophy [14], and a significant decrease in gastric cancer deaths in Japan [15]. These data indicated that a decrease in the prevalence of H. pylori and an increase in the attention to H. pylori might have a direct or indirect link to the reduction of gastric cancer deaths even in regions with high incidence of gastric cancer.
Epidemiology of resistance to antimicrobial agents in H. pylori
Considering effective treatment of H. pylori eradication, it is important to have a deep understanding of the epidemiology of resistance to antimicrobial agents.
Resistance to amoxicillin is either null or less than 1% [16], and no significant changes in resistance were observed [17]. Therefore, amoxicillin is, and will be, a key drug in the treatment of H. pylori. On the contrary, we reported unsuccessful eradication treatment increased resistance even to amoxicillin [18]; thus, rescue therapy should be also determined considering amoxicillin resistance.
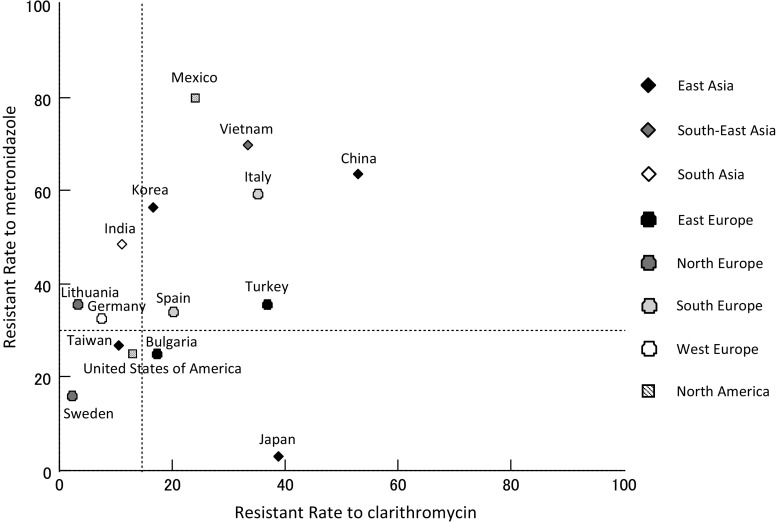
The geographic distribution of resistance to clarithromycin and metronidazole is shown in Fig. 2 [19–34]. Interestingly, there is only a limited relationship between geographic factors and resistance to clarithromycin and metronidazole. Among the countries in eastern Asia, high resistance to clarithromycin and low resistance to metronidazole are found in Japan; low resistance to clarithromycin and high resistance to metronidazole are found in Korea; and high resistance to both clarithromycin and metronidazole is found in China. In northern Europe, there is generally a low resistance to clarithromycin. In some countries, such as Italy, China, Vietnam, and Mexico, high resistance to both clarithromycin and metronidazole is reported. A significant increase in resistance to clarithromycin and metronidazole was noted over the last year [35, 36], suggesting that acquisition of resistance is related to high consumption rates of these antibiotics.
Fig. 2.
The geographic distribution of resistance to clarithromycin and metronidazole is described. There is only a limited relationship between geographic factor and the resistance to clarithromycin and metronidazole
The prevalence of primary resistance to quinolones has been reported to range from 2 to 22% [28]. The prevalence of quinolone resistance is reported to be relatively higher in Japan, Korea, and Italy (15–22%), and to be very low in China and Egypt (approximately 2%).
The prevalence of primary resistance to rifabutin, if rifamycins have not been used, is very low [37–39].
