Abstract
Through the course of over three decades, nonhuman primate (NHP) studies on cell-based therapies (CBTs) for Parkinson’s disease (PD) have provided insight into the feasibility, safety and efficacy of the approach, methods of cell collection and preparation, cell viability, as well as potential brain targets. Today, NHP research continues to be a vital source of information for improving cell grafts and analyzing how the host affects graft survival, integration and function. Overall, this article aims to discuss the role that NHP models of PD have played in CBT development and highlights specific issues that need to be considered to maximize the value of NHP studies for the successful clinical translation of CBTs.
Keywords: Parkinson’s disease, Nonhuman primates, Dopamine, Stem cells
Introduction
Since the early 1980s, scientists have relied on nonhuman primate (NHP) models to assess whether cell-based therapies (CBTs) can be beneficial for Parkinson’s disease (PD). CBT strategies and NHP models of PD emerged into the scientific arena simultaneously. In a way, the availability of the new NHP models fueled CBT progression towards clinical application.
As the main aim of CBTs for PD was and still is the replacement of neurons lost in the disease, PD animal models with neurotoxin-induced neuronal loss became an ideal platform to assess the approach. CBT studies in rodent models provided invaluable information on neuronal survival, migration and integration after grafting (Kim et al. 2013). Clinical translation of CBTs requires progressive evaluation in different species and as a first-in-class and invasive brain therapy, NHP experiments are a logical next step (Capitanio and Emborg 2008). Compared to rodents that are inbred, NHPs are outbred. Behavioral outcome measures such as fine motor skills, which are affected in PD, can be easily tested in NHPs but not in other large species, like pigs. Clinically relevant behavioral outcome measures are critical to determine the efficacy of the strategy, including the selection of intracerebral grafting targets. In that regard, NHPs and humans share a similar organization of the striatum, with the caudate nucleus and the putamen clearly delineated by the white matter tracts of the internal capsule. In rodents, transecting white matter tracts perforate throughout the striata, without presenting a physical barrier for cell distribution (Fig. 1).
Fig. 1.
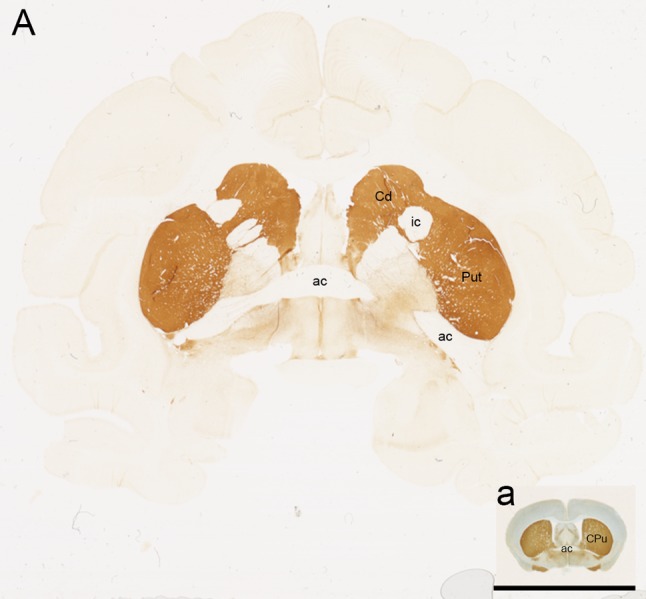
Coronal brain sections of A rhesus and a mouse brain immunostained against tyrosine hydroxylase (TH) highlighting the comparison of brain size and complexity. Scale bar 1 cm. Cd caudate, ic internal capsule, Put putamen, ac anterior commissure, CPu caudate and putamen
In this article, we aim to discuss the role of NHP models of PD in CBT development, keeping in perspective how the field of PD is evolving, analyze ongoing advances in CBTs and the issues that need to be considered to maximize the value of NHP studies for the successful clinical translation of CBTs for PD.
Parkinson’s disease: then and now
Parkinson’s disease (PD) is the 2nd most common neurodegenerative disorder affecting around 1% of the population over the age of 60 (Driver et al. 2009). When CBTs were first envisioned for actual clinical application, the conceptualization of PD suggested that it was an ideal candidate disease for brain repair. Patients were diagnosed by typical motor symptoms (resting tremor, rigidity, bradykinesia, and postural instability), which were associated with the loss of dopaminergic (DAergic) nigral neurons. As the symptoms responded to oral dopamine (DA) replacement therapy, it was expected that dopamine replacement with a cell source should be an efficient way to securely and locally deliver DA and, basically, cure PD. In addition, the brain was viewed as an immunoprivileged, postmitotic organ.
The understanding of PD and the brain has evolved over time, which affects the application of CBT strategies. Today, PD diagnoses still depend on the presence of typical motor symptoms, and postmortem confirmation of nigral DAergic neuron loss and the presence of intracytoplasmic neuronal inclusions termed Lewy bodies [LBs; (Vermilyea and Emborg 2015)]. Yet, PD is now recognized as a complex neurodegenerative disorder that includes non-motor symptoms (NMS). Depression, anxiety, loss of sense of smell, gastrointestinal dysfunction and cardiac dysautonomia are common PD NMS, which are associated with neurodegeneration in other areas of the central and peripheral nervous system (Chaudhuri and Odin 2010; Chaudhuri et al. 2011). Interestingly, NMS precede the onset of the movement disorder by decades and are now proposed as prodromal signs of the disease (Postuma et al. 2012). Earlier PD diagnoses would increase the chances of success of neuroprotective strategies. In that regard, brain immunoreactivity has been documented (Kordower et al. 1997; Roitberg et al. 2004; Tambur 2004) and neuro-inflammation has been linked to PD neurodegeneration, suggesting that immunomodulation can be neuroprotective (Kannarkat et al. 2013). Neurogenesis has been documented in the adult brain of rodents (Altman and Das 1965; Kaplan and Hinds 1977; Kempermann et al. 1997), NHPs (Gould et al. 1999) and humans (Eriksson et al. 1998), and directed neurogenesis has been discussed for self-brain repair (Rakic 2004).
Another chain of events that led to findings with great implications for PD and CBTs started in 1996, when mutations in the alpha-synuclein (α-syn) gene were found in familial forms of PD (Polymeropoulos et al. 1997). Subsequent studies identified α-syn as the main component of LBs (Spillantini et al. 1997, 1998). Then, in 2008 LBs were reported in dopaminergic fetal grafts of PD patients that were transplanted a decade earlier, suggesting that the grafts “caught PD” from the host (Li et al. 2008; Kordower et al. 2008). Since then, α-syn research has taken a center stage in PD research (Bendor et al. 2013; Vermilyea and Emborg 2015). Investigations on whether α-syn has prion-like activity revitalized the Braak and Braak hypothesis that PD may start in the brainstem and propagate through the neural axis (Braak et al. 2004; Chu and Kordower 2015; Hilker et al. 2011). Studies on protein aggregation followed (Luk et al. 2009), as well as the search for neuroprotective approaches aiming to prevent aggregation (Kalia et al. 2015). It should be noted that the cause of PD is still unclear and that the question of whether the early peripheral symptoms reflect where PD starts or less neuroplasticity is being debated (Engelender and Isacson 2016).
NHP models of PD used for CBT evaluation
Common marmoset, vervet and macaque monkeys are the most used NHP species for CBT studies. To the best of our knowledge, only neurotoxin-induced NHP models of PD have been used as testing platforms for CBTs, mainly by the administration of 6-hydroxydopamine (6-OHDA) or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), although new models are emerging (Table 1).
Table 1.
Comparison between NHP models of PD highlighting key features for evaluation of CBTs for DA cell replacement
| NHP PD model | PD motor symptoms | Nigrostriatal DA depletion | Typical LIDs | Synucleinopathy | Comments |
|---|---|---|---|---|---|
| 6-OHDA | Yes | Yes | No | Not reported | Unilateral model. Requires multiple intracerebral stereotaxic injections to induce a stable lesion |
| MPTP systemic | Yes | Yes | Yes | Upregulation of a-syn | Bilateral model. Needs to be individually titrated, and depending on dosing paradigm may require from one week to over a year to induce syndrome. PD symptoms may spontaneously recover |
| MPTP systemic+aging | Yes | Yes | Yes | Upregulation of α-syn, possible aggregates | Same as MPTP systemic plus animals may require more intensive care post intoxication |
| MPTP ICA | Yes | Yes | No | Not reported | Unilateral model. Requires surgical set up; Induces a stable and reproducible lesion |
| MPTP ICA+aging | Yes | Yes | No | Not reported | Same as MPTP ICA except MPTP dose needs to be 2/3 of younger animals. Monkeys may require more intensive care post intoxication |
| Aged NHPs | Yes | Yes | No | Translocation of α-syn | Not enough dopamine deficit to be responsive to l-DOPA treatment |
| Viral vector delivery of α-syn | Yes (Common marmosets) | Yes | Unknown | Overexpression of α-syn, aggregates | Unilateral model. Requires intracerebral stereotaxic injections |
| Lewy body extracts | No | Yes | Unknown | Overexpression of α-syn, aggregates | Unilateral model. Requires intracerebral stereotaxic injections |
| α-syn Transgenic | Mild (Cynomolgus 1.5–2 years old) | Unknown | Unknown | Unknown | Bilateral model |
6-OHDA 6 hydroxydopamine, α-syn alpha-synuclein, MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, ICA intracarotid artery, l -DOPA L-3,4-dihydroxyphenylalanine, LIDs l-DOPA induced dyskinesias
6-OHDA (Senoh and Witkop 1959) is preferentially used in common marmoset monkeys (Ungerstedt 1968; Blandini et al. 2008; Eslamboli et al. 2005). As 6-OHDA does not cross the blood–brain barrier (BBB), it is stereotaxically injected directly into the right or left striatum, medial forebrain bundle or substantia nigra, inducing unilateral motor impairments. The neurotoxin is selectively taken up by catecholaminergic neurons through monoamine transporters, and induces sympathetic neuronal loss by increasing the production of reactive oxygen species (ROS), and disrupting energy metabolism and neuronal activity (Blum et al. 2001).
MPTP (Davis et al. 1979; Langston et al. 1983) is most commonly administered to macaque monkeys, although it is also used in other old and new world NHP species (Fox and Brotchie 2010; Emborg 2007). Unlike 6-OHDA, MPTP crosses the BBB and is administered to NHPs via s.c., i.m., i.v. or intracarotid artery (ICA) injection. Severity of PD symptoms depends on route, dose and frequency of administration; dosing regimen varies between species (Emborg 2007). In the brain, MPTP is transformed into its toxic metabolite MPP+ by monoamine oxidase-B (MAO-B). MPP+ is then selectively taken up by the DA transporter into dopaminergic neurons where it disrupts normal mitochondrial respiration by acting as a mitochondrial complex I inhibitor, leading to oxidative stress and apoptosis (Przedborski and Vila 2001).
Although it can be argued that neurotoxic models are missing critical components of PD, such as “true” LB formation (Dauer and Przedborski 2003), several reasons justify their use to test CBTs as DA replacement/network restoration strategies: (1) neurotoxin-induced models present PD-like motor symptoms and dopaminergic nigrostriatal loss, (2) they are well characterized, and (3) they can be induced in a protracted period of time, which facilitates their use as testing platforms.
We agree with the concept that an ideal NHP model of PD should mimic the disease by replicating its etiology, which should induce the pathological mechanisms that give rise to the typical symptoms. Yet, although great progress has been made towards understanding the complexity of PD and possible pathways of neurodegeneration, its cause is still unknown. Neurotoxin-based models attempt to capitalize on the knowledge that exposure to environmental toxins is a PD risk factor. In that regard, both neurotoxins trigger mechanisms associated with neurodegeneration in PD. In addition to disrupting energy metabolism and increasing oxidative stress, dosing with 6-OHDA and MPTP induces inflammatory responses (Rodriguez-Pallares et al. 2007; Joglar et al. 2009; McGeer et al. 2003). MPTP dosing has also been shown to trigger an increase in α-syn expression and, in some cases, its accumulation (Halliday et al. 2009; Kowall et al. 2000; McCormack et al. 2008).
Other identified PD risk factors include aging and genetic mutations which are exploited for modeling purposes (Emborg 2007). Studies in aged NHPs with or without MPTP have been reported. Intact aged animals present subtle PD-like symptoms (e.g.: slowness and overall decreased amount of movement) and nigrostriatal dopaminergic loss, with individual variations. As the symptoms in aged monkeys do not respond to DA replacement therapies, the animals are not useful models to assess cell replacement strategies. In contrast, aged animals intoxicated with MPTP present the typical motor and pathological syndrome observed after neurotoxin, plus the background aged condition associated with PD, and could be useful testing platforms, yet CBT studies in models combining aging and neurotoxins have not been reported.
