Abstract
The hemostatic response requires the tightly regulated interaction of the coagulation system, platelets, other blood cells and components of the vessel wall at a site of vascular injury. The dysregulation of this response may result in excessive bleeding if the response is impaired, and pathologic thrombosis with vessel occlusion and tissue ischemia if the response is overly robust. Extensive studies over the past decade have sought to unravel the regulatory mechanisms that coordinate the multiple biochemical and cellular responses in time and space to ensure that an optimal response to vascular damage is achieved. These studies have relied in part on advances in in vivo imaging techniques in animal models, allowing for the direct visualization of various molecular and cellular events in real time during the hemostatic response. This review summarizes knowledge gained using these in vivo imaging and other approaches that provides new insights into the spatio-temporal regulation of coagulation and platelet activation at a site of vascular injury.
Introduction
The hemostatic response to vascular injury is a highly regulated and complex process that requires coagulation proteins, platelets and components of the vascular wall to form a localized hemostatic plug that prevents bleeding. Many aspects of this process have been well characterized at the molecular and cellular level in vitro, including detailed analyses of the coagulation system and the mechanisms of platelet activation. This work has been the subject of several extensive reviews and book chapters [1–5]. Additional investigations by many groups in recent years have sought to uncover the mechanisms by which key molecular and cellular events are regulated in the native environment of a blood vessel in vivo. In this setting, one must consider not only the biochemical pathways involved in the generation of thrombin and platelet activation, but also how these biochemical processes are influenced by other factors within the complex locale of a particular vasculature, including contributions from endothelial cells, other blood cells and local hemodynamic conditions. We need to understand how these various components of the hemostatic response are integrated in time and space in order to better understand how an optimal response to injury is achieved, and how perturbations of this regulation result in thrombosis.
The advent of genetically modified mice coupled with hemostasis/thrombosis models has proved to be extremely useful for the analysis of hemostasis in vivo. In particular, recent developments in intravital microscopy imaging approaches have yielded valuable insights into the complex hemostatic process in a physiological setting. Current technology enables direct visualization and quantitative analysis of multiple components of hemostatic plug formation in blood vessels of live animals, most commonly arterioles or venules within the mesentery or cremaster muscle of mice. These studies have been made possible both by advances in equipment technology, and by the development of an arsenal of fluorescent imaging tools, including fluorescently labeled antibodies, cells, coagulation factors and biochemical sensors, that are now available for detecting components of the biological process. Thus, it is possible to visualize distinct proteins or cell populations involved in the regulation of a hemostatic plug with high accuracy. This review summarizes recent efforts utilizing these technologies and other in vivo studies to gain insights into the spatio-temporal regulation of the hemostatic process, with a particular emphasis on mechanisms regulating thrombin generation and platelet activation.
Spatio-temporal regulation of thrombin generation following vascular injury in vivo
Initiation of thrombin generation in vivo
The blood coagulation response to vascular damage consists of a series of sequential biochemical reactions that lead to production of thrombin, which converts fibrinogen to insoluble fibrin, and activates platelets, other blood cells and the endothelium [5]. Thrombin generation at the site of the injury is triggered by tissue factor (TF) that forms a complex with factor VIIa and initiates the extrinsic pathway of coagulation [6]. Thus, the localization of tissue factor expression can be rationalized as an important determinant of the spatio-temporal distribution of subsequent thrombin generation and activity. TF is a transmembrane protein present on the surface of the cells that synthesized it. The classical view is that TF is abundant on different extravascular cells and is only exposed to blood following a breach in the vessel wall [7]. However, evidence continues to accumulate suggesting that alternative sources of TF exist within the vascular compartment that may play a role in thrombin generation in both normal and pathologic settings.
Quantifiable amounts of TF antigen were found in circulating blood [8] and, upon laser-injury, circulating TF antigen incorporated within the growing hemostatic plug [9]. This TF appears to originate from microparticles and seems to have an active role in early hemostatic events under physiological conditions. In support of this observation, leukocyte-derived microparticles containing TF infused into a wild-type mouse were recruited to the site of the injury through P-selectin/PSGL-1 interaction and contributed to fibrin propagation [10]. The question of how and whether endogenous TF-positive microparticles contribute to thrombus formation is still unresolved. More recently, neutrophils present at the site of injury have been considered as another source of TF [11–13]. Cultured endothelial cells readily expressed TF following laser-injury and an antibody to TF prevented fibrin deposition [14]; however, an in vivo study showed that vessel wall TF alone was not sufficient to support thrombin generation and fibrin formation within the developing hemostatic plug [10]. Thus, the extent that the vessel wall contributes to the overall pool of TF in vivo is not yet clear. In spite of these promising data, there is still debate regarding the significance of these sources of TF, because active TF cannot be detected in normal blood [15]. Some studies have proposed that additional mediators, such as protein disulfide isomerase (PDI) secreted following injury, might participate in the local activation of TF and fibrin formation [16, 17].
Apart from the TF-initiated coagulation cascade, thrombin generation may also be initiated through the contact pathway of blood coagulation, specifically factor XII and factor XI [18]. In this regard, FXI- or FXII-deficient mice had a severe defect in thrombus formation in multiple different thrombosis models [19, 20]. While these studies demonstrate a role for the contact pathway in thrombosis, the precise contribution of factors XI and XII to the spatio-temporal regulation of thrombin generation has yet to be determined.
Localization of procoagulant surfaces
The bulk of thrombin production occurs through cleavage of prothrombin by the prothrombinase complex (factor Xa / factor Va) on phosphatidylserine (PS) on the outer membrane leaflet of activated cells at the site of the injury. Such binding events localize coagulation enzyme assembly to the site of the injury and amplify the activity of coagulation proteases by several orders of magnitude [21, 22]. It is well established that negatively charged phospholipids exposed on activated platelets can provide a surface for the assembly of tenase and prothrombinase complexes [23]. According to the prevalent current model, activated platelets are considered the preeminent cell membrane surface required for the assembly of coagulation factor complexes that lead to thrombin generation and the formation of the fibrin network [24]. However, much of our understanding of the contribution of platelets to coagulation reactions is derived from in vitro/ex vivo studies that may not reliably reflect hemostatic events under the influence of the native environment.