Treatment of H. pylori infection
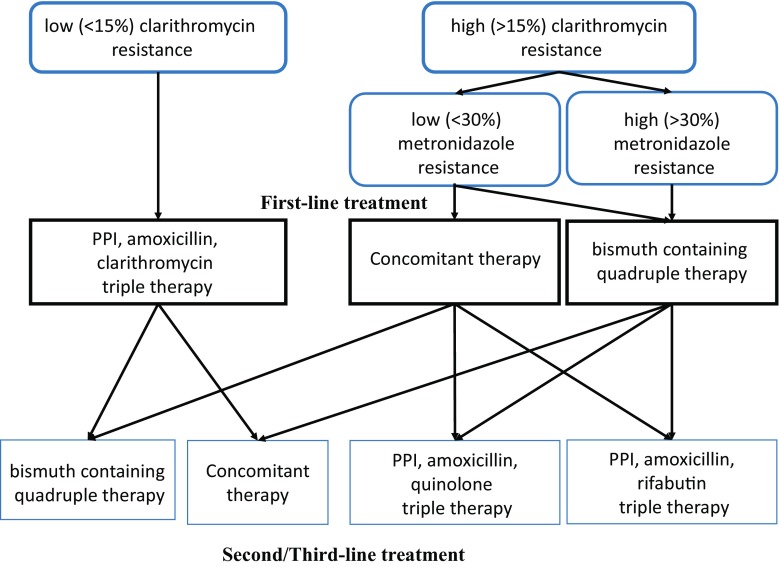
A strategy for treating H. pylori infection is shown in Fig. 3 in accordance with the Maastricht V/Florence Consensus Report, a novel European guideline for managing H. pylori infection [40]. At first, the standard triple regimen of proton pump inhibitor (PPI), amoxicillin, and clarithromycin is recommended when the clarithromycin resistance rate in the region is less than 15%. Murakami et al. showed standard triple regimen (lansoprazole 30 mg bid, amoxicillin 750 mg bid, clarithromycin 400 or 800 mg bid for 7 days) achieved a successful eradication rate of 97.3% when the strains are susceptible to clarithromycin [41]. In areas of high (> 15%) clarithromycin resistance, bismuth-containing quadruple therapy is recommended as a first-line treatment. In regions with high clarithromycin resistance but low to intermediate metronidazole resistance, non-bismuth quadruple concomitant therapy (PPI, amoxicillin, clarithromycin and metronidazole) can be an alternative treatment [40, 42]. In areas of high dual clarithromycin and metronidazole resistance, bismuth-containing quadruple therapy is the recommended first-line treatment. Ten-day bismuth quadruple therapy is more effective than 10-days standard triple therapy as first-line therapy for patients with H. pylori-induced chronic gastritis in China (86.1 vs 58.4%, ITT analysis) [43].
Fig. 3.
Strategy on treatment of H. pylori infection is shown in accordance with the Maastricht V/Florence Consensus Report [40]
After failure of the first-line treatment (triple or non-bismuth quadruple) and second-line treatment (quinolone-containing therapy), the bismuth-based quadruple therapy is recommended. Rescue therapy with bismuth-containing quadruple therapy in patients achieved 83% of successful eradications in France [44]. After failure of first-line treatment with bismuth quadruple and second-line treatment, it is recommended to use a quinolone-based triple or quadruple therapy [40]. In regions with high clarithromycin resistance but low to intermediate metronidazole resistance, triple therapy (amoxicillin, metronidazole, and PPI) is also effective when metronidazole was not used as a first-line regimen [45, 46].
Reports of third- and fourth-line treatment are described in Tables 1, 2, 3. Quinolone-based therapy is one of the most used third-line regimens. Levofloxacin have been widely used as H. pylori rescue therapy [47–51]. However, the effectiveness of levofloxacin-based therapies against gyrA mutation positive strains is insufficient; the eradication rates are approximately 40% [52, 53]. Sitafloxacin-containing treatment achieved up to 70% of successful eradications for gyrA mutation positive strains [54–57]. A randomized study revealed that the eradication rate of sitafloxacin-based triple regimens was better than that of levofloxacin-based triple regimens [49]. Therefore, sitafloxacin is currently the most useful fluoroquinolone, if available. However, resistance to quinolones is acquired easily, and some strains develop strong resistance via acquisition of double mutations in gyrA [58, 59].
Table 1.