Genetic-like NHP PD models have been induced by intracerebral injection of viral vectors encoding for mutated α-syn or administration of LB extracts [see review (Vermilyea and Emborg 2015)]. Adeno-associated viral (AAV) vector-induced nigral overexpression of human α-syn wild type and A53T has been shown to induce PD-like motor symptoms, significant nigral dopaminergic cell loss, and α-syn aggregates in common marmoset monkeys (Eslamboli et al. 2007; Kirik et al. 2003). AAV and lentiviral vectors encoding for A53T α-syn have also been used in cynomolgus (Koprich et al. 2016) and rhesus (Yang et al. 2015) monkeys. In both studies, A53T α-syn induced nigral cell loss and α-syn accumulation and aggregation; behavioral changes were not reported. A combination of AAV-induced overexpression of Parkin and A53T α-syn was reported in cynomolgus; although the animals had decreased striatal dopaminergic markers and α-syn accumulation and phosphorylation, no motor symptoms were observed. It should be noted that in vervet monkeys nigral injection of AAV expressing a short hairpin RNA (shRNA) to knock down α-syn induced a region-specific decrease in TH-positive nigral cell number and striatal innervation compared to animals that received scrambled shRNA; no behavioral changes were reported (Collier et al. 2016). Intracerebral inoculation of α-syn fibrils has been extensively used in rodents, but not yet in monkeys (e.g.: (Luk et al. 2012; Paumier et al. 2015)). Instead cadaveric LB extracts have been injected into the striatum or nigra of cynomolgus monkeys with or without previous MPTP, (Recasens et al. 2014). The extracts induced some decreases in striatal and nigral dopaminergic markers and increases in α-syn expression, yet PD motor symptoms were not detected. It should be noted that with exception of the AAV α-syn studies in marmosets, all the other reports in genetic-like models were performed in a few subjects; further characterization and validation of the models are needed before they are used as testing platforms for CBTs. Transgenic NHP models induced by oocyte injection of lentiviral vectors encoding for mutations of interest are emerging; transgenic rhesus monkeys overexpressing mutant A53T α-syn have been reported (Niu et al. 2015). The authors also reported some behavioral deficits after 1.5–2.5 years of age. New technologies such as CRISPR/Cas9 genomic editing present an opportunity to create NHP models with PD associated mutations expressed at physiological levels that may help clarify the disease onset process, including motor and non-motor symptoms (Gaj et al. 2016). Timely evaluation of CBTs in these novel NHP models may provide clues to understand α-syn-related problems during clinical translation and define the role of CBTs in global therapies.
CBTs for DA cell replacement: from rodents and monkeys to PD patients
Fetal mesencephalon and autologous adrenal medullary tissues were the first sources used to demonstrate the feasibility for DA cell replacement. In 1979, Bjorklund and Stenevi (Bjorklund and Stenevi 1979) reported positive effects of fetal grafts in circling behavior of 6-OHDA-treated rats, and in 1981, Freed et al. (Freed et al. 1981) showed adrenal graft survival in a similar model. In 1984, Morihisa and colleagues (Morihisa et al. 1984) transplanted adrenal medullary tissue and fetal mesencephalic cells into MPTP-treated parkinsonian rhesus monkeys and showed some survival of adrenal, but not fetal, cells. In follow-up experiments, poor survival of transplanted adrenal tissue was reported (Morihisa et al. 1987) yet as the PD signs ameliorated, researchers hypothesized that the intense host-derived dopaminergic sprouting in the transplanted area was responsible for the behavioral improvements. Improved graft survival was observed when methods were applied to minimize the endothelial components (Schueler et al. 1993). With regard to fetal mesencephalic grafts, between 1986 and 1994, 15 NHP reports were published (Bakay et al. 1987; Collier et al. 1987; Redmond et al. 1986, 1988; Sladek et al. 1986; Annett et al. 1990, 1994; Bankiewicz et al. 1990; Collier et al. 1994; Dubach et al. 1988; Elsworth et al. 1994; Fine et al. 1988; Sladek et al. 1993; Taylor et al. 1990, 1991) demonstrating the feasibility of the approach, as well as different degrees of antiparkinsonian effects. It should be noted that these studies were performed in a limited number of NHPs per treatment group, in most cases the area of the brain targeted was the caudate nucleus, the animals did not receive immunosuppression and the effect of antiparkinsonian medication was not evaluated (Fitzpatrick et al. 2009).
Both CBT approaches were rapidly translated to humans. In 1985, Backlund et al. reported two cases of patients receiving adrenal medulla grafts resulting in mild effects, and in 1987, Madrazo and colleagues showed the first dramatic improvement of PD symptoms in two patients (Madrazo et al. 1987). Following these promising results, several similar case studies were undertaken, with different, less favorable outcomes [see review: (Redmond 2002)]. Goetz et al. reported the results of a multi-center study of 19 patients in 1989 (Goetz et al. 1989). The grafted patients showed minimal temporary antiparkinsonian effects, described as decreased mean severity of “off” time (time when the positive effects of pharmacological treatment wear off) as assessed by both the Activities of Daily Living subscale of the Unified Parkinson’s Disease Rating Scale (UPDRS) and the Schwab and England scale. Yet, the patients’ antiparkinsonian medications could not be decreased and postoperative morbidity was considerable, due to the double surgery (abdominal and brain) required to harvest the adrenal medulla and then transplant the cells. Postmortem results revealed poor cell survival and localized host regional neuronal sprouting, similar to the results in the NHP experiments.
With regard to fetal mesencephalon grafting, after multiple case reports in PD patients [see (Freed et al. 1992; Redmond et al. 1993)] the National Institute of Health (NIH) funded two prospective, double blind, randomized control trials aiming to assess the efficacy and safety of transplanting fetal mesencephalic tissue to treat PD. The Freed et al. (Freed et al. 2001) trial consisted of 40 PD patients: 20 received bilateral post-commissural putaminal grafts of ventral mesencephalic fetal neurons, and 20 had a burr hole drilled into their skull as a sham procedure. Immunosuppression was not administered. The fetal cells were cultured for 1 week prior to transplantation. The primary endpoint was patient self-reports on activities of daily living (recorded at home for 12 months). The Olanow et al. trial (2003) consisted of 34 PD patients: 11 received bilateral post-commissural putaminal grafts of ventral mesencephalic fetal neurons from one donor fetus, 12 from four donor fetuses, and 11 received bilateral sham procedures. Oral cyclosporine (CsA) immunosuppression was administered to all patients starting 2 weeks prior to surgery and continued for 6 months after grafting. The primary endpoint was the UPDRS motor subscore. Both trials did not show significant differences between treatment groups, although further analysis revealed that patients younger than 60 years old or with less severe PD at baseline had improvements in their parkinsonian signs. Positron Emission Tomography (PET) imaging demonstrated increased [18F]fluorodopa uptake in the grafted areas suggesting graft survival that was later confirmed by postmortem examinations.
An unexpected outcome for both trials was the occurrence of what was then named “runaway” dyskinesias, also known as graft-induced dyskinesias (GIDs). Unlike typical PD dyskinesias that are induced by chronic long-term l-DOPA administration [see (Bezard et al. 2001)], these uncontrolled abnormal movements were not associated with antiparkinsonian medication and did not ameliorate with reduction or cessation of l-DOPA treatment. GIDs were observed in a quarter of the grafted patients in the Freed et al. and half the Olanow et al. trials.
Several issues may have contributed to the onset of GIDs. As patients that received the fetal mesencephalic grafts presented focal points of increased PET signal (Ma et al. 2002), it was proposed that the grafts produced “hot spot” regions in which DA was being excessively released. To answer the question of whether there was an association between GIDs and focal versus widespread distribution of cell grafts in the striatum (Maries et al. 2006), 6-OHDA-treated rats received either a focal striatal transplant of 200,000 cells, or the same number of cells across six different striatal locations. The experiment showed that GIDs were only present in the focal graft recipients. It also brought to light that the number of functional cells within a focal site might be of equal concern. Another proposed contributing factor for GIDs was that the chronic l-DOPA treatment preceding the transplantation may have “primed” the patients to have dyskinesias. In 6-OHDA-intoxicated rats, l-DOPA pre-treatment affected graft integration and functionality, although GIDs were present with or without l-DOPA pre-treatment (Steece-Collier et al. 2009). A study in 24 systemic MPTP-treated vervet monkeys (Chlorocebus sabaeus) assessed whether priming the animals to develop l-DOPA-induced dyskinesias (LIDs), and then injecting allogeneic fetal dopaminergic cells in a “spread” or “hotspot” pattern would affect the development of GIDs (Kordower et al. 2016). The investigators did not detect GIDs in any of the monkeys, regardless of cell distribution or condition. Possible species differences could be at play as well as methods of cell preparation. A critical limitation of this report is that the animals’ MPTP-induced parkinsonism spontaneously improved overtime. All the monkeys presented similar mild PD scores with no differences between grafted and control subjects. Thus, the graft’s antiparkinsonian efficacy or graft potential to produce true functional hotspots could not be evaluated.
Inflammation has also been proposed to promote the onset of GIDs through aberrant synapse formation between grafted neurons and host striatal medium spiny neurons [MSNs; (Soderstrom et al. 2008)]. This theory was derived from the observation of GIDs in patients who did not receive immunosuppression following fetal engraftment or those that had been recently taken off an immunosuppressive regimen. Interestingly, the evaluation of spine density maintenance in MSNs through administration of slow-release pellets of the calcium channel antagonist nimodipine has led to intriguing evidence about the importance of preserving MSNs for reducing LIDs and transiently reducing GIDs (Soderstrom et al. 2010). Patient and rodent studies have also highlighted that serotonergic and noradrenergic neurotransmission either by host innervation or by mixed-cell grafts may contribute to GIDs (Shin et al. 2012). As the cause of GIDs is being unraveled, new strategies aiming to prevent or decrease GIDs are being evaluated.
Another potential complication for CBTs was uncovered in 2008, when follow-up postmortem analysis of PD patients treated a decade earlier with fetal grafts found LB-like pathology in the grafted cells (Kordower et al. 2008; Li et al. 2008). Although it is unclear how much the aggregates affected the functionality of the grafts, the implication that PD could be transferred to the grafted cells reverberated throughout the field (see “Parkinson’s disease: then and now”). A preliminary confirmation of α-syn being transferred into grafted cells was obtained in rats injected with adeno-associated viral vector serotype 6 encoding for human α-syn into the striatum after fetal cell engraftment (Kordower et al. 2011).
New clinical trials utilizing fetal mesencephalic tissue for DA cell replacement are currently ongoing in Canada, Europe (Transeuro), South Korea and Mexico (listed in: clinical trials.gov and isrctn.com). The investigators leading these trials aim to optimize the approach by taking advantage of the knowledge gained from the clinical and preclinical studies described above.
Additional clinical trials using CBTs for DA cell replacement in PD
A number of alternative sources for dopaminergic neurons, including mesencephalic fetal porcine cells, cadaveric human retinal-pigmented epithelium (hRPE) and autologous sympathetic and carotid ganglia, have been investigated to avoid the surgical complication of using autologous adrenal medullary tissue and to overcome the practical and ethical limitations of using human fetal cells for large-scale clinical applications. NHP preclinical studies were only performed for hRPE. The DA-producing cells were attached to gel microcarriers (Spheramine®) and placed into the striatum of parkinsonian monkeys. The grafts improved motor function and postmortem analysis showed cell survival and a mild inflammatory reaction (Watts et al. 2003). Controlled clinical trials for hRPE as well as all the sources listed above failed to show a significant antiparkinsonian effect (Fitzpatrick et al. 2009).
Ex vivo gene therapy for PD
Ex vivo gene therapy originated as a method to engineer cells for delivery of therapeutic molecules (Raymon et al. 1997). The cells are typically genetically modified using viral vectors. The main advantage of this method compared to direct intracerebral viral vector delivery (in vivo gene therapy) is that the transfected cells can be monitored before transplantation for the effects of viral infection and the production of a foreign protein. Safety, genetically engineered, “tricks” have been developed to curtail unwanted side effects, such as regulatable promoters to stop gene expression and kill-switches to terminate the cells and completely stop product synthesis.