Recent advances in intravital video microscopy have shed a different light on the exclusive role of platelets and point to a more dynamic and complex interaction among coagulation factors, blood cells and the vessel wall to regulate thrombus formation than previously appreciated. A key observation was that platelets and fibrin accumulation appear simultaneously at the site of the injury in the cremaster arteriole model [9]. Another unexpected finding was that PAR-4 deficient mice (platelets lack the thrombin receptor, protease activated receptor 4) form robust fibrin clots without the accumulation of a large number of platelets after laser-induced injury [25]. In addition, pharmacologic inhibition of platelet accumulation using αIIbβ3 blockers had no significant effect on fibrin formation following injury in a cremaster mouse model [14, 25], or following an electrolytic injury in the mouse femoral vein [26]. These surprising findings indicate that sufficient amounts of thrombin can be formed in the absence of robust platelet accumulation and raises the question of what other biological surfaces can support coagulation reactions at a site of injury in vivo.
Contribution of the endothelium to fibrin formation
In addition to activated platelets, other PS-exposed membrane surfaces of different blood cells or the injured vessel wall might support thrombin generation. Whilst several pieces of evidence indicate that physiological membranes of leukocytes [27] and red cells [28] play a role in supporting fibrin formation, it is the endothelium that has recently received more attention. Apart from its anticoagulant role by triggering activation of protein C [29], recent evidence suggest that damaged or activated endothelium can support thrombin production by forming a procoagulant surface. One important indication comes from in vitro data where stimulated endothelial cells become PS-positive and have the ability to support binding of the components of the prothrombinase complex [30]. A critical question is whether the phenotype of cultured endothelial cells accurately imitates the extant features of the endothelium in vivo. The available in vivo data, largely from the cremaster mouse model, are consistent with a significant role played by endothelium in supporting blood coagulation reactions at a site of vascular injury [14, 31, 32].
Intravital microscopy coupled with fluorescently labeled antibodies has been employed to visualize the coagulation events at the site of the injury. For example, visualization of fibrin provides a readout of thrombin activity, but does not document in vivo distribution of the coagulation factors. Fibrin deposition is also subject to convective effects from blood flow [33, 34]. Moreover, Annexin V labeling permits visualization of PS-positive membranes, although it is possible not all Annexin V labeled structures support coagulation factor assembly, and further, Annexin V competes with coagulation factors for binding to PS-positive membranes, thus potentially altering the evolution of responses. The development of site-specific fluorescently labeled coagulation factor derivatives has started to address the localization of coagulation factors at the site of the injury in a more specific manner, and results suggest the possible contribution of the vessel wall to thrombin formation. For example, a study employing active site-labeled prothrombin in the cremaster arteriole model documented the accumulation of the fluorescent prothrombin species on the vessel wall at the site of the laser injury, prior to platelet accumulation [31]. This active site labeled prothrombin is catalytically inactive and thus has the potential to inhibit endogenous thrombin activity, however, it provides information regarding localization of procoagulant membranes at the site of the injury [31]. This observation suggests exposure of PS on membranes other than activated platelets, such as activated/damaged endothelium, which is consistent with a role of the endothelium in supporting clotting factor binding [31].
More recently, additional fluorescently labeled coagulation factor species have emerged for detecting enzyme constituents during thrombus evolution following vascular injury. Imaging of bound FXa has been accomplished by the development and use of Xa bearing Alexa647 tethered to the active site with a peptidyl chloromethyl ketone. The resulting derivative is catalytically inactive Xa (Xai) that retains the ability to assemble into prothrombinase. Since high concentrations of FXai can significantly inhibit thrombus formation, this derivative is used only as a tracer to visualize the spatial distribution of this coagulation factor at the site of the injury [32]. In contrast, the site-specific fluorescently labeled FV-810SYA, a B-domainless FVa (FVdes811-149 or FV-810) [35] is constitutively active and participates in thrombus formation in vivo [32, 36]. This derivative has three mutated cysteines (Cys) and one free Cys at position 539, which was labeled with a fluorescent probe.
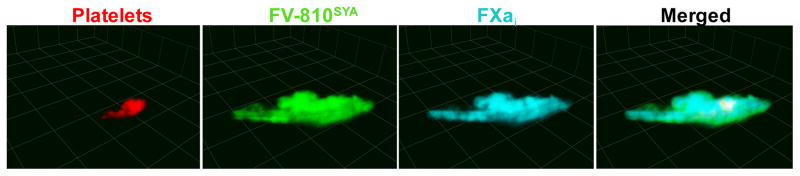
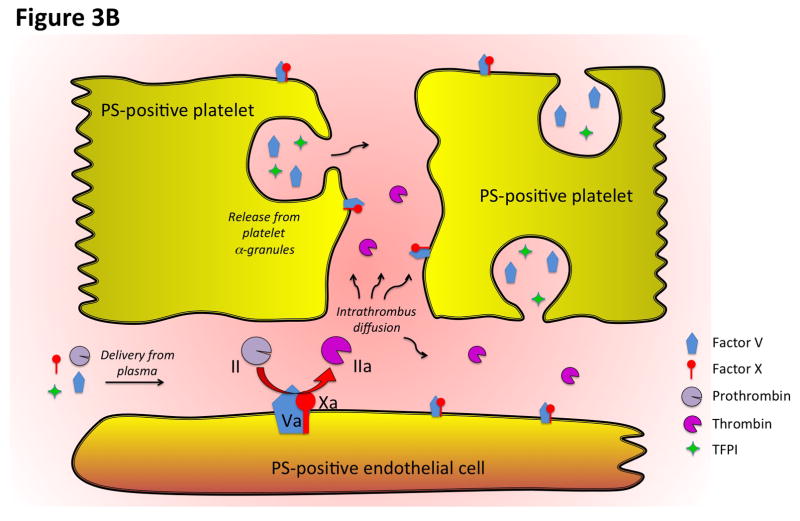
Thrombus formation at the site of the laser injury in hemophilia B (HB) mice can be restored by infusion of FVa [37]. In particular, the extent and localization of platelet and fibrin accumulation are similar in wild-type and HB mice infused with FV-810SYA [32], demonstrating that despite the lack of FIX-dependent FX activation, thrombin activity and localization are restored to normal levels in HB mice following infusion of FV-810SYA. Therefore, HB mice infused with FV-810SYA can be used as a platform to assess the localization of these site-specific fluorescent derivatives of Va and Xa in the growing thrombus. A surprising discovery was the spatio-temporal difference between the distribution of platelets and the constituents of prothrombinase at the injury site. Factors Va and Xa were imaged on surfaces surrounding the injury site and along the side of the vessel (Figure 1), and only on a small subset of platelets within the forming thrombus. The fact that coagulation factors FVa and FXa were not found on all adherent platelets at the injury site suggests that only a fraction of platelets localized at the core of the thrombus may provide a PS-positive surface. This is consistent with previous work describing that PS-exposed platelets were mainly localized in the center of the thrombus [38]. A more recent study has highlighted the dynamic nature of platelet recruitment to the site of the injury, where surprisingly not all adherent platelets were activated to the same extent [39]. Fibrin is only partially localized with platelets and FVa, and appears more distal to these components [32]. The rate of fibrin deposition reflects the rate of thrombin formation in the zone of vascular damage. Formation of thrombin proceeds through a tightly regulated series of reactions involving a number of pro- and anticoagulant proteins. At the site of the injury, tissue factor pathway inhibitor (TFPI) or FV released from adherent platelets can play a key role in modulating the amount of thrombin generated (Figure 3B).