Reports of quinolone containing third-line regimens
| Authors | Country | Publication (year) | Number of previous failed treatments | Therapy regimen | Duration | Eradication rate (%) |
|---|---|---|---|---|---|---|
| Zullo A et al. [47] | Italy | 2003 | 2 | RPZ 20 mg b.i.d., AMX 1 g b.i.d. and LVFX 250 mg b.i.d. | 10 | 83 |
| Gisbert JP et al. [48] | Spain | 2006 | 2 | OPZ 20 mg b.i.d., AMX 1 g b.i.d. and LVFX 500 mg b.i.d. | 10 | 85 |
| Murakami K et al. [49] | Japan | 2013 | 2 | LPZ 30 mg b.i.d., AMX 750 mg b.i.d. and LVFX 300 mg b.i.d. | 7 | 43 |
| Okimoto K et al. [50] | Japan | 2014 | 2 | RPZ 10 mg b.i.d., AMX 750 mg b.i.d. and LVFX 500 mg q.d.s. | 10 | 46 |
| Paoluzi OA et al. [51] | Italy | 2015 | 2 | EPZ 20 mg b.i.d., LVFX 500 mg b.i.d. and doxycycline 100 mg b.i.d. | 7 | 46 |
| Matsuzaki J et al. [54] | Japan | 2012 | 2 | RPZ 10 mg q.i.d., AMX 500 mg q.i.d. and STFX 100 mg bid for 7 days | 7 | 84 |
| Murakami K et al. [49] | Japan | 2013 | 2 | LPZ 30 mg b.i.d., AMX 750 mg b.i.d. and STFX 100 mg b.i.d. | 7 | 70 |
| Mori H et al. [55] | Japan | 2016 | 2 | EPZ 20 mg b.i.d., AMX 500 mg q.i.d. and STFX 100 mg b.i.d. | 10 | 82 |
| Mori H et al. [55] | Japan | 2016 | 2 | EPZ 20 mg b.i.d., MTZ 250 mg b.i.d. and STFX 100 mg b.i.d. | 10 | 76 |
RPZ Rabeprazole, OPZ omeprazole, LPZ lansoprazole, EPZ esomeprazole, AMX amoxicillin, LVFX levofloxacin, STFX sitafloxacin, MTZ metronidazole
Table 2.
Reports of rifabutin containing third- and fourth-line regimens
| Authors | Country | Publication (year) | Number of previous failed treatments | Therapy regimen | Duration | Eradication rate (%) |
|---|---|---|---|---|---|---|
| Miehlke S et al. [60] | Germany | 2006 | 2 | EPZ 20 mg b.i.d., AMX 1 g b.i.d. and RBT 150 mg b.i.d. | 7 | 74 |
| Gisbert JP et al. [61] | Spain | 2006 | 2 | OPZ 20 mg b.i.d., AMX 1 g b.i.d. and RBT 150 mg b.i.d. | 10 | 45 |
| Gisbert JP et al. [61] | Spain | 2012 | 3 | PPIs b.i.d., AMX 1 g b.i.d. and RBT 150 mg b.i.d. | 10 | 50 |
| Perri F et al. [62] | Italy | 2014 | 2 | LPZ 30 mg b.i.d., AMX 1 g t.i.d. and RBT 150 mg b.i.d. | 7 | 78 |
| Perri F et al. [62] | Italy | 2014 | 2 | LPZ 60 mg b.i.d., AMX 1 g t.i.d. and RBT 150 mg b.i.d. | 7 | 96 |
| Mori H et al. [63] | Japan | 2016 | ≥ 2 | EPZ 20 mg b.i.d., AMX 500 mg q.i.d. and RBT 300 mg q.d.s. | 10 | 83 |
| Mori H et al. [63] | Japan | 2016 | ≥ 2 | EPZ 20 mg b.i.d., AMX 500 mg q.i.d. and RBT 300 mg q.d.s. | 14 | 94 |
| Ciccaglione AF et al. [64] | Italy | 2016 | 2 | PPZ 20 mg b.i.d., AMX 1 g b.i.d. and RBT 150 mg b.i.d. | 10 | 67 |
| Ciccaglione AF et al. [64] | Italy | 2016 | 2 | PPZ 20 mg b.i.d., AMX 1 g b.i.d., RBT 150 mg b.i.d. and bismuth subcitrate 240 mg b.i.d. | 10 | 97 |
OPZ omeprazole, LPZ lansoprazole, EPZ esomeprazole, PPZ pantoprazole, AMX amoxicillin, RBT rifabutin
Table 3.
Reports of high-dose dual therapy for third-line regimens
| Authors | Country | Publication (year) | Number of previous failed treatments | Therapy regimen | Duration | Eradication rate (%) |
|---|---|---|---|---|---|---|
| Miehlke S et al. [60] | Germany | 2006 | 2 | OPZ 40 mg t.i.d. and AMX 1 g t.i.d. | 14 | 70 |
| Nishizawa T et al. [67] | Japan | 2012 | 2 | RPZ 10 mg q.i.d. and AMX 500 mg q.i.d. | 14 | 63 |
| Murakami K et al. [49] | Japan | 2013 | 2 | LPZ 30 mg q.i.d. and AMX 500 mg q.i.d. | 14 | 54 |
| Okimoto K et al. [50] | Japan | 2014 | 2 | RPZ 10 mg q.i.d. and AMX 500 mg q.i.d. | 14 | 64 |
OPZ omeprazole, RPZ rabeprazole, LPZ lansoprazole, AMX amoxicillin
On the contrary, rifabutin-containing therapy has been reported as a third-line treatment [48, 60–64]. Because the resistance to rifabutin is rare, rifabutin-containing therapy can overcome H. pylori strains with resistance to multiple antibiotics. However, there is a concern about side effects, such as leukopenia and thrombocytopenia, and occurrence of multi-resistant strains of Mycobacterium tuberculosis; thus, application of rifabutin-containing therapy should be chosen very carefully [65]. Regarding duration of rifabutin-containing therapy, 10-day or longer regimens were better than 7-day regimens [63, 65]. We reported 83.3% achieved successful eradication with 10-day rifabutin-containing regimens and 94.1% with 14-day regimens [63]. Regarding PPI dosage, rifabutin therapies with high dose PPI achieved more effective eradication than normal PPI dosing [66]. Bismuth had an additional effect on rifabutin-containing regimen [64].