Ex vivo gene therapy strategies have been developed for DA replacement and trophic factor delivery with variable results. MPTP-intoxicated rhesus monkeys received autologous fibroblasts genetically engineered to produce tyrosine hydroxylase [the rate-limiting enzyme for DA production; e.g., (Bankiewicz et al. 2000)] with minimal antiparkinsonian effects. Glial cell line-derived neurotrophic factor (GDNF) producing C2C12 cells were encapsulated and transplanted into MPTP-treated baboons (Kishima et al. 2004) inducing only temporary improvements of their parkinsonian symptoms, probably due to the low survival of encapsulated cells which led to a low and variable protein production. New cell sources for ex vivo gene therapy (see below) are emerging. For example, human neuroprogenitor cells (hNPCs) have been shown to survive and locally produce GDNF in the brain of parkinsonian immunosuppressed rats and monkeys (Behrstock et al. 2006; Emborg et al. 2008). Although the clinical translation for PD has not been pursued (probably due to the poor results of GDNF protein delivery trials; (Richardson et al. 2011)), a clinical trial for amyotrophic lateral sclerosis is currently ongoing (http://www.clinicaltrials.gov).
Stem cells as sources of cell lines
Biological research breakthroughs and development of new technologies have paved the way for the identification of stem cells (SCs) as new cell sources for regenerative medicine approaches. SCs are defined by their self-renewal capacity and their potential for becoming a different cell type with a specialized function. These properties allow researchers to create cell lines to be repurposed for in vitro studies and transplantation.
hNPCs are typically obtained from the germinal layer of a fetal brain. Although they are not pluripotent SCs (they are already fated toward a brain cell phenotype), in vitro hNPCs can be expanded and differentiated to a DA phenotype (Sanchez-Pernaute et al. 2001). In MPTP-treated monkeys, hNPCs induced improvements of PD signs and postmortem analysis has shown their survival and migration. Their cell progeny seemed to differentiate in vivo into DA-like neurons and glial phenotypes and overall have a “homeostatic” impact (Bjugstad et al. 2005, 2008; Redmond et al. 2007). It should be noted that NPCs have been found in the adult human and NHP brains (Eriksson et al. 1998; Gould et al. 1999). These findings, plus earlier studies in rodents, refuted the idea that the adult brain is incapable of forming new neurons (Altman and Das 1965; Buzanska et al. 2002; Kaplan and Hinds 1977; Kempermann et al. 1997). The option of recruiting resident hNPCs for brain repair has tantalizing possibilities but its utility has not yet been proven (Rakic 2004). Interestingly, one study grafted autologous adult cortical cells (cultured for a couple of weeks) into the caudate of MPTP-intoxicated vervet monkeys. Grafted cells were found 4 months post-surgery and their presence was associated with increased local levels of GDNF (Brunet et al. 2009).
Bone marrow, umbilical cord blood and adult adipose-derived stromal tissue (Fallahi-Sichani et al. 2007; Levy et al. 2008; McCoy et al. 2008) have been proposed as SC sources as they can be obtained for autologous grafts. Although NHP studies with these cells have not been performed, evaluation in rodents suggests that they produce trophic factors that could be beneficial for PD. Several clinical trials are currently ongoing to test the safety and efficacy after administration of mesenchymal stem cells through intravenous, intracarotid or intranasal routes (clinicaltrials.gov). Intravenous infusion of adipose-derived stromal vascular fraction cells is also being investigated for safety and efficacy, as well as for benefits to the quality of daily living in PD patients.
Embryonic stem cells (ESCs) obtained from blastocysts are pluripotent SCs, thus they have the potential to become any cell of the body. In 1995, Thomson et al. (Thomson et al. 1995) reported the isolation of ESCs from rhesus monkeys. Common marmoset ESCs were isolated in 1996 (Thomson et al. 1996), followed in 1998 by human ESCs [hESC; (Thomson et al. 1998)]. Differentiation of ESCs into a DA phenotype was first accomplished in mice (Lee et al. 2000), followed a few years later in human (Perrier et al. 2004; Yan et al. 2005) and rhesus (Takagi et al. 2005). Since then, investigators have been looking for more efficient ways to produce mesencephalic DA neurons, as well as to solve the problems of intracerebral graft survival and other challenges identified by the fetal tissue trials. The ethical dilemma of the cells’ origin triggered the 2001 restriction of USA federal funding for hESC research to studies performed on authorized cell lines, limiting the chances for creating new ones. The 8-year ban on federal funding for ESC research was lifted on March 9, 2009.
Parthenogenesis, somatic cell nuclear transfer and altered somatic cell nuclear transfer have been proposed as alternative sources of pluripotent SCs (Kastenberg and Odorico 2008). In NHPs, parthenogenesis has been used to generate an SC line from cynomolgus monkeys (cyno-1). Parthenogenesis is an asexual form of reproduction; although mammalian eggs cannot fully develop, they can provide blastocysts to generate parthenogenesis-derived ESCs. Using the cyno-1 cell line, investigators differentiated the cells into dopaminergic neurons (Perrier et al. 2004). These cells were successfully transplanted into 6-OHDA-treated rats and one MPTP-treated cynomolgus monkey (Sanchez-Pernaute et al. 2008). hNPCs generated from parthenogenetic hSCs (hpNPCs) have been evaluated in rats and MPTP-lesioned vervet monkeys immunosuppressed with a combination of cyclosporine, prednisone and azathioprine (Gonzalez et al. 2015, 2016). The investigators first demonstrated graft survival and increased DA striatal levels 3 months post-surgery in two NHPs (Gonzalez et al. 2015). Antiparkinsonian efficacy of hpNPCs was then evaluated in 18 monkeys (Gonzalez et al. 2016). The animals were matched according to disability and assigned to one of 3 treatment groups: vehicle (n = 6), low (n = 6) and high (n = 6) cell dosing. Cells were inoculated in the caudate, putamen and substantia nigra. Twelve months post-grafting, the low dose animals showed significant behavioral improvements compared to their baseline condition; however, no significant differences between vehicle and dosing groups were detected. A clinical trial to assess the safety and tolerability of hpNPCs in PD is currently ongoing in Australia (clinical trials.gov). Three patient groups (each to receive different cell number doses) of four patients each who have moderate to severe PD will receive between 30 and 70 million cells injected into the striatum and substantia nigra.
The development of induced pluripotent stem cells (iPSCs) from somatic cells (Takahashi et al. 2007a, b; Yu et al. 2007) has further facilitated the production of additional cell lines for regenerative medicine and disease modeling purposes. iPSCs represent a major advancement towards personalized medicine as cells can be generated from the prospective recipient. iPSCs have been generated from macaque (Deleidi et al. 2011; Liu et al. 2008) and marmoset (Tomioka et al. 2010; Wiedemann et al. 2012; Wu et al. 2010; Vermilyea et al. 2016a) monkeys. The derivation of iPSCs has been modified in recent years using expression plasmids that do not integrate into the host DNA, which increases their safety for clinical translation.
NHP studies assessing SC-derived DA cell replacement strategies
Over the past decade, several NHP studies have analyzed the potential use of SCs from different sources and at different stages of differentiation for DA cell replacement (Table 2).
Table 2.
Peer-reviewed publications in NHP models of PD evaluating SCs as sources for dopaminergic cell replacement
| References | Host species | Cell species; graft type | Cell type | Cell labeling | Transplant location | Model | n | Immunosup. | l-DOPA | Behavioral test | PET imaging | Duration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Takagi et al. (2005) | Cynomolgus (M. fascicularis) | Cynomolgus; allograft | ESC-derived DAergic progenitors | BrdU | Bilateral putamen; 3 tracts per side, each with 4 deposits | MPTP i.v. (0.4 mg/kg; twice weekly for 7 weeks) | 6 | CsA 10 mg/kg | None | Clinical rating | [18F]DOPA | 14 weeks |
| Cynomolgus (M. fascicularis) | N/A | Sham-operated | N/A | N/A | MPTP i.v. (0.4 mg/kg; twice weekly for 7 weeks) | 4 | CsA 10 mg/kg | None | Clinical rating | [18F]DOPA | 14 weeks | |
| Kikuchi et al. (2011) | Cynomolgus (M. fascicularis) | Human; xenograft | iPSC-derived DAergic progenitors | None | Bilateral putamen (d28 to right, d42 to left); 6 tracts per side, each with 4 deposits | MPTP i.v. (0.4 mg/kg; twice weekly until symptoms persisted) | 1 | FK506 0.05 mg/kg | None | Clinical rating, spontaneous movements, Gross Movements, Raisin pick-up test | [18F]DOPA, [11C]DTBZ, [11C]PE2I, [18F]FLT | 6 months |
| Daadi et al. (2012) | Green monkey (C. sabaeus) | Human; xenograft | ESC-derived DAergic progenitors | GFP | Caudate and SN | MPTP i.m. (2.25 mg/kg cumulative dose; administered over 5-day period) | 4 | CsA 5 mg/kg/day Prednisone 2 mg/kg tapering to 0.6 mg/kg/day Azathioprine 5 mg/kg/day reduced to 1 mg/kg/day | None | None | None | 2 months |
| Kriks et al. (2011) | Rhesus (M. mulatta) | Human; xenograft | ESC-derived DAergic progenitors | GFP | Bilateral posterior caudate, pre-commissural putamen and overlying white matter (GFP to one side, unmarked to other); 3 tracts per side | MPTP ICA (3 mg) followed by weekly i.v. administration (dose n.r.) | 2 | CsA 30 mg/kg tapered to 15 mg/kg | None | None | None | 1 month |
| Emborg et al. (2013b) | Rhesus (M. mulatta) | Human; xenograft | ESC-derived DAergic progenitors | GFP | Unilateral (ipsi-MPTP) rostral, medial and caudal caudate and putamen, and SN; 7 tracts total | MPTP ICA (3 mg) | 3 | CsA 40–50 mg/kg | None | None | None | 3 months |
| Emborg et al. (2013a) | Rhesus (M. mulatta) | Rhesus; autograft | iPSC-derived DAergic progenitors | GFP | Unilateral (ipsi-MPTP) precommisural and commissural caudate, pre-commisural, commissural and postcommisural putamen, and SN; 6 tracts total | MPTP ICA (3 mg) | 3 | None | None | None | None | 6 months |
| Wakeman et al. (2014b) | Green monkey (C. sabaeus) | Human; xenograft | ESC-derived DAergic progenitors | GFP | Caudate and SN | MPTP i.m. (2 mg/kg cumulative dose; administered over 5-day period) | 2 | Azathioprine, CsA and prednisolone | None | None | None | 6 weeks |
| Hallett et al. (2015) | Cynomolgus (M. fascicul ris) | Cynomolgus; autograft | iPSC-derived DAergic progenitors (Cooper et al. 2010 protocol) | None | Unilateral postcommissural putamen; 4 tracts total | MPTP i.v. (0.15–0.3 mg/kg) every 1–2 weeks | 1 | None | None | Clinical rating, activity monitoring, FMS | [11C]CFT | 2 years |
| Cynomolgus (M. fascicularis) | Cynomolgus; autograft | iPSC-derived DAergic progenitors (Sundberg et al. 2013 protocol) | None | Unilateral postcommissural putamen; 4 tracts total | MPTP i.v. (0.15–0.3 mg/kg) every 1–2 weeks | 2 | None | None | Clinical rating, activity monitoring, FMS | [11C]CFT | 2 years | |
| Cynomolgus (M. fascicularis) | Cynomolgus; allograft | ESC-derived DAergic progenitors (Sanchez-Pernaute et al. 2005 protocol) | None | Unilateral putamen | MPTP i.v. (0.15–0.3 mg/kg) every 1–2 weeks | 3 | None | None | Clinical rating, activity monitoring, FMS | [11C]CFT | 1 year | |
| Cynomolgus (M. fascicularis) | N/A | N/A | N/A | N/A | MPTP i.v. (0.15–0.3 mg/kg) every 1–2 weeks | 4 | None | None | Clinical rating, activity monitoring, FMS | [11C]CFT | 2 years | |
| Wang et al. (2015) | Cynomolgus (M. fascicularis) | Cynomolgus; autograft | iPSC-derived DAergic progenitors | GFP and Feridex IV | Unilateral (ipsi-MPTP) rostral, intermediate and caudal caudate and putamen, and SN; 7 tracts total | MPTP ICA (3 mg) | 1 | None | None | Clinical rating | None | 6 months |
| Cynomolgus (M. fascicularis) | N/A | Vehicle | N/A | Unilateral (ipsi-MPTP) rostral, intermediate and caudal caudate and putamen, and SN; 7 tracts total | MPTP ICA (3 mg) | 3 | None | None | Clinical rating | None | 6 months |
Immunosup immunosuppression, PET positron emission tomography, ESC embryonic stem cell, iPSC induced pluripotent stem cell, DAergic dopaminergic, BrdU 5-Bromo-2′-deoxyuriding, MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, L-DOPA L-3,4-dihydroxyphenylalanine, ipsi ipsilateral, i.v. intravenous, ICA intracarotid artery, mg milligram, kg kilogram, CsA Cyclosporine A, GFP green fluorescent protein, n.r. not reported, N/A not applicable, FMS fine motor skills, SN substantia nigra, [18 F]DOPA 6-[18 F]fluoro-l-3,4-dihydroxyphenylalanineresentatio, [11 C]DTBZ [11 C]dihydrotetrabenazine, [11 C]PE2I (E)-N-(3-iodoprop-2-enyl)-2β-carbo[11 C]methoxy-3β-(4-methylphenyl)nortropane, [18 F]FLT 3′-deoxy-3′3[18 F]fluorothymidine
Takagi et al. investigated monkey ESC-derived neural progenitors capable of producing dopaminergic neurons for transplantation into an NHP model of PD (Takagi et al. 2005). ESCs from a cynomolgus monkey (Macaca fascicularis) were differentiated on stromal cells with the addition of FGF2 and FGF20, and labeled in vitro with BrdU for postmortem identification. The ESC-derived dopaminergic progenitor cells were then transplanted into systemic MPTP-treated cynomolgus monkeys. Beginning at 10 weeks post-brain surgery, the neurological parkinsonian scores of animals receiving grafts (n = 6) decreased significantly (p < 0.05) compared with sham controls (n = 4) and were associated with a significant increase in striatal [18F]fluorodopa uptake observed by in vivo PET. Postmortem analysis at 14 weeks post-grafting verified survival of BrdU/TH colabeled neurons. Neither mitosis (identified by Ki67-immunoreactivity) nor tumor formation was observed in animals that received ESC-derived neuronal transplants.