Figure 1. Spatio-temporal distribution of the constituents of prothrombinase at a site of vascular injury.
3D reconstruction from confocal images acquired 4 minutes after laser-injury to the cremaster arteriole of hemophilic mice. Platelets were visualized using Alexa555-labeled rat anti-CD41 F(ab)2 antibody; the distribution of clotting factors were directly imaged with Alexa488-FV-810SYA and Alexa647-FXai; step size = 1μm in z-plane; total sections 35μm.
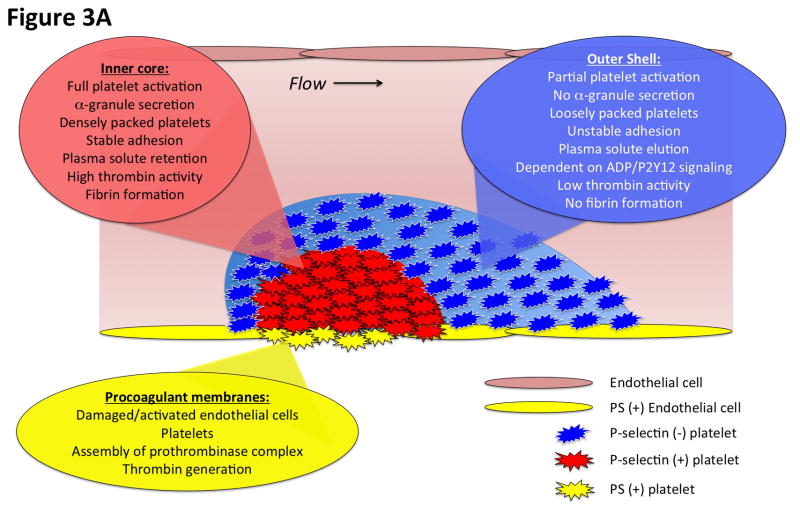
Figure 3. Diagrammatic representation of the spatial distribution of coagulation events and platelet activation at the cellular and molecular levels.
A) This diagram illustrates the heterogeneity of platelet activation at a site of vascular injury. Two regions, an inner core and an outer shell, may be defined based on a number of characteristics including platelet activation state, platelet packing density, porosity and level of thrombin activity. Localization of procoagulant surfaces and fibrin formation is also shown. B) This diagram illustrates coagulation events at the molecular level and how they are regulated by the local microenvironment. Assembly of the prothrombinase complex on PS-positive endothelial cell and platelet membranes localized at the site of injury leads to thrombin generation. The spatio-temporal distribution of thrombin activity is determined by its sites of generation, localization of inhibitors, and physical factors in the local microenvironment that regulate molecular transport. Molecular transport phenomena also regulate the delivery of additional pro- and anti-coagulant factors from the bulk flow and those released locally from platelet granules (e.g. factor V and TFPI).
The observation that FVa and Xa binding at and surrounding the site of injury only partially overlaps with platelets indicates that additional cellular membranes also support coagulation factor assembly. This conclusion is reinforced by the correlation of FXa localization with PS exposure on the vessel wall, visualized with Annexin V [32]. Of note, however, the PS-positive area of the vessel wall was restricted to the immediate vicinity of the injury, and does not extend to distant parts of the vessel wall. Surprisingly, inhibition of platelet accumulation had no major effect on fibrin deposition or the distribution of FVa and Xa at a site of injury, further suggesting that the endothelium participates in prothrombinase assembly/activity. Furthermore, infusion of fluorescently labeled Xa in animals with GFP expression controlled by the Tie2 promoter in vascular endothelial cells provided another approach to strengthen the conclusion that damaged/activated endothelium is capable of localizing coagulation factors and promoting thrombin and fibrin generation [32].
Taken together, recent studies using intravital technology to directly visualize coagulation factor complexes on PS-positive membranes in vivo following laser injury to the mouse cremaster arteriole have started to challenge the paradigm that activated platelets represent the primary source of membranes facilitating the assembly and function of tenase and prothrombinase complexes [40, 41]. The finding that components of the prothrombinase complex bound more prominently in the vicinity of the injury site on cellular surfaces other than activated platelets, as well as the finding that fibrin formation is normal following inhibition of platelet accumulation or activation, points to a role for the endothelium and possibly other blood cells in supporting enzyme coagulation reactions. These recent studies also demonstrate that the development of proper reagents that locally image coagulation factors in vivo are instrumental for identifying the mechanisms of normal and pathologic thrombus formation and may allow a more precise evaluation of pharmacological therapies directed towards thrombin production.
Spatio-temporal regulation of platelet activation following vascular injury in vivo
Platelet activation is heterogeneous in time and space
Intravital imaging studies in the microcirculation have demonstrated that platelet activation following vascular injury is heterogeneous [25, 39, 42–44]. This heterogeneity partly reflects graded temporal dynamics of distinct platelet activation events, but also reflects specific spatial localization of platelets with differing degrees of activation. Platelet accumulation at a site of injury is characterized by the rapid formation of a platelet aggregate. While some degree of platelet activation is likely necessary for platelet retention within the evolving platelet plug, the precise extent of platelet activation in this initial aggregate is unclear, and initial tethering of platelets may not require activation at all. Indeed, multiple studies have shown that upon initial adhesion to a growing aggregate, platelets may retain their discoid shape, which is generally considered a resting morphology [45–47]. That being said, platelet calcium signaling is dynamic during the initial stages of platelet recruitment and accumulation, with rapid calcium transients or oscillations observed in individual platelets [42, 44]. As the platelet mass evolves, a subpopulation of platelets close to the site of injury may then be observed with a more sustained elevation in cytosolic calcium [44]. Surface expression of the platelet α-granule protein P-selectin similarly lags substantially behind initial platelet accumulation [25, 39, 43]. P-selectin expression is spatially localized to a region adjacent to the site of injury, initially observed in a small population of platelets that slowly grows over several minutes [25, 39, 43]. We and others have referred to this region of stably adherent, P-selectin positive platelets as the thrombus or hemostatic plug “core”, which is generally overlaid by a “shell” of less activated, loosely adherent platelets (Figure 3A) [39]. As discussed in detail in the first part of this review, the distribution of phophatidylserine expressing membranes is spatio-temporally regulated as well [32, 38].