On the contrary, high-dose PPI and amoxicillin dual therapy is a useful option as an alternative third-line treatment regimen [49, 50, 60, 67]. We previously reported eradication rate of 63.0% with rabeprazole (10 mg qid) and amoxicillin (500 mg qid) for 14 days [67]. The eradication rates are not better than those of quinolone- or rifabutin-containing therapy; however, fewer concerns about some complications and acquisition of new drug resistance is an advantage for high-dose PPI and amoxicillin dual therapy.
Vonoprazan, a first-in-class potassium-competitive acid blocker that has a strong gastric acid secretion inhibitory effect, is spotlighted. Vonoprazan, amoxicillin, and clarithromycin triple regimen clearly improved the efficacy for eradicating clarithromycin-resistant strains when compared to the standard lansoprazole, amoxicillin, and clarithromycin triple regimen (82.0 vs. 40.0%) [41]. The reports of vonoprazan-containing regimens are limited to only the triple regimens [68]; therefore, other regimens including vonoprazan, such as bismuth-quadruple therapy or concomitant therapy, are expected.
Effectiveness of H. pylori eradication for gastric cancer prevention
A large-scale prospective study showed that gastric cancer develops in persons infected with H. pylori but not in uninfected persons [2]; thus, it was proven that infection with H. pylori is the most important cause of gastric carcinogenesis. However, the effect of eradication treatment on gastric cancer risk is not well evaluated. In an open-label, randomized, controlled trial in Japan, Fukase et al. revealed H. pylori eradication reduced the incidence of metachronous gastric cancer even after endoscopic resection of early gastric cancer during a 3-year follow-up period [69]. Meta-analysis also showed that the occurrence of metachronous gastric cancer after endoscopic resection of early gastric cancer is significantly lower in the H. pylori eradication group compared with the control non-eradicated group, and that the odds ratio for the incidence of metachronous gastric cancer in the eradication group was 0.42 [70]. Systematic review and meta-analysis of randomized controlled trials, of which six individual randomized controlled trials were included, also revealed that eradication therapy reduced the risk of primary gastric cancer in healthy asymptomatic infected individuals compared with control individuals, and that the relative risk was 0.66 [71]. Take et al. showed that eradication of H. pylori reduced the risk of developing gastric cancer in patients with peptic ulcer diseases; however, the risk is higher in the patients with severe atrophic gastritis than those with mild or moderate atrophic gastritis [72]. These data suggested that “the earlier, the better” in H. pylori eradication prevents gastric cancer.
Conclusions
H. pylori-associated gastritis leads to the development of gastric cancer. H. pylori should be eradicated in patients at a younger age, possibly after adolescence at the earliest, to prevent gastric cancer. However, even after endoscopic resection of early gastric cancer, H. pylori eradication reduces the incidence of metachronous gastric cancer. The incidence of gastric cancer is generally in direct proportion to the prevalence of H. pylori infection, even in eastern Asia where the prevalence of gastric cancer is relatively high. There are regional differences in resistance to clarithromycin, metronidazole, and quinolones. Resistance to amoxicillin and rifabutin is generally rare. To manage H. pylori infection, it is important to choose the appropriate regimen considering regional differences in resistance to clarithromycin and metronidazole. Quinolones or rifabutin-containing regimen are useful as third- or fourth-line rescue therapies.
Compliance with ethical standards
Conflict of interest
During the last 2 years, H.S. received scholarship funds for the research from Daiichi-Sankyo Co., Otsuka Pharmaceutical Co. Ltd., and Tsumura Co., and received service honoraria from Astellas Pharma Inc, AstraZeneca K.K., Daiichi-Sankyo Co., Otsuka Pharmaceutical Co. Ltd., Mylan EPD Co., Takeda Pharmaceutical Co., Ltd., and Zeria Pharmaceutical Co. Ltd.