Aiming for human translation, Kriks et al. differentiated floorplate-derived dopaminergic neurons from human ESCs (hESCs), labeled half of the cells with GFP and then transplanted the labeled cells into one side of the striatum, and unlabeled cells to the other, of two systemic MPTP-treated rhesus monkeys immunosuppressed by CsA administration (Kriks et al. 2011). At one-month post-transplantation, grafted cells were observed in the posterior caudate and pre-commissural putamen as well as Iba1+cells, suggesting immunoreaction by the host. Daadi et al. 2012 and Wakeman et al. 2014 tested hESC-derived DAergic neurons expressing GFP in the caudate and nigra of MPTP-intoxicated vervet monkeys immunosuppressed with cyclosporine, prednisone and azathioprine (Daadi et al. 2012; Wakeman et al. 2014b). The 4 monkeys treated by Daadi et al. showed a few TH-positive grafted cells, extending neurite outgrowth and expressing synaptic markers 2 months post-surgery; cell counts of grafts and immunological response were not reported. In the Wakeman et al. study, the two monkeys presented co-expression of GFP and βIII-tubulin positive cells but not TH, dopamine transporter (DAT) or other markers of midbrain floorplate differentiation 6 weeks after grafting, suggesting de-differentiation; evaluation of inflammatory markers was not reported. Emborg et al. also studied hESC-derived DAergic neurons; however, the brain evaluations were performed 3 months after grafting. The cells were genetically engineered to express GFP and grafted in the striatum and nigra of three rhesus monkeys that received MPTP by carotid artery injection. The three animals were immunosuppressed by daily oral dosing of cyclosporine that was started 48 h prior to grafting (Emborg et al. 2013b). Postmortem analysis at 3 months revealed graft survival in only one of the three monkeys. The graft was infiltrated with GFAP, CD68 and CD45 immunoreactive cells, suggesting ongoing immune reaction despite immunosuppression. These results further demonstrated that immunological issues are a major concern for xenografts and that allogeneic or autologous transplants may render better graft survival and integration.
Grafts of human iPSC (hiPSC)-derived dopaminergic neurons have also been attempted. In 2011, Kikuchi et al. reported their evaluation of grafting into one FK506 immunosuppressed MPTP-treated monkey (Kikuchi et al. 2011). The hiPSCs were differentiated into DAergic neural progenitors in feeder-free conditions but were not labeled. Day 28 (d28) and d42 neurospheres were transplanted into the right and left putamen, respectively. Graft size and function was evaluated at 1, 3 and 6 months by MRI and at 6 months by PET using the radioligands 6-[18F]fluoro-l-3,4-dihydroxyphenylalanine ([18F]DOPA), [11C]dihydrotetrabenazine ([11C]DTBZ), (E)-N-(3-iodoprop-2-enyl)-2β-carbo[11C]methoxy-3β-(4-methylphenyl)nortropane ([11C]PE2I), to assess for DA synthesis, vesicle transport, and DA reuptake, and with 3′-deoxy-3′3[18F]fluorothymidine ([18F]FLT) to visualize cell proliferation. Interestingly, MRI showed increase in graft size on the side of grafted d28 neurospheres compared to d42. In general, PET did not identify any meaningful graft-related uptake, with exception of increased binding of ([11C]PE2I) in the d42-grafted putamen, suggesting cell differentiation. Neurological evaluation throughout the study showed no behavioral recovery. Postmortem analysis 6 months after transplantation revealed graft survival; d42 spheres produced higher amounts of TH+cells compared with the d28 spheres, while also maintaining some progenitor populations, which explains the observed intracerebral graft growth; immunological response was not reported.
A major advantage of iPSC technology is that patient-specific cells can be generated for grafting, which minimizes host immune reaction and avoids immunosuppression. In 2013, Emborg et al. reported their findings in three rhesus monkeys that received an intracarotid artery injection of MPTP, followed 6 months later with autologous iPSC-derived neuroprogenitors into the striatum and nigra, without immunosuppression [Fig. 2; (Emborg et al. 2013a)]. For identification, the cells were genetically modified to express GFP. Six months post-surgery, brain analysis showed abundant GFP-positive neuron-like cells, which integrated with the host brain and expressed TH. Most importantly, infiltration of host immune cells observed by CD3 and CD8 reactivity was minimal while HLA-DR and GFAP were mild, similar to MPTP-induced inflammation. Although neurological scores were not reported, it was mentioned that the animals’ PD motor signs did not improve; the lack of recovery was probably due to low numbers of DAergic neurons grafted. Shortly after, Morizane et al. compared autologous and allogeneic transplantation of iPSC-derived neural cells in intact cynomolgus monkeys (Morizane et al. 2013). As expected, the allogeneic transplants elicited a marked immune response observed, with in vivo PET, as increased uptake of [11C]PK11195 compared to the autografts, further emphasizing the benefit of self-derived cells for translational applications.
Fig. 2.
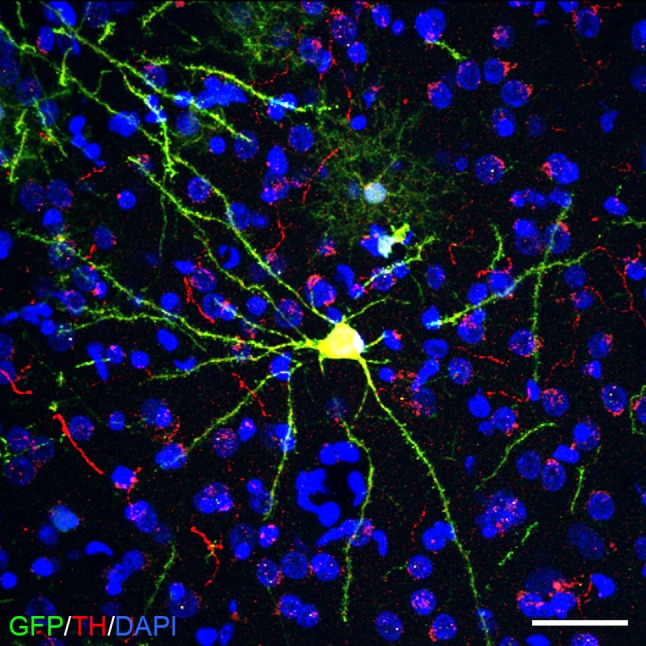
An example of a grafted autologous rhesus IPSC-derived dopaminergic neuron labeled with GFP (green) and immunostained against TH (red); DAPI (blue). Scale bar 50 μm
In 2015, Hallett et al. reported that the differentiation protocols used to generate iPSC-derived DAergic neurons could be a factor for survival and functional integration of grafted cells (Hallett et al. 2015). After ten cynomolgus monkeys were rendered stably parkinsonian through systemic MPTP administration, the animals received iPSC- or ESC-derived DAergic grafts differentiated following three different protocols: (Cooper et al. 2010) (n = 1), (Sundberg et al. 2013) (n = 2), and a modified (Sanchez-Pernaute et al. 2005) (n = 3; for ESCs); no transplanted animals (n = 4) were used as controls. The grafts derived from the Sanchez–Pernaute differentiation protocol did not survive and were thus separately analyzed as the non-surviving transplant group, compared with the Cooper- and Sundberg-derived grafts. Only the animal that received cells using the Cooper differentiation protocol presented functional recovery of daytime activity and movement analysis. The animal recovered to pre-MPTP levels for global activity, and improved in fine motor skills after 2 years. [11C]CFT PET imaging corroborated graft function with increased uptake around the transplant site. The lack of functional recovery and survival of the Sundberg and Sanchez-Pernaute grafts provide evidence that DAergic differentiation patterning may be critical for functional integration.
Wang et al. also assessed the feasibility of autologous iPSC-derived DAergic neuron grafts to one MPTP-treated cynomolgus monkey (Wang et al. 2015). Throughout the 6-month survival period, only between 6–8, and 22–24 weeks, the transplant monkey appeared to have an improved clinical rating compared with the control monkeys (n = 3). Postmortem analysis in the transplanted monkey showed graft survival and TH immunoreactivity.
An examination of these SC-based studies highlights that, similar to the fetal tissue reports in NHPs, they were mainly feasibility/safety experiments, performed in a few animals (with exception of Takagi et al. 2005) and focusing on assessing cell survival, cell proliferation/tumor formation and whether the grafted cells integrated and showed a DA phenotype. Some antiparkinsonian effects were described. Considering the ongoing improvements on SC research, this approach shows responsible use of the NHP resource and also underscores that the results should be kept in perspective with the limitations of the experimental design and outcome measures utilized.
Nigral vs. striatal targets
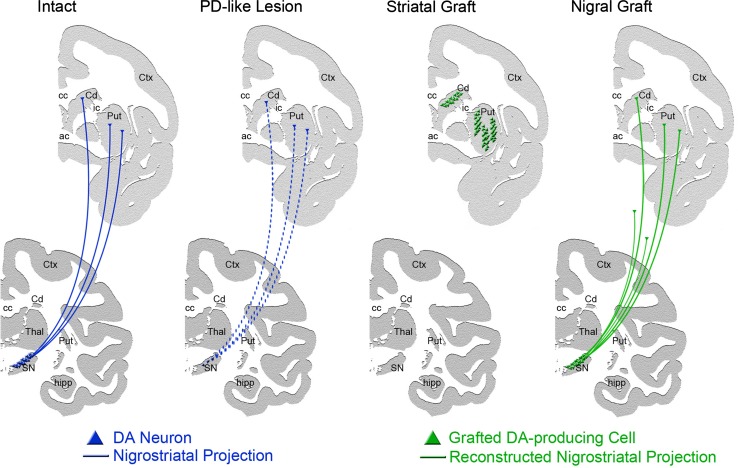
For DA cell replacement, the field strives to recreate and purify A9 DA nigral cells, yet in patients, the cells have been mainly transplanted in the postcommisural putamen [see review: (Redmond 2002)] with a few exceptions in which the fetal nigral cells were placed in the nigra and putamen [e.g.: (Mendez et al. 2002); Fig. 3]. Putaminal targeting aims to provide DA in the area where needed, while nigral grafting is proposed to restore the nigrostriatal pathway, including the regulation of grafted cell activity and restoring the DA tone in the substantia nigra pars reticulata.
Fig. 3.
Graphical depiction of a rhesus monkey brain hemisphere in intact and PD-like conditions and with striatal and nigral grafts. The main area of projection of nigral dopaminergic neurons is the striatum, which is composed by the caudate nucleus and putamen. In PD and PD-like conditions (e.g.: after MPTP intoxication), nigral dopaminergic neurons die; thus, striatal dopamine (DA) is decreased. Grafting of DA-producing cells into the striatum is envisioned as a way to ensure DA availability in the area of projection. Nigral grafts are proposed as a way to reconstruct the nigrostriatal pathway. Cd caudate, ic internal capsule, Put Putamen, ac anterior commissure, cc corpus callosum, Ctx cortex
Theoretically, the nigral location is a most optimal method for brain repair, yet an obstacle for its application is the long distance to be covered by the axons of cells inoculated in the nigra to reach their striatal targets. The successful reconstruction of the nigrostriatal pathway requires time for axonal extension, axonal guidance and ultimately recognition and synaptic connection with targets. In rodents, the feasibility and advantages of the approach have been reported using fetal cells (Mendez et al. 1996) and ESCs (Grealish et al. 2014), observing positive behavioral effects as soon as 6 weeks post-fetal grafting and 18 weeks with ESC-derived DA cells, with the latter requiring more time to mature and integrate in the host. In NHPs and humans, the nigrostriatal distance is greater, which means that the time needed after grafting to observe behavioral benefits may also increase; therefore, multisite grafting has been preferred.