While the above description of platelet activation heterogeneity following vascular injury comes largely from studies in the microcirculation, there is evidence showing a similar heterogeneity of platelet activation in other vascular beds and in larger blood vessels. Early studies of the ultrastructure of arterial thrombi examined using transmission electron microscopy (TEM) show heterogeneity of platelet activation, with regions of thrombi composed of fully degranulated platelets and other regions where platelet granules remain intact [48, 49]. More recently, TEM images of platelet rich thrombi formed following mild or severe injuries in mouse mesenteric arteries clearly show platelets with differing degrees of activation, with fully degranulated platelets present adjacent to the site of injury [50]. This architecture is not peculiar to laser injury models, as it is also seen following mechanical puncture injury [39, 44]. A similar architecture has also been observed with human platelets using a specialized in vitro flow chamber system that mimics a punctured blood vessel in vivo (with collagen and tissue factor exposed at the “injury site”) [51]. A number of important questions arise from such observations including how does such spatio-temporal heterogeneity of platelet activation happen, are distinct platelet agonists (e.g. thrombin, ADP, collagen) responsible for the different degrees of activation, and what are the implications for therapeutic approaches that target these specific agonist signaling pathways? Studies utilizing genetically modified mice and pharmacologic approaches have begun to address these questions, and the results reveal a complex integration of platelet signaling pathways during thrombus formation in vivo, as described below.
Spatio-temporal regulation of platelet activation by soluble agonists
The graded activation of platelets extending from the site of injury as observed in intravital imaging studies suggests that gradients of platelet agonists are established within the platelet mass as it evolves. Further, platelet activation pathways can serve distinct and/or redundant roles during platelet accumulation and activation. Here, we will consider the role of each of the major platelet agonist pathways in determining the spatio-temporal heterogeneity of the platelet response to injury in vivo.
Thrombin is a potent platelet activator acting on members of the protease activated receptor (PAR) family of g-protein coupled receptors (GPCRs, PAR-1 and -4 on human platelets, and PAR-3 and -4 on mouse platelets). Studies in PAR-4 deficient mice demonstrated that thrombin signaling is necessary for full platelet activation including α-granule secretion in the cremaster laser injury model [25]. Thrombin inhibition with direct thrombin inhibitors such as lepirudin and hirudin yielded similar results [9, 25, 39]. As full platelet activation with α-granule secretion is normally observed in a spatially distinct region emanating from the site of injury, these studies suggest that thrombin activity is localized within this region of a platelet mass. Indeed, fibrin accumulation, which may be considered a surrogate of thrombin activity, is localized to the core region of a platelet plug [9, 25, 39, 52], and we have also shown that thrombin activity is localized to the core region using a fluorogenic thrombin activity sensor bound to the surface of platelets [53]. There are multiple mechanisms by which thrombin activity is spatially restricted within the evolving hemostatic plug, including localization of thrombin generation on the surface of procoagulant membranes (discussed above), restricted diffusion of thrombin away from its site of generation (discussed in detail below), and binding of thrombin to local substrates such as fibrin.
ADP is a critical regulator of platelet activity released from platelet dense granules, acting via a pair of GPCRs on platelets, the Gq coupled P2Y1 receptor and Gi2 coupled P2Y12 receptor. P2Y12 receptor signaling in particular plays a critical role in platelet aggregation at a site of injury in vivo, as genetic deletion or pharmacologic inhibition of P2Y12 consistently results in attenuated platelet accumulation in a variety of injury models [54–57]. Conversely, genetic mutants in which Gi2α signaling is enhanced result in increased platelet accumulation [58]. The kinetics and localization of dense granule secretion at a site of injury in vivo are not well characterized due to the lack of specific markers, but we have nonetheless gained an appreciation for the role of ADP in the spatio-temporal regulation of platelet activation. Specifically, inhibition of P2Y12 signaling results in impaired platelet recruitment and retention in the outer shell region of a developing platelet plug, but has little impact on full platelet activation in the core region where thrombin activity is highest [39]. Similar results are also seen in mouse models of Hermansky-Pudlak syndrome in which dense granule secretion is abrogated [59, 60]. Thus, P2Y12 signaling and thrombin-dependent platelet activation appear to have distinct roles during the hemostatic response. These findings have important implications for the targeting of these two platelet activation pathways by anti-platelet agents.
Thromboxane A2 (TxA2) is generated and released by activated platelets providing feedback to reinforce platelet activation. TxA2 is generated via a cyclooxygenase-1 (COX-1) pathway in platelets, and when released, binds its receptors (TPα andβ) on the platelet surface. Studies in thromboxane receptor-deficient mice showed attenuated thrombus formation, demonstrating a role for TxA2 signaling in platelet activation following vascular injury [61]. A number of large clinical studies showing the efficacy of aspirin treatment in the prevention of cardiovascular events (i.e. myocardial infarction and stroke) [62] highlight the clinical relevance of this signaling pathway in thrombosis. The spatio-temporal distribution of TxA2 within a growing hemostatic plug is not yet well defined. As in the case of ADP release from platelet dense granules, TxA2 requires platelet activation for its generation. Thus, its localization will primarily be determined by its source (activated platelets) and its rapid metabolism in plasma to inactive metabolites. However, the precise contribution of TxA2 signaling to heterogeneous platelet activation remains to be determined.
Spatio-temporal regulation of platelet activation by adhesive ligands
A number of adhesive ligands, such as collagen and von Willebrand factor (vWf), can also contribute to platelet activation via binding to specific receptors on the platelet surface, in addition to their adhesive functions. The contribution of these signaling pathways to the spatio-temporal heterogeneity of platelet activation is largely dependent on the spatial localization of the ligand. For example, collagen mediated platelet activation via the GPVI receptor will only occur in platelets that encounter exposed collagen at the site of injury. Thus, the extent to which GPVI signaling contributes to platelet activation in vivo is highly context dependent, which may explain differences reported in studies of GPVI function performed in various injury models and vascular beds [63–67]. In addition, robust thrombin activity may mask GPVI-dependent effects, suggesting at least partial redundancy of these signaling pathways. Evidence for such redundancy comes from studies showing that inhibition of GPVI signaling has little or no effect in assays in which thrombin activity is intact, but inhibition of thrombin activity and GPVI signaling together has a greater effect than thrombin inhibition alone [65, 68, 69]. Thus, injury models in which thrombin activity contributes significantly to the hemostatic/thrombotic response are less likely to require GPVI signaling. The contribution of signaling from the GPIb-V-IX complex (the platelet vWf receptor) to platelet activation in vivo remains rather enigmatic. In vitro studies have suggested that GPIb-V-IX may act as a mechanosensor, including the demonstration of platelet calcium transients following GPIb complex engagement under flow conditions in vitro [70, 71]. However, these results have not been directly recapitulated in an in vivo system. In fact, vWf mediated signaling appears to be dispensable for platelet activation as measured by cytoplasmic calcium concentration in the mouse cremaster laser-injury model [42]. In contrast, a mouse line in which the cytoplasmic tail of GPIb-alpha was truncated showed defective thrombus formation in a FeCl3 injury model [72].