Footnotes
A comment to this article is available at https://doi.org/10.1007/s00535-018-1453-3.
References
- 1.Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15(3):306–316. doi: 10.1016/j.chom.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345(11):784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 3.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki H, Mori H. Helicobacter pylori: Helicobacter pylori gastritis-a novel distinct disease entity. Nat Rev Gastroenterol Hepatol. 2015;12(10):556–557. doi: 10.1038/nrgastro.2015.158. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Mori H. Different pathophysiology of gastritis between East and West? An Asian perspective. Inflamm Intest Dis. 2016;1(3):123–128. doi: 10.1159/000446301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 7.Adamu MA, Weck MN, Gao L, et al. Incidence of chronic atrophic gastritis: systematic review and meta-analysis of follow-up studies. Eur J Epidemiol. 2010;25(7):439–448. doi: 10.1007/s10654-010-9482-0. [DOI] [PubMed] [Google Scholar]
- 8.Jaskiewicz K, Louwrens HD. Chronic atrophic gastritis in a population at risk for gastric carcinoma. Anticancer Res. 1991;11(2):835–839. [PubMed] [Google Scholar]
- 9.Naylor GM, Gotoda T, Dixon M, et al. Why does Japan have a high incidence of gastric cancer? Comparison of gastritis between UK and Japanese patients. Gut. 2006;55(11):1545–1552. doi: 10.1136/gut.2005.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4(9):688–694. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 11.Higashi H, Tsutsumi R, Fujita A, et al. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci USA. 2002;99(22):14428–14433. doi: 10.1073/pnas.222375399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azuma T. Helicobacter pylori CagA protein variation associated with gastric cancer in Asia. J Gastroenterol. 2004;39(2):97–103. doi: 10.1007/s00535-003-1279-4. [DOI] [PubMed] [Google Scholar]
- 13.Higashi H, Tsutsumi R, Muto S, et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295(5555):683–686. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- 14.Kamada T, Haruma K, Ito M, et al. Time Trends in Helicobacter pylori Infection and Atrophic Gastritis Over 40 Years in Japan. Helicobacter. 2015;20(3):192–198. doi: 10.1111/hel.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuda M, Asaka M, Kato M, et al. Effect on Helicobacter pylori eradication therapy against gastric cancer in Japan. Helicobacter. 2017;22(5). 10.1111/hel.12415. [DOI] [PMC free article] [PubMed]
- 16.Megraud F. H. pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53(9):1374–1384. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi I, Murakami K, Kato M, et al. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol. 2007;45(12):4006–4010. doi: 10.1128/JCM.00740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishizawa T, Suzuki H, Tsugawa H, et al. Enhancement of amoxicillin resistance after unsuccessful Helicobacter pylori eradication. Antimicrob Agents Chemother. 2011;55(6):3012–3014. doi: 10.1128/AAC.00188-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashinaga M, Okimoto T, Kodama M, et al. Drug resistance in Japan; the present status of Helicobacter pylori; the totaled results of the resistant strain survey in 2013 and 2014, wagakunini okeru yakuzaitaisei Helicobacter pylori no genjo—2013–2014 nendo taiseikin surveillance no syuukei houkoku. Jpn J Helicobacter Res. 2016;17:45–49. [Google Scholar]
- 20.An B, Moon BS, Kim H, et al. Antibiotic resistance in Helicobacter pylori strains and its effect on H. pylori eradication rates in a single center in Korea. Ann. Lab Med. 2013;33(6):415–419. doi: 10.3343/alm.2013.33.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang YX, Zhou LY, Song ZQ, et al. Primary antibiotic resistance of Helicobacter pylori strains isolated from patients with dyspeptic symptoms in Beijing: a prospective serial study. World J Gastroenterol. 2015;21(9):2786–2792. doi: 10.3748/wjg.v21.i9.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang WL, Sheu BS, Cheng HC, et al. Resistance to metronidazole, clarithromycin and levofloxacin of Helicobacter pylori before and after clarithromycin-based therapy in Taiwan. J Gastroenterol Hepatol. 2009;24(7):1230–1235. doi: 10.1111/j.1440-1746.2009.05829.x. [DOI] [PubMed] [Google Scholar]