The feasibility of grafting fetal midbrain cells in the nigra (Collier et al. 2002) or in combination with fetal striatal grafts for axonal guidance (Sladek et al. 2008) has been demonstrated in MPTP-treated vervet monkeys; in both studies axonal extension through the nigrostriatal pathway and some modest caudal putamen reinnervation was observed at 6 months; although follow-up studies of co-grafting fetal nigra and striatum had limited effect (Redmond et al. 2009). Localized delivery of trophic factors has been proposed as a method to stimulate and guide axonal growth. Striatal GDNF overexpression via AAV2 vectors was reported to enhance the survival and outgrowth of fetal dopamine neurons implanted in the striatum (Elsworth et al. 2008). A follow-up comparison between MPTP-intoxicated monkeys treated with AAV5 GDNF in the caudate nucleus or solid fetal midbrain grafts in the caudate and putamen or a combination of AAV-GDNF and fetal grafts or buffered saline solution did not reveal greater functional improvement in the AAV-GDNF and fetal grafts monkeys during the 8 months of observation (Redmond et al. 2013). AAV2-induced overexpression of GDNF in the caudate supported outgrowth of fetal midbrain grafts maximally observed at 22 months post-grafting (Redmond et al. 2009), as well as stimulated neurite extension and dopaminergic differentiation of hNC grafts, both placed in the nigra of MPTP monkeys (Wakeman et al. 2014a). The studies suggest that reconstruction of the nigrostriatal pathway can be achieved and that research on methods aiming to promote and guide axonal growth is needed.
The future of CBTs and NHP studies
NHP studies have paved the way for the first clinical trials using CBTs for PD. Although the number of monkey experiments and/or total number of animals was a fraction compared to rodent studies, they provided key evidence that CBTs could be used to treat PD. NHP experiments have facilitated the optimization of cell collection and preparation methods, cell viability, as well as identify potential brain targets. Today, old approaches are being re-evaluated and optimized and new ones are being developed; some are being translated into the clinic (Barker 2014). NHP research can provide an even more crucial insight into CBTs, but as a limited resource, prioritization of issues to be evaluated will be critical. How cells are prepared and stored affect engraftment, the methods of delivery, the choice of targets and related timelines of recovery are basic questions that still need to be solved and tested in NHPs to improve CBT outcome. The response of the host to the candidate CBT encompasses a different set of topics related to efficacy, safety (e.g.: prevention of GIDs), immunomodulation and inflammatory response and propagation of α-syn. Each of these issues can also benefit from careful NHP research.
Several groups are invested in the development of the optimal cell for transplantation, working on generating cell lines of “superdonors” with immunological compatibility and improving the methods of cryoprotection to facilitate clinical application, as creating a cell line per individual to be treated would be a daunting task. While the idea of testing in NHPs the same cell lines to be grafted to humans is compelling, current data presented in this review highlight the limitations of the xenograft approach with current immunosuppression paradigms. Thus, a more parsimonious approach would be to produce equivalent cells derived from the same species, at least until the NHP equivalent of severe combined immune deficiency (SCID) mice becomes available (Sato et al. 2016).
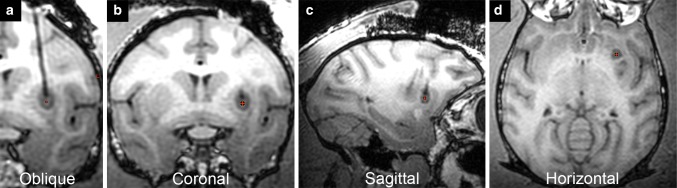
Due to the brain volume and complexity of NHPs, questions regarding intracerebral targets and graft distribution will benefit from NHP studies and noninvasive imaging approaches. For example, great strides have been made towards the development and improvement of intraoperative MRI (iMRI) methods (Mislow et al. 2009). Silvestrini et al. (Silvestrini et al. 2015) used real-time iMRI (RT-iMRI) for cell transplantation into a swine and cadaveric human head as a concept for application in the human brain. The platform technology utilizes a radially branched deployment strategy to access multi-directional deposit sites along a single cannula insertion tract. Our group modified an RT-iMRI delivery system that has a pivot point base, a clear silica cannula and inline pressure monitoring system (Emborg et al. 2010, 2014) for the in vivo delivery of DAergic progenitor cell spheres into the putamen of a rhesus monkey [Fig. 4; (Vermilyea et al. 2016b)]. Malloy et al. (2016) used an MRI-compatible delivery system for MRI monitoring of the distribution of cells pre-labeled with a contrast agent into a baboon basal ganglia. These new MRI-based imaging methods can increase the safety and accuracy of the grafting procedures and facilitate the evaluation of different targeting sites. In that regard, imaging technologies, such as diffusion tensor imaging (Hall et al. 2016), can be applied to preclinical studies to evaluate circuit reconstruction, complementary to traditional PET imaging with DA-related radioligands. Application of imaging methods overtime to monitor graft integration and function would be critical to reduce the number of NHPs groups needed per experiment to understand recovery timelines.
Fig. 4.
Real-time intraoperative MRI for intracerebral delivery of stem cells provides feedback of cannula placement and infusion site: a oblique, b coronal, c sagittal and d horizontal planes
At this time, GIDs cannot be prevented or cured; NHP safety studies on methods aiming to modulate grafted cell activity are needed. Optogenetics is a technique that uses light to control cells that have been genetically modified to express light-sensitive ion channels (Lerner et al. 2016). Optogenetic approaches have been applied to control electrophysiological and neurochemical properties of grafted SC-derived DA neurons in rodent models (Chen et al. 2015; Steinbeck et al. 2015), yet its clinical translation is not recommended as the patients would require the intracerebral placement of a probe to locally deliver the appropriate light wavelength. Compared to optogenetics, the designer receptors exclusively activated by designer drugs (DREADDs) technology use noninvasive methods to exert its effects, as it depends on designer drugs to modulate the activity of cells that are genetically modified to express the corresponding receptor. DREADDs have been used to modulate human PSC-derived DA neurons (Chen et al. 2016). There has been a lot of discussion regarding CBT safety centered on defining an adequate cell number and volume for intracerebral delivery. The use of DREADDs or an equivalent technology would allow more flexibility as, theoretically, cells can be further excited if behavioral recovery is not observed, or inhibited if serious GIDs occur. Basically, it would provide a strategy for patient-specific graft modulation that could be monitored by a combination of clinical and PET imaging tools.
How can the value of the next generation of NHP studies be maximized for the successful clinical translation of CBTs for PD? We propose that first and foremost, the investigators should use NHP PD models that match the question at hand (Table 1). As we previously discussed, until now all monkey studies of CBTs for PD have been performed in neurotoxic models. Many basic questions regarding feasibility, efficacy, and safety can be answered in these models. Yet, the same way that only old animals can provide insight on grafting in the aging brain, studies in models of synucleinopathies are needed to assess the impact of protein aggregation in graft efficacy. In that regard, the field is anxiously waiting for the validation of current genetic models and the availability of transgenic and genomic edited PD monkeys. Second, a well-designed feasibility study in a few monkeys presents an opportunity to learn if an approach is worth pursuing, with the caution that improvement of PD symptoms could be due to individual variability or spontaneous recovery. Demonstration of efficacy requires properly powered NHP experiments with blind group assignment and evaluation. Third, the surgical method of cell delivery is critical to ensuring appropriate targets, optimized cell survival and distribution and should not be minimized. Fourth, the animals should be assessed with multiple outcome measures with clinical impact to maximize the knowledge to be extracted from the study. Behavioral evaluations complemented by in vivo imaging methods can facilitate postsurgical follow-up, especially for CBT studies taken several years to be completed. Postmortem analysis should include unbiased cell counts and evaluation of host immunological response in order to inform about the safety, efficacy and limitations of the approach. Fifth, grafted cells should be pre-labeled to facilitate identification from host cells. Images of grafts should be in low and high magnification to assess the extent of cell survival and interaction with host. Sixth, there are no bad results, but poorly designed experiments. Publication of positive as well as negative experimental results in NHPs should be encouraged for the overall evolution of the field.
To conclude, NHP PD research plays a small but critical role in CBT clinical translation. Ultimately, investigators should remember that whether CBTs for PD work will depend on the benefits outweighing the patients’ risk and that NHPs have unique characteristics to help identify and solve these problems.
Acknowledgements
This research was supported by NIH grants R24 OD019803, R01NS076352, P51OD011106-53S2 (Wisconsin National Primate Research Center, University of Wisconsin-Madison), NINDS T32-Neuroscience Training Program (S.C.V.), Departments of Radiology and Medical Physics at UW-Madison, WARF Accelerator Program, and funding from the UW-Madison Vice Chancellor for Research and Graduate Education.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Annett LE, Dunnett SB, Martel FL, Rogers DC, Ridley RM, Baker HF, Marsden CD. A functional assessment of embryonic dopaminergic grafts in the marmoset. Prog Brain Res. 1990;82:535–542. doi: 10.1016/S0079-6123(08)62644-8. [DOI] [PubMed] [Google Scholar]
- Annett LE, Martel FL, Rogers DC, Ridley RM, Baker HF, Dunnett SB. Behavioral assessment of the effects of embryonic nigral grafts in marmosets with unilateral 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 1994;125(2):228–246. doi: 10.1006/exnr.1994.1026. [DOI] [PubMed] [Google Scholar]
- Backlund EO, Granberg PO, Hamberger B, Knutsson E, Martensson A, Sedvall G, Seiger A, Olson L. Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trials. J Neurosurg. 1985;62(2):169–173. doi: 10.3171/jns.1985.62.2.0169. [DOI] [PubMed] [Google Scholar]
- Bakay RA, Barrow DL, Fiandaca MS, Iuvone PM, Schiff A, Collins DC. Biochemical and behavioral correction of MPTP Parkinson-like syndrome by fetal cell transplantation. Ann N Y Acad Sci. 1987;495:623–640. doi: 10.1111/j.1749-6632.1987.tb23705.x. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Plunkett RJ, Jacobowitz DM, di Porrino L, Porzio U, London WT, Kopin IJ, Oldfield EH. The effect of fetal mesencephalon implants on primate MPTP-induced parkinsonism. Histochemical and behavioral studies. J Neurosurg. 1990;72(2):231–244. doi: 10.3171/jns.1990.72.2.0231. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Bringas J, Pivirotto P, Kutzscher E, Nagy D, Emborg ME. Technique for bilateral intracranial implantation of cells in monkeys using an automated delivery system. Cell Transplant. 2000;9(5):595–607. doi: 10.1177/096368970000900505. [DOI] [PubMed] [Google Scholar]
- Barker RA. Developing stem cell therapies for Parkinson’s disease: waiting until the time is right. Cell Stem Cell. 2014;15(5):539–542. doi: 10.1016/j.stem.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Behrstock S, Ebert A, McHugh J, Vosberg S, Moore J, Schneider B, Capowski E, Hei D, Kordower J, Aebischer P, Svendsen CN. Human neural progenitors deliver glial cell line-derived neurotrophic factor to parkinsonian rodents and aged primates. Gene Ther. 2006;13(5):379–388. doi: 10.1038/sj.gt.3302679. [DOI] [PubMed] [Google Scholar]