Intrathrombus solute transport shapes agonist distribution
As platelets accumulate at a site of injury, dense platelet packing resulting from retractile processes creates sheltered microenvironments in the spaces between platelets. These microenvironments are important harbors for plasma solutes and platelet releasates, protected from the effects of bulk flow in the vessel lumen [73]. As with other platelet activation events, the packing density of platelets within a hemostatic plug is heterogeneous, with platelets closer to the site of injury more densely packed than those in the outer shell region. This heterogeneity in the physical architecture of the platelet mass has important consequences for the transport of plasma solutes into and out of the spaces between platelets, which contributes to the establishment of platelet agonist gradients and further determines the extent of platelet activation.
A number of physical parameters determine the movement of plasma solutes within a platelet mass, including the volume of the spaces between platelets (i.e. porosity), the connectivity and tortuosity of the pore space, solute size, and plasma convection. Computational studies showed that the platelet aggregate acts as an obstacle to bulk flow in the lumen of the vessel such that plasma velocities in the spaces between platelets are reduced by orders of magnitude compared to velocity in the lumen [73]. As a result, solute transport in the spaces between platelets, especially in the densely packed core region, is determined primarily by diffusion, rather than convection [73]. Measurements from in vivo studies in the mouse cremaster laser-injury model showed that porosity within the core region is reduced compared to the shell, resulting in a gradient of platelet packing density that correlates spatially with the extent of platelet activation [39]. Dense platelet packing in the core region also results in increased pore space tortuosity [74]. These physical characteristics result in plasma solutes moving within the platelet mass through hindered diffusion.
The physical architecture and hindered diffusion of plasma solutes within the spaces between platelets is likely to impact the spatio-temporal regulation of thrombin generation and platelet activation in a number of ways. Multiple in vitro and computational studies have demonstrated that delivery of coagulation factors, inhibitors and fibrinolytics to the inside of a thrombus or fibrin clot is diminished [75–77]. Studies performed in vivo showed the size-dependent exclusion of a large plasma solute from the hemostatic plug core region [39]. Thus, in addition to molecular based anti-hemostatic mechanisms, including tissue factor pathway inhibitor (TFPI) released from platelets, protein C generated on the endothelial cell surface and tPA dependent fibrinolysis, the impaired delivery of plasma proteins, including coagulation factors, to the inside of a thrombus may be one mechanism by which continued thrombus growth is inhibited. Further, hindered diffusion increases the retention time of plasma proteins in the core region, thus increasing their effective concentration locally [77, 78]. With regard to thrombin generated at the site of injury, this would be expected to increase platelet activation and fibrin generation in the core region [73], which is consistent with experimental evidence regarding the spatial localization of these components of the hemostatic response [39]. As a further demonstration of the importance of the physical architecture of the platelet mass in determining thrombin distribution, experiments in which platelet retraction was impaired showed decreased platelet packing density and increased plasma solute transport in the thrombus core region, resulting in decreased local thrombin activity and platelet activation [79]. Taken together, these studies highlight the role that physical attributes of the platelet mass play in regulating the spatio-temporal distribution of thrombin and other platelet agonists at a site of injury in vivo.
Conclusions
The remarkable advances in intravital imaging techniques in the last decade and the availability of a broad array of fluorescent-labeled antibodies, cells and coagulation factors make it possible to explore the dynamic events of the hemostatic process in vivo that is not possible using conventional in vitro methods. Studies using these approaches have started to change our understanding of the interactions between the coagulation system, vessel wall and blood cells as they occur in the native environment of a live animal. The ability to quantify the components of hemostatic response in vivo opens many possibilities for investigating blood disorders as well as therapeutic interventions at the site of vascular damage. Many questions remain, however. For example, is the spatio-temporal regulation of hemostatic responses different in different vascular beds, in arteries vs. veins, or in the macrocirculation as compared to the microcirculation? How is the spatio-temporal regulation of responses altered in pathologic conditions resulting in either hemorrhage or thrombosis? How is the distribution of coagulation inhibitors and components of the fibrinolytic system regulated and varied in these different settings? Studies to address these questions and others are now possible, and will further increase our understanding of hemostasis and thrombosis.
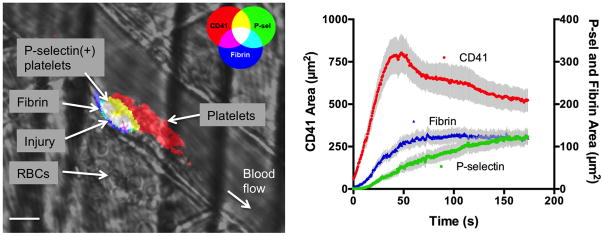
Figure 2. Spatio-temporal regulation of platelet activation following vascular injury.
A) Photomicrograph shows platelet accumulation, activation and fibrin deposition following laser-induced injury in a mouse cremaster arteriole. Note the leakage of RBCs into the extravascular space as a result of the injury, and the spatial localization of P-selectin positive platelets adjacent to the site of injury. Scale bar is 10 μm. B) Graph depicts typical kinetics of platelet accumulation, activation and fibrin deposition following laser-induced injury in cremaster arterioles of C57Bl/6 mice. Platelet accumulation is shown in red (left axis), P-selectin in green and fibrin in blue (right axis). Values are mean±SEM for n=27 thrombi.
Acknowledgments
The authors gratefully acknowledge funding from the American Heart Association (11SDG5720011 to T.J.S.) and the National Heart, Lung and Blood Institute (P01HL40387, R01HL119070 and P01HL120846 to T.J.S.).