- 23.Binh TT, Shiota S, Nguyen LT, et al. The incidence of primary antibiotic resistance of Helicobacter pylori in Vietnam. J Clin Gastroenterol. 2013;47(3):233–238. doi: 10.1097/MCG.0b013e3182676e2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gehlot V, Mahant S, Mukhopadhyay AK, et al. Antimicrobial susceptibility profiles of Helicobacter pylori isolated from patients in North India. J Glob Antimicrob Res. 2016;5:51–56. doi: 10.1016/j.jgar.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Caliskan R, Tokman HB, Erzin Y, et al. Antimicrobial resistance of Helicobacter pylori strains to five antibiotics, including levofloxacin, in Northwestern Turkey. Rev Soc Bras Med Trop. 2015;48(3):278–284. doi: 10.1590/0037-8682-0027-2015. [DOI] [PubMed] [Google Scholar]
- 26.Saracino IM, Zullo A, Holton J, et al. High prevalence of primary antibiotic resistance in Helicobacter pylori isolates in Italy. J Gastrointestin Liver Dis. 2012;21(4):363–365. [PubMed] [Google Scholar]
- 27.Molina-Infante J, Pazos-Pacheco C, Vinagre-Rodriguez G, et al. Nonbismuth quadruple (concomitant) therapy: empirical and tailored efficacy versus standard triple therapy for clarithromycin-susceptible Helicobacter pylori and versus sequential therapy for clarithromycin-resistant strains. Helicobacter. 2012;17(4):269–276. doi: 10.1111/j.1523-5378.2012.00947.x. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki H, Nishizawa T, Hibi T. Helicobacter pylori eradication therapy. Future Microbiol. 2010;5(4):639–648. doi: 10.2217/fmb.10.25. [DOI] [PubMed] [Google Scholar]
- 29.Boyanova L, Gergova G, Nikolov R, et al. Prevalence and evolution of Helicobacter pylori resistance to 6 antibacterial agents over 12 years and correlation between susceptibility testing methods. Diagn Microbiol Infect Dis. 2008;60(4):409–415. doi: 10.1016/j.diagmicrobio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Storskrubb T, Aro P, Ronkainen J, et al. Antimicrobial susceptibility of Helicobacter pylori strains in a random adult Swedish population. Helicobacter. 2006;11(4):224–230. doi: 10.1111/j.1523-5378.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 31.Kupcinskas J, Leja M. Management of Helicobacter pylori-related diseases in the Baltic States. Dig Dis. 2014;32(3):295–301. doi: 10.1159/000357862. [DOI] [PubMed] [Google Scholar]
- 32.Duck WM, Sobel J, Pruckler JM, et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis. 2004;10(6):1088–1094. doi: 10.3201/eid1006.030744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres J, Camorlinga-Ponce M, Perez-Perez G, et al. Increasing multidrug resistance in Helicobacter pylori strains isolated from children and adults in Mexico. J Clin Microbiol. 2001;39(7):2677–2680. doi: 10.1128/JCM.39.7.2677-2680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisig JN, Silva FM, Barbuti RC, et al. Helicobacter pylori antibiotic resistance in Brazil: clarithromycin is still a good option. Arq Gastroenterol. 2011;48(4):261–264. doi: 10.1590/S0004-28032011000400008. [DOI] [PubMed] [Google Scholar]
- 35.Nishizawa T, Suzuki H, Hibi T. Quinolone-Based Third-Line Therapy for Helicobacter pylori Eradication. J Clin Biochem Nutr. 2009;44(2):119–124. doi: 10.3164/jcbn.08-220R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawai T, Takahashi S, Suzuki H, et al. Changes in the first line Helicobacter pylori eradication rates using the triple therapy-a multicenter study in the Tokyo metropolitan area (Tokyo Helicobacter pylori study group) J Gastroenterol Hepatol. 2014;29(Suppl 4):29–32. doi: 10.1111/jgh.12796. [DOI] [PubMed] [Google Scholar]
- 37.Heep M, Beck D, Bayerdorffer E, et al. Rifampin and rifabutin resistance mechanism in Helicobacter pylori. Antimicrob Agents Chemother. 1999;43(6):1497–1499. doi: 10.1128/aac.43.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishizawa T, Suzuki H, Matsuzaki J, et al. Helicobacter pylori resistance to rifabutin in the last 7 years. Antimicrob Agents Chemother. 2011;55(11):5374–5375. doi: 10.1128/AAC.05437-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki S, Suzuki H, Nishizawa T, et al. Past rifampicin dosing determines rifabutin resistance of Helicobacter pylori. Digestion. 2009;79(1):1–4. doi: 10.1159/000191204. [DOI] [PubMed] [Google Scholar]