- Bendor JT, Logan TP, Edwards RH. The function of alpha-synuclein. Neuron. 2013;79(6):1044–1066. doi: 10.1016/j.neuron.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Brotchie JM, Gross CE. Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat Rev Neurosci. 2001;2(8):577–588. doi: 10.1038/35086062. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res. 1979;177(3):555–560. doi: 10.1016/0006-8993(79)90472-4. [DOI] [PubMed] [Google Scholar]
- Bjugstad KB, Redmond DE, Jr, Teng YD, Elsworth JD, Roth RH, Blanchard BC, Snyder EY, Sladek JR., Jr Neural stem cells implanted into MPTP-treated monkeys increase the size of endogenous tyrosine hydroxylase-positive cells found in the striatum: a return to control measures. Cell Transplant. 2005;14(4):183–192. doi: 10.3727/000000005783983098. [DOI] [PubMed] [Google Scholar]
- Bjugstad KB, Teng YD, Redmond DE, Jr, Elsworth JD, Roth RH, Cornelius SK, Snyder EY, Sladek JR., Jr Human neural stem cells migrate along the nigrostriatal pathway in a primate model of Parkinson’s disease. Exp Neurol. 2008;211(2):362–369. doi: 10.1016/j.expneurol.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandini F, Armentero MT, Martignoni E. The 6-hydroxydopamine model: news from the past. Parkinsonism Relat Disord. 2008;14(Suppl 2):S124–S129. doi: 10.1016/j.parkreldis.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol. 2001;65(2):135–172. doi: 10.1016/S0301-0082(01)00003-X. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Redmond DE, Jr, Bloch J. Primate adult brain cell autotransplantation, a pilot study in asymptomatic MPTP-treated monkeys. Cell Transplant. 2009;18(7):787–799. doi: 10.3727/096368909X470847. [DOI] [PubMed] [Google Scholar]
- Buzanska L, Machaj EK, Zablocka B, Pojda Z, Domanska-Janik K. Human cord blood-derived cells attain neuronal and glial features in vitro. J Cell Sci. 2002;115(Pt 10):2131–2138. doi: 10.1242/jcs.115.10.2131. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Emborg ME. Contributions of non-human primates to neuroscience research. Lancet. 2008;371(9618):1126–1135. doi: 10.1016/S0140-6736(08)60489-4. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Odin P. The challenge of non-motor symptoms in Parkinson’s disease. Prog Brain Res. 2010;184:325–341. doi: 10.1016/S0079-6123(10)84017-8. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Odin P, Antonini A, Martinez-Martin P. Parkinson’s disease: the non-motor issues. Parkinsonism Relat Disord. 2011;17(10):717–723. doi: 10.1016/j.parkreldis.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xiong M, Zhang SC. Illuminating Parkinson’s therapy with optogenetics. Nat Biotechnol. 2015;33(2):149–150. doi: 10.1038/nbt.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Xiong M, Dong Y, Haberman A, Cao J, Liu H, Zhou W, Zhang SC. Chemical control of grafted human PSC-derived neurons in a mouse model of Parkinson’s disease. Cell Stem Cell. 2016;18(6):817–826. doi: 10.1016/j.stem.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Kordower JH. The prion hypothesis of Parkinson’s disease. Curr Neurol Neurosci Rep. 2015;15(5):28. doi: 10.1007/s11910-015-0549-x. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Redmond DE, Jr, Sladek CD, Gallagher MJ, Roth RH, Sladek JR., Jr Intracerebral grafting and culture of cryopreserved primate dopamine neurons. Brain Res. 1987;436(2):363–366. doi: 10.1016/0006-8993(87)91680-5. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Elsworth JD, Taylor JR, Sladek JR, Jr, Roth RH, Redmond DE., Jr Peripheral nerve-dopamine neuron co-grafts in MPTP-treated monkeys: augmentation of tyrosine hydroxylase-positive fiber staining and dopamine content in host systems. Neuroscience. 1994;61(4):875–889. doi: 10.1016/0306-4522(94)90410-3. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Sortwell CE, Elsworth JD, Taylor JR, Roth RH, Sladek JR, Jr, Redmond DE., Jr Embryonic ventral mesencephalic grafts to the substantia nigra of MPTP-treated monkeys: feasibility relevant to multiple-target grafting as a therapy for Parkinson’s disease. J Comp Neurol. 2002;442(4):320–330. doi: 10.1002/cne.10108. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Redmond DE, Jr, Steece-Collier K, Lipton JW, Manfredsson FP. Is alpha-synuclein loss-of-function a contributor to parkinsonian pathology? Evidence from non-human primates. Front Neurosci. 2016;10:12. doi: 10.3389/fnins.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper O, Hargus G, Deleidi M, Blak A, Osborn T, Marlow E, Lee K, Levy A, Perez-Torres E, Yow A, Isacson O. Differentiation of human ES and Parkinson's disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol Cell Neurosci. 2010;45(3):258–266. doi: 10.1016/j.mcn.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daadi MM, Grueter BA, Malenka RC, Redmond DE, Jr, Steinberg GK. Dopaminergic neurons from midbrain-specified human embryonic stem cell-derived neural stem cells engrafted in a monkey model of Parkinson’s disease. PLoS One. 2012;7(7):e41120. doi: 10.1371/journal.pone.0041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Davis GC, Williams AC, Markey SP, Ebert MH, Caine ED, Reichert CM, Kopin IJ. Chronic Parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979;1(3):249–254. doi: 10.1016/0165-1781(79)90006-4. [DOI] [PubMed] [Google Scholar]
- Deleidi M, Hargus G, Hallett P, Osborn T, Isacson O. Development of histocompatible primate-induced pluripotent stem cells for neural transplantation. Stem Cells. 2011;29(7):1052–1063. doi: 10.1002/stem.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver JA, Logroscino G, Gaziano JM, Kurth T. Incidence and remaining lifetime risk of Parkinson disease in advanced age. Neurology. 2009;72(5):432–438. doi: 10.1212/01.wnl.0000341769.50075.bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubach M, Schmidt RH, Martin R, German DC, Bowden DM. Transplant improves hemiparkinsonian syndrome in nonhuman primate: intracerebral injection, rotometry, tyrosine hydroxylase immunohistochemistry. Prog Brain Res. 1988;78:491–496. doi: 10.1016/S0079-6123(08)60322-2. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Al-Tikriti MS, Sladek JR, Jr, Taylor JR, Innis RB, Redmond DE, Jr, Roth RH. Novel radioligands for the dopamine transporter demonstrate the presence of intrastriatal nigral grafts in the MPTP-treated monkey: correlation with improved behavioral function. Exp Neurol. 1994;126(2):300–304. doi: 10.1006/exnr.1994.1068. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Redmond DE, Jr, Leranth C, Bjugstad KB, Sladek JR, Jr, Collier TJ, Foti SB, Samulski RJ, Vives KP, Roth RH. AAV2-mediated gene transfer of GDNF to the striatum of MPTP monkeys enhances the survival and outgrowth of co-implanted fetal dopamine neurons. Exp Neurol. 2008;211(1):252–258. doi: 10.1016/j.expneurol.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg ME. Nonhuman primate models of Parkinson’s disease. ILAR J. 2007;48(4):339–355. doi: 10.1093/ilar.48.4.339. [DOI] [PubMed] [Google Scholar]
- Emborg ME, Ebert AD, Moirano J, Peng S, Suzuki M, Capowski E, Joers V, Roitberg BZ, Aebischer P, Svendsen CN. GDNF-secreting human neural progenitor cells increase tyrosine hydroxylase and VMAT2 expression in MPTP-treated cynomolgus monkeys. Cell Transplant. 2008;17(4):383–395. [PubMed] [Google Scholar]
- Emborg ME, Joers V, Fisher R, Brunner K, Carter V, Ross C, Raghavan R, Brady M, Raschke J, Kubota K, Alexander A. Intraoperative intracerebral MRI-guided navigation for accurate targeting in nonhuman primates. Cell Transplant. 2010;19(12):1587–1597. doi: 10.3727/096368910X514323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg ME, Liu Y, Xi J, Zhang X, Yin Y, Lu J, Joers V, Swanson C, Holden JE, Zhang SC. Induced pluripotent stem cell-derived neural cells survive and mature in the nonhuman primate brain. Cell Rep. 2013;3(3):646–650. doi: 10.1016/j.celrep.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg ME, Zhang Z, Joers V, Brunner K, Bondarenko V, Ohshima S, Zhang SC. Intracerebral transplantation of differentiated human embryonic stem cells to hemiparkinsonian monkeys. Cell Transplant. 2013;22(5):831–838. doi: 10.3727/096368912X647144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg ME, Hurley SA, Joers V, Tromp do PM, Swanson CR, Ohshima-Hosoyama S, Bondarenko V, Cummisford K, Sonnemans M, Hermening S, Blits B, Alexander AL. Titer and product affect the distribution of gene expression after intraputaminal convection-enhanced delivery. Stereotact Funct Neurosurg. 2014;92(3):182–194. doi: 10.1159/000360584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelender S, Isacson O. The threshold theory for Parkinson’s disease. Trends Neurosci. 2016 doi: 10.1016/j.tins.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Eslamboli A, Georgievska B, Ridley RM, Baker HF, Muzyczka N, Burger C, Mandel RJ, Annett L, Kirik D. Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson’s disease. J Neurosci Off J Soc Neurosci. 2005;25(4):769–777. doi: 10.1523/JNEUROSCI.4421-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslamboli A, Romero-Ramos M, Burger C, Bjorklund T, Muzyczka N, Mandel RJ, Baker H, Ridley RM, Kirik D. Long-term consequences of human alpha-synuclein overexpression in the primate ventral midbrain. Brain J Neurol. 2007;130(Pt 3):799–815. doi: 10.1093/brain/awl382. [DOI] [PubMed] [Google Scholar]
- Fallahi-Sichani M, Soleimani M, Najafi SM, Kiani J, Arefian E, Atashi A. In vitro differentiation of cord blood unrestricted somatic stem cells expressing dopamine-associated genes into neuron-like cells. Cell Biol Int. 2007;31(3):299–303. doi: 10.1016/j.cellbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Fine A, Hunt SP, Oertel WH, Nomoto M, Chong PN, Bond A, Waters C, Temlett JA, Annett L, Dunnett S, et al. Transplantation of embryonic marmoset dopaminergic neurons to the corpus striatum of marmosets rendered parkinsonian by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Prog Brain Res. 1988;78:479–489. doi: 10.1016/S0079-6123(08)60321-0. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick KM, Raschke J, Emborg ME. Cell-based therapies for Parkinson’s disease: past, present, and future. Antioxid Redox Signal. 2009;11(9):2189–2208. doi: 10.1089/ars.2009.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SH, Brotchie JM. The MPTP-lesioned non-human primate models of Parkinson’s disease. Past, present, and future. Prog Brain Res. 2010;184:133–157. doi: 10.1016/S0079-6123(10)84007-5. [DOI] [PubMed] [Google Scholar]
- Freed WJ, Morihisa JM, Spoor E, Hoffer BJ, Olson L, Seiger A, Wyatt RJ. Transplanted adrenal chromaffin cells in rat brain reduce lesion-induced rotational behaviour. Nature. 1981;292(5821):351–352. doi: 10.1038/292351a0. [DOI] [PubMed] [Google Scholar]
- Freed CR, Breeze RE, Rosenberg NL, Schneck SA, Kriek E, Qi JX, Lone T, Zhang YB, Snyder JA, Wells TH, et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson’s disease. N Engl J Med. 1992;327(22):1549–1555. doi: 10.1056/NEJM199211263272202. [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344(10):710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Gaj T, Sirk SJ, Shui SL, Liu J. Genome-editing technologies: principles and applications. Cold Spring Harb Perspect Biol. 2016 doi: 10.1101/cshperspect.a023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Olanow CW, Koller WC, Penn RD, Cahill D, Morantz R, Stebbins G, Tanner CM, Klawans HL, Shannon KM. Multicenter study of autologous adrenal medullary transplantation to the corpus striatum in patients with advanced Parkinson’s disease. N Engl J Med. 1989;320(6):337–341. doi: 10.1056/NEJM198902093200601. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Garitaonandia I, Crain A, Poustovoitov M, Abramihina T, Noskov A, Jiang C, Morey R, Laurent LC, Elsworth JD, Snyder EY, Redmond DE, Jr, Semechkin R. Proof of concept studies exploring the safety and functional activity of human parthenogenetic-derived neural stem cells for the treatment of Parkinson’s disease. Cell Transplant. 2015;24(4):681–690. doi: 10.3727/096368915X687769. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Garitaonandia I, Poustovoitov M, Abramihina T, McEntire C, Culp B, Attwood J, Noskov A, Christiansen-Weber T, Khater M, Mora-Castilla S, To C, Crain A, Sherman G, Semechkin A, Laurent LC, Elsworth JD, Sladek J, Snyder EY, Redmond DE, Jr, Kern RA. Neural stem cells derived from human parthenogenetic stem cells engraft and promote recovery in a nonhuman primate model of Parkinsons disease. Cell Transplant. 2016;25(11):1945–1966. doi: 10.3727/096368916X691682. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286(5439):548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- Grealish S, Diguet E, Kirkeby A, Mattsson B, Heuer A, Bramoulle Y, Van Camp N, Perrier AL, Hantraye P, Bjorklund A, Parmar M. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell. 2014;15(5):653–665. doi: 10.1016/j.stem.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JM, Ehgoetz Martens KA, Walton CC, O’Callaghan C, Keller PE, Lewis SJ, Moustafa AA. Diffusion alterations associated with Parkinson’s disease symptomatology: a review of the literature. Parkinsonism Relat Disord. 2016;33:12–26. doi: 10.1016/j.parkreldis.2016.09.026. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Deleidi M, Astradsson A, Smith GA, Cooper O, Osborn TM, Sundberg M, Moore MA, Perez-Torres E, Brownell AL, Schumacher JM, Spealman RD, Isacson O. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell Stem Cell. 2015;16(3):269–274. doi: 10.1016/j.stem.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday G, Herrero MT, Murphy K, McCann H, Ros-Bernal F, Barcia C, Mori H, Blesa FJ, Obeso JA. No Lewy pathology in monkeys with over 10 years of severe MPTP Parkinsonism. Move Disord Off J Move Disord Soc. 2009;24(10):1519–1523. doi: 10.1002/mds.22481. [DOI] [PubMed] [Google Scholar]