Literature Cited
- 1.Brass LF, Stalker TJ, Zhu L, Woulfe DS. Signal transduction during initiation, extension and perpetuation of platelet plug formation. In: Michelson AD, editor. Platelets. 2. Academic Press; 2006. [Google Scholar]
- 2.Stalker TJ, Newman DK, Ma P, Wannemacher KM, Brass LF. Platelet signaling. Handb Exp Pharmacol. 2012:59–85. doi: 10.1007/978-3-642-29423-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93:327–58. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- 4.Mintz KP, Mann KG. Detection of Procollagen Biosynthesis Using Peptide-Specific Antibodies. Matrix. 1990;10:186–99. doi: 10.1016/s0934-8832(11)80168-x. [DOI] [PubMed] [Google Scholar]
- 5.Mann KG, Jenny RJ, Krishnaswamy S. Cofactor proteins in the assembly and expression of blood clotting enzyme complexes. Annu Rev Biochem. 1988;57:915–56. doi: 10.1146/annurev.bi.57.070188.004411. [DOI] [PubMed] [Google Scholar]
- 6.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–49. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 7.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–97. [PMC free article] [PubMed] [Google Scholar]
- 8.Giesen PL, Rauch U, Bohrmann B, Kling D, Roque M, Fallon JT, Badimon JJ, Himber J, Riederer MA, Nemerson Y. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci U S A. 1999;96:2311–5. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8:1175–81. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
- 10.Chou J, Mackman N, Merrill-Skoloff G, Pedersen B, Furie BC, Furie B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood. 2004;104:3190–7. doi: 10.1182/blood-2004-03-0935. [DOI] [PubMed] [Google Scholar]
- 11.Darbousset R, Thomas GM, Mezouar S, Frere C, Bonier R, Mackman N, Renne T, Dignat-George F, Dubois C, Panicot-Dubois L. Tissue factor-positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood. 2012;120:2133–43. doi: 10.1182/blood-2012-06-437772. [DOI] [PubMed] [Google Scholar]
- 12.Darbousset R, Mezouar S, Dignat-George F, Panicot-Dubois L, Dubois C. Involvement of neutrophils in thrombus formation in living mice. Pathol Biol (Paris) 2014;62:1–9. doi: 10.1016/j.patbio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Osterud B. Tissue factor/TFPI and blood cells. Thromb Res. 2012;129:274–8. doi: 10.1016/j.thromres.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 14.Atkinson BT, Jasuja R, Chen VM, Nandivada P, Furie B, Furie BC. Laser-induced endothelial cell activation supports fibrin formation. Blood. 2010;116:4675–83. doi: 10.1182/blood-2010-05-283986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood. 2005;105:2764–70. doi: 10.1182/blood-2004-09-3567. [DOI] [PubMed] [Google Scholar]
- 16.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118:1123–31. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–22. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gailani D, Renne T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:2507–13. doi: 10.1161/ATVBAHA.107.155952. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Cheng Q, Xu L, Feuerstein GZ, Hsu MY, Smith PL, Seiffert DA, Schumacher WA, Ogletree ML, Gailani D. Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. doi: 10.1111/j.1538-7836.2005.01236.x. [DOI] [PubMed] [Google Scholar]
- 20.Renne T, Pozgajova M, Gruner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–81. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann KG, Nesheim ME, Church WR, Haley P, Krishnaswamy S. Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood. 1990;76:1–16. [PubMed] [Google Scholar]
- 22.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–32. [PubMed] [Google Scholar]
- 23.Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol. 2002;22:1381–9. doi: 10.1161/01.atv.0000031340.68494.34. [DOI] [PubMed] [Google Scholar]
- 24.Heemskerk JW, Mattheij NJ, Cosemans JM. Platelet-based coagulation: different populations, different functions. J Thromb Haemost. 2013;11:2–16. doi: 10.1111/jth.12045. [DOI] [PubMed] [Google Scholar]
- 25.Vandendries ER, Hamilton JR, Coughlin SR, Furie B, Furie BC. Par4 is required for platelet thrombus propagation but not fibrin generation in a mouse model of thrombosis. Proc Natl Acad Sci U S A. 2007;104:288–92. doi: 10.1073/pnas.0610188104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooley BC. Platelet integrin beta-3 deletion or pharmacologic blockade augments fibrin in large-vein thrombosis. J Thromb Haemost. 2011;9:618. [Google Scholar]
- 27.Bouchard BA, Tracy PB. Platelets, leukocytes, and coagulation. Curr Opin Hematol. 2001;8:263–9. doi: 10.1097/00062752-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Whelihan MF, Mann KG. The role of the red cell membrane in thrombin generation. Thromb Res. 2013;131:377–82. doi: 10.1016/j.thromres.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Esmon CT, Esmon NL. The Link Between Vascular Features and Thrombosis. Annu Rev Physiol. 2011;73:503–14. doi: 10.1146/annurev-physiol-012110-142300. [DOI] [PubMed] [Google Scholar]
- 30.Hackeng TM, van’t Veer C, Meijers JC, Bouma BN. Human protein S inhibits prothrombinase complex activity on endothelial cells and platelets via direct interactions with factors Va and Xa. J Biol Chem. 1994;269:21051–8. [PubMed] [Google Scholar]
- 31.Kroh HK, Panizzi P, Tchaikovski S, Baird TR, Wei N, Krishnaswamy S, Tans G, Rosing J, Furie B, Furie BC, Bock PE. Active site-labeled prothrombin inhibits prothrombinase in vitro and thrombosis in vivo. J Biol Chem. 2011;286:23345–56. doi: 10.1074/jbc.M111.230292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivanciu L, Krishnaswamy S, Camire RM. New insights into the spatiotemporal localization of prothrombinase in vivo. Blood. 2014;124:1705–14. doi: 10.1182/blood-2014-03-565010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guy RD, Fogelson AL, Keener JP. Fibrin gel formation in a shear flow. Math Med Biol. 2007;24:111–30. doi: 10.1093/imammb/dql022. [DOI] [PubMed] [Google Scholar]
- 34.Neeves KB, Illing DA, Diamond SL. Thrombin flux and wall shear rate regulate fibrin fiber deposition state during polymerization under flow. Biophys J. 2010;98:1344–52. doi: 10.1016/j.bpj.2009.12.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toso R, Camire RM. Removal of B-domain sequences from factor V rather than specific proteolysis underlies the mechanism by which cofactor function is realized. J Biol Chem. 2004;279:21643–50. doi: 10.1074/jbc.M402107200. [DOI] [PubMed] [Google Scholar]