- 40.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66(1):6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 41.Murakami K, Sakurai Y, Shiino M, et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65(9):1439–1446. doi: 10.1136/gutjnl-2015-311304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molina-Infante J, Gisbert JP. Optimizing clarithromycin-containing therapy for Helicobacter pylori in the era of antibiotic resistance. World J Gastroenterol. 2014;20(30):10338–10347. doi: 10.3748/wjg.v20.i30.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Lin Z, Chen S, et al. Ten-day bismuth-containing quadruple therapy is effective as first-line therapy for Helicobacter pylori-related chronic gastritis: a prospective randomized study in China. Clin Microbiol Infect. 2017;23(6):391–395. doi: 10.1016/j.cmi.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 44.Muller N, Amiot A, Le Thuaut A, et al. Rescue therapy with bismuth-containing quadruple therapy in patients infected with metronidazole-resistant Helicobacter pylori strains. Clin Res Hepatol Gastroenterol. 2016;40(4):517–524. doi: 10.1016/j.clinre.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Nishizawa T, Maekawa T, Watanabe N, et al. Clarithromycin versus metronidazole as first-line Helicobacter pylori eradication: a multicenter, prospective, randomized controlled study in Japan. J Clin Gastroenterol. 2015;49(6):468–471. doi: 10.1097/MCG.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 46.Asaoka D, Nagahara A, Matsuhisa T, et al. Trends of second-line eradication therapy for Helicobacter pylori in Japan: a multicenter study in the Tokyo metropolitan area. Helicobacter. 2013;18(6):468–472. doi: 10.1111/hel.12063. [DOI] [PubMed] [Google Scholar]
- 47.Zullo A, Esposito G, Ridola L, et al. Prevalence of lesions detected at upper endoscopy: an Italian survey. Eur J Intern Med. 2014;25(8):772–776. doi: 10.1016/j.ejim.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 48.Gisbert JP, Gisbert JL, Marcos S, et al. Third-line rescue therapy with levofloxacin is more effective than rifabutin rescue regimen after two Helicobacter pylori treatment failures. Aliment Pharmacol Ther. 2006;24(10):1469–1474. doi: 10.1111/j.1365-2036.2006.03149.x. [DOI] [PubMed] [Google Scholar]
- 49.Murakami K, Furuta T, Ando T, et al. Multi-center randomized controlled study to establish the standard third-line regimen for Helicobacter pylori eradication in Japan. J Gastroenterol. 2013;48(10):1128–1135. doi: 10.1007/s00535-012-0731-8. [DOI] [PubMed] [Google Scholar]
- 50.Okimoto K, Arai M, Saito K, et al. Efficacy of Levofloxacin Based Triple and High-Dose PPI-Amoxicillin Dual Eradication Therapy for Helicobacter pylori after Failures of First- and Second-Line Therapies. Int Sch Res Not. 2014;2014:631501. doi: 10.1155/2014/631501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paoluzi OA, Del Vecchio Blanco G, Visconti E, et al. Low efficacy of levofloxacin-doxycycline-based third-line triple therapy for Helicobacter pylori eradication in Italy. World J Gastroenterol. 2015;21(21):6698–6705. doi: 10.3748/wjg.v21.i21.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liou JM, Chang CY, Sheng WH, et al. Genotypic resistance in Helicobacter pylori strains correlates with susceptibility test and treatment outcomes after levofloxacin- and clarithromycin-based therapies. Antimicrob Agents Chemother. 2011;55(3):1123–1129. doi: 10.1128/AAC.01131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perna F, Zullo A, Ricci C, et al. Levofloxacin-based triple therapy for Helicobacter pylori re-treatment: role of bacterial resistance. Dig Liver Dis. 2007;39(11):1001–1005. doi: 10.1016/j.dld.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 54.Matsuzaki J, Suzuki H, Nishizawa T, et al. Efficacy of sitafloxacin-based rescue therapy for Helicobacter pylori after failures of first- and second-line therapies. Antimicrob Agents Chemother. 2012;56(3):1643–1645. doi: 10.1128/AAC.05941-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mori H, Suzuki H, Matsuzaki J, et al. Efficacy of 10-day sitafloxacin-containing third-line rescue therapies for Helicobacter pylori strains containing the gyrA mutation. Helicobacter. 2016;21(4):286–294. doi: 10.1111/hel.12286. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki H, Nishizawa T, Muraoka H, et al. Sitafloxacin and garenoxacin may overcome the antibiotic resistance of Helicobacter pylori with gyrA mutation. Antimicrob Agents Chemother. 2009;53(4):1720–1721. doi: 10.1128/AAC.00049-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuzaki J, Suzuki H, Tsugawa H, et al. Homology model of the DNA gyrase enzyme of Helicobacter pylori, a target of quinolone-based eradication therapy. J Gastroenterol Hepatol. 2010;25(Suppl 1):S7–S10. doi: 10.1111/j.1440-1746.2010.06245.x. [DOI] [PubMed] [Google Scholar]