- Hilker R, Brotchie JM, Chapman J. Pros and cons of a prion-like pathogenesis in Parkinson’s disease. BMC Neurol. 2011;11:74. doi: 10.1186/1471-2377-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joglar B, Rodriguez-Pallares J, Rodriguez-Perez AI, Rey P, Guerra MJ, Labandeira-Garcia JL. The inflammatory response in the MPTP model of Parkinson’s disease is mediated by brain angiotensin: relevance to progression of the disease. J Neurochem. 2009;109(2):656–669. doi: 10.1111/j.1471-4159.2009.05999.x. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Kalia SK, Lang AE. Disease-modifying strategies for Parkinson’s disease. Move Disord Off J Move Disord Soc. 2015;30(11):1442–1450. doi: 10.1002/mds.26354. [DOI] [PubMed] [Google Scholar]
- Kannarkat GT, Boss JM, Tansey MG. The role of innate and adaptive immunity in Parkinson’s disease. J Parkinson’s Dis. 2013;3(4):493–514. doi: 10.3233/JPD-130250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197(4308):1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kastenberg ZJ, Odorico JS. Alternative sources of pluripotency: science, ethics, and stem cells. Transplant Rev. 2008;22(3):215–222. doi: 10.1016/j.trre.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Morizane A, Doi D, Onoe H, Hayashi T, Kawasaki T, Saiki H, Miyamoto S, Takahashi J. Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson’s disease. J Parkinson’s Dis. 2011;1(4):395–412. doi: 10.3233/JPD-2011-11070. [DOI] [PubMed] [Google Scholar]
- Kim SU, Lee HJ, Kim YB. Neural stem cell-based treatment for neurodegenerative diseases. Neuropathol Off J Jpn Soc Neuropathol. 2013;33(5):491–504. doi: 10.1111/neup.12020. [DOI] [PubMed] [Google Scholar]
- Kirik D, Annett LE, Burger C, Muzyczka N, Mandel RJ, Bjorklund A. Nigrostriatal alpha-synucleinopathy induced by viral vector-mediated overexpression of human alpha-synuclein: a new primate model of Parkinson’s disease. Proc Natl Acad Sci USA. 2003;100(5):2884–2889. doi: 10.1073/pnas.0536383100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishima H, Poyot T, Bloch J, Dauguet J, Conde F, Dolle F, Hinnen F, Pralong W, Palfi S, Deglon N, Aebischer P, Hantraye P. Encapsulated GDNF-producing C2C12 cells for Parkinson’s disease: a pre-clinical study in chronic MPTP-treated baboons. Neurobiol Dis. 2004;16(2):428–439. doi: 10.1016/j.nbd.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Koprich JB, Johnston TH, Reyes G, Omana V, Brotchie JM. Towards a non-human primate model of alpha-synucleinopathy for development of therapeutics for Parkinson’s disease: optimization of AAV1/2 delivery parameters to drive sustained expression of alpha synuclein and dopaminergic degeneration in Macaque. PLoS One. 2016;11(11):e0167235. doi: 10.1371/journal.pone.0167235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Styren S, Clarke M, DeKosky ST, Olanow CW, Freeman TB. Fetal grafting for Parkinson’s disease: expression of immune markers in two patients with functional fetal nigral implants. Cell Transplant. 1997;6(3):213–219. doi: 10.1177/096368979700600304. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14(5):504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Dodiya HB, Kordower AM, Terpstra B, Paumier K, Madhavan L, Sortwell C, Steece-Collier K, Collier TJ. Transfer of host-derived alpha synuclein to grafted dopaminergic neurons in rat. Neurobiol Dis. 2011;43(3):552–557. doi: 10.1016/j.nbd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Vinuela A, Chu Y, Isacson O, Redmond DE., Jr Parkinsonian monkeys with prior levodopa-induced dyskinesias followed by fetal dopamine precursor grafts do not display graft-induced dyskinesias. J Comp Neurol. 2016 doi: 10.1002/cne.24081. [DOI] [PubMed] [Google Scholar]
- Kowall NW, Hantraye P, Brouillet E, Beal MF, McKee AC, Ferrante RJ. MPTP induces alpha-synuclein aggregation in the substantia nigra of baboons. NeuroReport. 2000;11(1):211–213. doi: 10.1097/00001756-200001170-00041. [DOI] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480(7378):547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18(6):675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- Lerner TN, Ye L, Deisseroth K. Communication in neural circuits: tools, opportunities, and challenges. Cell. 2016;164(6):1136–1150. doi: 10.1016/j.cell.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy YS, Bahat-Stroomza M, Barzilay R, Burshtein A, Bulvik S, Barhum Y, Panet H, Melamed E, Offen D. Regenerative effect of neural-induced human mesenchymal stromal cells in rat models of Parkinson’s disease. Cytotherapy. 2008;10(4):340–352. doi: 10.1080/14653240802021330. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, Widner H, Revesz T, Lindvall O, Brundin P. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14(5):501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhu F, Yong J, Zhang P, Hou P, Li H, Jiang W, Cai J, Liu M, Cui K, Qu X, Xiang T, Lu D, Chi X, Gao G, Ji W, Ding M, Deng H. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell. 2008;3(6):587–590. doi: 10.1016/j.stem.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci USA. 2009;106(47):20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Feigin A, Dhawan V, Fukuda M, Shi Q, Greene P, Breeze R, Fahn S, Freed C, Eidelberg D. Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Ann Neurol. 2002;52(5):628–634. doi: 10.1002/ana.10359. [DOI] [PubMed] [Google Scholar]
- Madrazo I, Drucker-Colin R, Diaz V, Martinez-Mata J, Torres C, Becerril JJ. Open microsurgical autograft of adrenal medulla to the right caudate nucleus in two patients with intractable Parkinson’s disease. N Engl J Med. 1987;316(14):831–834. doi: 10.1056/NEJM198704023161402. [DOI] [PubMed] [Google Scholar]
- Malloy KE, Li J, Choudhury GR, Torres A, Gupta S, Kantorak C, Goble T, Fox PT, Clarke GD, Daadi MM. Magnetic resonance imaging-guided delivery of neural stem cells into the basal ganglia of nonhuman primates reveals a pulsatile mode of cell dispersion. Stem Cells Transl Med. 2016 doi: 10.5966/sctm.2016-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maries E, Kordower JH, Chu Y, Collier TJ, Sortwell CE, Olaru E, Shannon K, Steece-Collier K. Focal not widespread grafts induce novel dyskinetic behavior in parkinsonian rats. Neurobiol Dis. 2006;21(1):165–180. doi: 10.1016/j.nbd.2005.07.002. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Mak SK, Shenasa M, Langston WJ, Forno LS, Di Monte DA. Pathologic modifications of alpha-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated squirrel monkeys. J Neuropathol Exp Neurol. 2008;67(8):793–802. doi: 10.1097/NEN.0b013e318180f0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Martinez TN, Ruhn KA, Wrage PC, Keefer EW, Botterman BR, Tansey KE, Tansey MG. Autologous transplants of adipose-derived adult stromal (ADAS) cells afford dopaminergic neuroprotection in a model of Parkinson’s disease. Exp Neurol. 2008;210(1):14–29. doi: 10.1016/j.expneurol.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Schwab C, Parent A, Doudet D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann Neurol. 2003;54(5):599–604. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- Mendez I, Sadi D, Hong M. Reconstruction of the nigrostriatal pathway by simultaneous intrastriatal and intranigral dopaminergic transplants. J Neurosci Off J Soc Neurosci. 1996;16(22):7216–7227. doi: 10.1523/JNEUROSCI.16-22-07216.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez I, Dagher A, Hong M, Gaudet P, Weerasinghe S, McAlister V, King D, Desrosiers J, Darvesh S, Acorn T, Robertson H. Simultaneous intrastriatal and intranigral fetal dopaminergic grafts in patients with Parkinson disease: a pilot study. Report of three cases. J Neurosurg. 2002;96(3):589–596. doi: 10.3171/jns.2002.96.3.0589. [DOI] [PubMed] [Google Scholar]
- Mislow JM, Golby AJ, Black PM. Origins of intraoperative MRI. Neurosurg Clin N Am. 2009;20(2):137–146. doi: 10.1016/j.nec.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihisa JM, Nakamura RK, Freed WJ, Mishkin M, Wyatt RJ. Adrenal medulla grafts survive and exhibit catecholamine-specific fluorescence in the primate brain. Exp Neurol. 1984;84(3):643–653. doi: 10.1016/0014-4886(84)90211-5. [DOI] [PubMed] [Google Scholar]
- Morihisa JM, Nakamura RK, Freed WJ, Mishkin M, Wyatt RJ. Transplantation techniques and the survival of adrenal medulla autografts in the primate brain. Ann N Y Acad Sci. 1987;495:599–605. doi: 10.1111/j.1749-6632.1987.tb23703.x. [DOI] [PubMed] [Google Scholar]
- Morizane A, Doi D, Kikuchi T, Okita K, Hotta A, Kawasaki T, Hayashi T, Onoe H, Shiina T, Yamanaka S, Takahashi J. Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a non-human primate. Stem Cell Rep. 2013;1(4):283–292. doi: 10.1016/j.stemcr.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Guo X, Chen Y, Wang CE, Gao J, Yang W, Kang Y, Si W, Wang H, Yang SH, Li S, Ji W, Li XJ. Early Parkinson’s disease symptoms in alpha-synuclein transgenic monkeys. Hum Mol Genet. 2015;24(8):2308–2317. doi: 10.1093/hmg/ddu748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54(3):403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- Paumier KL, Luk KC, Manfredsson FP, Kanaan NM, Lipton JW, Collier TJ, Steece-Collier K, Kemp CJ, Celano S, Schulz E, Sandoval IM, Fleming S, Dirr E, Polinski NK, Trojanowski JQ, Lee VM, Sortwell CE. Intrastriatal injection of pre-formed mouse alpha-synuclein fibrils into rats triggers alpha-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol Dis. 2015;82:185–199. doi: 10.1016/j.nbd.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2004;101(34):12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Aarsland D, Barone P, Burn DJ, Hawkes CH, Oertel W, Ziemssen T. Identifying prodromal Parkinson’s disease: pre-motor disorders in Parkinson’s disease. Move Dis Off J Move Dis Soc. 2012;27(5):617–626. doi: 10.1002/mds.24996. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Vila M. MPTP: a review of its mechanisms of neurotoxicity. Clin Neurosci Res. 2001;1(6):407–418. doi: 10.1016/S1566-2772(01)00019-6. [DOI] [Google Scholar]
- Rakic P. Neuroscience: immigration denied. Nature. 2004;427(6976):685–686. doi: 10.1038/427685a. [DOI] [PubMed] [Google Scholar]
- Raymon HK, Thode S, Gage FH. Application of ex vivo gene therapy in the treatment of Parkinson’s disease. Exp Neurol. 1997;144(1):82–91. doi: 10.1006/exnr.1996.6392. [DOI] [PubMed] [Google Scholar]
- Recasens A, Dehay B, Bove J, Carballo-Carbajal I, Dovero S, Perez-Villalba A, Fernagut PO, Blesa J, Parent A, Perier C, Farinas I, Obeso JA, Bezard E, Vila M. Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol. 2014;75(3):351–362. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- Redmond DE., Jr Cellular replacement therapy for Parkinson’s disease—where we are today? Neurosci Rev J Neurobiol Neurol Psychiatry. 2002;8(5):457–488. doi: 10.1177/107385802237703. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Sladek JR, Jr, Roth RH, Collier TJ, Elsworth JD, Deutch AY, Haber S. Fetal neuronal grafts in monkeys given methylphenyltetrahydropyridine. Lancet. 1986;1(8490):1125–1127. doi: 10.1016/S0140-6736(86)91839-8. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Jr, Naftolin F, Collier TJ, Leranth C, Robbins RJ, Sladek CD, Roth RH, Sladek JR., Jr Cryopreservation, culture, and transplantation of human fetal mesencephalic tissue into monkeys. Science. 1988;242(4879):768–771. doi: 10.1126/science.2903552. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Jr, Robbins RJ, Naftolin F, Marek KL, Vollmer TL, Leranth C, Roth RH, Price LH, Gjedde A, Bunney BS, et al. Cellular replacement of dopamine deficit in Parkinson’s disease using human fetal mesencephalic tissue: preliminary results in four patients. Res Publ Assoc Res Nerv Ment Dis. 1993;71:325–359. [PubMed] [Google Scholar]