- 36.Ivanciu L, Krishnaswamy S, Camire RM. Imaging coagulation reactions in vivo. Thromb Res. 2012;129(Suppl 2):S54–6. doi: 10.1016/j.thromres.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlachterman A, Schuettrumpf J, Liu JH, Furlan Freguia C, Toso R, Poncz M, Camire RM, Arruda VR. Factor V Leiden improves in vivo hemostasis in murine hemophilia models. J Thromb Haemost. 2005;3:2730–7. doi: 10.1111/j.1538-7836.2005.01639.x. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi T, Mogami H, Murakami Y, Nakamura T, Kanayama N, Konno H, Urano T. Real-time analysis of platelet aggregation and procoagulant activity during thrombus formation in vivo. Pflugers Arch. 2008;456:1239–51. doi: 10.1007/s00424-008-0466-9. [DOI] [PubMed] [Google Scholar]
- 39.Stalker TJ, Traxler EA, Wu J, Wannemacher KM, Cermignano SL, Voronov R, Diamond SL, Brass LF. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121:1875–85. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haynes LM, Bouchard BA, Tracy PB, Mann KG. Prothrombin activation by platelet-associated prothrombinase proceeds through the prethrombin-2 pathway via a concerted mechanism. J Biol Chem. 2012;287:38647–55. doi: 10.1074/jbc.M112.407791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berny MA, Munnix ICA, Auger JM, Schols SEM, Cosemans JMEM, Panizzi P, Bock PE, Watson SP, McCarty OJT, Heemskerk JWM. Spatial Distribution of Factor Xa, Thrombin, and Fibrin(ogen) on Thrombi at Venous Shear. Plos One. 2010;5:e10415. doi: 10.1371/journal.pone.0010415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubois C, Panicot-Dubois L, Gainor JF, Furie BC, Furie B. Thrombin-initiated platelet activation in vivo is vWF independent during thrombus formation in a laser injury model. J Clin Invest. 2007;117:953–60. doi: 10.1172/JCI30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gross PL, Furie BC, Merrill-Skoloff G, Chou J, Furie B. Leukocyte-versus microparticle-mediated tissue factor transfer during arteriolar thrombus development. J Leukoc Biol. 2005;78:1318–26. doi: 10.1189/jlb.0405193. [DOI] [PubMed] [Google Scholar]
- 44.van Gestel MA, Heemskerk JW, Slaaf DW, Heijnen VV, Sage SO, Reneman RS, oude Egbrink MG. Real-time detection of activation patterns in individual platelets during thromboembolism in vivo: differences between thrombus growth and embolus formation. J Vasc Res. 2002;39:534–43. doi: 10.1159/000067208. [DOI] [PubMed] [Google Scholar]
- 45.Maxwell MJ, Westein E, Nesbitt WS, Giuliano S, Dopheide SM, Jackson SP. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood. 2007;109:566–76. doi: 10.1182/blood-2006-07-028282. [DOI] [PubMed] [Google Scholar]
- 46.Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, Carberry J, Fouras A, Jackson SP. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15:665–73. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 47.Nishimura S, Manabe I, Nagasaki M, Kakuta S, Iwakura Y, Takayama N, Ooehara J, Otsu M, Kamiya A, Petrich BG, Urano T, Kadono T, Sato S, Aiba A, Yamashita H, Sugiura S, Kadowaki T, Nakauchi H, Eto K, Nagai R. In vivo imaging visualizes discoid platelet aggregations without endothelium disruption and implicates contribution of inflammatory cytokine and integrin signaling. Blood. 2012;119:e45–56. doi: 10.1182/blood-2011-09-381400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jorgensen L, Rowsell HC, Hovig T, Mustard JF. Resolution and organization of platelet-rich mural thrombi in carotid arteries of swine. Am J Pathol. 1967;51:681–719. [PMC free article] [PubMed] [Google Scholar]
- 49.Stehbens WE, Biscoe TJ. The ultrastructure of early platelet aggregation in vivo. Am J Pathol. 1967;50:219–43. [PMC free article] [PubMed] [Google Scholar]
- 50.Hechler B, Nonne C, Eckly A, Magnenat S, Rinckel JY, Denis CV, Freund M, Cazenave JP, Lanza F, Gachet C. Arterial thrombosis: relevance of a model with two levels of severity assessed by histologic, ultrastructural and functional characterization. J Thromb Haemost. 2010;8:173–84. doi: 10.1111/j.1538-7836.2009.03666.x. [DOI] [PubMed] [Google Scholar]
- 51.Muthard RW, Diamond SL. Side view thrombosis microfluidic device with controllable wall shear rate and transthrombus pressure gradient. Lab Chip. 2013;13:1883–91. doi: 10.1039/c3lc41332b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamocka MM, Mu J, Liu X, Chen N, Zollman A, Sturonas-Brown B, Dunn K, Xu Z, Chen DZ, Alber MS, Rosen ED. Two-photon intravital imaging of thrombus development. J Biomed Opt. 2010;15:016020. doi: 10.1117/1.3322676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welsh JD, Colace TV, Muthard RW, Stalker TJ, Brass LF, Diamond SL. Platelet-targeting sensor reveals thrombin gradients within blood clots forming in microfluidic assays and in mouse. J Thromb Haemost. 2012;10:2344–53. doi: 10.1111/j.1538-7836.2012.04928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andre P, Delaney SM, LaRocca T, Vincent D, DeGuzman F, Jurek M, Koller B, Phillips DR, Conley PB. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest. 2003;112:398–406. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJ, Jr, Wiekowski MT, Abbondanzo SJ, Cook DN, Bayne ML, Lira SA, Chintala MS. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–8. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gachet C. The platelet P2 receptors as molecular targets for old and new antiplatelet drugs. Pharmacol Ther. 2005;108:180–92. doi: 10.1016/j.pharmthera.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 57.Stolla M, Stefanini L, Roden RC, Chavez M, Hirsch J, Greene T, Ouellette TD, Maloney SF, Diamond SL, Poncz M, Woulfe DS, Bergmeier W. The kinetics of alphaIIbbeta3 activation determines the size and stability of thrombi in mice: implications for antiplatelet therapy. Blood. 2011;117:1005–13. doi: 10.1182/blood-2010-07-297713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Signarvic RS, Cierniewska A, Stalker TJ, Fong KP, Chatterjee MS, Hess PR, Ma P, Diamond SL, Neubig RR, Brass LF. RGS/Gi2alpha interactions modulate platelet accumulation and thrombus formation at sites of vascular injury. Blood. 