- 58.Gisbert JP. “Rescue” regimens after Helicobacter pylori treatment failure. World J Gastroenterol. 2008;14(35):5385–5402. doi: 10.3748/wjg.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mori H, Suzuki H, Matsuzaki J, et al. Acquisition of double mutation in gyrA caused high resistance to sitafloxacin in Helicobacter pylori after unsuccessful eradication with sitafloxacin-containing regimens. United Eur Gastroenterol J. 2017 doi: 10.1177/2050640617737215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miehlke S, Hansky K, Schneider-Brachert W, et al. Randomized trial of rifabutin-based triple therapy and high-dose dual therapy for rescue treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Aliment Pharmacol Ther. 2006;24(2):395–403. doi: 10.1111/j.1365-2036.2006.02993.x. [DOI] [PubMed] [Google Scholar]
- 61.Gisbert JP, Castro-Fernandez M, Perez-Aisa A, et al. Fourth-line rescue therapy with rifabutin in patients with three Helicobacter pylori eradication failures. Aliment Pharmacol Ther. 2012;35(8):941–947. doi: 10.1111/j.1365-2036.2012.05053.x. [DOI] [PubMed] [Google Scholar]
- 62.Perri F, Festa V, Clemente R, et al. Randomized study of two “rescue” therapies for Helicobacter pylori-infected patients after failure of standard triple therapies. Am J Gastroenterol. 2001;96(1):58–62. doi: 10.1111/j.1572-0241.2001.03452.x. [DOI] [PubMed] [Google Scholar]
- 63.Mori H, Suzuki H, Matsuzaki J, et al. Rifabutin-based 10-day and 14-day triple therapy as a third-line and fourth-line regimen for Helicobacter pylori eradication: a pilot study. United Eur Gastroenterol J. 2016;4(3):380–387. doi: 10.1177/2050640615618043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ciccaglione AF, Tavani R, Grossi L, et al. Rifabutin containing triple therapy and rifabutin with bismuth containing quadruple therapy for third-line treatment of Helicobacter pylori infection: two pilot studies. Helicobacter. 2016;21(5):375–381. doi: 10.1111/hel.12296. [DOI] [PubMed] [Google Scholar]
- 65.Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;35(2):209–221. doi: 10.1111/j.1365-2036.2011.04937.x. [DOI] [PubMed] [Google Scholar]
- 66.Lim HC, Lee YJ, An B, et al. Rifabutin-based high-dose proton-pump inhibitor and amoxicillin triple regimen as the rescue treatment for Helicobacter pylori. Helicobacter. 2014;19(6):455–461. doi: 10.1111/hel.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishizawa T, Suzuki H, Maekawa T, et al. Dual therapy for third-line Helicobacter pylori eradication and urea breath test prediction. World J Gastroenterol. 2012;18(21):2735–2738. doi: 10.3748/wjg.v18.i21.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishizawa T, Suzuki H, Fujimoto A, et al. Effects of patient age and choice of antisecretory agent on success of eradication therapy for Helicobacter pylori infection. J Clin Biochem Nutr. 2017;60(3):208–210. doi: 10.3164/jcbn.16-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372(9636):392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 70.Yoon SB, Park JM, Lim CH, et al. Effect of Helicobacter pylori eradication on metachronous gastric cancer after endoscopic resection of gastric tumors: a meta-analysis. Helicobacter. 2014;19(4):243–248. doi: 10.1111/hel.12146. [DOI] [PubMed] [Google Scholar]
- 71.Ford AC, Forman D, Hunt RH, et al. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. doi: 10.1136/bmj.g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Take S, Mizuno M, Ishiki K, et al. The long-term risk of gastric cancer after the successful eradication of Helicobacter pylori. J Gastroenterol. 2011;46(3):318–324. doi: 10.1007/s00535-010-0347-9. [DOI] [PubMed] [Google Scholar]