- Redmond DE, Jr, Bjugstad KB, Teng YD, Ourednik V, Ourednik J, Wakeman DR, Parsons XH, Gonzalez R, Blanchard BC, Kim SU, Gu Z, Lipton SA, Markakis EA, Roth RH, Elsworth JD, Sladek JR, Jr, Sidman RL, Snyder EY. Behavioral improvement in a primate Parkinson’s model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci USA. 2007;104(29):12175–12180. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond DE, Jr, Elsworth JD, Roth RH, Leranth C, Collier TJ, Blanchard B, Bjugstad KB, Samulski RJ, Aebischer P, Sladek JR., Jr Embryonic substantia nigra grafts in the mesencephalon send neurites to the host striatum in non-human primate after overexpression of GDNF. J Comp Neurol. 2009;515(1):31–40. doi: 10.1002/cne.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond DE, Jr, McEntire CR, Kingsbery JP, Leranth C, Elsworth JD, Bjugstad KB, Roth RH, Samulski RJ, Sladek JR., Jr Comparison of fetal mesencephalic grafts, AAV-delivered GDNF, and both combined in an MPTP-induced nonhuman primate Parkinson’s model. Mol Ther J Am Soc Gene Ther. 2013;21(12):2160–2168. doi: 10.1038/mt.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RM, Kells AP, Rosenbluth KH, Salegio EA, Fiandaca MS, Larson PS, Starr PA, Martin AJ, Lonser RR, Federoff HJ, Forsayeth JR, Bankiewicz KS. Interventional MRI-guided putaminal delivery of AAV2-GDNF for a planned clinical trial in Parkinson’s disease. Mol Ther J Am Soc Gene Ther. 2011;19(6):1048–1057. doi: 10.1038/mt.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pallares J, Parga JA, Munoz A, Rey P, Guerra MJ, Labandeira-Garcia JL. Mechanism of 6-hydroxydopamine neurotoxicity: the role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. J Neurochem. 2007;103(1):145–156. doi: 10.1111/j.1471-4159.2007.04699.x. [DOI] [PubMed] [Google Scholar]
- Roitberg B, Urbaniak K, Emborg M. Cell transplantation for Parkinson’s disease. Neurol Res. 2004;26(4):355–362. doi: 10.1179/016164104225017604. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Studer L, Bankiewicz KS, Major EO, McKay RD. In vitro generation and transplantation of precursor-derived human dopamine neurons. J Neurosci Res. 2001;65(4):284–288. doi: 10.1002/jnr.1152. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Studer L, Ferrari D, Perrier A, Lee H, Vinuela A, Isacson O. Long-term survival of dopamine neurons derived from parthenogenetic primate embryonic stem cells (cyno-1) after transplantation. Stem Cells. 2005;23(7):914–922. doi: 10.1634/stemcells.2004-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Lee H, Patterson M, Reske-Nielsen C, Yoshizaki T, Sonntag KC, Studer L, Isacson O. Parthenogenetic dopamine neurons from primate embryonic stem cells restore function in experimental Parkinson’s disease. Brain J Neurol. 2008;131(Pt 8):2127–2139. doi: 10.1093/brain/awn144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Oiwa R, Kumita W, Henry R, Sakuma T, Ito R, Nozu R, Inoue T, Katano I, Sato K, Okahara N, Okahara J, Shimizu Y, Yamamoto M, Hanazawa K, Kawakami T, Kametani Y, Suzuki R, Takahashi T, Weinstein EJ, Yamamoto T, Sakakibara Y, Habu S, Hata J, Okano H, Sasaki E. Generation of a nonhuman primate model of severe combined immunodeficiency using highly efficient genome editing. Cell Stem Cell. 2016;19(1):127–138. doi: 10.1016/j.stem.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Schueler SB, Ortega JD, Sagen J, Kordower JH. Robust survival of isolated bovine adrenal chromaffin cells following intrastriatal transplantation: a novel hypothesis of adrenal graft viability. J Neurosci Off J Soc Neurosci. 1993;13(10):4496–4510. doi: 10.1523/JNEUROSCI.13-10-04496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoh S, Witkop B. Non-enzymatic conversions of dopamine to norepinephrine and trihydroxyphenethylamines. J Am Chem Soc. 1959;81(23):6222–6231. doi: 10.1021/ja01532a028. [DOI] [Google Scholar]
- Shin E, Garcia J, Winkler C, Bjorklund A, Carta M. Serotonergic and dopaminergic mechanisms in graft-induced dyskinesia in a rat model of Parkinson’s disease. Neurobiol Dis. 2012;47(3):393–406. doi: 10.1016/j.nbd.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Silvestrini MT, Yin D, Martin AJ, Coppes VG, Mann P, Larson PS, Starr PA, Zeng X, Gupta N, Panter SS, Desai TA, Lim DA. Interventional magnetic resonance imaging-guided cell transplantation into the brain with radially branched deployment. Mol Ther J Am Soc Gene Ther. 2015;23(1):119–129. doi: 10.1038/mt.2014.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek JR, Jr, Collier TJ, Haber SN, Roth RH, Redmond DE., Jr Survival and growth of fetal catecholamine neurons transplanted into primate brain. Brain Res Bull. 1986;17(6):809–818. doi: 10.1016/0361-9230(86)90092-4. [DOI] [PubMed] [Google Scholar]
- Sladek JR, Jr, Elsworth JD, Roth RH, Evans LE, Collier TJ, Cooper SJ, Taylor JR, Redmond DE., Jr Fetal dopamine cell survival after transplantation is dramatically improved at a critical donor gestational age in nonhuman primates. Exp Neurol. 1993;122(1):16–27. doi: 10.1006/exnr.1993.1103. [DOI] [PubMed] [Google Scholar]
- Sladek JR, Jr, Bjugstad KB, Collier TJ, Bundock EA, Blanchard BC, Elsworth JD, Roth RH, Redmond DE., Jr Embryonic substantia nigra grafts show directional outgrowth to cografted striatal grafts and potential for pathway reconstruction in nonhuman primate. Cell Transplant. 2008;17(4):427–444. [PubMed] [Google Scholar]
- Soderstrom KE, Meredith G, Freeman TB, McGuire SO, Collier TJ, Sortwell CE, Wu Q, Steece-Collier K. The synaptic impact of the host immune response in a parkinsonian allograft rat model: influence on graft-derived aberrant behaviors. Neurobiol Dis. 2008;32(2):229–242. doi: 10.1016/j.nbd.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom KE, O’Malley JA, Levine ND, Sortwell CE, Collier TJ, Steece-Collier K. Impact of dendritic spine preservation in medium spiny neurons on dopamine graft efficacy and the expression of dyskinesias in parkinsonian rats. Euro J Neurosci. 2010;31(3):478–490. doi: 10.1111/j.1460-9568.2010.07077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998;95(11):6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steece-Collier K, Soderstrom KE, Collier TJ, Sortwell CE, Maries-Lad E. Effect of levodopa priming on dopamine neuron transplant efficacy and induction of abnormal involuntary movements in parkinsonian rats. J Comp Neurol. 2009;515(1):15–30. doi: 10.1002/cne.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D, Mosharov EV, Studer L. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat Biotechnol. 2015;33(2):204–209. doi: 10.1038/nbt.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg M, Bogetofte H, Lawson T, Jansson J, Smith G, Astradsson A, Moore M, Osborn T, Cooper O, Spealman R, Hallett P, Isacson O. Improved cell therapy protocols for Parkinson's disease based on differentiation efficiency and safety of hESC-, hiPSC-, and non-human primate iPSC-derived dopaminergic neurons. Stem Cells. 2013;31(8):1548–1562. doi: 10.1002/stem.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Investig. 2005;115(1):102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2(12):3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tambur AR. Transplantation immunology and the central nervous system. Neurol Res. 2004;26(3):243–255. doi: 10.1179/016164104225013932. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Roth RH, Collier TJ, Sladek JR, Jr, Redmond DE., Jr Improvements in MPTP-induced object retrieval deficits and behavioral deficits after fetal nigral grafting in monkeys. Prog Brain Res. 1990;82:543–559. doi: 10.1016/S0079-6123(08)62645-X. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Roth RH, Sladek JR, Jr, Collier TJ, Redmond DE., Jr Grafting of fetal substantia nigra to striatum reverses behavioral deficits induced by MPTP in primates: a comparison with other types of grafts as controls. Exp Brain Res. 1991;85(2):335–348. doi: 10.1007/BF00229411. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci USA. 1995;92(17):7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Hearn JP. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol Reprod. 1996;55(2):254–259. doi: 10.1095/biolreprod55.2.254. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tomioka I, Maeda T, Shimada H, Kawai K, Okada Y, Igarashi H, Oiwa R, Iwasaki T, Aoki M, Kimura T, Shiozawa S, Shinohara H, Suemizu H, Sasaki E, Okano H. Generating induced pluripotent stem cells from common marmoset (Callithrix jacchus) fetal liver cells using defined factors, including Lin28. Genes Cells Devot Mol Cell Mech. 2010;15(9):959–969. doi: 10.1111/j.1365-2443.2010.01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5(1):107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- Vermilyea SC, Emborg ME. alpha-Synuclein and nonhuman primate models of Parkinson’s disease. J Neurosci Methods. 2015;255:38–51. doi: 10.1016/j.jneumeth.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermilyea SC, Guthrie S, Meyer M, Smuga-Otto K, Braun K, Howden S, Thomson JA, Zhang SC, Golos TG, Emborg ME. Common marmoset iPSC-derived midbrain floorplate dopaminergic neurons. Cell Transplant. 2016;25(4):775–776. [Google Scholar]
- Vermilyea SC, Lu J, Olsen M, Guthrie S, Tao Y, Fekete EM, Riedel MK, Brunner K, Boettcher C, Bondarenko V, Brodsky E, Block WF, Alexander A, Zhang SC, Emborg ME. Real-time intraoperative MRI intracerebral delivery of induced pluripotent stem cell-derived neurons. Cell Transplant. 2016 doi: 10.3727/096368916X692979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman DR, Redmond DE, Jr, Dodiya HB, Sladek JR, Jr, Leranth C, Teng YD, Samulski RJ, Snyder EY. Human neural stem cells survive long term in the midbrain of dopamine-depleted monkeys after GDNF overexpression and project neurites toward an appropriate target. Stem Cells Transl Med. 2014;3(6):692–701. doi: 10.5966/sctm.2013-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman DR, Weiss S, Sladek JR, Elsworth JD, Bauereis B, Leranth C, Hurley PJ, Roth RH, Redmond DE. Survival and integration of neurons derived from human embryonic stem cells in MPTP-lesioned primates. Cell Transplant. 2014;23(8):981–994. doi: 10.3727/096368913X664865. [DOI] [PubMed] [Google Scholar]
- Wang S, Zou C, Fu L, Wang B, An J, Song G, Wu J, Tang X, Li M, Zhang J, Yue F, Zheng C, Chan P, Zhang YA, Chen Z. Autologous iPSC-derived dopamine neuron transplantation in a nonhuman primate Parkinson’s disease model. Cell Discov. 2015;1:15012. doi: 10.1038/celldisc.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts RL, Raiser CD, Stover NP, Cornfeldt ML, Schweikert AW, Allen RC, Subramanian T, Doudet D, Honey CR, Bakay RA. Stereotaxic intrastriatal implantation of human retinal pigment epithelial (hRPE) cells attached to gelatin microcarriers: a potential new cell therapy for Parkinson’s disease. J Neural Transm Suppl. 2003;65:215–227. doi: 10.1007/978-3-7091-0643-3_14. [DOI] [PubMed] [Google Scholar]
- Wiedemann A, Hemmer K, Bernemann I, Gohring G, Pogozhykh O, Figueiredo C, Glage S, Schambach A, Schwamborn JC, Blasczyk R, Muller T. Induced pluripotent stem cells generated from adult bone marrow-derived cells of the nonhuman primate (Callithrix jacchus) using a novel quad-cistronic and excisable lentiviral vector. Cellular Reprogr. 2012;14(6):485–496. doi: 10.1089/cell.2012.0036. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang Y, Mishra A, Tardif SD, Hornsby PJ. Generation of induced pluripotent stem cells from newborn marmoset skin fibroblasts. Stem cell Res. 2010;4(3):180–188. doi: 10.1016/j.scr.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23(6):781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Wang G, Wang CE, Guo X, Yin P, Gao J, Tu Z, Wang Z, Wu J, Hu X, Li S, Li XJ. Mutant alpha-synuclein causes age-dependent neuropathology in monkey brain. J Neurosci Off J Soc Neurosci. 2015;35(21):8345–8358. doi: 10.1523/JNEUROSCI.0772-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]