2010;116:6092–100. doi: 10.1182/blood-2010-05-283846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meng R, Wu J, Harper DC, Wang Y, Kowalska MA, Abrams CS, Brass LF, Poncz M, Stalker TJ, Marks MS. Defective release of alpha granule and lysosome contents from platelets in mouse Hermansky-Pudlak syndrome models. Blood. 2015;125:1623–32. doi: 10.1182/blood-2014-07-586727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharda A, Kim SH, Jasuja R, Gopal S, Flaumenhaft R, Furie BC, Furie B. Defective PDI release from platelets and endothelial cells impairs thrombus formation in Hermansky-Pudlak syndrome. Blood. 2015;125:1633–42. doi: 10.1182/blood-2014-08-597419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas DW, Mannon RB, Mannon PJ, Latour A, Oliver JA, Hoffman M, Smithies O, Koller BH, Coffman TM. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J Clin Invest. 1998;102:1994–2001. doi: 10.1172/JCI5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lauer MS. Clinical practice. Aspirin for primary prevention of coronary events. N Engl J Med. 2002;346:1468–74. doi: 10.1056/NEJMcp012672. [DOI] [PubMed] [Google Scholar]
- 63.Bender M, Hagedorn I, Nieswandt B. Genetic and antibody-induced glycoprotein VI deficiency equally protects mice from mechanically and FeCl(3) -induced thrombosis. J Thromb Haemost. 2011;9:1423–6. doi: 10.1111/j.1538-7836.2011.04328.x. [DOI] [PubMed] [Google Scholar]
- 64.Dubois C, Panicot-Dubois L, Merrill-Skoloff G, Furie B, Furie BC. Glycoprotein VI-dependent and -independent pathways of thrombus formation in vivo. Blood. 2006;107:3902–6. doi: 10.1182/blood-2005-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mangin P, Yap CL, Nonne C, Sturgeon SA, Goncalves I, Yuan Y, Schoenwaelder SM, Wright CE, Lanza F, Jackson SP. Thrombin overcomes the thrombosis defect associated with platelet GPVI/FcRgamma deficiency. Blood. 2006;107:4346–53. doi: 10.1182/blood-2005-10-4244. [DOI] [PubMed] [Google Scholar]
- 66.Massberg S, Gawaz M, Gruner S, Schulte V, Konrad I, Zohlnhofer D, Heinzmann U, Nieswandt B. A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo. J Exp Med. 2003;197:41–9. doi: 10.1084/jem.20020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bender M, May F, Lorenz V, Thielmann I, Hagedorn I, Finney BA, Vogtle T, Remer K, Braun A, Bosl M, Watson SP, Nieswandt B. Combined in vivo depletion of glycoprotein VI and C-type lectin-like receptor 2 severely compromises hemostasis and abrogates arterial thrombosis in mice. Arterioscler Thromb Vasc Biol. 2013;33:926–34. doi: 10.1161/ATVBAHA.112.300672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bynagari-Settipalli YS, Cornelissen I, Palmer D, Duong D, Concengco C, Ware J, Coughlin SR. Redundancy and interaction of thrombin- and collagen-mediated platelet activation in tail bleeding and carotid thrombosis in mice. Arterioscler Thromb Vasc Biol. 2014;34:2563–9. doi: 10.1161/ATVBAHA.114.304244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nonne C, Lenain N, Hechler B, Mangin P, Cazenave JP, Gachet C, Lanza F. Importance of platelet phospholipase Cgamma2 signaling in arterial thrombosis as a function of lesion severity. Arterioscler Thromb Vasc Biol. 2005;25:1293–8. doi: 10.1161/01.ATV.0000163184.02484.69. [DOI] [PubMed] [Google Scholar]
- 70.Mazzucato M, Pradella P, Cozzi MR, De Marco L, Ruggeri ZM. Sequential cytoplasmic calcium signals in a 2-stage platelet activation process induced by the glycoprotein Ibalpha mechanoreceptor. Blood. 2002;100:2793–800. doi: 10.1182/blood-2002-02-0514. [DOI] [PubMed] [Google Scholar]
- 71.Nesbitt WS, Kulkarni S, Giuliano S, Goncalves I, Dopheide SM, Yap CL, Harper IS, Salem HH, Jackson SP. Distinct glycoprotein Ib/V/IX and integrin alpha IIbbeta 3-dependent calcium signals cooperatively regulate platelet adhesion under flow. J Biol Chem. 2002;277:2965–72. doi: 10.1074/jbc.M110070200. [DOI] [PubMed] [Google Scholar]
- 72.Jain S, Zuka M, Liu J, Russell S, Dent J, Guerrero JA, Forsyth J, Maruszak B, Gartner TK, Felding-Habermann B, Ware J. Platelet glycoprotein Ib alpha supports experimental lung metastasis. Proc Natl Acad Sci U S A. 2007;104:9024–8. doi: 10.1073/pnas.0700625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tomaiuolo M, Stalker TJ, Welsh JD, Diamond SL, Sinno T, Brass LF. A systems approach to hemostasis: 2. Computational analysis of molecular transport in the thrombus microenvironment. Blood. 2014;124:1816–23. doi: 10.1182/blood-2014-01-550343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Voronov RS, Stalker TJ, Brass LF, Diamond SL. Simulation of intrathrombus fluid and solute transport using in vivo clot structures with single platelet resolution. Ann Biomed Eng. 2013;41:1297–307. doi: 10.1007/s10439-013-0764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim OV, Xu Z, Rosen ED, Alber MS. Fibrin networks regulate protein transport during thrombus development. PLoS Comput Biol. 2013;9:e1003095. doi: 10.1371/journal.pcbi.1003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hathcock JJ, Nemerson Y. Platelet deposition inhibits tissue factor activity: in vitro clots are impermeable to factor Xa. Blood. 2004;104:123–7. doi: 10.1182/blood-2003-12-4352. [DOI] [PubMed] [Google Scholar]
- 77.Leiderman K, Fogelson AL. The influence of hindered transport on the development of platelet thrombi under flow. Bull Math Biol. 2013;75:1255–83. doi: 10.1007/s11538-012-9784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Welsh JD, Stalker TJ, Voronov R, Muthard RW, Tomaiuolo M, Diamond SL, Brass LF. A systems approach to hemostasis: 1. The interdependence of thrombus architecture and agonist movements in the gaps between platelets. Blood. 2014;124:1808–15. doi: 10.1182/blood-2014-01-550335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stalker TJ, Welsh JD, Tomaiuolo M, Wu J, Colace TV, Diamond SL, Brass LF. A systems approach to hemostasis: 3. Thrombus consolidation regulates intrathrombus solute transport and local thrombin activity. Blood. 2014;124:1824–31. doi: 10.1182/blood-2014-01-550319. [DOI] [PMC free article] [PubMed] [Google Scholar]